Abstract
Targeting the ubiquitin-proteasome pathway has emerged as a rational approach in the treatment of human cancer. Based on positive preclinical and clinical studies, bortezomib was subsequently approved for the clinical use as a front-line treatment for newly diagnosed multiple myeloma patients and for the treatment of relapsed/refractory multiple myeloma and mantle cell lymphoma, for which this drug has become the staple of treatment. The approval of bortezomib by the US Food and Drug Administration (FDA) represented a significant milestone as the first proteasome inhibitor to be implemented in the treatment of malignant disease. Bortezomib has shown a positive clinical benefit either alone or as a part of combination therapy to induce chemo-/radio-sensitization or overcome drug resistance. One of the major mechanisms of bortezomib associated with its anticancer activity is through upregulation of NOXA, which is a proapoptotic protein, and NOXA may interact with the anti-apoptotic proteins of Bcl-2 subfamily Bcl-XL and Bcl-2, and result in apoptotic cell death in malignant cells. Another important mechanism of bortezomib is through suppression of the NF-κB signaling pathway resulting in the down-regulation of its anti-apoptotic target genes. Although the majority of success achieved with bortezomib has been in hematological malignancies, its effect toward solid tumors has been less than encouraging. Additionally, the widespread clinical use of bortezomib continues to be hampered by the appearance of dose-limiting toxicities, drug-resistance and interference by some natural compounds. These findings could help guide physicians in refining the clinical use of bortezomib, and encourage basic scientists to generate next generation proteasome inhibitors that broaden the spectrum of efficacy and produce a more durable clinical response in cancer patients. Other desirable applications for the use of proteasome inhibitors include the development of inhibitors against specific E3 ligases, which act at an early step in the ubiquitin-proteasome pathway, and the discovery of less toxic and novel proteasome inhibitors from natural products and traditional medicines, which may provide more viable drug candidates for cancer chemoprevention and the treatment of cancer patients in the future.
Keywords: The ubiquitin-proteasome pathway, proteasome inhibitors, bortezomib, chemotherapy, multiple myeloma
INTRODUCTION
The ubiquitin-proteasome pathway plays an essential role in regulating homeostatic and various cellular events including those involved in tumorigenesis [1–4]. Since aberrant proteasome-dependent proteolysis appears to be associated with the pathophysiology of some malignancies, it was suggested that pharmacological inhibition of proteasome function may prove useful as a novel class of anticancer drugs. Thus targeting key features of protein function responsible for the growth and progression of cancer has been the subject of intense investigation by many groups for drug discovery purposes. Proteasome inhibition leads to the accumulation of pro-apoptotic proteins in tumorigenic cells but not normal tissue [5–6].
Bortezomib (Velcade, Millennium Pharmaceuticals, Inc., Cambridge, MA, and Johnson & Johnson Pharmaceutical Research & Development, L.L.C., Raritan, NJ) is the first proteasome inhibitor approved by the US FDA for the treatment of newly diagnosed multiple myeloma and relapsed/refractory multiple myeloma and mantle cell lymphoma [7–8]. Although the mechanisms of its anticancer activity by proteasome inhibition are not fully elucidated, it is clear that multiple mechanisms are involved. Proteasome inhibition could promote degradation of anti-apoptotic proteins and prevent degradation of pro-apoptotic proteins, resulting in programmed cell death in malignant cells.
The possibility of targeting the proteasome was initially doubted due to the essential role the ubiquitin-proteasome pathway plays in critical biological processes. However, preclinical results showed proteasome inhibitors to be well tolerated with activity against in vivo models bearing human malignancies [9]. These outstanding results paved the way for the introduction of bortezomib into phase I clinical trials to test for safety based on different dose schedules [10]. The data from these trials showed an acceptable level of toxicity coupled with significant clinical benefit toward hematological malignancies [11]. The FDA approval of bortezomib for the treatment of multiple myeloma provided “proof of concept” that targeting the ubiquitin-proteasome pathway is a viable route for the treatment of human cancer [6, 12]. Bortezomib has emerged as not only important in the treatment of multiple myeloma, but is also being investigated in the treatment of other hematological malignancies and solid tumors as a single agent or as a part of combination therapy [5, 13–14]. However, some toxic side effects associated with bortezomib treatment were observed. Furthermore, bortezomib alone showed minimal effects for the treatment of solid tumors and bortezomib combination could not improve patients’ responses to currently used chemotherapy or radiation in solid tumors [6, 12]. Bortezomib resistance in solid tumors further encouraged researchers to develop novel proteasome inhibitors, which act differently from bortezomib, as well as novel natural compounds with proteasome-inhibitory activity as chemo-/radio-sensitizers. Recently, marizomib (NPI-0052) [15–17] and carfilzomib [18–19], the two representatives of a new generation of proteasome inhibitors have been developed, and are currently being evaluated in clinical trials. In this review we attempted to summarize the discovery and development of bortezomib and the impact it has made in cancer therapy, in addition to highlighting persistent problems encountered with using this proteasome inhibitor drug in the clinical setting.
STRUCTURAL AND FUNCTIONAL CHARACTERISTICS OF THE UBIQUITIN-PROTEASOME PATHWAY
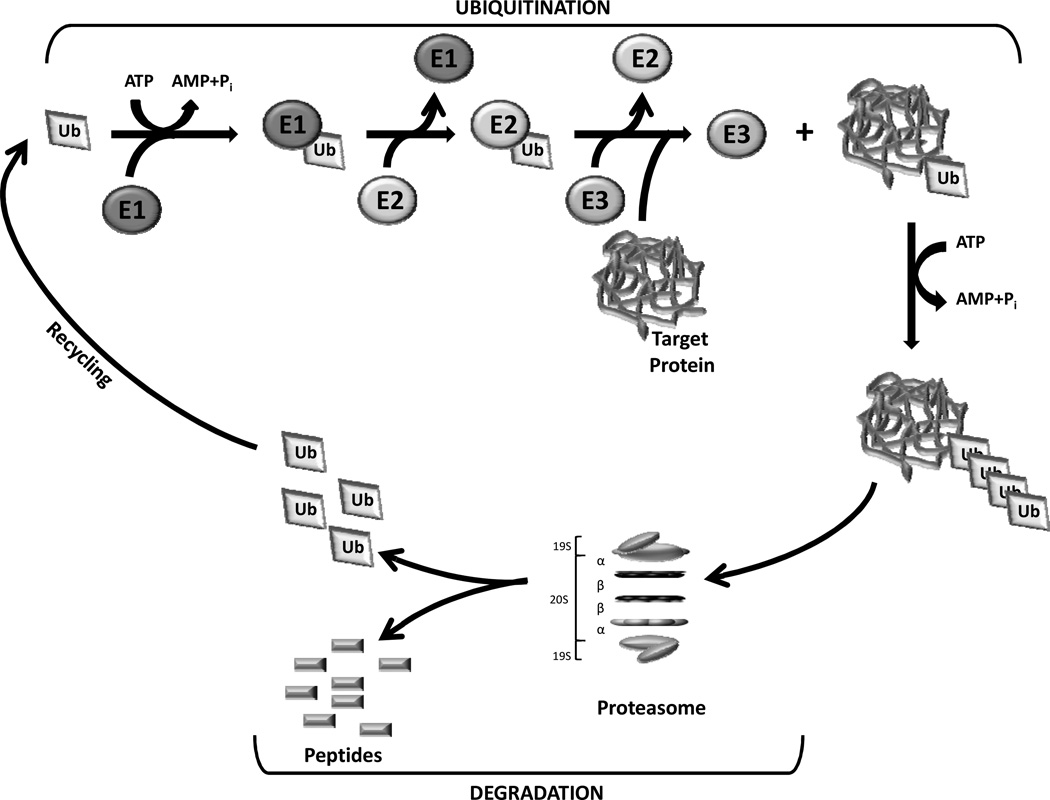
The ubiquitin-proteasome pathway is responsible for the degradation of misfolded and mutated proteins as well as many proteins involved in the regulation of development, differentiation, cell proliferation, signal transduction, and apoptosis [2–3, 20]. By governing these important functions, the ubiquitin-proteasome pathway plays a vital role in regulating protein stability and thus normal cellular function. The majority of intracellular protein degradation is facilitated through the ubiquitin-proteasome pathway, which is the predominant means of proteolysis in eukaryotic cells [21–22]. The ubiquitin-proteasome pathway involves two distinct, critical steps: covalent attachment of multiple ubiquitin molecules to a protein substrate, and degradation of the tagged substrate protein by the 26S proteasome [23] (Fig. 1).
Fig. (1).
The ubiquitin-proteasome pathway. The ubiquitination of target proteins is mediated by Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes. Substrate proteins tagged with a multiple-ubiquitin chain are then degraded by the 26S proteasome which is composed of a 20S catalytic core and two 19S subunits.
The 26S proteasome is a multisubunit protease (2.5 MDa) that is localized in the nucleus and cytosol of eukaryotic cells and selectively degrades unnecessary intracellular proteins. The 26S proteasome contains the 20S proteasome that serves as the catalytic core and two 19S regulatory subunits, which act as a recognition and entry site for proteins destined for proteolysis [1, 24]. The 20S core resembles a barrel-like structure with a central cavity consisting of 4 heptameric rings composed of a total of 28 subunits [25–27]. These stacked rings include two identical non-catalytic α rings (14 α subunits) outside of two identical catalytic β rings (14 β subunits), and together form a cylindrical structure (Fig. 1). The α subunits guard the entrance to the active site of the complex by allowing access to only unfolded proteins. The proteolytic activities are confined to the β subunits that are responsible for mediating the enzymatic activity of the proteasome complex [27]. These unique features are best represented by the β1, β2, and β5 subunits which are responsible for the caspase- or peptidyl-glutamyl peptide-hydrolyzing-like (PGPH) (cleavage after acidic residues), trypsin-like (cleavage after basic residues), and chymotrypsin-like (cleavage after hydrophobic residues) proteolytic activities of the 20S proteasome, respectively [27–28]. In all three β-subunit, Thr1 at the amino terminal is responsible for catalysis, which is achievable through nucleophilic attack [26, 29].
In addition, the 19S proteasome (700 kDa) serves as the regulatory subunits of the 26S proteasome and consists of six ATPase and at least eight non-ATPase subunits which are required for recognition, deubiquitination, unfolding and translocation of marked proteins before granting access to the 20S core [30–31].
Prior to degradation, proteins are first recognized and tagged with multiple ubiquitin molecules. Ubiquitin is a highly conserved 76 amino acid protein that serves as a tag for substrate proteins that are destined for degradation through the proteasome. The ubiquitin (Ub) system is characterized by three distinct enzymes, Ub-activating (E1), Ubconjugating (E2), and Ub-ligating (E3) which links to protein substrates through covalent modification in a multistep process [32] (Fig. 1). E1 activates ubiquitin in an ATP-requiring step by forming a high energy thiol-ester bond at its C-terminus. Activated ubiquitin is then transferred from E1 to one of several distinct E2 enzymes through an additional thiol-ester intermediate. From the E2- to the E3-bound substrate, the activated ubiquitin can then be transferred directly or via a third high energy thiol-ester intermediate [22, 33]. Therefore, the target proteins tagged with polyubiquitins will be recognized by the 19S proteasome, transferred into the 20S proteasome core and digested into peptides (Fig. 1). It has been well recognized that the ubiquitin conjugating system plays a critical role in the regulation of protein turnover by controlling the precise degradation of intracellular proteins. However, it has also been found that several proteasome target proteins, including ornithine decarboxylase (ODC), p21, IκB-β, retinoblastoma protein (pRB) and hypoxia-inducible factor, may be degraded by the proteasome without the requirement of ubiquitin [34–35].
The proteasome is an important cellular contributor to many pathological disorders including cancer, in which some regulatory proteins are either stabilized or degraded [23]. Many important proteasome target proteins have also been identified as important mediators in tumorigenesis, including, but not limited to, cyclins [36–38], tumor suppressor protein p53 [39], pRB [40], pro-apoptotic protein Bax [41], cyclin-dependent kinase inhibitor (CKI) p27 [42], and the NF-κB inhibitor, IκB-α [43].
Accumulating evidence highlights the potential use of targeting the proteasome as a favorable target in cancer therapy. This revelation was supported by experimental results demonstrating that increased proteasome activity is associated with malignant disease, including those of the colon [44], prostate [41], and leukemia [45]. Empirical findings indicate that many types of actively proliferating tumor cells are more sensitive to proteasome inhibitors than benign or pre-malignant cells [46–47]. Various studies have demonstrated that pharmacological inhibition of the proteasome results in increased levels of pro-apoptotic proteins, coupled with decreased levels of anti-apoptotic proteins, leading to cell cycle arrest and apoptosis [41, 48–49]. Importantly, it has been shown that proteasome inhibitors exhibit more effective apoptosis-inducing capability compared to standard cytotoxic agents when tested in various human tumor cells [50–51]. It has even been shown that proteasome inhibition is an effective strategy for chemo-/radio-sensitization and overcoming resistance by selectively targeting cancer cells with minimal collateral damage to normal tissue [21]. Thus, these observations provide compelling evidence that targeting the ubiquitin-proteasome pathway with bortezomib and other pharmacological inhibitors represents a potent arsenal in the treatment of human malignancy.
PROPERTIES, PHARMACOKINETICS, BIOLOGICAL EFFECTS AND POSIBLE MECHANISMS OF BORTEZOMIB
Bortezomib is a dipeptide boronic acid derivative which contains pyrazinoic acid, phenylalanine and leucine with boronic acid in its structure (Fig. 2). Bortezomib has demonstrated considerable apoptotic inducing activity in a range of tumor cell lines and animal models [10, 52–53]. Originally, bortezomib was synthesized in 1995 by Myogenics Company and termed MG-341. After promising results from in vitro and in vivo studies, it was tested in a small Phase I clinical trial on patients with multiple myeloma cancer and then named PS-341. In 1999, Millennium Pharmaceuticals bought this potential anticancer drug from the previous company and performed extensive clinical trials. In 2003, seven years after the initial synthesis, it was finally approved in the USA by the FDA with the name of bortezomib (brand name Velcade®) for the treatment of multiple myeloma patients.
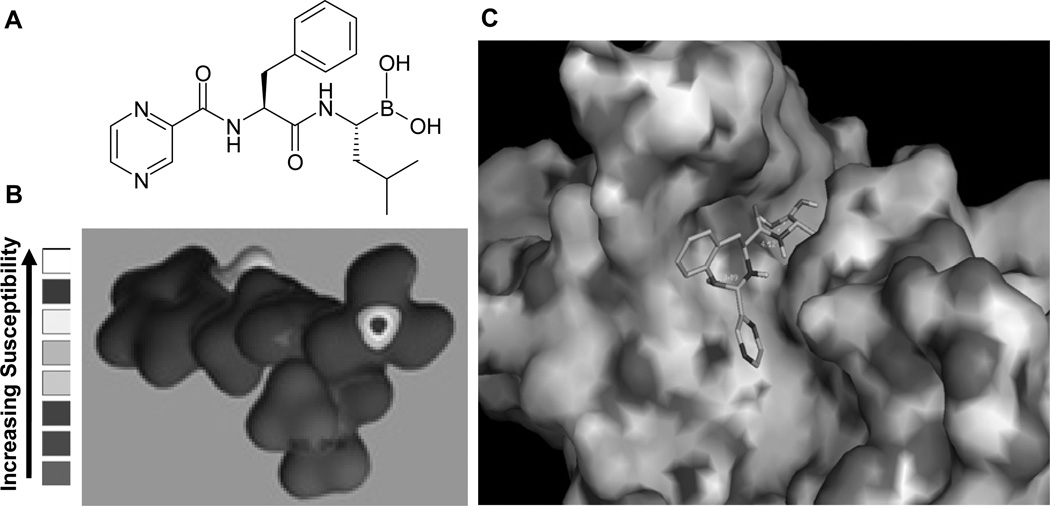
Fig. (2).
Chemical structure, nucleophilic susceptibility of bortezomib and its computational docking in the β5 subunit of the proteasome. A, The chemical structure of bortezomib. B, Nucleophilic susceptibility of bortezomib analyzed using CAChe software. Higher susceptibility was shown mainly at the boron in bortezomib. This highly susceptible atom for nucleophilic attack was shown by a red “bull’s-eye”. C, Bortezomib was docked into the β5 subunit of the proteasome by using AutoDock 3.0 and visualized by using PyMol v 0.99. The resultant image showed that bortezomib fits into and blocks the S1 pocket of β5 subunit of the proteasome.
The molecular formula of bortezomib is C19H25BN4O4 and its chemical IUPAC name is [3-methyl-1-(3-phenyl-2-pyrazin-2-ylcarbonylamino-propanoyl) amino-butyl] boronic acid. The pharmacokinetics of bortezomib were investigated in two phase I trials in cancer patients who received combination therapy of bortezomib and other anticancer agents [14, 54–55]. After intravenous (IV) injection, bortezomib quickly distributes into tissues from the plasma within 10 minutes [54], where its half life is more than 40 hours [54]. Bortezomib is able to distribute into nearly all tissues except adipose and brain tissue [56, 57]. Bortezomib is metabolized primarily through intracellular oxidative deboronation mediated by multiple cytochrome p450 enzymes [7]. Pharmacodynamic studies showed that when patients received bortezomib in doses ranging from 0.13 to 2.0 mg/m2 (mg per square meter of body-surface area), a dose-dependent inhibition of proteasome activity was observed. A peak of ≥75% of proteasome inhibition in whole blood samples was observed within one hour after dosing [14, 58]. According to another Phase I clinical trial, proteasomal activity in blood samples was inhibited 70% in patients with solid tumors or lymphomas who were treated with bortezomib at 0.25 to 1.9 mg/m2 every two weeks [59].
Bortezomib operates as a reversible inhibitor of the 26S proteasome. Usually it becomes undetectable 72 hours after administration and the inhibited proteasome activity recovers [54]. The boronic acid group in bortezomib can bind and form a complex with the active site of threonine hydroxyl group in the β5-subunit and block the chymotrypsin-like activity of the proteasome (Fig. 2), which is responsible for its cell-death inducing capabilities [60–61]. After the major success achieved with bortezomib in hematological malignancies, its effect toward solid tumors was also investigated, giving less than encouraging results [62–63]. Besides being tested as a single agent, the antitumor effects of bortezomib have been evaluated in a combination with other conventional therapeutic drugs or radiation as a means to induce chemo-/radio-sensitization or overcome the resistance in various malignancies [5, 64–69]. Promising results were observed from these experiments (see below sections).
Preclinical data have proved useful in the identification of the involved mechanisms of bortezomib. One of the possible mechanisms of bortezomib associated with its anticancer activity is suppression of the NF-κB signaling pathway resulting in the down-regulation of its anti-apoptotic target genes [1] (Fig. 3). NF-κB is a p50/p65 heterodimeric transcription factor and in the majority of cells NF-κB exists in an inactive form in the cytoplasm bound to the inhibitory protein IκB. Activation of NF-κB is initiated by degradation of IκB proteins (Fig. 3). This occurs primarily via activation of IκB kinase (IKK) and activated IKK phosphorylates IκB. The phosphorylation of IκB leads to IκB ubiquitination and degradation by the proteasome [70]. With the degradation of IκB, the NF-κB complex then translocates into the nucleus where it can stimulate the expression of specific genes including cytokines (IL-6, TNF-α), survival factors (IAPs, Bcl-XL), and insulin-like growth factor-I (IGF-I), resulting in cell proliferation, resistance to induction of apoptosis and drug-resistance in cancer cells [71] (Fig. 3). It has been demonstrated that IL-6 and IGF promote the survival of multiple myeloma cells by blocking apoptosis triggered by the conventional anticancer agent Dexamethasone [71]. The drug-sensitive multiple myeloma cells show lower NF-κB activity than drug-resistant multiple myeloma cells [72]. The multiple myeloma cells derived from relapsed patients have elevated NF-κB levels [73]. All of these findings indicate that NF-κB is a key regulator of growth and survival of multiple myeloma cells. Importantly, treatment of multiple myeloma with bortezomib can prevent degradation of IκB, and in turn block not only NF-κB activation but also suppress related cytokine and survival factor productions in multiple myeloma cells under some experimental conditions.
Fig. (3).
Inactivation of NF-κB pathway by bortezomib. In response to external stress, the IκB becomes phosphorylated by IKK and degraded by the proteasome. With the degradation of IκB, the NF-κB complex then translocates into the nucleus and promotes transcription of a series of pro-survival genes. Proteasome inhibition prevents the activation of NF-κB and increases the susceptibility of malignant cells to chemotherapeutic drugs.
However increasingly more studies have shown that NF-κB may not be a key mechanism of bortezomib’s anticancer activity. Hideshima et al. reported that treatment with bortezomib could not inhibit NF-κB p65 nuclear translocation in a murine xenograft model bearing human MM cells, but instead was associated with NF-κB activation [74]. They found that bortezomib significantly down-regulated IκB-α expression and triggered NF-κB activation in MM cell lines and primary tumor cells from MM patients. The involved mechanism was possibly the result of bortezomib-mediated phosphorylation of IKK-β and its upstream receptor-interacting protein 2. This hypothesis was confirmed by the finding that IKK-β inhibitor MLN120B could block bortezomib-induced IkB-α down-regulation and NF-κB activation. This finding indicates that receptor-interacting protein 2/ IKK-β signaling could play a critical role in bortezomib-induced NF-κB activation [74].
It has also been reported that NOXA appears to be another key mechanism of bortezomib associated with its anti-cancer activity [75]. NOXA (Latin for damage) is a pro-apoptotic member of the Bcl-2 protein family [76]. NOXA has been shown to be involved in p53-mediated apoptosis and expression of the Noxa gene is associated with direct activation of its promoter by tumor suppressor p53 [76]. The involved apoptotic pathway mediated by chemotherapeutic drugs or x-ray irradiation is through upregulation of p53 expression and subsequent Noxa gene expression. Upregulation of NOXA is known to induce apoptosis by selectively interacting with the anti-apoptotic proteins of the Bcl-2 subfamily Bcl-XL and Bcl-2 or by stimulating other apoptosis-promoting factors [76–78]. Qin et al. found that bortezomib induced-apoptotic cell death in myeloma and melanoma cell lines was associated with p53-independent induction of the BH3-only protein NOXA [75]. Blocking NOXA induction using an antisense oligonucleotide could reduce the apoptotic response by 30% to 50% [75].
Nikiforov et al. identified that the oncogene c-MYC was a direct modulator of NOXA mRNA, but not p53, HIF-1α, or E2F-1 [79]. The human NOXA promoter contains a p53 binding site. However NOXA can be induced by bortezomib in a variety of tumor cell lines with defective p53 signaling [79], and a variety of clinical studies indicate that bortezomib can block tumor growth in a p53-independent manner [80–81]. Although p53 is not strictly required for transcription of NOXA mRNA, it can still contribute as a cofactor to the accumulation of NOXA protein in bortezomib-treated cells. There are MYC-binding sites identified at the NOXA promoter [79]. NOXA protein is a proapoptotic factor induced by bortezomib preferentially in cancer cells but not in normal cells [75, 82]. Bortezomib could selectively promote a 20- to 60-fold induction of NOXA in a variety of melanoma cells, whereas levels in normal melanocytes remained unchanged [75, 82–83]. Proteasome inhibitors have the property of promoting a dramatic induction of the proapoptotic protein NOXA in a tumor cell-restricted manner and the induction of NOXA by bortezomib is directly dependent on the oncogene c-MYC. Depletion of c-MYC by RNA interference blocks the tumor cell-selective induction of NOXA by bortezomib [79].
PRECLINICAL STUDIES OF BORTEZOMIB
Preclinical studies demonstrate that bortezomib is very potent against a broad range of cancer cell lines in vitro and in various animal xenograft models. Bortezomib was found to potently inhibit cell proliferation in a standard National Cancer Institute screen of 60 cell lines derived from human tumors [10]. Bortezomib-induced cell growth inhibition and apoptosis induction were observed in vitro in many different kinds of malignant cells including multiple myeloma, prostate cancer, pancreatic cancer, renal cell carcinoma, and squamous cell carcinoma [84–89]. Hideshima et al. reported that bortezomib could potently inhibit cell proliferation in different multiple myeloma cell lines with IC50 values of 3–20 nanomolar (nM) [53]. Moreover, through in vitro testing of bortezomib in doxorubicin (Dox)-, mitoxantrone (Mit)-, and melphalan (Mel)-sensitive and -resistant RPMI-8226 human multiple myeloma cells, the IC50 values of bortezomib were 40, 20, 20 and 30 nM, respectively [53]. These results indicate that bortezomib possesses potent growth inhibitory effects on both drug-sensitive and -resistant human multiple myeloma cells [53].
Bortezomib showed its antitumor and sensitization activities against chemoresistant and chemosensitive myeloma cells through blocking NF-κB activity [72]. The sensitivity of chemoresistant myeloma cells to chemotherapeutic agents was markedly increased (100,000–1,000,000-fold) when combined with a noncytotoxic dose of bortezomib without affecting normal hematopoietic cells [72]. At nontoxic doses, bortezomib could also sensitize the chemoresistant multiple myeloma cell lines to the conventional therapeutic drugs melphalan, doxorubicin, and mitoxantrone, three of which became cytotoxic to chemoresistant cells at concentrations 10,000- to 100,000-fold lower than the dose of regular usage [90].
Results from an in vitro study of four different human ovarian carcinoma cell lines and three prostate carcinoma cell lines treated with bortezomib demonstrate that bortezomib could have equal cell killing effect on cells derived from solid tumors and hematological malignancies [52]. Bortezomib was also shown to significantly inhibit the growth of human multiple myeloma xenografts in mice [91]. The treatment of dexamethasone-resistant multiple myeloma-xenografted mice with bortezomib resulted in significant tumor growth inhibition after as early as five days of treatment. Complete tumor regression was seen in some mice receiving 0.5 or 1.0 mg/kg of bortezomib. Importantly, prolonged median survival (>40%) of mice tumor inhibition was observed as well [91].
Preclinical studies have demonstrated that bortezomib has multiple targets in malignant cells, besides NOXA and NF-κB signaling pathways, including (i) inhibition of angiogenesis in human squamous cell carcinoma, myeloma, and pancreatic tumor xenografts [89, 92]; (ii) disruption of the interaction between tumor cells and dendritic cells since this interaction promotes clonogenic growth of tumors [93]; (iii) activating both caspase-8 and caspase-9 in malignant cells leading to induction of extrinsic and intrinsic apoptotic pathways [94–95]; (iv) induction of endoplasmic reticulum (ER) stress and generation of reactive oxygen species (ROS) [96–97]; and (vi) activation of the p38 mitogen-activated protein kinase (MAPK) pathway [98]. Generally, one or more molecular targets may play major roles in inducing apoptosis by bortezomib in certain kinds of cancer cells while different targets may be critical in other malignant cells. Thus, bortezomib is able to target multiple cancer survival pathways and networks, tipping the balance toward the direction of cell growth inhibition and pro-apoptosis in malignant cells.
BORTEZOMIB IN CLINICAL TRIALS
Phase I Clinical Trials
Following preclinical findings that have demonstrated the correlation of proteasome inhibition by bortezomib with malignant cell death, clinical trials evaluating bortezomib for the treatment of multiple myeloma patients were initiated. A tolerable and efficacious dose-defining Phase I trial enrolled 27 patients with refractory multiple myeloma. Bortezomib was used as a single agent and the results showed that bortezomib induced a dose-dependent inhibition of 20S proteasome activity from 36%, 60%, 65%, to 74%, after 1 hour treatment of bortezomib at 0.40-, 1.04-, 1.20-, and 1.38-mg/m2 doses, respectively [11]. The clinical result confirmed the preclinical finding that the proteasome activity could be inhibited by bortezomib in a dose- and time-dependent manner. The dose at 1.04 mg/m2 of bortezomib was well tolerated. Regarding the antitumor activity of bortezomib in this clinical trial, twelve patients with plasma cell dyscrasias were treated with bortezomib as a single agent, nine of whom completed at least one full cycle and were assessable for response. One of the patients had complete response and eight patients showed improvement in paraprotein levels and marrow plasmacytosis [11].
In another Phase I clinical trial, bortezomib was investigated in combination with doxorubicin to treat 42 patients with advanced hematologic malignancies. The purpose of this Phase I trial was to obtain preliminary response data, and to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of bortezomib when combined with doxorubicin. The most frequent adverse effects in the patients included fatigue (88%), thrombocytopenia (69%), lymphopenia (64%), nausea (64%), constipation (60%), peripheral neuropathy (55%), and anemia (52%) [67]. The maximum tolerated dose of bortezomib was suggested as 1.30 mg/m2. The results from a clinical trial to test 22 evaluable multiple myeloma patients with the combinational treatment of bortezomib and doxorubicin showed that 8 of 22 patients had a complete response (CR, 36%) or near-CR, and another 8 of 22 had partial responses (PRs, 36%) [67].
Some phase I clinical studies on solid tumors also showed promising data when bortezomib was used either as a single agent or as a part of combination therapy. Bortezomib alone showed anti-tumor activity in patients with advanced androgen-independent prostate cancer [14], and the combination of bortezomib and carboplatin elicited an overall response rate of 47% in recurrent ovarian or primary peritoneal cancer [58].
The clinical trials related to aggressive metastatic breast cancer and neuroendocrine tumor patients demonstrated no significant response rates with bortezomib alone [63, 99–100]. Similarly, combinational treatment of bortezomib with docetaxel [101] or prednisone [102] did not show any significant antitumor effects in patients with hormone refractory prostate cancer and with castration-resistant metastatic prostate cancer [103]. Multiple clinical trials in patients with non-small cell lung carcinoma (NSCLC) were conducted based on preclinical and phase I data.
Phase II Clinical Trials
Based on the results of phase I trials, a large Phase II trial, SUMMIT (Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy), was performed [104]. 202 pretreated patients with relapsed and refractory myeloma were enrolled and treated with bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of a 3-week cycle for up to eight cycles. The overall response rate (complete response + partial response + minimal response) was 35% for bortezomib treatment alone, including 4% patients with complete response (negative by measurement of immunofixation), 6% patients with a near-complete response (positive by measurement of immunofixation) and 18% patients with partial response [104].
Sixty-seven patients with relapsed or refractory myeloma were tested in the CREST (Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma) Phase II trial [105]. The patients were randomly divided into two groups and received 1.0 or 1.3 mg/m2 of bortezomib. The anticancer activity was observed in patients treated with either dose. The results confirmed that the efficacious dose of bortezomib could be reduced to 1.0 mg/m2 for relapsing multiple myeloma patients with attenuating dose-associated toxicity [105].
The combination of bortezomib with other conventional anticancer drugs was tested in two Phase II clinical trials. Oakervee and colleagues treated relapsed multiple myeloma with the combination therapy of bortezomib, doxorubicin and dexamethasone [106]. The results showed that 20 of 21 patients (95%) achieved at least a partial response (PR), including CR in 43%, near CR in 14%, very good PR in 24%, and PR in 14% [106]. In another Phase II trial, Jagannath et al. tested bortezomib as a single agent and in combination with dexamethasone in 32 consecutive patients with untreated symptomatic multiple myeloma [85]. The response rate (CR + PR) was 88%, with CR in 6%, and 19% patients with near CR. All 32 patients completed the first two cycles of bortezomib alone, and 3% patients achieved CR, 9% near CR, and 28% patients with PR. Among 32 patients, 22 patients received combinational therapy with dexamethasone, leading to improved responses in 15 patients [85].
Clinical studies have demonstrated that bortezomib also presents promising treatment effects on patients with mantle cell lymphoma and non-Hodgkin’s lymphoma. The results from a Phase II study of bortezomib in mantle cell lymphoma showed that treatment with bortezomib (1.3 mg/m2 given on days 1, 4, 8 and 11 every 21 days) resulted in 46.2% and 46.7% response rate in mantle cell lymphoma patients with prior chemotherapeutic treatment and no prior treatment, respectively, indicating that bortezomib is pretty effective in treating patients with mantle cell lymphoma [107]. Another phase II clinical trial showed that 58% overall response rate to treatment with bortezomib was observed in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma [108].
A phase II clinical study was conducted to evaluate the efficacy and safety of bortezomib and pemetrexed alone or in combination, in patients with previously treated advanced NSCLC. Results showed no statistically significant response or survival advantage, but when given in combination with pemetrexed as compared to alone, bortezomib was well tolerated [109]. Various studies with bortezomib for treatment of advanced solid tumors have proven disappointing while numerous other clinical trials are currently being conducted to investigate the potential treatment for NSCLC.
Phase III Clinical Trials
A large international Phase III trial was performed to compare efficacy of bortezomib with high-dose dexamethasone in patients with multiple myeloma who had relapsed after 1–3 prior therapies [87]. The 669 patients received either an intravenous administration of 1.3 mg/m2 bortezomib (twice weekly for 2 weeks followed by a 1 week rest), or high-dose dexamethasone (40 mg orally). The results showed that the combined complete and partial response rates were 38% for bortezomib and 18% for dexamethasone (P<0.001). Median times to progression in the bortezomib and dexamethasone groups were 6.22 months and 3.49 months, respectively. The one-year survival rate was 80% among patients taking bortezomib and 66% among patients taking dexamethasone (P=0.003) [87]. In this Phase III trial, bortezomib demonstrated superiority over dexamethasone in terms of response rate, time to progression, and survival [87].
In the Phase III APEX (Assessment of Proteasome Inhibition for Extending Remissions) study of patients with relapsed myeloma, the impact of a dose-modification guideline on peripheral neuropathy severity and reversibility was assessed [110]. After receiving bortezomib 1.3 mg/m2 for eight 21-day cycles and then three 35-d cycles, 37% patients (124/331) had peripheral neuropathy. However dose modification using a specific guideline in this trial could improve peripheral neuropathy management without adversely affecting outcome [110]. Therefore bortezomib-associated peripheral neuropathy is manageable and reversible in most patients with relapsed myeloma.
A Phase IIIb study of 638 patients with relapsed or refractory multiple myeloma (≥2 prior lines of treatment) has been completed. In this study, patients received 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 of a maximum of eight 3-week cycles, with median completion of five cycles [111]. A 67% overall response rate was observed, with CR in 11%, near CR in 22%, PR in 18%, and minimal response in 16%. After ≥2 cycles for progressive disease or ≥4 cycles for stable disease, on the day of and day after each bortezomib dose, 20 mg/d dexamethasone was added. A total of 208 patients (33%) received dexamethasone and 34% (70 patients) showed enhanced response. The most common adverse effects were thrombocytopenia (39%), neutropenia (16%), anemia (12%), diarrhea (7%), and peripheral neuropathy (5%). Overall, this study demonstrated that bortezomib is safe and effective, alone or in combination with dexamethasone, for the treatment of relapsed or refractory multiple myeloma in patients with ≥2 prior lines of treatment [111]. The efficacy of combination of bortezomib with other conventional chemotherapeutic drugs was investigated in another Phase III trial [112]. This study was investigating the use of bortezomib in combination with dexamethasone (VD) or with dexamethasone and lenalidomide (VRD) as primary first-line treatment for multiple myeloma. Patients who have received previous dexamethasone-based treatments have been enrolled to test whether switching to a proteasome inhibitor (VD arm) or adding a proteasome inhibitor (VRD arm) results in better prolonged disease control. Complete results have not yet been published [112].
Another large Phase III trial was conducted at 151 centers in 22 countries with recruitment of 682 previously untreated patients with multiple myeloma. Of the patients, 30% were age 75 or older. The patients randomly received either the combination of bortezomib plus melphalan–prednisone or melphalan–prednisone alone as the control group [113]. The results of this trial showed that the proportions of patients with a partial response or better were 71% in the bortezomib group and 35% in the control group. Complete-response rates were 30% and 4%, respectively (P<0.001). The median duration of the response was 19.9 months in the bortezomib group and 13.1 months for the controls. At a median follow-up of 16.3 months, 45 patients in the bortezomib group and 76 of the controls had died (P=0.008) [113]. The findings of this study suggest that bortezomib plus melphalan-prednisone is a valuable front-line treatment for myeloma patients age 65 or older and melphalan prednisone alone can no longer be considered the standard of care for multiple myeloma patients age 65 or older [113].
In the most recent phase III VISTA trial, bortezomib plus melphalan and prednisone compared with melphalan and prednisone were tested in previously untreated multiple myeloma. Analyses of the data from this trial following prolonged follow-up (median 36.7 months) were conducted. This follow-up validated the original findings of the study, as well as indicating that the use of bortezomib-based drugs as first-line treatments affords a greater survival advantage than treatment with conventional drugs followed by bortezomib-based treatments for salvage [114]. It also showed that patients initially treated with bortezomib are not more resistant to subsequent therapies as compared to patients initially treated with traditional chemotherapeutics. Additionally, after prolonged follow-up, the rate of improvement of peripheral neuropathy in patients treated with bortezomib plus melphalan-prednisone was 79%, indicating that this is a generally reversible adverse side effect [114].
ADVANTAGES AND DISADVANTAGES OF BORTEZOMIB AS AN ANTICANCER AGENT
Advantages of Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor drug tested in human patients for the treatment of relapsed and refractory multiple myeloma [6–7]. Bortezomib has also been tested in clinical trials against other hematological malignancy, such as non-Hodgkins lymphoma [115], and solid tumors [14], both as a single agent and in combination with other conventional anticancer drugs [85]. Proteasome inhibitors including bortezomib possess the following advantages in cancer therapy.
The Proteasome, a Novel Molecular Target in Cancer Cells for Chemotherapy
It has been demonstrated that malignant cells harbor elevated proteasome activity compared with normal cells [116–117]. Besides solid tumor cells, it has been reported that expression of proteasome and the mRNA levels are consistently increased to much higher levels in a variety of malignant human hematopoietic cell lines compared with peripheral lymphocytes and monocytes from healthy adults [45]. Cancer cells are more dependent on proteasome activity for their survival and drug resistance; therefore, malignant cells should be more sensitive to treatment with proteasome inhibitors such as bortezomib than normal cells, a hypothesis which has been supported by many studies [118–122].
Effectiveness of Bortezomib
The results from clinical trials demonstrate that bortezomib is very efficacious in treatment of multiple myeloma patients. Multiple myeloma is a hematologic malignancy characterized by the accumulation of clonal plasma cells at multiple sites in the bone marrow. The majority of the patients respond to initial chemotherapy but most of them eventually become refractory and relapse due to the proliferation of resistant malignant cells [123]. Clinical trials have shown that the response rate to bortezomib was, surprisingly, up to 35% in the enrolled multiple myeloma patients, who in majority had been treated with three or more cytotoxic drugs against myeloma and were refractory to these agents [124].
Selectivity of Bortezomib
The preferential induction of apoptosis by bortezomib has been well established in myeloma cell lines. Multiple myeloma cell lines are more sensitive to treatment with bortezomib compared with bone marrow cells or peripheral blood mononuclear cells from healthy individuals. Hideshima et al. reported that myeloma cell lines or patient-derived myeloma cells were at least 170-fold more sensitive to bortezomib compared with peripheral blood mononuclear cells (PBMCs) from normal volunteers [53]. It indicates that by inhibition of proteasome activity, bortezomib causes cancer cells to die. Normal cells are affected too, but to a lesser extent.
Suppression of Target Genes and Cell-Cell Interactions
Bortezomib appears to not only have activity against multiple myeloma cells, but also to inhibit blood vessel development and suppress interactions of multiple myeloma cells with bone marrow stem cells in the bone marrow microenvironment, which may contribute to multiple myeloma cell drug resistance [124].
Sensitization of Resistant Cancer Cells to Chemotherapy and Radiotherapy
Bortezomib is capable of enhancing the sensitivity of cancer cells to conventional chemotherapeutic agents, and appears to overcome drug resistance [53, 125]. Bortezomib also increases radiation sensitivity in cancer cells in vitro and in vivo. Consistently, combination of bortezomib and radiotherapy can also result in significantly reduced tumor growth in animal models [64–65, 126–127].
Disadvantages of Bortezomib
Significant challenges remain for bortezomib as an anticancer drug, as observed especially in clinical trials.
Severe Side Effects
Although bortezomib has achieved significant benefit for multiple myeloma patients in clinical trials, its effectiveness and administration have been limited by toxic side effects. The most frequent side effects (incidence >30%) associated with bortezomib in clinical trials include asthenic conditions (such as fatigue, generalized weakness), gastrointestinal events (nausea, diarrhea, vomiting, poor appetite, etc.), hematological toxicity (low platelet and erythrocytes counts), peripheral neuropathy characterized by decreased sensation, paresthesia (numbness and tingling of the hands and feet), and a high rate of shingles [87, 106]. The less common side effects (occurring in about 10–29%) of bortezomib include headache, insomnia, joint pain, arthralgia, myalgias, edema of the face, hands, feet or legs, as well as low white blood cell count (which can increase risk of infection, shortness of breath, dizziness, rash, etc.) [128]. The mechanisms of bortezomib induced-side effects are still not clear. Bortezomib is able to activate the mitochondrial-based apoptotic pathway [129] and therefore mitochondrial and endoplasmic reticulum damage may play a key role in bortezomib-induced side effects. This is supported by an in vitro study in which the mitochondrial-mediated dysregulation of calcium homeostasis is a critical regulator of bortezomib cytotoxicity [130].
Drug Resistance
Bortezomib is particularly effective in multiple myeloma and mantle cell lymphoma compared with other cancers. However, only about 40 – 50% of mantle cell lymphoma patients and 35% of multiple myeloma patients are sensitive to bortezomib, indicating that up to, or more than half, of patients possess intrinsic resistance to proteasome inhibition, which is a critical limitation of bortezomib therapy [13, 104, 108, 131]. A clinical usage of bortezomib is also hampered by acquired resistance to this drug, which appears to be associated with proteasome subunit β5 mutation and overexpression [132–135].
Reduction of Bortezomib’s Efficacy due to its Interactions with Some Natural Compounds
Some natural compounds can interfere, rather than synergize the anticancer effect of bortezomib. Most recently, it has been reported that the proteasome-inhibitory and anticancer activity of bortezomib and other boronic acid-based proteasome inhibitors can be blocked by green tea polyphenols (GTPs) [136–137]. This is due to the interaction between the boronic acid structure of bortezomib and GTPs' catechol structure to form a borate ester. Similarly, it has been found that dietary flavonoids and ascorbic acid (Vitamin C) also inhibit the anticancer effects of the proteasome inhibitor bortezomib via similar drug-drug interaction mechanism [68, 138]. The involved mechanism is probably due to that polyphenols or other compounds containing 1,2-benzenediol moieties, such as EGCG, quercetin, myricetin and ascorbic acid, could interact with the structure of a boronic acid in boronic acid-based proteasome inhibitors (bortezomib, MG262, PS-IX, etc.), to form boronic ester complexes. Since the boronic acid is an active site in bortezomib (Fig. 2), as long as the site is blocked, bortezomib is not able to fit and bind with the active site in the S1 pocket of the β5 subunit of the proteasome. This hypothesis has been supported by pre-clinical evidence in which polyphenols containing 1,2-benzenediol moieties were unable to inhibit the apoptotic effects induced by non-boronic acid-based proteasome inhibitors [136–138]. Moreover, a direct chemical interaction of bortezomib with polyphenols containing 1,2-benzenediol moieties was confirmed by nuclear magnetic resonance (NMR) spectroscopy [137].
Unsatisfied Efficacy in Treatment of Solid Tumors
From their clinical trials on 12 enrolled metastatic breast cancer patients, Yang et al. [63] found no objective responses. One patient had stable disease, but 11 other patients experienced disease progression. The median survival time was only 4.3 months. The results of this trial suggested that bortezomib was well tolerated but showed limited clinical activity against metastatic breast cancer when used as a single agent [63]. Results from a Phase II study of bortezomib in patients with metastatic neuroendocrine tumors showed that of 16 patients, not one achieved a partial or complete remission and single-agent bortezomib did not induce any objective responses [139]. Kondagunta et al. [86] reported, according to a Phase II clinical trial using bortezomib in 37 patients with advanced renal cell carcinoma, that a partial response was observed in 4 patients (11%) and stable disease in 14 patients (38%). They concluded that the small proportion of patients who achieved a partial response does not support routine use in patients with advanced renal cell carcinoma [86].
The observations from laboratory studies and clinical trials suggest that the anticancer potency for the treatment of solid tumors might be increased if bortezomib is combined with some conventional anticancer agents [140]. Clinical trials have indicated that combination of bortezomib with pegylated liposomal doxorubicin [141], melphalan [5], dexamethasone [142], cyclophosphamide [143], thalidomide [144], or arsenic trioxide [145] was significantly more effective than bortezomib alone. However, conclusions from another Phase II study of 155 patients with advanced non–small cell lung cancer (NSCLC) treated with bortezomib alone or in combination with docetaxel showed that bortezomib has modest single-agent activity in patients with relapsed or refractory advanced NSCLC, with minor enhancement in combination with docetaxel [146].
CONCLUSION AND FUTURE PERSPECTIVES
Promising preclinical and clinical studies suggest the ubiquitin-proteasome pathway as a unique target for the treatment of human cancer, and these validate the clinical use of proteasome inhibition as a promising anticancer strategy. Bortezomib is the first approved proteasome inhibitor drug for the clinical treatment of cancer, and acts by reversibly inhibiting the proteasomal activity in addition to multiple oncogenic pathways in tumor cells. The antitumor activity of bortezomib has been thoroughly investigated in Phase I, II and III clinical trials. From these clinical studies, it was revealed that bortezomib possesses chemo-/radio-sensitizing activities as a means for overcoming resistance when combined with conventional therapeutic agents or radiation. However, many disadvantages of bortezomib exist owing to its severe side effects, decreased efficacy toward solid tumors, interactions with numerous natural products and the acquisition of drug-resistance in a large portion of cancer patients. These findings could help guide physicians in refining its clinical use, and encourage basic scientists to generate next generation proteasome inhibitors that broaden the spectrum of activity and produce a more durable clinical response. The next generation of proteasome inhibitors are currently undergoing evaluation in clinical trials, and include marizomib (NPI-0052) [15–17, 147–148], carfilzomib (PR171, Proteolix) [18–19], and ONX 0912 (PR-047, http://www.onyx-pharm.com).
The discovery of novel, less toxic proteasome inhibitors from natural products and traditional medicines could provide more effective anticancer drug candidates for the treatment of cancer. It has been reported that many natural compounds have proteasome-inhibitory and anti-tumor activity, which include tea polyphenol EGCG [149–150], soy isoflavone genistein [151], turmeric polyphenol curcumin [152–153] and dietary flavone apigenin [120, 154]. Scientists have modified and synthesized various analogs based on the structures of natural compounds and these synthetic compounds have shown greater potency of anticancer activity and better bioavailability than their natural counterparts [155–159].
Another desirable direction may lie in the development of inhibitors against specific E3 ligases. These ligases act on an early step in the ubiquitin-proteasome system (Fig. 1) and could be specifically targeted in many cancer related proteins. E3 inhibitors have been found to selectively suppress tumor related E3 ligases, including the cell cycle regulatory E3 ubiquitin ligases [160], apoptosis related E3 ubiquitin ligases [161] and the murine double minute (mdm2) E3 ubiquitin ligases [162]. Pre-clinical studies of an E3 inhibitor MLN4924 have shown interesting positive results [163]. The first Phase I clinical trial of E3 inhibitor MLN4924 for treatment of acute myelogenous leukemia and high-grade myelodysplastic syndrome is ongoing and will be completed in October 2011 (http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00911066). Thus, it is conceivable that inhibitors of E3 ubiquitin ligases may represent a rational approach in cancer treatment. Therefore, targeting the tumor ubiquitin-proteasome degradation pathway continues to be a promising strategy for human cancer therapies.
ACKNOWLEDGEMENTS
The research was partially supported by grants from National Cancer Institute (1R01CA120009, 3R01CA120009-04S1, and 1R21CA139386-01 to Q.P. Dou).
ABBREVIATIONS
- CKI
Cyclin-dependent kinase inhibitor
- CR
Complete response
- DLTs
Dose-limiting toxicities
- ER
Endoplasmic reticulum
- FDA
Food and Drug Administration
- HIF-1α
Hypoxia-inducible factor 1 alpha
- IAP
Inhibitor of Apoptosis
- IGF-I
Insulin-like growth factor-I
- IKK
IκB kinase
- IL-6
Interleukin-6
- IUPAC
International Union of Pure and Applied Chemistry
- IκB
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor
- MAPK
Mitogen-activated protein kinase
- MTD
Maximum tolerated dose
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMR
Nuclear magnetic resonance
- NOXA
Phorbol-12-myristate-13-acetate-induced protein 1
- NSCLC
Non-small cell lung carcinoma
- ODC
Ornithine decarboxylase
- PBMCs
Peripheral blood mononuclear cells
- PGPH
Peptidyl-glutamyl peptide-hydrolyzing
- pRB
Retinoblastoma protein
- PRs
Partial responses
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-alpha
REFERENCES
- 1.Adams J. The proteasome: a suitable antineoplastic target. Nat. Rev. Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr. Opin. Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 4.Orlowski RZ, Dees EC. The role of the ubiquitination-proteasome pathway in breast cancer: applying drugs that affect the ubiquitin-proteasome pathway to the therapy of breast cancer. Breast Cancer Res. 2003;5:1–7. doi: 10.1186/bcr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, Purner M, Lee SP, Wilson J, Morrison B, Adams J, Schenkein D, Swift R. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J. Clin. Oncol. 2006;24:937–944. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- 6.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 7.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 8.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin. Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 9.Orlowski RZ, Eswara JR, Lafond-Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human Burkitt's lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–4348. [PubMed] [Google Scholar]
- 10.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 11.Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, Guerciolini R, Anderson JK, Depcik-Smith ND, Bhagat R, Lehman MJ, Novick SC, O'Connor OA, Soignet SL. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J. Clin. Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 12.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 13.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 14.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, Kim J, Adams J, Elliott P, Esseltine D, Petrusich A, Dieringer P, Perez C, Logothetis CJ. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J. Clin. Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 15.Potts BC, Lam KS. Generating a generation of proteasome inhibitors: from microbial fermentation to total synthesis of salinosporamide a (marizomib) and other salinosporamides. Mar. Drugs. 2010;8:835–880. doi: 10.3390/md8040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh AV, Palladino MA, Lloyd GK, Potts BC, Chauhan D, Anderson KC. Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI-0052 (marizomib) in a human plasmacytoma xenograft murine model. Br. J. Haematol. 2010;149:550–559. doi: 10.1111/j.1365-2141.2010.08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenical W, Jensen PR, Palladino MA, Lam KS, Lloyd GK, Potts BC. Discovery and development of the anticancer agent salinosporamide A (NPI-0052) Bioorg. Med. Chem. 2009;17:2175–2180. doi: 10.1016/j.bmc.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin. Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 21.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin. Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 22.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters JM, Cejka Z, Harris JR, Kleinschmidt JA, Baumeister W. Structural features of the 26 S proteasome complex. J. Mol. Biol. 1993;234:932–937. doi: 10.1006/jmbi.1993.1646. [DOI] [PubMed] [Google Scholar]
- 25.Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 26.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 27.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. J. Biol. Chem. 1999;274:22123–22126. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 30.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 31.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J. Biosci. 2006;31:137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 32.Hershko A, Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 33.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev. 2003;29 Suppl 1:3–9. doi: 10.1016/s0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 34.Hoyt MA, Coffino P. Ubiquitin-free routes into the proteasome. Cell Mol Life Sci. 2004;61:1596–1600. doi: 10.1007/s00018-004-4133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong X, Alvarez-Castelao B, Lin Z, Castano JG, Caro J. Constitutive/hypoxic degradation of HIF-alpha proteins by the proteasome is independent of von Hippel Lindau protein ubiquitylation and the transactivation activity of the protein. J. Biol. Chem. 2007;282:15498–15505. doi: 10.1074/jbc.M700704200. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Lee J, Cho SY, Fine HA. Proteasome-mediated destruction of the cyclin a/cyclin-dependent kinase 2 complex suppresses tumor cell growth in vitro and in vivo. Cancer Res. 2004;64:3949–3957. doi: 10.1158/0008-5472.CAN-03-3906. [DOI] [PubMed] [Google Scholar]
- 37.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 38.Won KA, Reed SI. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]
- 39.Blagosklonny MV. P53: an ubiquitous target of anticancer drugs. Int. J. Cancer. 2002;98:161–166. doi: 10.1002/ijc.10158. [DOI] [PubMed] [Google Scholar]
- 40.Kalejta RF, Shenk T. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA. 2003;100:3263–3268. doi: 10.1073/pnas.0538058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 43.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 45.Kumatori A, Tanaka K, Inamura N, Sone S, Ogura T, Matsumoto T, Tachikawa T, Shin S, Ichihara A. Abnormally high expression of proteasomes in human leukemic cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7071–7075. doi: 10.1073/pnas.87.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist. Updat. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 47.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 48.Sun J, Nam S, Lee CS, Li B, Coppola D, Hamilton AD, Dou QP, Sebti SM. CEP1612, a dipeptidyl proteasome inhibitor, induces p21WAF1 and p27KIP1 expression and apoptosis and inhibits the growth of the human lung adenocarcinoma A-549 in nude mice. Cancer Res. 2001;61:1280–1284. [PubMed] [Google Scholar]
- 49.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 50.Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 51.Wojcik C. Proteasomes in apoptosis: villains or guardians? Cell Mol. Life Sci. 1999;56:908–917. doi: 10.1007/s000180050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin. Cancer Res. 2000;6:3719–3728. [PubMed] [Google Scholar]
- 53.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 54.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park) 2004;18:14–21. [PubMed] [Google Scholar]
- 55.Suh KS, Goy A. Bortezomib in mantle cell lymphoma. Future Oncol. 2008;4:149–168. doi: 10.2217/14796694.4.2.149. [DOI] [PubMed] [Google Scholar]
- 56.Montagut C, Rovira A, Albanell J. The proteasome: a novel target for anticancer therapy. Clin. Transl. Oncol. 2006;8:313–317. doi: 10.1007/s12094-006-0176-8. [DOI] [PubMed] [Google Scholar]
- 57.Utecht KN, Kolesar J. Bortezomib: a novel chemotherapeutic agent for hematologic malignancies. Am. J. Health Syst. Pharm. 2008;65:1221–1231. doi: 10.2146/ajhp070272. [DOI] [PubMed] [Google Scholar]
- 58.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin. Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 59.Hamilton AL, Eder JP, Pavlick AC, Clark JW, Liebes L, Garcia-Carbonero R, Chachoua A, Ryan DP, Soma V, Farrell K, Kinchla N, Boyden J, Yee H, Zeleniuch-Jacquotte A, Wright J, Elliott P, Adams J, Muggia FM. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J. Clin. Oncol. 2005;23:6107–6116. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 60.Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, Irvine AE. Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res. 2006;66:6379–6386. doi: 10.1158/0008-5472.CAN-06-0605. [DOI] [PubMed] [Google Scholar]
- 61.Chen D, Dou QP. The ubiquitin-proteasome system as a prospective molecular target for cancer treatment and prevention. Curr. Protein Pept. Sci. 2010;11:459–470. doi: 10.2174/138920310791824057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engel RH, Brown JA, Von Roenn JH, O'Regan RM, Bergan R, Badve S, Rademaker A, Gradishar WJ. A phase II study of single agent bortezomib in patients with metastatic breast cancer: a single institution experience. Cancer Invest. 2007;25:733–737. doi: 10.1080/07357900701506573. [DOI] [PubMed] [Google Scholar]
- 63.Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, Esseltine D, Stec J, Broglio KR, Islam R, Hortobagyi GN, Cristofanilli M. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann. Oncol. 2006;17:813–817. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 64.Edelman MJ. The potential role of bortezomib in combination with chemotherapy and radiation in non-small-cell lung cancer. Clin. Lung Cancer. 2005;7 Suppl 2:S64–S66. doi: 10.3816/clc.2005.s.011. [DOI] [PubMed] [Google Scholar]
- 65.Russo SM, Tepper JE, Baldwin AS, Jr, Liu R, Adams J, Elliott P, Cusack JC., Jr Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int. J. Radiat. Oncol. Biol. Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 66.Messersmith WA, Baker SD, Lassiter L, Sullivan RA, Dinh K, Almuete VI, Wright JJ, Donehower RC, Carducci MA, Armstrong DK. Phase I trial of bortezomib in combination with docetaxel in patients with advanced solid tumors. Clin. Cancer Res. 2006;12:1270–1275. doi: 10.1158/1078-0432.CCR-05-1942. [DOI] [PubMed] [Google Scholar]
- 67.Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, Johri AR, Jones PE, Ivanova A, Van Deventer HW, Gabriel DA, Shea TC, Mitchell BS, Adams J, Esseltine DL, Trehu EG, Green M, Lehman MJ, Natoli S, Collins JM, Lindley CM, Dees EC. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 68.Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, Santo L, Cirstea D, Raje N, Chauhan D, Baccarani M, Cavo M, Anderson KC. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23:1679–1686. doi: 10.1038/leu.2009.83. [DOI] [PubMed] [Google Scholar]
- 69.Ryan DP, O'Neil BH, Supko JG, Rocha Lima CM, Dees EC, Appleman LJ, Clark J, Fidias P, Orlowski RZ, Kashala O, Eder JP, Cusack JC., Jr A Phase I study of bortezomib plus irinotecan in patients with advanced solid tumors. Cancer. 2006;107:2688–2697. doi: 10.1002/cncr.22280. [DOI] [PubMed] [Google Scholar]
- 70.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 71.Chauhan D, Anderson KC. Mechanisms of cell death and survival in multiple myeloma (MM): Therapeutic implications. Apoptosis. 2003;8:337–343. doi: 10.1023/a:1024164700094. [DOI] [PubMed] [Google Scholar]
- 72.Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E, Neeser J, Mikail A, Adams J, Sjak-Shie N, Vescio RA, Berenson JR. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin. Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- 73.Feinman R, Koury J, Thames M, Barlogie B, Epstein J, Siegel DS. Role of NF-kappaB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood. 1999;93:3044–3052. [PubMed] [Google Scholar]
- 74.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, Anderson KC. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, Bennett F, Pollock PM, Trent JM, Hendrix MJ, Rizzo P, Miele L, Nickoloff BJ. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 76.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 77.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 78.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 79.Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 81.Caravita T, de Fabritiis P, Palumbo A, Amadori S, Boccadoro M. Bortezomib: efficacy comparisons in solid tumors and hematologic malignancies. Nat. Clin. Pract. Oncol. 2006;3:374–387. doi: 10.1038/ncponc0555. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, Lowe SW, Soengas MS. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 83.Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced killing of melanoma cells by simultaneously targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–9645. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 84.Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr. Opin. Chem. Biol. 2002;6:493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 85.Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br. J. Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 86.Kondagunta GV, Drucker B, Schwartz L, Bacik J, Marion S, Russo P, Mazumdar M, Motzer RJ. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J. Clin. Oncol. 2004;22:3720–3725. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 87.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 88.Shah SA, Potter MW, McDade TP, Ricciardi R, Perugini RA, Elliott PJ, Adams J, Callery MP. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J. Cell Biochem. 2001;82:110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- 89.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 90.Berenson JR, Ma HM, Vescio R. The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma. Semin. Oncol. 2001;28:626–633. doi: 10.1016/s0093-7754(01)90036-3. [DOI] [PubMed] [Google Scholar]
- 91.LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 92.Nawrocki ST, Bruns CJ, Harbison MT, Bold RJ, Gotsch BS, Abbruzzese JL, Elliott P, Adams J, McConkey DJ. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol. Cancer Ther. 2002;1:1243–1253. [PubMed] [Google Scholar]
- 93.Kukreja A, Hutchinson A, Mazumder A, Vesole D, Angitapalli R, Jagannath S, O'Connor OA, Dhodapkar MV. Bortezomib disrupts tumour-dendritic cell interactions in myeloma and lymphoma: therapeutic implications. Br. J. Haematol. 2007;136:106–110. doi: 10.1111/j.1365-2141.2006.06369.x. [DOI] [PubMed] [Google Scholar]
- 94.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strauss SJ, Higginbottom K, Juliger S, Maharaj L, Allen P, Schenkein D, Lister TA, Joel SP. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 96.Fribley A, Wang CY. Proteasome inhibitor induces apoptosis through induction of endoplasmic reticulum stress. Cancer Biol. Ther. 2006;5:745–748. doi: 10.4161/cbt.5.7.2971. [DOI] [PubMed] [Google Scholar]
- 97.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol. Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lioni M, Noma K, Snyder A, Klein-Szanto A, Diehl JA, Rustgi AK, Herlyn M, Smalley KS. Bortezomib induces apoptosis in esophageal squamous cell carcinoma cells through activation of the p38 mitogen-activated protein kinase pathway. Mol. Cancer Ther. 2008;7:2866–2875. doi: 10.1158/1535-7163.MCT-08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Markovic SN, Geyer SM, Dawkins F, Sharfman W, Albertini M, Maples W, Fracasso PM, Fitch T, Lorusso P, Adjei AA, Erlichman C. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 100.Voortman J, Smit EF, Honeywell R, Kuenen BC, Peters GJ, van de Velde H, Giaccone G. A parallel dose-escalation study of weekly and twice-weekly bortezomib in combination with gemcitabine and cisplatin in the first-line treatment of patients with advanced solid tumors. Clin. Cancer Res. 2007;13:3642–3651. doi: 10.1158/1078-0432.CCR-07-0061. [DOI] [PubMed] [Google Scholar]
- 101.Dreicer R, Petrylak D, Agus D, Webb I, Roth B. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin. Cancer Res. 2007;13:1208–1215. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 102.Hainsworth JD, Meluch AA, Spigel DR, Barton J, Jr, Simons L, Meng C, Gould B, Greco FA. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network phase II trial. Clin. Genitourin. Cancer. 2007;5:278–283. doi: 10.3816/CGC.2007.n.004. [DOI] [PubMed] [Google Scholar]
- 103.Morris MJ, Kelly WK, Slovin S, Ryan C, Eicher C, Heller G, Scher HI. A phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J. Urol. 2007;178:2378–2383. doi: 10.1016/j.juro.2007.08.015. discussion 2383–2374. [DOI] [PubMed] [Google Scholar]
- 104.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 105.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine DL, Schenkein DP, Anderson KC. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br. J. Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 106.Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, Agrawal S, Stec J, Schenkein D, Esseltine DL, Cavenagh JD. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br. J. Haematol. 2005;129:755–762. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 107.Belch A, Kouroukis CT, Crump M, Sehn L, Gascoyne RD, Klasa R, Powers J, Wright J, Eisenhauer EA. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann. Oncol. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 108.O'Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 109.Scagliotti GV, Germonpre P, Bosquee L, Vansteenkiste J, Gervais R, Planchard D, Reck M, De Marinis F, Lee JS, Park K, Biesma B, Gans S, Ramlau R, Szczesna A, Makhson A, Manikhas G, Morgan B, Zhu Y, Chan KC, von Pawel J. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer. 2010;68:420–426. doi: 10.1016/j.lungcan.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 110.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Blade J, Boccadoro M, Cavenagh JD, Boral AL, Esseltine DL, Wen PY, Amato AA, Anderson KC, San Miguel J. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br. J. Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 111.Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, Petrucci MT, Musto P, Komarnicki M, Stewart AK. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br. J. Haematol. 2009;144:169–175. doi: 10.1111/j.1365-2141.2008.07409.x. [DOI] [PubMed] [Google Scholar]
- 112.Fonseca R, Rajkumar SV. Consolidation therapy with bortezomib/lenalidomide/dexamethasone versus bortezomib/dexamethasone after a dexamethasone-based induction regimen in patients with multiple myeloma: a randomized phase III trial. Clin. Lymphoma Myeloma. 2008;8:315–317. doi: 10.3816/CLM.2008.n.046. [DOI] [PubMed] [Google Scholar]
- 113.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 114.Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Esseltine DL, Liu K, Cakana A, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J. Clin. Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 115.Orlowski RZ. The ubiquitin proteasome pathway from bench to bedside. Hematology Am. Soc. Hematol. Educ. Program. 2005:220–225. doi: 10.1182/asheducation-2005.1.220. [DOI] [PubMed] [Google Scholar]
- 116.Arlt A, Bauer I, Schafmayer C, Tepel J, Muerkoster SS, Brosch M, Roder C, Kalthoff H, Hampe J, Moyer MP, Folsch UR, Schafer H. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2) Oncogene. 2009;28:3983–3996. doi: 10.1038/onc.2009.264. [DOI] [PubMed] [Google Scholar]
- 117.Ma W, Kantarjian H, Bekele B, Donahue AC, Zhang X, Zhang ZJ, O'Brien S, Estey E, Estrov Z, Cortes J, Keating M, Giles F, Albitar M. Proteasome enzymatic activities in plasma as risk stratification of patients with acute myeloid leukemia and advanced-stage myelodysplastic syndrome. Clin. Cancer Res. 2009;15:3820–3826. doi: 10.1158/1078-0432.CCR-08-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih Ie M, Roden RB. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66:3754–3763. doi: 10.1158/0008-5472.CAN-05-2321. [DOI] [PubMed] [Google Scholar]
- 119.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 120.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 121.Ovaa H, Kessler BM, Rolen U, Galardy PJ, Ploegh HL, Masucci MG. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2253–2258. doi: 10.1073/pnas.0308411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soligo D, Servida F, Delia D, Fontanella E, Lamorte G, Caneva L, Fumiatti R, Lambertenghi Deliliers G. The apoptogenic response of human myeloid leukaemia cell lines and of normal and malignant haematopoietic progenitor cells to the proteasome inhibitor PSI. Br. J. Haematol. 2001;113:126–135. doi: 10.1046/j.1365-2141.2001.02683.x. [DOI] [PubMed] [Google Scholar]
- 123.Anderson KC, Kyle RA, Dalton WS, Landowski T, Shain K, Jove R, Hazlehurst L, Berenson J. Multiple Myeloma: New Insights and Therapeutic Approaches. Hematology Am. Soc. Hematol. Educ. Program. 2000:147–165. doi: 10.1182/asheducation-2000.1.147. [DOI] [PubMed] [Google Scholar]
- 124.Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–369. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- 125.Landis-Piwowar KR, Milacic V, Chen D, Yang H, Zhao Y, Chan TH, Yan B, Dou QP. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist. Updat. 2006;9:263–273. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 126.Goktas S, Baran Y, Ural AU, Yazici S, Aydur E, Basal S, Avcu F, Pekel A, Dirican B, Beyzadeoglu M. Proteasome inhibitor bortezomib increases radiation sensitivity in androgen independent human prostate cancer cells. Urology. 2010;75:793–798. doi: 10.1016/j.urology.2009.07.1215. [DOI] [PubMed] [Google Scholar]
- 127.Pervan M, Pajonk F, Sun JR, Withers HR, McBride WH. Molecular pathways that modify tumor radiation response. Am. J. Clin. Oncol. 2001;24:481–485. doi: 10.1097/00000421-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 128.Curran MP, McKeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs. 2009;69:859–888. doi: 10.2165/00003495-200969070-00006. [DOI] [PubMed] [Google Scholar]
- 129.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. Cancer Res. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 130.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 131.Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M, Broglio K, Samuels B, Gilles F, Sarris AH, Hart S, Trehu E, Schenkein D, Cabanillas F, Rodriguez AM. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 132.Lu S, Chen Z, Yang J, Chen L, Gong S, Zhou H, Guo L, Wang J. Overexpression of the PSMB5 gene contributes to bortezomib resistance in T-lymphoblastic lymphoma/leukemia cells derived from Jurkat line. Exp. Hematol. 2008;36:1278–1284. doi: 10.1016/j.exphem.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 133.Lu S, Yang J, Chen Z, Gong S, Zhou H, Xu X, Wang J. Different mutants of PSMB5 confer varying bortezomib resistance in T lymphoblastic lymphoma/leukemia cells derived from the Jurkat cell line. Exp. Hematol. 2009;37:831–837. doi: 10.1016/j.exphem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 134.Lu S, Yang J, Song X, Gong S, Zhou H, Guo L, Song N, Bao X, Chen P, Wang J. Point mutation of the proteasome beta5 subunit gene is an important mechanism of bortezomib resistance in bortezomib-selected variants of Jurkat T cell lymphoblastic lymphoma/leukemia line. J. Pharmacol. Exp. Ther. 2008;326:423–431. doi: 10.1124/jpet.108.138131. [DOI] [PubMed] [Google Scholar]
- 135.Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, van der Heijden JW, Ylstra B, Peters GJ, Kaspers GL, Dijkmans BA, Scheper RJ, Jansen G. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112:2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- 136.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schonthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 137.Kim TY, Park J, Oh B, Min HJ, Jeong TS, Lee JH, Suh C, Cheong JW, Kim HJ, Yoon SS, Park SB, Lee DS. Natural polyphenols antagonize the antimyeloma activity of proteasome inhibitor bortezomib by direct chemical interaction. Br. J. Haematol. 2009;146:270–281. doi: 10.1111/j.1365-2141.2009.07752.x. [DOI] [PubMed] [Google Scholar]
- 138.Liu FT, Agrawal SG, Movasaghi Z, Wyatt PB, Rehman IU, Gribben JG, Newland AC, Jia L. Dietary flavonoids inhibit the anticancer effects of the proteasome inhibitor bortezomib. Blood. 2008;112:3835–3846. doi: 10.1182/blood-2008-04-150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shah MH, Young D, Kindler HL, Webb I, Kleiber B, Wright J, Grever M. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin. Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 140.Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin. Investig. Drugs. 2009;18:957–971. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J. Clin. Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 142.Popat R, Oakervee H, Williams C, Cook M, Craddock C, Basu S, Singer C, Harding S, Foot N, Hallam S, Odeh L, Joel S, Cavenagh J. Bortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myeloma. Br. J. Haematol. 2009;144:887–894. doi: 10.1111/j.1365-2141.2008.07572.x. [DOI] [PubMed] [Google Scholar]
- 143.Reece DE, Rodriguez GP, Chen C, Trudel S, Kukreti V, Mikhael J, Pantoja M, Xu W, Stewart AK. Phase I-II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J. Clin. Oncol. 2008;26:4777–4783. doi: 10.1200/JCO.2007.14.2372. [DOI] [PubMed] [Google Scholar]
- 144.Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N, Eleftherakis-Papaiakovou E, Tsionos K, Croucher P, Dimopoulos MA. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22:2247–2256. doi: 10.1038/leu.2008.235. [DOI] [PubMed] [Google Scholar]
- 145.Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin. Cancer Res. 2007;13:1762–1768. doi: 10.1158/1078-0432.CCR-06-1812. [DOI] [PubMed] [Google Scholar]
- 146.Fanucchi MP, Fossella FV, Belt R, Natale R, Fidias P, Carbone DP, Govindan R, Raez LE, Robert F, Ribeiro M, Akerley W, Kelly K, Limentani SA, Crawford J, Reimers HJ, Axelrod R, Kashala O, Sheng S, Schiller JH. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 147.Bhatnagar I, Kim SK. Marine antitumor drugs: status, shortfalls and strategies. Mar. Drugs. 2010;8:2702–2720. doi: 10.3390/md8102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chauhan D, Hideshima T, Anderson KC. A novel proteasome inhibitor NPI-0052 as an anticancer therapy. Br. J. Cancer. 2006;95:961–965. doi: 10.1038/sj.bjc.6603406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen D, Milacic V, Chen MS, Wan SB, Lam WH, Huo C, Landis-Piwowar KR, Cui QC, Wali A, Chan TH, Dou QP. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008;23:487–496. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 151.Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem. Pharmacol. 2003;66:965–976. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 152.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J. Biol. Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 153.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen D, Landis-Piwowar KR, Chen MS, Dou QP. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007;9:R80. doi: 10.1186/bcr1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 157.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm. Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Padhye S, Yang H, Jamadar A, Cui QC, Chavan D, Dominiak K, McKinney J, Banerjee S, Dou QP, Sarkar FH. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009;26:1874–1880. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang H, Sun DK, Chen D, Cui QC, Gu YY, Jiang T, Chen W, Wan SB, Dou QP. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010;292:48–53. doi: 10.1016/j.canlet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pray TR, Parlati F, Huang J, Wong BR, Payan DG, Bennett MK, Issakani SD, Molineaux S, Demo SD. Cell cycle regulatory E3 ubiquitin ligases as anticancer targets. Drug Resist. Updat. 2002;5:249–258. doi: 10.1016/s1368-7646(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 161.Jia L, Yang J, Hao X, Zheng M, He H, Xiong X, Xu L, Sun Y. Validation of SAG/RBX2/ROC2 E3 ubiquitin ligase as an anticancer and radiosensitizing target. Clin. Cancer Res. 2010;16:814–824. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 163.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]