Abstract
In earlier work, we compared the amount of newly fixed nitrogen (N, as synthetic fertilizer and biologically fixed N) entering agricultural systems globally to the total emission of nitrous oxide (N2O). We obtained an N2O emission factor (EF) of 3–5%, and applied it to biofuel production. For ‘first-generation’ biofuels, e.g. biodiesel from rapeseed and bioethanol from corn (maize), that require N fertilizer, N2O from biofuel production could cause (depending on N uptake efficiency) as much or more global warming as that avoided by replacement of fossil fuel by the biofuel. Our subsequent calculations in a follow-up paper, using published life cycle analysis (LCA) models, led to broadly similar conclusions. The N2O EF applies to agricultural crops in general, not just to biofuel crops, and has made possible a top-down estimate of global emissions from agriculture. Independent modelling by another group using bottom-up IPCC inventory methodology has shown good agreement at the global scale with our top-down estimate. Work by Davidson showed that the rate of accumulation of N2O in the atmosphere in the late nineteenth and twentieth centuries was greater than that predicted from agricultural inputs limited to fertilizer N and biologically fixed N (Davidson, E. A. 2009 Nat. Geosci. 2, 659–662.). However, by also including soil organic N mineralized following land-use change and NOx deposited from the atmosphere in our estimates of the reactive N entering the agricultural cycle, we have now obtained a good fit between the observed atmospheric N2O concentrations from 1860 to 2000 and those calculated on the basis of a 4 per cent EF for the reactive N.
Keywords: nitrous oxide, biofuels, reactive N, agriculture, N2O emission factor, life cycle analysis
1. Introduction
Data from ice-core analysis show that for thousands of years, atmospheric N2O mixing ratios (i.e. the concentrations in dry air) were close to 270 ppbv [1]. In more recent times, however, a 20 per cent increase has occurred. The increase began around AD 1850 and the concentration now exceeds 320 ppbv. Since the 1960s, the annual increase has been about 0.7 ppbv [2]. The Intergovernmental Panel on Climate Change (IPCC) Third Assessment Report [3] concluded that the primary driver of this increase was enhanced by microbial production of N2O in expanding and fertilized agricultural lands. This production requires nitrogen (N) in a reactive form, Nr [4], as substrate—virtually any form other than dinitrogen, N2. Reactive N is introduced into the natural terrestrial environment primarily by biological N fixation, together with a smaller contribution as NOx formed by lightning. Galloway et al. [5] estimated this input in 1860, at the beginning of the industrial age, at ca 140 Tg (million metric tonnes) per year of Nr. Within the past few decades, human activities have roughly doubled this supply, mainly through synthetic fixation of N by the Haber–Bosch process [6], and also through industrial processes and as a by-product of fossil fuel combustion.
The main microbial reactions involved in the production of N2O are nitrification (oxidation of ammonium to nitrite) and denitrification (reduction of nitrate, via N2O, to N2). Nitrification is essentially an aerobic process and denitrification an anaerobic process. The main source of emissions is N-fertilized agricultural land—both directly from the soil surface and also from the considerable portion of the N additions to the land that is lost to the environment through runoff, leaching of nitrate and ammonia volatilization and subsequently nitrified and denitrified [7,8].
Two general methods used to estimate soil N2O emissions can be broadly considered as either (i) bottom-up approaches based on estimates of emission rates from land and waters or (ii) top-down approaches based on changes in the atmospheric concentration of N2O and estimates of sink size. Here, we describe how we used a global top-down approach to calculate the fraction of all newly fixed N entering terrestrial systems that is converted to N2O, and how we applied the outcome to determine the extent to which a reduction of global warming by the use of crop-based liquid biofuels instead of fossil fuels would be offset by the associated N2O emissions. We also examine the impact of more complete life cycle analyses (LCAs) on our initial conclusions, the implications of generalizing the results derived for biofuel crops to agriculture in general, and the extent to which the global N2O emission factor (EF) obtained by our approach can be reconciled with those derived by Davidson [9] and the IPCC [7,8].
2. Biofuels and global warming
We [10] used the data in table 1 to calculate the global EFs for N2O for the newly fixed N entering terrestrial and coastal ecosystems in 1860 when there had not yet been a major perturbation resulting from human activities, and also for 2000 when the annual quantity of new reactive N had more or less doubled. The results showed very similar ranges for the EFs: 4.4–5.1% for 1860 and 3.8–5.1% for the year 2000 (table 1).
Table 1.
Reactive N inputs to the biosphere and atmosphere, atmospheric N2O mixing ratios and associated N2O emission factors in 1860 and 2000 (after Crutzen et al. [10]).
| source/process | annual rate/ EF | reference |
|---|---|---|
| 1860 (atmospheric mixing ratio = 270 ppbv) | ||
| [A] total N2O source | 10.2 Tg N2O-N yr−1 | [11] |
| [B] land and coastal zone source | 6.2–7.2 Tg N2O-N yr−1 | [11] |
| [C] input of new reactive N | 141 Tg N yr−1 | [5] |
| [D] N2O emission factor (i.e. [B]/[C]) | 4.4–5.1% | |
| 2000 (atmospheric mixing ratio = 315 ppbv) | ||
| [E] atmospheric increase | 3.9 Tg N2O-N yr−1 | [11] |
| [F] photochemical loss (stratosphere) | 11.9 Tg N2O-N yr−1 | [11] |
| [G] total N2O source (= [E] + [F]) | 15.8 Tg N2O-N yr−1 | |
| [H] decrease in natural source | 0–0.9 Tg N2O-N yr−1 | [12] |
| [I] total anthropogenic source (i.e. [G] − [A] + [H]) | 5.6–6.5 Tg N2O-N yr−1 | |
| [J] non-biological anthropogenic source | 0.7–1.3 Tg N2O-N yr−1 | [11] |
| [K] land/coastal zone biological source (=[I] − [J]) | 4.3–5.8 Tg N2O-N yr−1 | |
| [L] new terrestrial anthropogenic N input | 114 Tg N yr−1 | [5,13] |
| [M] N2O emission factor for new N (i.e. [K]/[L]) | 3.8–5.1% |
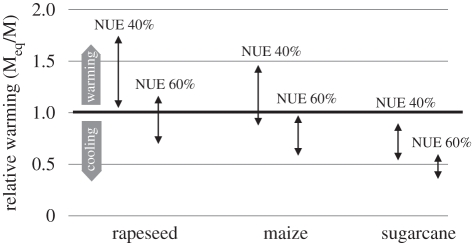
We adopted a conservative lower limit of 3 per cent for the EF in 2000, rather than the calculated value of 3.8 per cent, and rounded the upper limit down to 5 per cent. We applied these values to the fertilizer N input for the production of maize and sugarcane for bioethanol and rapeseed oil for biodiesel—together with the global average N-use efficiency (NUE) by crops of 40 per cent [5,14,15]—and found that for rapeseed and maize, the global warming impact of the resulting N2O emissions matched or even exceeded the corresponding ‘cooling’ achieved by the reduction in CO2 emissions resulting from the replacement of fossil fuels by the biofuels, as shown in figure 1. There are substantial differences in NUE between regions and farming systems, and figure 1 also shows the pro-rata reduction in calculated global warming by the biofuels if the NUE is set at 60 per cent.
Figure 1.
Global warming effect of N2O emissions, relative to cooling effect of replacement of fossil fuel, for ‘first-generation’ biofuels rapeseed biodiesel, corn (maize) bioethanol and sugarcane bioethanol; black arrows show range for global N2O EFs of 3% (bottom) to 5% (top), at two levels of nitrogen-use efficiency (NUE) by the crop: 40% and 60%. Bold horizontal line indicates transition between net warming and net cooling. Based on data in Crutzen et al. [10].
Although figure 1 indicates net cooling for sugarcane bioethanol, in contrast with rapeseed- and maize-based fuels, the overall balance may not be quite so favourable. Lisboa et al. [16] report that the mean EF for direct N2O emissions from sugarcane fields, based on published data, is about 3.9 per cent, even with the exclusion of very high emissions from sites with unusual soil conditions. If one were to include the inevitable associated indirect emissions, the total EF for this dataset would be of the order of the 5 per cent used to model the upper end of the relative warming range shown in figure 1, and thus any global cooling achieved by the use of sugarcane ethanol would be very dependent on the NUE of the crop. Smeets et al. [17] also concluded that N2O emissions can have an important impact on the overall greenhouse gas (GHG) balance of biofuels. This conclusion was reached even though their analysis included only some aspects of N2O emissions and did not account for locations, such as those discussed by Lisboa et al. [16] or Weier [18], where N2O emissions from sugarcane fields were large.
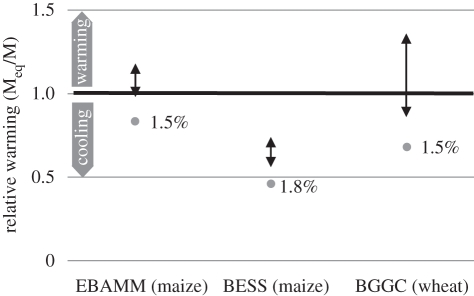
Our original study [10] only took into consideration the N2O emissions associated with the biofuel crop production, thus ignoring the additional GHG emissions associated with fertilizer production, transport and energy use on the farm and in the biofuel refinery. Conversely, no account was taken of the energy saving associated with the biofuel coproducts that can replace grain and soya bean meal as animal feed. However, in subsequent work [19], we introduced the global N2O EF range of 3–5% into three existing LCA models that included GHG emissions from the various stages of crop production and fuel refining: two for maize in the USA and one for wheat in the UK. The EBAMM model [20] includes GHG emissions from fertilizer manufacture, crop production, transport of feedstock to the refinery and production, distribution and use of the biofuel; the EF for N2O is set at 1.5 per cent of fertilizer N input. The model also takes account of coproducts that can replace other sources of animal feed. The BESS model [21] also analyses GHG emissions for maize bioethanol production, including those associated with crop production, the refinery and cattle on feedlots that use the coproduct animal feed produced. The total N2O emissions used in the model are ca 1.8 per cent of fertilizer N input. The Bioethanol GHG Calculator (BGGC) [22,23] calculates the emissions associated with the whole production train for bioethanol from wheat in the UK, and as in EBAMM, the EF for N2O is set at 1.5 per cent of fertilizer N input. The insertion of the higher EFs for N2O (3 and 5%) changed the GHG balance significantly for all three models; for EBAMM and BGGC, the predicted net outcomes in terms of relative warming/cooling were broadly similar to those predicted by the original method of Crutzen et al. [10], although the BESS model showed a greater GHG saving (figure 2).
Figure 2.
Comparison of global warming effect of N2O emissions, relative to cooling effect of replacement of fossil fuel, using the EBAMM model [20] and the BESS model [21] for maize-based ethanol production in the USA, and the Bioethanol GHG Calculator (BGGC) for wheat-based bioethanol production in the UK [22,23]. Grey circles show cooling effects using the respective internal model N2O emission estimates (1.5, 1.8 and 1.5% of N input, respectively); black arrows show ranges of warming/cooling effects using global N2O EFs of 3% (bottom) to 5% (top), taken from Crutzen et al. [10]. Bold horizontal line indicates transition between net warming and net cooling, as in figure 1. Based on data in Mosier et al. [19].
Based on the EBAMM analyses, net GHG emissions from the average US Corn Belt maize-ethanol production do not fulfil the requirement in the US Energy Independence and Security Act [24] for a 20 per cent reduction compared with emissions from fossil fuels for renewable fuel production, whereas in all cases tested BESS estimates indicate that maize ethanol decreases net GHG emissions by more than the EISA minimum requirement. In the European Union, the current requirement for GHG savings using biofuels is that total GHG emissions must be at least 35 per cent below fossil fuel emissions [25], and the calculations with BGGC show that wheat-based bioethanol just fails to comply at an N2O EF of 1.5 per cent, and the GHG balance becomes even more unfavourable at the higher EFs (figure 2).
Recent LCA calculations for bioethanol production from locally grown wheat in Sweden, using actual N2O emission data for two farms on mineral soils, show a similar picture, with only a 50 per cent chance of emissions reductions meeting the EU minimum requirement [26]. Of the arable land in Sweden, 9 per cent is on organic soils, where stored N is released by mineralization, causing emissions of the order of 10 kg N2O-N ha−1 yr−1 when cultivated for cereal crops [27]; 28 per cent of the organic soils in Sweden are used in this way [28]. There is no regulation controlling which soil type can be used for biofuel production. Taking the emissions from organic soils into account increases the regional average emission by almost 1 kg N2O-N ha−1 yr−1, making biofuel production from arable crops impossible under the EU's 35 per cent GHG savings rule [26].
The global warming implications of biofuel production extend beyond those resulting from making full allowance for N2O emissions. The additional demand for grains, oilseeds and sugars brought about by increased biofuel production is expected to indirectly bring about the conversion of land currently under forest or other natural ecosystems into agriculture, with the concomitant release into the atmosphere of carbon stored in trees and soil. This land is not necessarily in the EU or the USA, but may be anywhere in the developing world. The increase in global warming engendered by the carbon release from this so-called indirect land-use change will cancel out any benefit derived from the biofuel for decades or even centuries to come [29,30]. Using linked economic and terrestrial biogeochemistry models, Melillo et al. [31] predicted that indirect land use will be responsible for substantially more carbon loss than direct land use; however, because of predicted increases in fertilizer use, N2O emissions will become more important than carbon losses themselves in terms of warming potential. Erisman et al. [32] analysed the current knowledge of fertilizer N use and global biofuel production and concluded that criteria for sustainable biofuel production should include the disturbance of the N cycle for biomass options that require additional fertilizer inputs.
3. N2O emissions from global agriculture
The top-down approach [10] for estimating the impact of newly fixed N on N2O emissions was developed in the context of biofuel crop production, but applies equally to agriculture in general. The basis of our methodology is that the newly fixed N entering agricultural systems (synthetic fertilizer N and N from biological nitrogen fixation (BNF)) is regarded as the source of all agriculture-related N2O emissions, including: direct emissions from N fertilizer added to soils and N mineralized from crop residues following cultivation or grassland renewal; emissions from dung and urine from livestock (both grazing and housed) fed variously on N-fertilized grain crops and on feeds containing BNF-N; and indirect emissions from leached N leaving agricultural fields and entering water systems, and from volatilized N deposited onto natural ecosystems.
In contrast, in the IPCC approach [8], emissions from crop residues and mineralization are included in the direct emissions and are assigned the same EF—a default value of 1 per cent; separate EFs are used for emissions from grazing animals (2%), and the N source here is quantified on the basis of the N excreted, and essentially is treated as an additional N source, not as fertilizer- or BNF-derived N. Indirect emissions from N lost by leaching, runoff or volatilization are estimated at 0.3–0.4% of the N applied to the land. Summing the individual sources gives the total emission from agriculture. Each of the source terms in the bottom-up IPCC method is very uncertain. However, their sum is consistent with the total derived by the top-down methodology, as shown by Del Grosso et al. [33], who calculated that approximately 5.8 Tg of N as N2O is currently emitted annually from agricultural systems at the global scale. This is close to the middle of the range (4.2–7.0 Tg N2O-N yr−1) given by our top-down approach [10]. Del Grosso et al. concluded that ‘the convergence of top-down and bottom-up approaches increases confidence in emissions estimates because the methods are based on different assumptions, and this convergence suggests that we have at least a rudimentary understanding of the factors that control emissions at large spatial and temporal scales’. In a similar way, Corazza et al. [34] apply atmospheric measurements and inverse modelling and conclude that there is ‘good agreement with the bottom-up emission inventories reported to the United Nations Framework Convention on Climate Change’ at least for Northwestern and Eastern Europe, where a sufficiently dense measurement network is available.
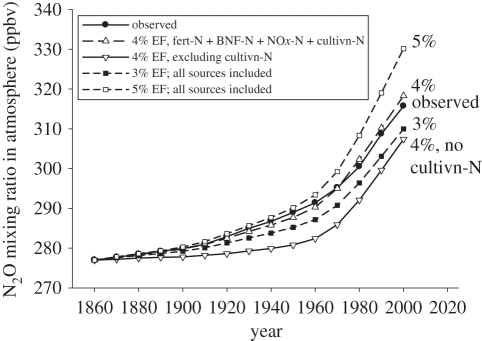
Davidson [9] reported that, although the calculation of a global EF of 4 ± 1% [10] fits the atmospheric concentration data well for 1860 and the 1990s, it underestimates the emissions that must have taken place in the late nineteenth and early twentieth centuries. He argued that other sources of N2O were important in that period, in particular, the previously plant-unavailable N released from old soil organic matter following land-use change from natural forest or grassland to cultivated agricultural land—the ‘mining’ of soil N. Davidson [9] achieved a good match to the rise in atmospheric concentration by combining emissions from fertilizer N with an EF of 2.5 per cent and those from manure N with an EF of 2 per cent. It is clear that N mining would have been important in the years in question as they coincided with the major expansion of crop and grazing land in, for example, the USA, Canada, Argentina, South Africa, Australia, New Zealand and the former USSR. Likewise, mineral nitrates (Chile saltpetre) were another source of reactive N [35]. Accordingly, we have extended the original concept of ‘newly fixed N’ that we used in Crutzen et al. [10] to include all reactive N (Nr) entering the terrestrial N cycle, i.e. by adding the N mineralized as a result of land-use change and N deposited as NOx to the estimated inputs from synthetic fertilizer N and BNF-N, and we show here that the observed upward trend in the atmospheric concentration of N2O over the 140 years between 1860 and 2000 is very closely matched when an overall EF of 4 per cent (i.e. the average of the 3–5% range) is applied to all this Nr (figure 3).
Figure 3.
Comparison between observed increase in atmospheric concentration of N2O over the period 1860–2000 (black circles) and calculated increase based on 4% EF for new reactive N, including N from synthetic fertilizers, from additional N from BNF above the rate of fixation in 1860, from NOx-N, and from N mineralized from soil organic matter as a result of land-use change to agriculture (white triangles). Bottom curve (inverted triangles) includes all these sources except mineralized N. Black and white squares indicate the increase for EFs of 3 and 5%, respectively. Note: values for synthetic fertilizer N, N from BNF and NOx-N, and also net contribution to atmospheric concentration change from N2O release from biomass burning, fossil fuel combustion and nylon production, and decrease in forest soil emissions, all taken from the electronic supplementary material in the study of Davidson [9]. N mineralization based on an estimate of soil C release from land-use change, 1850–1990, of 35 Pg [36]; corresponding N release would have been ca 3 Pg, or an average of 21 Tg yr−1. Mineralization-N included in graph set at 10 Tg yr−1 1860–1890, 15 Tg yr−1 1890–1910 and 20 Tg yr−1 1910–1990.
As a contribution to the European Nitrogen Assessment, Butterbach-Bahl et al. [37] recently analysed the multitude of effects related to Nr specifically for Europe. Because of data availability, they focused on European Union member countries. Applying approaches as cited above, as well as referring to other relevant literature, they also concluded that the overall emissions of N2O seem to be well understood, even if use of IPCC default EFs may lead to underestimation (owing to implicit negligence of the full N cycle, but also because indirect emissions from soil seem to be at least a factor of two higher than suggested by IPCC). Their paper also attempted to look at additional climate effects, such as carbon sequestration facilitated by N inputs, ozone formation owing to atmospheric NOx and cooling effects owing to aerosol formation. For the European situation, the conclusion was that the carbon sink and aerosol formation, as a consequence of NOx and NH3 emissions, possibly outweigh in their cooling effects the global warming caused by N2O emissions. At this stage quantification of the agricultural effects alone (NOx predominantly derives from combustion), and an extension beyond Europe, are not possible.
4. Conclusions
By extending our original definition of ‘newly fixed N’ entering the terrestrial N cycle beyond fertilizer N and BNF-N to include the N mineralized as a result of land-use change and N deposited as NOx, the observed upward trend in the atmospheric concentration of N2O over the 140 years between 1860 and 2000 is very closely matched when an overall EF of 4 per cent (i.e. the average of the 3–5% range) is applied.
Agriculture (and the creation of new arable land for its expansion) is the activity mainly responsible for the additional N2O emissions over the past century and a half. Its expansion in recent years to meet the demand for biofuels has resulted in additional emissions; on the basis of the likely EFs associated with first-generation biofuel crops, these crops can exacerbate, rather than alleviate, global warming. It is thus important to avoid biofuel production based on crops with a high N demand and use those that can be grown with little or no fertilizer N requirement: the so-called ‘second-generation’ biofuel crops such as willow and Miscanthus.
For all biofuels, a complete LCA, including the effect of fossil carbon used in biofuel production as well as the N2O emission, and the energy equivalent of by-products, is needed to get the full picture, but the application of existing LCA has to date given broadly similar outcomes to our earlier conclusion based simply on the global N2O EF.
References
- 1.Flückiger J. M., Monnin E., Stauffer B., Schwander J., Stocker T. F., Chappellaz J., Raynaud D., Barnola J. M. 2002. High-resolution Holocene N2O ice core record and its relationship with CH4 and CO2. Glob. Biogeochem. Cycl. 16, 1010. 10.1029/2001GB001417 (doi:10.1029/2001GB001417) [DOI] [Google Scholar]
- 2.Mosier A., Kroeze C. 2000. Potential impact on the global atmospheric N2O budget of the increased nitrogen input required to meet future global food demands. Chemosphere 2, 465–473 10.1016/S1465-9972(00)00039-8 (doi:10.1016/S1465-9972(00)00039-8) [DOI] [Google Scholar]
- 3.IPCC 2001. Climate change 2001: the scientific basis. In Contribution of Working Group I to the 3rd Assessment Report (eds Houghton J. T., Ding Y., Griggs D. J., Noguer M., van der Linden P. J., Dai X., Maskell K., Johnson C. A.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Galloway J. N., Aber J. D., Erisman J. W., Seitzinger S. P., Howarth R. H., Cowling E. B., Cosby B. J. 2003. The nitrogen cascade. Bioscience 53, 341–356 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2 (doi:10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2) [DOI] [Google Scholar]
- 5.Galloway J. N., et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 10.1007/s10533-004-0370-0 (doi:10.1007/s10533-004-0370-0) [DOI] [Google Scholar]
- 6.Erisman J. W., Sutton M. A., Galloway J., Klimont Z., Winiwarter W. 2008. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 10.1038/ngeo325 (doi:10.1038/ngeo325) [DOI] [Google Scholar]
- 7.IPCC (Intergovernmental Panel on Climate Change) 1997. Greenhouse gas inventory reference manual, vol. 3, Section 4.5, Agriculture (eds Houghton J. T., Meira Filho L. G., Lim B., et al.). Bracknell, UK: Meteorological Office [Google Scholar]
- 8.IPCC 2006. N2O emissions from managed soils and CO2 emissions from lime and urea application. In IPCC guidelines for national greenhouse gas inventories, vol. 4 (eds Eggleston H. S., Buendia L., Miwa K., Ngara T., Tanabe K.), ch. 11 Hayama, Japan: IGES [Google Scholar]
- 9.Davidson E. A. 2009. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2, 659–662 10.1038/ngeo608 (doi:10.1038/ngeo608) [DOI] [Google Scholar]
- 10.Crutzen P. J., Mosier A. R., Smith K. A., Winiwarter W. 2008. N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 8, 389–395 10.5194/acp-8-389-2008 (doi:10.5194/acp-8-389-2008) [DOI] [Google Scholar]
- 11.Prather M., et al. 2001. Atmospheric chemistry and greenhouse gases. In Climate change 2001: the scientific basis (eds Houghton J. T., Ding Y., Griggs D. J., Noguer M., van der Linden P. J., Dai X., Maskell K., Johnson C. A.), pp. 239–287 Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Klein Goldewijk C. G. M. 2001. Estimating global land use change over the past 300 years: the HYDE data base. Glob. Biogeochem. Cycl. 15, 415–434 [Google Scholar]
- 13.Smeets E., Bouwman A. F., Stehfest E. 2007. Interactive comment on ‘N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels’ by P. J. Crutzen et al. Atmos. Chem. Phys. Discuss. 7, S4937–S4941 [Google Scholar]
- 14.Cassman K. G., Dobermann A., Walters D. T. 2002. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31, 132–140 [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanian V., Alves B., Aulakh M., Bekunda M., Cai Z., Drinkwater L., Mugendi D., van Kessel C., Oenema O. 2004. Crop, environmental, and management factors affecting nitrogen use efficiency. In Agriculture and the nitrogen cycle (ed. Mosier A. R., Syers J. K., Freney J.), SCOPE 65, pp. 19–33 Washington, DC: Island Press [Google Scholar]
- 16.Lisboa C. C., Butterbach-Bahl K., Mauder M., Kiese R. 2011. Bioethanol production from sugarcane and emissions of greenhouse gases—knowns and unknowns. Glob. Change Biol. Bioenergy 3, 277–292 (doi:10.1111/j.1757–1707.2011.01095.x) [DOI] [Google Scholar]
- 17.Smeets E. M. W., Bouwman L. F., Stehfest E., van Vuuren D. P., Posthuma A. 2009. Contribution of N2O to the greenhouse gas balance of first-generation biofuels. Glob. Change Biol. 15, 1–23 10.1111/j.1365-2486.2008.01704.x (doi:10.1111/j.1365-2486.2008.01704.x) [DOI] [Google Scholar]
- 18.Weier K. L. 1996. Trace gas emissions from a trash blanketed sugarcane field in tropical Australia. In Sugarcane: research towards efficient and sustainable production (eds Wilson J. R., Hogarth D. M., Campbell J. A., Garside A. L.), pp. 271–272 Brisbane, Australia: CSIRO Division of Tropical Crops and Pastures [Google Scholar]
- 19.Mosier A. R., Crutzen P. J., Smith K. A., Winiwarter W. 2009. Nitrous oxide's impact on net greenhouse gas savings from biofuels: life-cycle analysis comparison. Int. J. Biotechnol. 11, 60–74 10.1504/IJBT.2009.028100 (doi:10.1504/IJBT.2009.028100) [DOI] [Google Scholar]
- 20.Farrell A. E., Plevin R. J., Turned B. T., Jones A. D., O'Hare M., Kammen D. A. 2006. Ethanol can contribute to energy and environmental goals. Science 31, 506–508 10.1126/science.1121416 (doi:10.1126/science.1121416) [DOI] [PubMed] [Google Scholar]
- 21.Liska A. J., Yang H. S., Bremer V. R., Klopfenstein T. J., Walters D. T., Erickson G. E., Cassman K. G. 2009. Improvements in life cycle energy efficiency and greenhouse gas emissions for corn-ethanol. J. Ind. Ecol. 13, 58–74 10.1111/j.1530-9290.2008.00105.x (doi:10.1111/j.1530-9290.2008.00105.x) [DOI] [Google Scholar]
- 22.Woods J., Brown G., Estrin A. 2005. Bioethanol greenhouse gas calculator. Home-Grown Cereals Authority. See www.hgca.com.
- 23.Smith T. C., Kindred D. R., Brosnan J. M., Weightman R. M., Shepherd M., Sylvester-Bradley R. 2006. Wheat as a feedstock for alcohol production. Research Review No. 61, p. 89 London, UK: Home-Grown Cereals Authority [Google Scholar]
- 24.EISA (Energy Independence & Security Act) 2007. Energy security through increased production of biofuels. Signed 19 December 2007 by President G. W. Bush, p. 310
- 25.European Commission 2009. Directive 2009/30/EC amending Directive 98/70/EC on fuel Quality. Brussels, Belgium: European Commission [Google Scholar]
- 26.Kasimir Klemedtsson Å., Smith K. A. 2011. The significance of nitrous oxide emission from biofuel crops on arable land: a Swedish perspective. Biogeosci. 8, 3581–3591 10.5194/bg-8-3581-2011 (doi:10.5194/bg-8-3581-2011) [DOI] [Google Scholar]
- 27.Kasimir Klemedtsson Å., Weslien P., Klemedtsson L. 2009. Methane and nitrous oxide fluxes from a farmed Swedish Histosol. Eur. J. Soil Sci. 60, 321–331 10.1111/j.1365-2389.2009.01124.x (doi:10.1111/j.1365-2389.2009.01124.x) [DOI] [Google Scholar]
- 28.Berglund Ö., Berglund K. 2008. Odlad organogen jord I Sverige 2003 (Farmed organic soil in Sweden 2003). Uppsala, Sweden: Swedish University of Agricultural Sciences, Division of Hydrotechnics; ISSN 1653–6797.pub-epsilon.slu.se:8080/197/01/ rapport7.pdf [Google Scholar]
- 29.Fargione J., Hill J., Tilman D., Polasky S., Hawthorne P. 2008. Land clearing and the biofuel carbon debt. Science 319, 1235–1238 10.1126/science.1152747 (doi:10.1126/science.1152747) [DOI] [PubMed] [Google Scholar]
- 30.Searchinger T., Heimlich R., Houghton R. A., Dong F. X., Elobeid A., Fabiosa J., Hayes D., Yu T. 2008. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319, 1238–1240 10.1126/science.1151861 (doi:10.1126/science.1151861) [DOI] [PubMed] [Google Scholar]
- 31.Melillo J. M., et al. 2009. Indirect emissions from biofuels: how important? Science 326, 1397–1399 10.1126/science.1161525 (doi:10.1126/science.1161525) [DOI] [PubMed] [Google Scholar]
- 32.Erisman J. W., van Grinsven H., Leip A., Mosier A., Bleeker A. 2010. Nitrogen and biofuels; an overview of the current state of knowledge. Nutr. Cycl. Agroecosys. 86, 211–223 10.1007/s10705-009-9285-4 (doi:10.1007/s10705-009-9285-4) [DOI] [Google Scholar]
- 33.Del Grosso S. J., Wirth T., Ogle S. M., Parton W. J. 2008. Estimating agricultural nitrous oxide emissions. Eos (Trans. Am. Geophys. Union) 89, 529–540 10.1029/2008EO510001 (doi:10.1029/2008EO510001) [DOI] [Google Scholar]
- 34.Corazza M., et al. 2011. Inverse modelling of European N2O emissions: assimilating observations from different networks. Atmos. Chem. Phys. 11, 2381–2398 10.5194/acp-11-2381-2011 (doi:10.5194/acp-11-2381-2011) [DOI] [Google Scholar]
- 35.Smil V. 2001. Enriching the earth: Fritz Haber, Carl Bosch, and the transformation of food production Cambridge, MA: MIT Press [Google Scholar]
- 36.Butterbach-Bahl K., et al. 2011. Nitrogen as a threat to the European greenhouse balance. In The European nitrogen assessment (eds Sutton M. A., Howard C. M., Erisman J. W., Billen G., Bleeker A., Grennfelt P., van Grinsven H., Grizzetti B.), pp. 434–462 Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Houghton R. A. 1999. The annual net flux of carbon to the atmosphere from changes in land use 1850–1990. Tellus B 51, 298–313 10.1034/j.1600-0889.1999.00013.x (doi:10.1034/j.1600-0889.1999.00013.x) [DOI] [Google Scholar]