Abstract
Studies suggest that occupational voice users have a greater incidence of vocal issues than the general population. Women have been found to experience vocal health problems more frequently than men, regardless of their occupation. Traditionally, it has been assumed that differences in the laryngeal system are the cause of this disproportion. Nevertheless, it is valuable to identify other potential gender distinctions which may make women more vulnerable to voice disorders. A search of the literature was conducted for gender-specific characteristics which might impact the vocal health of women. This search can be used by healthcare practitioners to help female patients avoid serious vocal health injuries, as well as to better treat women who already suffer from such vocal health issues.
Keywords: Occupational groups, occupational voice disorder, risk, singers, teachers
INTRODUCTION
Many studies have focused on the vocal health issues of occupational voice users, or those whose professional performance depends on good vocal quality and health 1. While many occupational voice users are at risk for vocal health issues 2–5, much of the current research has focused on school teachers. One survey of teachers in the United States (N=237) found that over 50% of teachers experienced three or more voice symptoms that negatively affected their teaching ability 6. In a survey of 550 school teachers in Ireland, only 20% of teachers reported no voice concerns, with 27% reporting voice issues and 53% reporting ‘intermittent’ voice issues 7.
Preliminary research suggests that women have more voice disorders than men. Women may be nearly twice as likely to report a history of voice problems as men 8 and represent up to 76% of voice clinicians referrals 9. Moreover, 10% more women than men (regardless of occupation) reported a history of a prolonged voice disorder, or a voice problem lasting more than four weeks 10. This gender distinction extends to occupational voice users (e.g., teachers 11, 12, customer service workers 13, and singers 14), a distinction which becomes particularly significant for such professions that are predominantly female (e.g., teaching, aerobic instructors, telemarketers).
METHODS
The information in this discussion paper was collected by first examining studies and reports published in English using the PubMed database through July 2009. Key words that were used, either in combination or singly, included: voice, gender, laryngeal anatomy, occupational, voice disorders, vocal health, hormones, endocrine, puberty, contraception, menses, menopause, blood flow, and laryngopharyngeal reflux. The reference list in each of these papers was then examined to identify additional references and key words. For example, when conducting the initial review of the effect of hormones on the laryngeal system, one identified paper included a reference for a study examining the effects of hormones on the voice in individuals who have undergone gender reassignment. The key words “gender reassignment” had not been initially included in the search parameters, but were subsequently included based on the identified reference. In this particular example, this methodology of expanding search parameters facilitated the inclusion of key words and references that would have been otherwise excluded, providing valuable insight into the effects of isolated hormones on various physiological systems. Thus, it should be noted that the purpose of this paper was not to conduct a traditional, replicable literature review. Such a review would likely be limited in scope due to the initial restriction of key words. Rather, this study was undertaken to ultimately identify as many potential factors as possible that might influence the female voice.
RESULTS
Voice production and vocal health is a complex issue with a range of physiological and non-physiological risk factors. A general lack of awareness of these risks may ultimately increase an occupational voice user’s vulnerability to voice disorders 15. However, there has not yet been a comprehensive presentation of gender differences from the perspective of voice production and vocal risk. To answer why women are more vulnerable to vocal health issues than men, the anatomical differences in their respective laryngeal systems will be highlighted. Second, differences in less obvious systems, beginning with the impact of the endocrine system, will be explored. Next, other non-laryngeal related differences, many of which are influenced by these endocrine differences, will be examined. Finally, gender-related non-physiological and behavioral differences which may cause vocal health issues to be more predominant in women will be surveyed.
LARYNGEAL DIFFERENCES
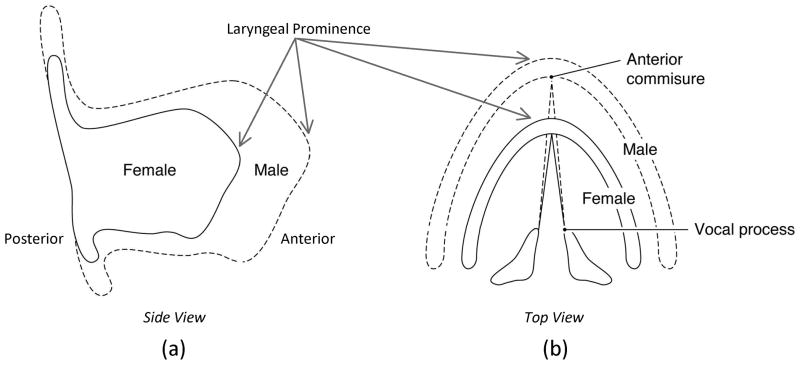
The pre-puberty laryngeal systems (Figure 1) of males and females are quite similar. However, the influx of testosterone during puberty creates structural changes to produce the male vocal gender. This rapid structural growth during puberty is a key source of teenage male vocal instability. Gender differences in laryngeal geometry affect many aspects of voice production such as the intensity of the voice 16, 17, pitch 18, 19, and voice onset and offset 20.
Figure 1.
Elongation of the male thyroid cartilage results in a longer vocal fold (after Titze, 1989).
Elongation of the male thyroid cartilage during puberty without an equal widening narrows the angle between the thyroid lamina and forms the distinctive laryngeal prominence (i.e., Adam’s apple) seen in the male thyroid cartilage 21, 22 (Figure 2). Because the female thyroid cartilage changes much less during puberty compared to the male, the adult female thyroid cartilage is approximately 20% smaller anteriorly-posteriorly than the male 23, 24. The vocal folds, which reside within the thyroid cartilage, show similar gender differences. The female vocal folds are on average 60% shorter anteriorly-posteriorly 25 which is one of the primary reasons for women’s higher average fundamental frequency (F0) (190 Hz, female, vs. 120 Hz, male) 26. Simply put, smaller structures correlate with higher vibratory frequencies, as do shorter structures, like strings or beams. Additionally, shorter structures increase the effect of material bending contributions that can result in further increases in string-like vibratory frequency 27. This difference in F0 may increase women’s risk for voice disorders because a higher F0 results in more vocal fold oscillations and collisions for an equal amount of voicing (on average, 70 more collisions per second, or 190 cycles per second minus 120 cycles per second). In addition to being longer, the male vocal folds are also thicker, primarily because the muscle within the vocal folds (i.e., thyroarytenoid muscle) is thickened by testosterone during puberty (Figure 3). In contrast, female vocal folds thicken only slightly during puberty, making them about 20–30% thinner than male vocal folds25. This difference in thickness also contributes to the difference in average fundamental frequency difference and its corresponding increased risk for voice disorders. Further, thinner vocal folds may increase women’s risk because there would be less tissue to damp/absorb vibratory forces 10, 24.
Figure 2.
The muscle within the male vocal folds is enlarged by testosterone during puberty thickening the vocal fold (after Titze, 1989).
While the primary reason for the gender difference in pitch is vocal fold length and thickness, the primary mechanism for pitch control is the variation in elongation of the vocal folds. Higher tensile stress on the vocal fold mucosa and vocal ligament, which lies along the medial edge of the vocal folds parallel with the thyroarytenoid muscle, correlates with higher pitch. Studies have uncovered gender differences in the amount and density of collagen, the primary tensile stress bearing structure 28 , within the vocal fold. Female vocal folds have been shown to have less tensile stress than a male for a given percent elongation (from 2 to 5 times less) 29, possibly because they have approximately 59% of the collagen found in adult male vocal folds 30,31. Thus, with the female vocal fold significantly less stiff during equal elongation or strain, the female tissue may require a larger percent elongation in order to obtain an equivalent stiffness range and thus an equivalent pitch range. This difference would require increased effort and, likely, more fatigue.
Within the vocal fold extracellular matrices is hyaluronic acid (HA), and its distribution appears to also vary based on gender. Found throughout the body, HA is important in developing tissues such as the vocal folds, as well as non-laryngeal tissues like the umbilical cord, dermis, subcutaneous tissue, and cartilages. Significantly, it is usually found in high concentrations in the body in areas of high shock absorption 32. Further, it controls viscosity and stiffness of the extracellular matrix, possibly by regulating the water content 32–34. Studies suggest that HA also plays a vital role in the laryngeal system specifically; for example, HA has been shown to be important in preventing vocal fold scarring 35. Therefore, the presence of HA potentially affects the damping and absorption of vocal fold collisions, likely helping to protect the vocal folds from phonotrauma during high vocal demand and excessive vibration as well as aiding in vocal recovery. While men seem to have a fairly stable distribution of HA throughout the depth of the vocal folds’ lamina propria, women have less HA in the superficial layer and more in the deep layer 36. Thus, reduced amounts of HA in the superficial layer may predispose females to increased vocal fold injury and increased scarring.
ENDOCRINE DIFFERENCES
As discussed above, most structural gender differences appear as the male laryngeal system changes with the onset of puberty. However, studies examining the effects of hormone therapy during gender reassignments provided details about the impact on the voice of isolated hormones. The primary goals of such therapy are to diminish the hormonally induced secondary sexual characteristics of the original gender, while inducing the secondary sexual characteristics of the intended gender. For example, one study showed that androgen therapy altered the adult female larynx, lowering the mean F0 from a pretreatment of 228.45Hz to 116.52Hz between the 3rd and 4th month of treatment 37. However, in male-female gender reassignment procedures, hormone therapy and voice coaching alone produced only minimal pitch changes because the male laryngeal structure was already altered at puberty. Such studies once again underline the gender-based physiological differences.
A key reason for the impact of hormones on the laryngeal system is the location of hormone receptors in the cytoplasm and nucleus of vocal fold cells 38. Studies suggest that these receptors make the human laryngeal system significantly responsive to variations in gonadal hormones. For example, the laryngeal system is affected by hormone fluctuations during menses and menopause 39–44. Indeed, epithelial swabs taken from the larynx during menses and menopause appear to mimic the fluctuations seen in the vagina 45.
Specifically, studies have found a variety of vocal health issues associated with hormone fluctuations during menses, including increased vocal fatigue, decreased range, and loss of vocal power and high harmonics. Some studies suggest that the new generation of oral contraceptives, which reduce hormonal fluctuations during menses, also seem to increase a female’s vocal stability (e.g., lower jitter and shimmer values) when compared to women not using any oral contraceptives 46–48. Perhaps even more significantly, the impact of these hormone fluctuations during menses is reported to be greater in occupational voice users versus non-occupational voice users 49. Further, studies of vocal fold changes during menses have suggested that non-optimal voice use or even vocal misuse (e.g., excessive vibration, poor vocal hygiene, inadequate breath management) may exacerbate the effect of menstruation on the voice 50.
The negative effects of menopause on the female voice have also been documented perceptually and acoustically (e.g. Raj, et al.51), supporting the positive effects of estrogen on voice quality in post-menopausal women. The lower amount of estrogen also causes a breakdown in their connective tissues, precluding optimal vocal health43. In a review of the potential effects of these hormone changes during menopause on the female larynx, D’haeseleer, et al.52 gives more detail than can be discussed here.
OTHER NON-LARYNGEAL PHYSIOLOGICAL DIFFERENCES
Gender differences in other major physiological systems may also impact the laryngeal system directly or indirectly. Some are systems tightly integrated with voice, such as the respiratory system, while others are more independent, such as the cardiovascular system.
Nervous System
Responses to pain sensitivity seem to have gender-related differences 53, 54. Pain is the body’s way to identify real or potential tissue damage. While sensitivity to painful stimuli appears to fluctuate during the menstrual cycle, women appear to be more sensitive to pain than men after the onset of puberty. Studies suggest that men have a greater pain threshold (i.e., the point at which a person becomes aware of pain) and tolerance for pain, while women generally report painful stimuli as more painful than men 55–57. Chronic pain is also more frequent in females than males 58,59, and changes with the hormone cycle 60,61. Gender reassignment studies have highlighted how hormones affect the experience of pain. In an examination of changes in chronic pain (e.g., headache) in two groups undergoing gender reassignment (with estrogen and anti-androgens administered to male-female subjects and androgens administered to female-male subjects), approximately 50 percent of the female-male subjects who had previously experienced chronic headaches experienced an improvement in their pain after receiving testosterone therapy, while 33 percent of the male-female subjects who were given estrogen and anti-androgen therapy experienced an onset in chronic pain 62. Whether women actual experience more pain or are more sensitive to pain generally, the gender difference in the pain experience could also account for a portion of the higher incidence of reported voice problems by women over men.
Respiratory System
Because sufficient and coordinated respiratory control and breath management (e.g., adequate sub-glottal pressure and airflow) is required to manage voice production, the respiratory system is also crucial in vocal health. During phonation, air pressure acts upon the vocal folds to help start and maintain their oscillation 63, control changes in vocal loudness 17, and affect vocal fold vibratory modes 64. For maximum aerodynamic-to-acoustic energy conversion and minimum disturbance of the natural vibratory patterns of the vocal folds, the interaction between respiratory support and vocalization should be optimized. Generally, a breathy voice results when the average airflow is excessive, and a pressed voice results when there is insufficient airflow. Neither are optimal healthy vocalization styles 65. In a comparison of laryngeal function and respiratory system coordination between the genders 66, the male lungs were reported to have a higher static recoil during exhalation. This finding suggests that women require a higher percentage of lung volume use to create an equivalent lung pressure, a necessary driving force vocal fold vibration. Further, females in the study 66 generally used a higher initial percentage of rib cage volume than males for comfortable speech possibly to try to compensate for the difference in lung volume or to create more equivalent static recoil. Thus, women may use more inhalation respiratory effort to vocalize on par with men. This pattern would likely either fatigue the respiratory muscles in females earlier than males or require the females to use increased respiratory muscular effort during speaking, which would also speed fatigue as well as require more breaths per words than males. Alternatively, women who did not compensate in one of these two ways but instead used a comparable initial percentage of rib cage volume as men would have insufficient airflow. Then to maintain voicing with insufficient airflow, these women would then have to compensate with increased laryngeal adduction, creating more contact force per unit area on the medial edges of the folds, which is ultimately a less healthy vocalization style. The consistent use of unusually low lung volumes in speech may be a risk factor for nodules due to the link between vocal fold adduction and impact stress during vibration 67,68. Specifically, women with vocal nodules have been shown to initiate and terminate speech breathing at lower lung volumes than healthy control subjects 69. Therefore, differences in the respiratory system could be one of the factors contributing to the higher instance of vocal nodules in female compared to male teachers 70.
Digestive System
Gender differences in the digestive system may also increase the risk for laryngeal health problems in women. The average ambulatory gastric residence time (i.e., time necessary for food to pass from the stomach to the small intestine) has been measured to be longer for females: 4.6 hours compared to 3.4 hours 71. This may be important as many patients with voice problems that precipitate a visit to a voice clinic are also diagnosed with laryngopharyngeal reflux 72,73, which is the backflow or regurgitation of stomach gasses up the esophagus into the pharynx. Such voice problems include minor throat irritations, chronic throat clearing or cough, hoarseness or sore throat (particularly in the morning), and laryngospasm 73,74. While it has been traditionally assumed that more males suffer from reflux, more females actually suffer from endoscopy-negative reflux disease, whose symptoms are similar but without visible endoscopic evidence 75,76. Further, in an examination of emergency room patients at Brigham & Women's Hospital in Boston, 57% of patients with Non-Cardiac Chest Pain (NCCP) were diagnosed with Gastroesophageal Reflux Disease, with more women than men presenting with NCCP. These women tended to have reflux both during supine (sleeping) and upright (awake) periods whereas men tended to experience the symptoms when upright 77. If these results are substantiated and could be expanded to included laryngopharyngeal reflux, such conditions could impede nightly vocal recovery 78 for a female occupational voice user (i.e., reflux while both supine and upright) more than a male (i.e., reflux while upright).
Whole Body Hydration
Gender differences in the body’s water content may also play a role. The adult female body’s water content is lower (female=50%; male= 59%). This difference is related to the disparate fat levels 79. Systemic tissue hydration is vital to healthy vocal performance and, to some extent, self-perceived vocal effort 80–83. Studies suggest that laryngeal tissue viscosity may decrease and vocal fold thickness increase with adequate systemic hydration 84,85. Therefore, if all other things were equal, the lower water content in females may make them more vocally at risk. This disparity might be somewhat mitigated as, when systemic hydration levels are low, the vocal tract, as well as the respiratory system at large, seems to preserve hydration levels longer than other parts of the body 86. However, subclinical systemic dehydration is not uncommon in the general population as hydration levels fluctuate and returning hydration levels to optimal is gradual. Thus, because females have a lower water content percentage than males, they may be more sensitive to these common fluctuations leaving ample windows for voice performance reduction.
Interestingly, higher F0 may also increase sensitivity to hydration. For example, in a fatiguing singing task with non-professional karaoke singers, the effect of hydration and short (1-minute) vocal rests on vocal health was examined 87. The female subjects in the non-hydration/non-rest group had a significant decrease in the highest sustained F0 (i.e., pitch) which could be sung; this decrease was not seen in the other study groups (female hydration/rest, male non-hydration/non-rest, male hydration/rest). While no attempt was made to explain this gender distinction, the absence of vocal rests was probably not the sole cause of the loss of F0; likely hydration played at least a partial role. The potential influence of dehydration at higher pitches is substantiated by a hydration study conducted by Verdolini et al. which used phonation threshold pressure and perceived phonatory effort to demonstrate a progressive sensitivity to dehydration with increasing F0 83. Although this point was not made by the authors of the paper, such a result could imply a greater sensitivity to dehydration (and, thus, increased effort) in females more than males because of their generally higher pitch.
NON-PHYSIOLOGICAL AND BEHAVIORAL DIFFERENCES
Non-physiological and behavioral differences might be another source for women’s increased risk. For example, one aspect which would obviously affect vocal health is gender differences in amount of vocal vibration exposure from vocalizing. Traditionally, women are believed to speak more although no study to date can be found which substantiate this claim with statistical significance. For example, in a widely reported study of 396 university students, no statistically significant gender difference was found: women spoke approximately 16,215 words daily and men spoke approximately 15,669 words 88. However, the dynamics of young adults in university classrooms and social situations may not accurately reflect a typical adult population. Additionally, one recent preliminary study conducted by Hunter and Titze 89 reported a similar trend with teachers, again without statistical significance. Phonation time dose, average loudness, and F0 were calculated using the National Center for Voice and Speech voice dosimetry databank. Occupational voice use (9am–3pm) and non-occupational voice use (4pm–10pm, weekends) were compared from two-week study portions for each subject (8400+ hours). Two key gender-related vocalization trends were presented. First, female teachers vocalized 10% more than males at work. Further, female teachers’ non-occupational vocalization was 7% more than male teachers, reinforcing the need to quantify women’s additional non-occupational vocal load, particularly as the primary caregivers for children in their homes 90.
Another potentially non-physiological difference[1] which may explain the gender disparity in voice disorders is a higher reported incidence of symptoms of depression and anxiety in females in the general population 91–93. Research contends that these symptoms appear to begin at puberty and continue through the child-bearing years 94,95. Wilhelm et al. tracked a group of professional voice users for 30 years (1978 n=170; 2003 n=154) and found that the women reported significantly more “social, simple and combined anxiety disorders” during the study 95. Such stress, anxiety, and depression may have a direct effect on voice production 96. Clinicians have been urged to recognize emotional factors to certain nonorganic voice disorders, which may be caused in part by increased muscle tension from vocal misuse and emotional stress 96. For example, high stress level is one factor contributing to muscle tension dysphonia, associated with tissue changes that affect the biomechanical properties of the vocal folds 97. Muscle tension dysphonia can be experienced as laryngeal discomfort and may lead to other harmful vocal behaviors in the belief they will “clear” the voice (e.g., coughing, throat clearing). Further, emotional stress often causes whole-body musculoskeletal tension, which often extends to the extrinsic laryngeal muscles (neck strap muscles); this tension can change the position of the larynx and/or vocal folds, which would result in a non-optimal position or mechanical stress distribution of the vocal folds 15,98,99. Baker suggests that studies must be conducted to determine the “degree to which the underlying emotional stresses contribute to onset and perpetuation of the excessive laryngeal tension” 100.
Another behavioral difference which may play into gender vocal health differences is the preponderance of eating disorders in females as compared to males: approximately 90–95% of all cases of anorexia nervosa, 80% of bulimia nervosa, and 65% of binge-eating (as opposed to overeating) disorders. Although few studies have examined the direct impact of such disorders on the voice, voluntary vomiting negatively impacts the larynx and studies have demonstrated the increased risk for reflux caused by eating disorders 101,102. As mentioned, many voice problems stem from various types of reflux, including minor throat irritations, chronic throat clearing or cough, hoarseness or sore throat (particularly in the morning), and laryngospasms 73,74. Another result of anorexia is a patulous (or chronically open) Eustachian tube 103, which is likely the reason other studies suggest that anorexia causes autophonia, the hyperperception of one's own voice and breathing 104. With such an effect changing aural feedback of one’s voice, this could impact how often and in what manner one’s voice is used by modifying the type and loudness of phonation—potentially changing to a less optimal production.
Finally, it is possible that gender differences in vocal disorders may reflect the well-documented tendency for women to report vocal symptoms more than men 8, 10, as well as distinct gender approaches to reporting symptoms and completing questionnaires generally. In the 30-year study conducted by Wilhelm et al. described above, while there were no gender differences in the number and type of significant life events were reported, there was a significant difference in how these events were experienced: female subjects judged “unpleasant life events” as more unpleasant than their male counterparts 95. Such a study suggests that it may be possible that some portion of the reported female propensity for voice disorders might be caused by males underreporting their voice issues.
CONCLUSIONS
Throughout voice literature, evidence has accumulated that women are more inclined to vocal health issues than men. In this review, potential causes for this gender difference were reviewed using both voice-specific literature and general medical and biological literature. These causes included differences related to laryngeal physiology, hormone differences, other non-laryngeal physiology, and non-physiological and/or behavioral characteristics. Thus, the voice, with its close proximity and connections to the major lifelines of the body, seems to be susceptible to many physiological systems and functions which on first glance would appear unrelated to the voice.
This review is intended to increase awareness of gender differences as they relate to voice disorders to guide clinical care. Further, it is meant to identify potential avenues of future gender-based studies. Specifically, gender-specific studies of risk factors for various types of voice disorders, treatment development and response, and voice disorder prevention are required. To this end, specific terms commonly used in describing vocal health (e.g., vocal fatigue or phonotrauma) and vocal behaviors (e.g., vocal abuse and misuse, or inadequate breath support) also need to be more rigorously defined with specific, widely accepted characteristics. These research priorities are necessary steps toward improving vocal health in women.
Acknowledgments
Funding for this work was in part provided by the National Institute on Deafness and Other Communication Disorders, grant number 1R01 DC04224. We express appreciation to The University of Utah Vice President for Research for support of the National Center for Voice and Speech.
Footnotes
While we recognize that depression and anxiety may have a physiologic basis, they also have such a vast array of potential causes – both non-physiologic and physiologic – that we have chosen to place it with the non-physiologic category for convenience sake alone.
References
- 1.Titze IR, Lemke J, Montequin D. Populations in the U.S. workforce who rely on voice as a primary tool of trade: a preliminary report. J Voice. 1997 September;11(3):254–9. doi: 10.1016/s0892-1997(97)80002-1. [DOI] [PubMed] [Google Scholar]
- 2.McHenry MA, Carlson HK. The vocal health of auctioneers. Logoped Phoniatr Vocol. 2004;29(1):41–7. doi: 10.1080/14015430310022545. [DOI] [PubMed] [Google Scholar]
- 3.Heidel SE, Torgerson JK. Vocal problems among aerobic instructors and aerobic participants. J Commun Disord. 1993 September;26(3):179–91. doi: 10.1016/0021-9924(93)90007-w. [DOI] [PubMed] [Google Scholar]
- 4.Verdolini K, Ramig LO. Review: occupational risks for voice problems. Logoped Phoniatr Vocol. 2001;26(1):37–46. [PubMed] [Google Scholar]
- 5.Williams NR. Occupational groups at risk of voice disorders: a review of the literature. Occup Med (Lond) 2003 October;53(7):456–60. doi: 10.1093/occmed/kqg113. [DOI] [PubMed] [Google Scholar]
- 6.Sapir S, Keidar A, Mathers-Schmidt B. Vocal attrition in teachers: survey findings. Eur J Disord Commun. 1993;28(2):177–85. doi: 10.3109/13682829309041465. [DOI] [PubMed] [Google Scholar]
- 7.Munier C, Kinsella R. The prevalence and impact of voice problems in primary school teachers. Occup Med (Lond) 2008 January;58(1):74–6. doi: 10.1093/occmed/kqm104. [DOI] [PubMed] [Google Scholar]
- 8.Roy N, Merrill RM, Gray SD, Smith EM. Voice disorders in the general population: prevalence, risk factors, and occupational impact. Laryngoscope. 2005 November;115(11):1988–95. doi: 10.1097/01.mlg.0000179174.32345.41. [DOI] [PubMed] [Google Scholar]
- 9.Morton V, Watson DR. The teaching voice: problems and perceptions. Log Phon Vocol. 1998;23(3):133–9. [Google Scholar]
- 10.Roy N, Merrill RM, Thibeault S, Parsa RA, Gray SD, Smith EM. Prevalence of voice disorders in teachers and the general population. Journal of Speech and Hearing Research. 2004 April;47(2):281–93. doi: 10.1044/1092-4388(2004/023). [DOI] [PubMed] [Google Scholar]
- 11.Smith E, Kirchner HL, Taylor M, Hoffman H, Lemke JH. Voice problems among teachers: differences by gender and teaching characteristics. J Voice. 1998 September;12(3):328–34. doi: 10.1016/s0892-1997(98)80022-2. [DOI] [PubMed] [Google Scholar]
- 12.Sliwinska-Kowalska M, Niebudek-Bogusz E, Fiszer M, et al. The prevalence and risk factors for occupational voice disorders in teachers. Folia Phoniatr Logop. 2006;58(2):85–101. doi: 10.1159/000089610. [DOI] [PubMed] [Google Scholar]
- 13.Jones K, Sigmon J, Hock L, Nelson E, Sullivan M, Ogren F. Prevalence and risk factors for voice problems among telemarketers. Arch Otolaryngol Head Neck Surg. 2002 May;128(5):571–7. doi: 10.1001/archotol.128.5.571. [DOI] [PubMed] [Google Scholar]
- 14.Miller MK, Verdolini K. Frequency and risk factors for voice problems in teachers of singing and control subjects. J Voice. 1995 December;9(4):348–62. doi: 10.1016/s0892-1997(05)80197-3. [DOI] [PubMed] [Google Scholar]
- 15.Thomas G, de Jong FI, Cremers CW, Kooijman PG. Prevalence of voice complaints, risk factors and impact of voice problems in female student teachers. Folia Phoniatr Logop. 2006;58(2):65–84. doi: 10.1159/000089609. [DOI] [PubMed] [Google Scholar]
- 16.Titze IR, Sundberg J. Vocal intensity in speakers and singers. J Acoust Soc Am. 1992 May;91(5):2936–46. doi: 10.1121/1.402929. [DOI] [PubMed] [Google Scholar]
- 17.Murry T, Xu JJ, Woodson GE. Glottal configuration associated with fundamental frequency and vocal register. J Voice. 1998;12(1):44–9. doi: 10.1016/s0892-1997(98)80074-x. [DOI] [PubMed] [Google Scholar]
- 18.Hirano M, Vennard W, Ohala J. Regulation of register, pitch and intensity of voice. An electromyographic investigation of intrinsic laryngeal muscles. Folia Phoniatr (Basel) 1970;22(1):1–20. doi: 10.1159/000263363. [DOI] [PubMed] [Google Scholar]
- 19.Honda K. Variability analysis of laryngeal muscle activities. In: Titze IR, Scherer RC, editors. Vocal fold physiology: biomechanics, acoustics and phonatory control. Denver, CO: The Denver Center for the Performing Arts; 1983. pp. 127–37. [Google Scholar]
- 20.Yoshioka H. Laryngeal adjustments in the production of the fricative consonants and devoiced vowels in Japanese. Phonetica. 1981;38(4):236–51. doi: 10.1159/000260027. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Hunter EJ, Titze IR. Comparison of human, canine, and ovine laryngeal dimensions. Ann Otol Rhinol Laryngol. 2004 January;113(1):60–8. doi: 10.1177/000348940411300114. [DOI] [PubMed] [Google Scholar]
- 22.Hunter EJ, Titze IR. Individual subject laryngeal dimensions of multiple mammalian species for biomechanical models. Ann Otol Rhinol Laryngol. 2005 October;114(10):809–18. doi: 10.1177/000348940511401012. [DOI] [PubMed] [Google Scholar]
- 23.Kahane JC. A morphological study of the human prepubertal and pubertal larynx. Am J Anat. 1978 January;151(1):11–9. doi: 10.1002/aja.1001510103. [DOI] [PubMed] [Google Scholar]
- 24.Titze IR. Physiologic and Acoustic Differences Between Male and Female Voices. J Acoust Soc Am. 1989 April;85(4):1699–707. doi: 10.1121/1.397959. [DOI] [PubMed] [Google Scholar]
- 25.Titze IR. Physiology of the Female Larynx. J Acoust Soc Am. 1988 [Google Scholar]
- 26.Baken RJ, Orlikoff RF. Clinical Measurement of Speech and Voice. 2. San Diego: Singular Publishing Group; 2000. [Google Scholar]
- 27.Titze IR, Hunter EJ. Normal vibration frequencies of the vocal ligament. J Acoust Soc Am. 2004 May;115(5 Pt 1):2264–9. doi: 10.1121/1.1698832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000 October;109(10 Pt 1):913–20. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 29.Chan RW, Fu M, Young L, Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng. 2007 August;35(8):1471–83. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- 30.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988 April;83(4):1536–52. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 31.Titze IR, Jiang J, Druker DG. Preliminaries to the body-cover model of pitch control. J Voice. 1988;1(4):314–9. [Google Scholar]
- 32.Ward PD, Thibeault SL, Gray SD. Hyaluronic acid: its role in voice. J Voice. 2002 September;16(3):303–9. doi: 10.1016/s0892-1997(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 33.Chan RW, Titze IR. Hyaluronic acid (with fibronectin) as a bioimplant for the vocal fold mucosa. Laryngoscope. 1999 July;109(7 Pt 1):1142–9. doi: 10.1097/00005537-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001 June;124(6):607–14. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 35.Thibeault SL, Rousseau B, Welham NV, Hirano S, Bless DM. Hyaluronan levels in acute vocal fold scar. Laryngoscope. 2004 April;114(4):760–4. doi: 10.1097/00005537-200404000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001 May;111(5):907–11. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Damrose EJ. Quantifying the impact of androgen therapy on the female larynx. Auris Nasus Larynx. 2009 February;36(1):110–2. doi: 10.1016/j.anl.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Newman SR, Butler J, Hammond EH, Gray SD. Preliminary report on hormone receptors in the human vocal fold. J Voice. 2000 March;14(1):72–81. doi: 10.1016/s0892-1997(00)80096-x. [DOI] [PubMed] [Google Scholar]
- 39.Amir O, Biron-Shental T. The impact of hormonal fluctuations on female vocal folds. Curr Opin Otolaryngol Head Neck Surg. 2004 June;12(3):180–4. doi: 10.1097/01.moo.0000120304.58882.94. [DOI] [PubMed] [Google Scholar]
- 40.Chae SW, Choi G, Kang HJ, Choi JO, Jin SM. Clinical analysis of voice change as a parameter of premenstrual syndrome. J Voice. 2001 June;15(2):278–83. doi: 10.1016/S0892-1997(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 41.Davis CB, Davis ML. The effects of premenstrual syndrome (PMS) on the female singer. J Voice. 1993 December;7(4):337–53. doi: 10.1016/s0892-1997(05)80257-7. [DOI] [PubMed] [Google Scholar]
- 42.Mendes-Laureano J, Sa MF, Ferriani RA, et al. Comparison of fundamental voice frequency between menopausal women and women at menacme. Maturitas. 2006 September 20;55(2):195–9. doi: 10.1016/j.maturitas.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Schneider B, van TM, Hanke G, Bigenzahn W, Huber J. Voice impairment and menopause. Menopause. 2004 March;11(2):151–8. doi: 10.1097/01.gme.0000094192.24934.46. [DOI] [PubMed] [Google Scholar]
- 44.Van Lierde KM, Claeys S, De BM, Van CP. Response of the female vocal quality and resonance in professional voice users taking oral contraceptive pills: a multiparameter approach. Laryngoscope. 2006 October;116(10):1894–8. doi: 10.1097/01.mlg.0000235917.06088.b1. [DOI] [PubMed] [Google Scholar]
- 45.Abitbol J, Abitbol P, Abitbol B. Sex hormones and the female voice. J Voice. 1999;13(3):424–46. doi: 10.1016/s0892-1997(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 46.Amir O, Kishon-Rabin L, Muchnik C. The effect of oral contraceptives on voice: preliminary observations. J Voice. 2002 June;16(2):267–73. doi: 10.1016/s0892-1997(02)00096-6. [DOI] [PubMed] [Google Scholar]
- 47.Amir O, Kishon-Rabin L. Association between birth control pills and voice quality. Laryngoscope. 2004 June;114(6):1021–6. doi: 10.1097/00005537-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Amir O, Biron-Shental T, Shabtai E. Birth control pills and nonprofessional voice: acoustic analyses. J Speech Lang Hear Res. 2006 October;49(5):1114–26. doi: 10.1044/1092-4388(2006/080). [DOI] [PubMed] [Google Scholar]
- 49.Silverman EM, Zimmer CH. Effect of the menstrual cycle on voice quality. Arch Otolaryngol. 1978 January;104(1):7–10. doi: 10.1001/archotol.1978.00790010011003. [DOI] [PubMed] [Google Scholar]
- 50.Chernobelsky SI. A study of menses-related changes to the larynx in singers with voice abuse. Folia Phoniatr Logop. 2002 January;54(1):2–7. doi: 10.1159/000048591. [DOI] [PubMed] [Google Scholar]
- 51.Raj A, Gupta B, Chowdhury A, Chadha S. A Study of Voice Changes in Various Phases of Menstrual Cycle and in Postmenopausal Women. J Voice. 2009 January 28; doi: 10.1016/j.jvoice.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.D'haeseleer E, Depypere H, Claeys S, Van BJ, Van LK. The menopause and the female larynx, clinical aspects and therapeutic options: a literature review. Maturitas. 2009 September 20;64(1):27–32. doi: 10.1016/j.maturitas.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Arendt-Nielsen L, Bajaj P, Drewes AM. Visceral pain: gender differences in response to experimental and clinical pain. Eur J Pain. 2004 October;8(5):465–72. doi: 10.1016/j.ejpain.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003 February;101(3):259–66. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 55.Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995 June;61(3):435–9. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- 56.Feine JS, Bushnell MC, Miron D, Duncan GH. Sex differences in the perception of noxious heat stimuli. Pain. 1991 March;44(3):255–62. doi: 10.1016/0304-3959(91)90094-E. [DOI] [PubMed] [Google Scholar]
- 57.McEwen BS, Alves SE, Bulloch K, Weiland NG. Clinically relevant basic science studies of gender differences and sex hormone effects. Psychopharmacol Bull. 1998;34(3):251–9. [PubMed] [Google Scholar]
- 58.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997 September;20(3):371–80. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 59.Lipton RB, Stewart WF. Migraine in the United States: a review of epidemiology and health care use. Neurology. 1993 June;43(6 Suppl 3):S6–10. [PubMed] [Google Scholar]
- 60.Ostensen M, Rugelsjoen A, Wigers SH. The effect of reproductive events and alterations of sex hormone levels on the symptoms of fibromyalgia. Scand J Rheumatol. 1997;26(5):355–60. doi: 10.3109/03009749709065698. [DOI] [PubMed] [Google Scholar]
- 61.Silberstein SD. Sex hormones and headache. Rev Neurol (Paris) 2000;156(Suppl 4):4S30–41. [PubMed] [Google Scholar]
- 62.Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007 November;132(Suppl 1):S60–S67. doi: 10.1016/j.pain.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Thorpe CW, Cala SJ, Chapman J, Davis PJ. Patterns of breath support in projection of the singing voice. J Voice. 2001 March;15(1):86–104. doi: 10.1016/S0892-1997(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 64.Berry DA, Titze IR. Normal modes in a continuum model of vocal fold tissues. J Acoust Soc Am. 1996 November;100(5):3345–54. doi: 10.1121/1.416975. [DOI] [PubMed] [Google Scholar]
- 65.Leanderson R, Sundberg J. Breathing for singing. J Voice. 1988;2(1):2–12. [Google Scholar]
- 66.Stathopoulos ET, Sapienza C. Respiratory and laryngeal function of women and men during vocal intensity variation. J Speech Hear Res. 1993 February;36(1):64–75. doi: 10.1044/jshr.3601.64. [DOI] [PubMed] [Google Scholar]
- 67.Berry D. Mechanism of modal and non-modal phonation. J Phonetics. 2001;29:431–50. [Google Scholar]
- 68.Jiang JJ, Titze IR. Measurement of vocal fold intraglottal pressure and impact stress. J Voice. 1994 June;8(2):132–44. doi: 10.1016/s0892-1997(05)80305-4. [DOI] [PubMed] [Google Scholar]
- 69.Sapienza C, Stathopoulos ET, Brown WS., Jr Speech breathing during reading in women with vocal nodules. J Voice. 1997;11(2):195–201. doi: 10.1016/s0892-1997(97)80078-1. [DOI] [PubMed] [Google Scholar]
- 70.Preciado-Lopez J, Perez-Fernandez C, Calzada-Uriondo M, Preciado-Ruiz P. Epidemiological study of voice disorders among teaching professionals of La Rioja, Spain. J Voice. 2008 July;22(4):489–508. doi: 10.1016/j.jvoice.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Mojaverian P, Vlasses PH, Kellner PE, Rocci ML., Jr Effects of gender, posture, and age on gastric residence time of an indigestible solid: pharmaceutical considerations. Pharm Res. 1988 October;5(10):639–44. doi: 10.1023/a:1015922903843. [DOI] [PubMed] [Google Scholar]
- 72.Selby JC, Gilbert HR, Lerman JW. Perceptual and acoustic evaluation of individuals with laryngopharyngeal reflux pre- and post-treatment. J Voice. 2003 December;17(4):557–70. doi: 10.1067/s0892-1997(03)00017-1. [DOI] [PubMed] [Google Scholar]
- 73.Sereg-Bahar M, Jansa R, Hocevar-Boltezar I. Voice disorders and gastroesophageal reflux. Log Phon Vocol. 2005;30(3–4):120–4. doi: 10.1080/14015430500320182. [DOI] [PubMed] [Google Scholar]
- 74.Oguz H, Tarhan E, Korkmaz M, et al. Acoustic analysis findings in objective laryngopharyngeal reflux patients. J Voice. 2007 March;21(2):203–10. doi: 10.1016/j.jvoice.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Fujiwara Y, Higuchi K, Shiba M, et al. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am J Gastroenterol. 2005 April;100(4):754–8. doi: 10.1111/j.1572-0241.2005.40966.x. [DOI] [PubMed] [Google Scholar]
- 76.Chey WD. Endoscopy-negative reflux disease: concepts and clinical practice. Am J Med. 2004 September 6;117(Suppl 5A):36S–43S. doi: 10.1016/j.amjmed.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Thompson SE, Liu JJ, Saltzman JR, Hua L, Zane R. Prevalence of Gastroesophageal Reflux Disease in Patients with Non-Cardiac Chest Pain Presenting to the Emergency Department. The American Journal of Gastroenterology. 2007;102:S119–S156. [Google Scholar]
- 78.Hunter EJ, Titze IR. Quantifying vocal fatigue recovery: Dynamic vocal recovery trajectories after a vocal loading exercise. Ann Otol Rhinol Laryngol. 2009 doi: 10.1177/000348940911800608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000 April;80(2):649–80. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 80.Solomon NP, DiMattia MS. Effects of a vocally fatiguing task and systemic hydration on phonation threshold pressure. J Voice. 2000 September;14(3):341–62. doi: 10.1016/s0892-1997(00)80080-6. [DOI] [PubMed] [Google Scholar]
- 81.Verdolini-Marston K, Titze I, Druker D. Changes in phonation threshold pressure with induced conditions of hydration. J Voice. 1990;4:142–51. [Google Scholar]
- 82.Solomon NP, Glaze LE, Arnold RR, van Mersbergen M. Effects of a vocally fatiguing task and systemic hydration on men's voices. J Voice. 2003 March;17(1):31–46. doi: 10.1016/s0892-1997(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 83.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. Journal of Speech and Hearing Research. 1994 October;37(5):1001–7. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 84.Ori Y, Sabo R, Binder Y, et al. Effect of hemodialysis on the thickness of vocal folds: a possible explanation for postdialysis hoarseness. Nephron Clin Pract. 2006;103(4):c144–c148. doi: 10.1159/000092911. [DOI] [PubMed] [Google Scholar]
- 85.Gunter HE. Modeling mechanical stresses as a factor in the etiology of benign vocal fold lesions. Journal of Biomechanics. 2004 July;37(7):1119–24. doi: 10.1016/j.jbiomech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Verdolini K, Min Y, Titze IR, et al. Biological mechanisms underlying voice changes due to dehydration. Journal of Speech, Language, & Hearing Research. 2002 April;45(2):268–81. doi: 10.1044/1092-4388(2002/021). [DOI] [PubMed] [Google Scholar]
- 87.Yiu EM, Chan RM. Effect of hydration and vocal rest on the vocal fatigue in amateur karaoke singers. J Voice. 2003 June;17(2):216–27. doi: 10.1016/s0892-1997(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 88.Mehl MR, Vazire S, Ramirez-Esparza N, Slatcher RB, Pennebaker JW. Are women really more talkative than men? Science. 2007 July 6;317(5834):82. doi: 10.1126/science.1139940. [DOI] [PubMed] [Google Scholar]
- 89.Hunter EJ, Titze IR. Variations in intensity, fundamental frequency, and voicing for teachers in occupational versus non-occupational settings. Journal of Speech, Language, & Hearing Research. 2010 doi: 10.1044/1092-4388(2009/09-0040). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vilkman E. Occupational safety and health aspects of voice and speech professions. Folia Phoniatrica et Logopaedica. 2004 July;56(4):220–53. doi: 10.1159/000078344. [DOI] [PubMed] [Google Scholar]
- 91.Dietrich M, Verdolini AK, Gartner-Schmidt J, Rosen CA. The frequency of perceived stress, anxiety, and depression in patients with common pathologies affecting voice. J Voice. 2008 July;22(4):472–88. doi: 10.1016/j.jvoice.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Jurado D, Gurpegui M, Moreno O, Fernandez MC, Luna JD, Galvez R. Association of personality and work conditions with depressive symptoms. Eur Psychiatry. 2005 May;20(3):213–22. doi: 10.1016/j.eurpsy.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993 October;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 94.Jorm AF. Sex and age differences in depression: a quantitative synthesis of published research. Aust N Z J Psychiatry. 1987 March;21(1):46–53. doi: 10.3109/00048678709160898. [DOI] [PubMed] [Google Scholar]
- 95.Wilhelm K, Parker G, Geerligs L, Wedgwood L. Women and depression: a 30 year learning curve. Aust N Z J Psychiatry. 2008 January;42(1):3–12. doi: 10.1080/00048670701732665. [DOI] [PubMed] [Google Scholar]
- 96.Seifert E, Kollbrunner J. Stress and distress in non-organic voice disorder. Swiss Med Wkly. 2005 July 9;135(27–28):387–97. doi: 10.4414/smw.2005.10346. [DOI] [PubMed] [Google Scholar]
- 97.Hsiao TY, Liu CM, Hsu CJ, Lee SY, Lin KN. Vocal fold abnormalities in laryngeal tension-fatigue syndrome. J Formos Med Assoc. 2001 December;100(12):837–40. [PubMed] [Google Scholar]
- 98.Altman KW, Atkinson C, Lazarus C. Current and emerging concepts in muscle tension dysphonia: a 30-month review. J Voice. 2005 June;19(2):261–7. doi: 10.1016/j.jvoice.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 99.Kooijman PG, de Jong FI, Oudes MJ, Huinck W, van Acht H, Graamans K. Muscular tension and body posture in relation to voice handicap and voice quality in teachers with persistent voice complaints. Folia Phoniatr Logop. 2005 May;57(3):134–47. doi: 10.1159/000084134. [DOI] [PubMed] [Google Scholar]
- 100.Baker J. Psychogenic voice disorders--heroes or hysterics? A brief overview with questions and discussion. Logoped Phoniatr Vocol. 2002;27(2):84–91. doi: 10.1080/140154302760409310. [DOI] [PubMed] [Google Scholar]
- 101.Bartlett D. Intrinsic causes of erosion. Monogr Oral Sci. 2006;20:119–39. doi: 10.1159/000093359. [DOI] [PubMed] [Google Scholar]
- 102.Hayashi M, Shimizu K, Takeshige F, Ebisu S. Restoration of erosion associated with gastroesophageal reflux caused by anorexia nervosa using ceramic laminate veneers: a case report. Oper Dent. 2007 May;32(3):306–10. doi: 10.2341/06-102. [DOI] [PubMed] [Google Scholar]
- 103.Karwautz A, Hafferl A, Ungar D, Sailer H. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999 April;25(3):353–5. doi: 10.1002/(sici)1098-108x(199904)25:3<353::aid-eat16>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 104.Finsten RM, Faguet RA. Autophonia associated with an atypical eating disorder. J Clin Psychiatry. 1983 May;44(5):191. [PubMed] [Google Scholar]