Abstract
Nitrous oxide (N2O) emissions from wastewater treatment plants vary substantially between plants, ranging from negligible to substantial (a few per cent of the total nitrogen load), probably because of different designs and operational conditions. In general, plants that achieve high levels of nitrogen removal emit less N2O, indicating that no compromise is required between high water quality and lower N2O emissions. N2O emissions primarily occur in aerated zones/compartments/periods owing to active stripping, and ammonia-oxidizing bacteria, rather than heterotrophic denitrifiers, are the main contributors. However, the detailed mechanisms remain to be fully elucidated, despite strong evidence suggesting that both nitrifier denitrification and the chemical breakdown of intermediates of hydroxylamine oxidation are probably involved. With increased understanding of the fundamental reactions responsible for N2O production in wastewater treatment systems and the conditions that stimulate their occurrence, reduction of N2O emissions from wastewater treatment systems through improved plant design and operation will be achieved in the near future.
Keywords: emissions, greenhouse gases, nitrous oxide, nitrogen removal, wastewater treatment
1. Introduction
Nitrous oxide (N2O) is a potent greenhouse gas, which accounts for 7.9 per cent of the global anthropogenic greenhouse gas emissions in 2004 [1]. It is also predicted to be the most dominant ozone-depleting substance in the twenty-first century [2]. Since 1750, the atmospheric N2O concentration has increased by about 16 per cent, from around 270 ppb, to 319 ppb in 2005. Human activity has been responsible for 40–50% of the annual increase in N2O emissions over its pre-industrial levels [1]. While agriculture is the major contributor accounting for 80 per cent of the anthropogenic N2O source, other contributors include biomass and fossil combustion, manure management, adipic acid and nitric acid production and waste management [1,3].
Since the first published data by Czepiel et al. [4], reporting N2O emissions from a wastewater treatment plant, awareness and concern of N2O emissions during wastewater treatment have grown significantly among urban water authorities. Owing to the complexity involved in measuring N2O emissions from full-scale plants and the lack of standardized measurement methods, N2O emissions for the wastewater sector have been estimated based on models without the input of measured data. The Environmental Protection Agency of the United States [5] reported that N2O from the wastewater sector accounts for about 3 per cent of N2O emissions from all sources and ranks as the sixth largest contributor. Similarly, the Intergovernmental Panel on Climate Change also reports that N2O emissions from wastewater account for approximately 2.8 per cent of the total anthropogenic sources [1]. Global N2O emissions from wastewater treatment are expected to increase by approximately 13 per cent between 2005 and 2020.
N2O is mainly released during biological nitrogen removal in biological nutrient removal (BNR) plants. There are various configurations of BNR plants that can achieve high levels of nitrogen removal from wastewater by promoting nitrification and denitrification in different reaction zones. N2O is a known obligatory intermediate in the heterotrophic denitrification pathway and is also produced by autotrophic nitrifying bacteria, mainly ammonia-oxidizing bacteria (AOB) [6], as a by-product.
The microbial nitrogen transformation processes in a wastewater treatment plant are fundamentally the same as in other environments such as soil, marine and freshwater habitats. However, unlike most other environments, wastewater treatment plants are engineered systems designed to achieve high nitrogen conversion rates. There are several key features that distinguish these plants from other environments:
— Domestic wastewater usually contains relatively high concentrations of nitrogen, around 20–70 mg l−1 total nitrogen as N. In order to attain almost complete nitrogen removal within 3–8 h, high nitrogen loading rates are applied, incurring relatively high nitrification and denitrification rates [7]. These are expected to impact on the rate of N2O production.
— Bacterial communities in the plants are subjected to rapid changes in process conditions that are applied to promote aerobic or anoxic microbial reactions. Such rapid changes in environmental conditions probably cause physiological stress to both the nitrifying and denitrifying communities, with the potential to induce transient behaviours.
— Active aeration is used to induce aerobic conditions. The aeration systems are engineered to efficiently provide oxygen to the bioreactor, which also enables efficient transfer of N2O from the liquid phase to the gas phase. Therefore, any temporary imbalance between N2O production and consumption could result in accumulation and then stripping of N2O during aeration.
— Given that wastewater treatment systems are highly engineered systems, there are opportunities to mitigate N2O emissions by improving process design and/or operational conditions.
In this paper, we review the key outcomes arising from the research on N2O production and emissions from wastewater systems. Following a brief description of the design and operation of wastewater treatment systems, the methods for measuring N2O in wastewater systems and the emission rates thus far measured are summarized. This is followed by discussions on the key metabolic pathways contributing to N2O production, and the most important influencing factors. Finally possible mitigation strategies are discussed.
2. Design and operation of biological wastewater treatment plants
(a). Activated sludge systems for biological wastewater treatment
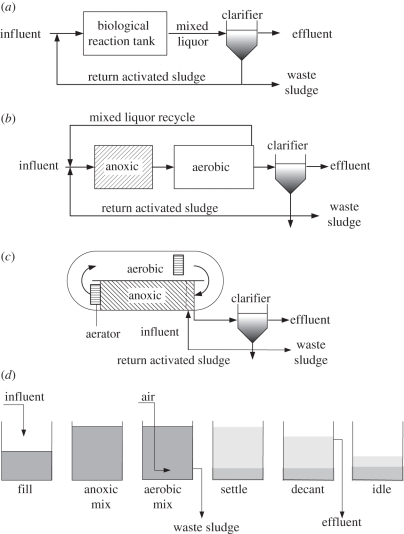
Activated sludge is the most widely used process for biological treatment of wastewater. This process uses a microbial community suspended in wastewater to metabolize the biodegradable organic and inorganic components. The microbial community usually clumps together forming three-dimensional aggregates or flocs, known as activated sludge. The sludge and wastewater mixture is called the mixed liquor and the treatment process takes place in a biological reaction tank (bioreactor). At the end of the biological treatment process, the mixed liquor is passed into the clarifier where the sludge is settled and separated from the treated water (figure 1a) [7]. The latter is discharged as the effluent. Most of the settled sludge is returned to the bioreactor, with a hydraulic flow rate that is comparable with that of the influent flow. A small fraction of the sludge, called the waste activated sludge, is removed and disposed of after several steps of sludge treatment (figure 1a). The rate of sludge wastage determines the average amount of time that the sludge is retained in the activated sludge system, and is termed the solids retention time. The key components typically removed during the activated sludge treatment process are solids, organic carbon compounds, nitrogen and phosphorus.
Figure 1.
Diagram of (a) a conventional activated sludge system; (b) a modified Ludzack–Ettinger system; (c) an oxidation ditch; and (d) a sequencing batch reactor system.
(b). Biological processes for nitrogen removal
Nitrogen in wastewater is present in the form of complex organic nitrogen compounds, ammonium (NH4+), and low (often negligible) levels of nitrite (NO2−) and nitrate (NO3−) [7]. The organic nitrogen fraction such as amino acids, amino sugars and proteins are readily converted to NH4+ by microbial degradation in sewer systems and in the bioreactor. In conventional BNR plants, NH4+ is first converted to NO2− and NO3− via autotrophic nitrification, which is followed by the reduction of NO3− and NO2− by heterotrophic denitrification to form N2.
The bioreactor used for nitrogen removal provides conditions enabling both nitrification and denitrification. Aerobic conditions are required for nitrification, whereas a sufficient amount of organic carbon compound is required to support denitrification under anoxic conditions. To achieve this, conventional BNR plants are usually configured as continuous systems whereby wastewater flows through the denitrification and nitrification processes, which are separated into either different compartments or zones.
In a typical modified Ludzack–Ettinger configuration, an anoxic compartment/zone precedes the aerobic compartment (figure 1b) [7]. At the end of the aerobic compartment, the nitrified wastewater containing NO3− is re-circulated back to the anoxic compartment with a flow rate that is a few times that of the wastewater influent. Wastewater is also fed to the anoxic compartment, which provides the organic carbon for denitrification. A wide range of solids retention time (10–30 days) depending on treatment needs can be applied. There are many variants of this configuration. For example, another pair of anoxic and aerobic compartments can be added to the end of the bioreactor shown in figure 1b, to form a four-stage anoxic–aerobic–anoxic–aerobic process. With this design, wastewater is often fed to both the first and second anoxic compartments, thus resulting in a step-feed process. In all cases, the sludge, where micro-organisms reside, is passed between anoxic and aerobic conditions frequently (in hours and in many cases even less than an hour). The dissolved oxygen (DO) concentration in the aerobic compartment is typically within the range of 0.5–2 mg l−1. Although, in some cases could be outside of this range, particularly when the DO is not controlled automatically. In comparison, DO in the anoxic compartment is usually not detectable. However, a limited amount of oxygen is brought into the anoxic compartment through the recirculation stream(s) and through natural surface oxygen transfer.
Figure 1c shows an oxidation ditch system, which is also commonly used for BNR [7]. Oxidation ditches are usually equipped with horizontal brush aerators to provide aeration and also to move the mixed liquor along the ditch at a relatively high velocity (0.25–0.35 m s−1) [8]. Each pass of mixed liquor in the ditch typically lasts for several minutes. A relatively high DO concentration is obtained at or close to the aerator and anoxic conditions develop further away from the aerator. The high recirculation flow and the large tank volume dampen the load variations, giving rise to more stable operating conditions in comparison with the modified Ludzack–Ettinger configuration (figure 1b). A further feature of an oxidation ditch is that the DO is typically low (e.g. around 0.5 mg l−1), favouring simultaneous nitrification and denitrification. Low DO conditions allow a buildup of an oxygen concentration gradient within the microbial flocs as a result of diffusion limitation. Nitrifiers reside at the outer layer of the flocs where there is sufficient oxygen supply, whereas denitrifiers can remain active in the anoxic zone of the flocs allowing nitrification and denitrification to occur simultaneously [9].
Unlike continuous flow systems outlined above, sequencing batch reactors can also be used to achieve the removal of nitrogen and organic carbon. Aerobic and anoxic conditions are separated by time instead of space [10] (figure 1d). All the phases in continuous systems that are spatially separated are provided in a single reactor. A sequencing batch reactor mimics a plug-flow continuous system producing significant concentration gradients of substrates and products with time. This clearly contrasts with the operational conditions found in an oxidation ditch. When a low DO (e.g. 0.5 mg l−1) is provided during an aerobic period of a sequencing batch reactor cycle, simultaneous nitrification and denitrification can also be encouraged.
3. Nitrous oxide measurement in wastewater treatment processes
(a). Gas-phase nitrous oxide measurement
In full-scale wastewater treatment plants, the N2O emitted from activated sludge tanks is usually captured using a closed floating chamber. This technique was originally adapted from emission measurements of solid surfaces. It was first used to measure N2O flux from liquid surfaces in a municipal wastewater treatment plant located in Durham, New Hampshire in USA [4]. During aeration, dissolved N2O was stripped from the liquid phase into the gas; during non-aerated phases, air was blown into the headspace of the chamber for sampling. Owing to the lack of online N2O measurement at that time, samples were grabbed from the headspace of the chamber into 20 ml nylon syringes at specific time intervals. Analysis for N2O was accomplished using a gas chromatograph (GC) with an electron capture detector. A similar approach was applied in full-scale studies [11] of an intermittent activated sludge process in Japan. An air pump was used to collect part of the emitted gas from a capture chamber into a gas sampling bag. During the anoxic period, argon was supplied into the chamber as a sweeping gas.
Although the emitted N2O can be captured through the floating chamber, the off-line sampling (grab samples) do not capture the dynamic changes in the N2O emission profiles, as will be further discussed. This can result in over- or underestimation of the N2O emissions. Therefore, online, continuous monitoring of N2O has been employed in recent years for accurate quantification of N2O emissions from wastewater treatment systems. The types of online sensors include an infrared analyser [12–15], chemiluminescence [6], a Fourier transform infrared analyser [16] and mass spectrometry [17,18]. Among these, the infrared analyser with a broad N2O measurement range of up to 2000 ppm is the most commonly used method. However, chemiluminescence has a higher sensitivity with a detection limit at parts per trillion levels.
In addition to temporal variations, spatial variations in N2O emissions should also be considered, especially for continuous processes (figure 1b,c). Ideally, multiple hoods should be used to measure N2O emissions from all zones simultaneously. Although not desirable, variations could also be reasonably captured by moving the single hood between zones. For sequencing batch reactor systems (figure 1d), a single location is theoretically adequate, although in practice multiple locations are also preferred to cover possible spatial variation of fluxes.
The N2O emission factor is typically represented as the ratio between the mass of emitted N2O-N (kg-N d−1) and the amount of influent total Kjeldahl nitrogen load (kg-N d−1). In some cases, the emission factors are represented as the ratio between the mass of N2O-N emitted and the amount of N removed through nitrification and denitrification in the treatment plant. The mass of emitted N2O-N is calculated from the measured N2O concentration, the gas flow rate out of the chamber and the covered cross-sectional area [19]. For aerated zones, the gas flow out of the chamber is equal to the air flow for aeration and is usually recorded by each plant. For non-aerated zones, the gas flow through the chamber can be recorded with a rotameter.
(b). Liquid-phase nitrous oxide measurement
Measurement of liquid-phase N2O using off-line grab samples followed by GC analysis has been used in both laboratory scale reactors and full-scale plants [4,15,20–22]. A liquid sample containing N2O is injected into a vacuum vial and allowed to reach liquid–gas equilibrium. The gas-phase N2O concentration (Cgas) in the vial is then measured and the liquid-phase N2O (Cliquid) concentration is calculated based on Henry's law. The total N2O concentration in the sample is obtained by dividing the total amount of N2O in both the gas and liquid phases by the total liquid volume.
Continuous monitoring of the dissolved N2O concentration can be done using N2O microsensors. Kampschreur et al. [6] used a modified Clark electrode (Unisense, Denmark) to measure the liquid-phase N2O in two laboratory scale reactors. Foley et al. [23] measured the liquid-phase N2O in seven full-scale plants in Australia using the same type of microsensor. The Clark-type sensor has an internal reference and a guard cathode. During measurement, N2O penetrates through the sensor tip membrane and is reduced at the metal cathode surface. The sensor is connected to a high-sensitivity pico-ammeter, which converts the resulting reduction current to a signal. The online signal can be recorded on a laptop. The response of the electrochemical microsensor is known to be linear in the range of 0–1.2 mM [24].
While N2O microsensors have a low detection limit, the high sensitivity can render it susceptible to interferences especially in full-scale measurements. Combining the analyses of both the microsensor and the GC-vial methods significantly increases the reliability of data.
Similar to the gas-phase analysis, liquid-phase detection at multiple locations is needed to capture the spatial variation in N2O concentration.
N2O flux is determined using the liquid-phase measurement [23]. However, this requires the estimation of the mass transfer coefficient between the liquid and gas phases, which is not a straightforward task in full-scale plants [23]. Consequently, the liquid-phase N2O data are primarily used for understanding N2O production and emission processes rather than for quantification purposes.
Other parameters such as pH, DO, temperature, total suspended solids and volatile suspended solids (VSS) are often measured at sampling locations and at the wastewater influent for mass balance, correlation analysis of N2O emission fluxes and for model development.
4. Full-scale emission data
The N2O emission factor (amount of N2O-N emitted relative to the nitrogen load) reported thus far for full-scale plants varies substantially, ranging from 0 to 25% (table 1). It should be noted that an emission factor of 1 per cent would already increase the carbon footprint of a wastewater treatment plant by approximately 30 per cent [29]. The large variation in N2O emissions among the investigated plants was probably owing to the different configurations and operational conditions applied. Additionally, the different monitoring and quantification methods used could have been a contributing factor. The large variation also implies that N2O emissions from a treatment plant can be reduced through proper plant design and operation. Foley et al. [23] concluded that plants achieving high-level nitrogen removal would emit less N2O in comparison with nitrifying plants achieving no or low levels of nitrogen removal. This implies that improved water quality and reduced N2O emissions can be achieved simultaneously.
Table 1.
Nitrous oxide (N2O) emission factors reported for several full-scale wastewater treatment plants.
| type of plant | N2O emission (% of N-influent) | sampling method | remarks | reference |
|---|---|---|---|---|
| activated sludge plant—primary and secondary treatment (aeration only; 4 ml d−1) | 0.035–0.05 | weekly grab samples for 15 weeks | N2O was emitted in aerated areas, low N2O flux at non-aerated areas | New Hampshire, USA [4] |
| activated sludge plant | 0.001 | grab samples in alternate weeks for 1 year | N2O emissions increased with nitrite and nitrate concentrations | Germany [25] |
| anoxic–aerobic activated sludge plant (78 Ml d−1) | 0.001–0.04 | grab samples | N2O emission was dependent on COD:N | Germany [26] |
| intermittent activated sludge plant (0.2 Ml d−1) | 0.01–0.08 | collecting gas-phase N2O samples using air bags during four aeration cycles (2 h) | N2O emission decreased with shorter aeration periods | Japan [11] |
| intermittent activated sludge treatment of municipal sewerage (2.5 and 31 Ml d−1) | 0.47 (0.01) | — | — | France [27] |
| nitritation–anammox sludge digestion liquor treatment | 2.3 | online measurement during 4 days | N2O emissions increased with decreasing oxygen concentration (aerated stage) and increasing nitrite concentration (anoxic stage) | Netherlands [22] |
| seven BNR plants | 0.6–25 (3.5 + 2.7% average) | grab samples | correlation between N2O emissions and nitrite accumulation was observed | Australia [23] |
| four treatment plants (completely mixed, plug-flow, membrane bioreactor) | 0–0.3 | online measurement | NH4-N and DO had impact on N2O emission | France [28] |
| partial nitritation–anammox sequencing batch reactor (three plants, five reactors) | 0.4–0.6 | online measurement | N2O emissions were slightly higher than in conventional nitrogen-removal systems | Switzerland [16] |
| 12 BNR plants | 0.003–2.59 | online measurement | aerobic zones contributed substantially more to N2O fluxes than anoxic zones | USA [14] |
| four-stage floc-based partial nitritation and anammox process | 5.1–6.6 | online measurement | high N2O emissions may be partly inherent to a separate nitritation step | Belgium [15] |
Many studies show that N2O is primarily emitted from the aerated zones [14]. Although N2O is an obligatory intermediate in denitrification, N2O formed in anoxic zones will largely be dissolved in the liquid phase and this is converted to N2 through N2O reduction before it is transferred to the gas phase. In contrast, N2O formed in aerobic periods is found to be stripped quickly owing to intensive aeration, forming the primary source of N2O emitted from wastewater treatment systems [14].
5. Nitrous oxide production pathways
N2O is produced in BNR systems during autotrophic nitrification and heterotrophic denitrification. Although the nitrification step involves both AOB and nitrite-oxidizing bacteria (NOB), it is widely accepted that NOB does not contribute to N2O production. The key metabolic pathways involved in N2O production by AOB and denitrifying bacteria in BNR systems are reviewed in this section.
(a). Nitrifier denitrification
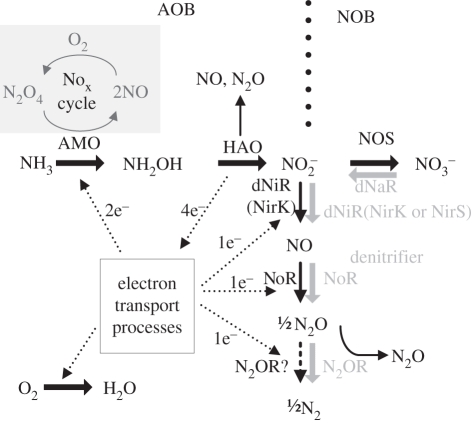
Nitrifier denitrification involves the reduction of NO2− to NO, N2O and N2 by autotrophic AOB. However, only genes encoding NO2− and NO reductase (nirK and nor) are found in the genome of AOB but not N2O reductase [30–36]. This suggests that N2O rather than N2 is the endproduct of the nitrifier denitrification pathway (figure 2). Hydroxylamine (NH2OH) [38], hydrogen (H2) [38] and ammonia (NH3) [39,40] can serve as electron donors for NO2− and NO reduction by AOB.
Figure 2.
Nitrogen transformation pathways of ammonia-oxidizing bacteria (AOB), nitrite-oxidizing bacteria (NOB) and denitrifying bacteria (modified from Kim et al. [37]). AOB and NOB pathways divided by large dotted line and denitrifying pathway shown in grey.
The nitrifier denitrification pathway plays a key role in N2O production by AOB, especially under anoxic to suboxic conditions [6,22,41]. Experiments conducted with full-scale sludge show that nitrifier denitrification can contribute up to 83 per cent of the N2O emissions and this depends on the DO level [42]. Kim et al. [37] also report that the denitrification activity by AOB is the predominant source of N2O in an activated sludge under nitrifying conditions and they detected simultaneous expression of nirK by AOB.
(b). Autotrophic ammonia oxidation
NH3 rather than NH4+ is shown to be the true substrate for AOB [43]. Aerobic NH3 oxidation to NO2− is a two-step process. NH3 is first converted to NH2OH catalysed by a membrane-bound ammonia mono-oxygenase (AMO). This first step requires molecular oxygen and a pair of electrons. The subsequent step is carried out by hydroxylamine oxidoreductase (HAO) in the periplasm to form NO2−, generating two pairs of electrons. One pair is used to support the first step of NH3 oxidation and the remaining pair is used for energy generation [44].
Extended studies by Igarashi et al. [45], to characterize the structure and function of HAO, suggest that the NH2OH oxidation is further split into two reactions to allow two electrons to be accepted and transferred simultaneously. The concurrent reaction involves: (i) conversion of NH2OH to a nitrosyl radical (NOH); and (ii) conversion of NOH to NO2− [46].
N2O and NO can be formed from the activity of HAO through the unstable NOH intermediate (figure 2). NO is generated as an intermediate during the enzymatic splitting of NOH to NO2− [44,47], whereas N2O is produced through the unstable breakdown of NOH [46].
Despite the fact that this pathway had been postulated for a long time, its relevance to wastewater treatment processes has not been fully confirmed. However, strong evidence demonstrating the potentially significant contribution of this pathway to N2O production during nitrification is emerging. Increased N2O production induced by the transition from anoxic to aerobic conditions [48] and high pH [49] are attributed to an increase in NH3 oxidation rate by AOB. The relationship between the NH3 oxidation rate and N2O production rate by AOB was further characterized by Law et al. [50], whereby N2O production rate was shown to be exponentially correlated to the NH3 oxidation rate (figure 3). This exponential correlation could be represented by a metabolic model based on N2O production through the chemical degradation of NOH. This provides evidence that N2O is produced during increased NH3 oxidation rates and is most likely produced from the unstable breakdown of NOH during NH2OH oxidation. This suggestion requires confirmation with further experimental studies.
Figure 3.
Correlation between the specific N2O production rate and the specific ammonia oxidation rate. Symbols represent experimental data under various conditions. Solid lines are predictions by a model based on the NOH pathway (adapted from Law et al. [50]).
In addition to the chemical breakdown of NOH, biological reduction of NO generated during NH2OH oxidation could also be a potential source of N2O. Two molecules of cytochrome c are expressed in AOB for transfer of electrons during NH2OH oxidation to the electron transport chain [51]. One of the two cytochromes, c554, can also act as an NO reductase in vitro [52] and is suggested to produce N2O from NO generated by the enzyme HAO [53]. NO generated during NH2OH oxidation could also be reduced by homologue NO reductases (NOR), namely NorS [53]. Indeed, genes encoding NorS are detected in the genome of most AOB [53].
As wastewater treatment systems feature high nitrogen conversion and high nitrogen loading, N2O production during NH2OH oxidation either through direct decomposition of NOH or the subsequent reduction of the generated NO could play a crucial role in full-scale systems. In addition, sudden process perturbations leading to transiently increased NH3 oxidation rates may potentially cause increased N2O production. However, the relevance of this pathway to the natural environment remains to be verified.
Besides aerobic NH3 oxidation by AOB, dinitrogen tetroxide (N2O4)-dependent NH3 oxidation is proposed as an alternative pathway for Nitrosomonas to oxidize NH3 [54]. Catalysed by AMO, NH3 oxidation to NH2OH is coupled to N2O4 reduction (figure 2) [55,56]. Two moles of NO are formed and released from the cell per mole of N2O4 reduced. This enables NH3 oxidation to proceed under complete anoxic conditions. Similarly, NH2OH oxidation is also catalysed by HAO, using NO2− as an electron acceptor to form N2 as a final product [57]. Under aerobic conditions, molecular oxygen is postulated to have an indirect role by re-oxidizing the NO to form N2O4. This NO to N2O4/NO2 conversion is called the NOx cycle [56]. It is proposed that nitrifier denitrification under oxic conditions plays a role in supplying NO for the NOx cycle [54].
(c). Heterotrophic denitrification
Heterotrophic denitrification is an enzyme-mediated sequential reduction of NO3− to N2 coupled to the oxidation of organic substrates (figure 2). N2O is an obligate intermediate of heterotrophic denitrification. Under typical denitrifying conditions found in a biological wastewater treatment process, NO and N2O reductases have higher maximum nitrogen turnover than NO3− and NO2− reductases [58]. Wicht [59] estimates that the maximum N2O reduction rate is almost four times faster than the NO3− and NO2− reduction rates. This indicates that N2O could be completely reduced under anoxic/anaerobic conditions without the occurrence of its accumulation or emission.
However, fluctuations in environmental conditions have been found to lead to inhibition of the N2O reductase and accumulation of N2O (see §6). Also, denitrification enzymes are induced during exposure to anaerobic conditions. Under most circumstances, the induction of N2O reductase appears to lag behind the others resulting in transient accumulation of N2O [60]. In addition, N2O has been found to be the principal product for some denitrifiers as there is only approximately a 20 per cent difference in energy loss if denitrification does not proceed to completion [61,62].
The accumulation of N2O has been found not to result in significant emissions of N2O because of the lack of active aeration in the anoxic zones. Under such conditions, the air–liquid interface is limited to the surface area of the reactor, which would lead to limited N2O emission (table 1) given the relatively high solubility of N2O. However, the accumulated N2O that is carried over into the aerobic zone will be stripped quickly [14,63]. This emission can be minimized by providing enough anoxic time to allow the temporarily accumulated N2O to be removed.
6. Key process conditions leading to nitrous oxide emissions
The key process conditions affecting the N2O production from full-scale wastewater treatment plants are summarized in table 1. These, and a range of other process conditions leading to N2O emissions are further discussed in this section.
(a). Stripping owing to aeration
In contrast to freshwater, marine or soil environments, N2O emission from wastewater treatment plants is substantially enhanced owing to the stripping that is induced by active aeration. N2O is a relatively soluble gas in water with a Henry's law constant of 24 mM atm−1 (at 25°C and 0% salinity) [64] in comparison with 1.3 mM atm−1 (at 25°C and 0% salinity) for oxygen [65]. This implies that N2O could accumulate to relatively high levels in the liquid phase in the absence of active stripping. For example, Law et al. [49] observed negligible N2O emission from a nitrifying reactor in non-aerated periods despite its accumulation to 0.5 mg N l−1 in the liquid phase. In contrast, the liquid-phase N2O was in the range of 0.01–0.03 mg N l−1 in the aerated periods. Here, vigorous aeration employed to promote the activity of nitrifying bacteria resulted in stripping of the dissolved N2O. Gas-phase N2O measurements in full-scale plants also show that N2O emissions are two to three orders of magnitude higher in aerated zones than in non-aerated zones [66]. The emitted N2O can be either produced under aerobic conditions or accumulated during anoxic conditions preceding the aeration.
(b). Transition between anoxic and aerobic conditions
As described in §2, anoxic and aerobic compartments/periods are engineered in a wastewater treatment system to achieve nitrification and denitrification, respectively. However, a single sludge process consisting of both nitrifiers and denitrifiers is normally employed. The activated sludge is re-circulated between anoxic and aerobic compartments/periods and this would result in exposure of the mixed bacterial community to repeatedly changing conditions. Fluctuations within a compartment can also occur, for example, the DO concentration may decrease owing to increased loading or limitation of the aeration capacity [12,13,67]. Transient changes in DO concentration are shown to cause immediate increase in N2O production especially from AOB [68–70].
(i). Imposition of anoxia on nitrifying bacteria
It is widely reported that N2O production from nitrifying cultures is significantly increased during oxygen limitation. Maximum N2O production rates are observed between DO concentrations of 0.1 and 0.3 mg O2 l−1 [41,42,71,72]. The response of a nitrifying culture to the transition from aerobic to anoxic conditions was demonstrated by Kampschreur et al. [68]. The N2O and NO production rates increased instantly upon the imposition of anoxia from fully aerobic condition. The NO production also increased immediately when tested with NO2− and NH4+ pulsing under both aerobic and anoxic conditions [68]. Nitrifier denitrification by AOB is suggested to be the main pathway contributing to the production of N2O and NO as both NH4+ and NO2− were required to be present.
Yu & Chandran [73] further investigated the response of AOB to low DO coupled to NO2− accumulation at the gene expression and transcription level. During the exponential growth phase of the Nitrosomonas europaea batch culture, mRNA concentrations for ammonia monooxygenase (amoA) and hydroxylamine oxidoreductase (hao) were higher in cultures cultivated at lower DO. In addition, the presence of 280 mg NO2−-N l−1 resulted in elevated concentrations of nirK and norB mRNA for NO2− reductase and NO reductase, respectively. They postulate that N. europaea increases the efficiency to metabolize NH3 and NH2OH under oxygen limitation and also promote the reduction of NO2− for detoxification purposes when NO2− accumulates. However, such responses are not observed in stationary phase cells suggesting that the efficiency to metabolize substrate and to detoxify is probably dependent on the physiological growth state of the N. europaea culture.
(ii). Recovery of nitrifying bacteria from anoxic condition
In contrast to the above study, Yu et al. [70] report that it is the recovery from anoxia rather than the transition to anoxia that causes N2O production from AOB. Such observation was also reported in various full-scale wastewater treatment plants [66]. In an N. europaea pure culture grown in chemostat, NO accumulated under anoxic conditions, however N2O was produced only during the recovery from anoxic to aerobic conditions [70]. The N2O production during the transient recovery period correlated positively to the accumulation of NH4+ during anoxia, and the oxygen concentration upon recovery. In addition, the increased N2O production during the recovery period did not correlate with changes in the gene-expression level. It was therefore concluded that the tendency of nitrifying cultures to produce N2O is owing to a shift in metabolism from a low specific activity (q < qmax) towards the maximum specific activity (qmax).
Various other studies also report increased N2O production during increased aeration rate. Sümer et al. [25] found that increased N2O production coincides with increased oxygen concentration in the activated sludge process. Kampschreur et al. [68] also observed that N2O production by AOB in a nitritation–anammox process decreased with decreased DO concentrations. However, the mechanisms leading to these observations were not identified.
(iii). Nitrous oxide reduction by denitrifying bacteria during transient aerobic and anaerobic conditions
Similar to nitrifier denitrification, N2O emission from heterotrophic denitrification is also shown to be the highest under low DO concentrations of around 0.1–0.3 mg O2 l−1 [18,42,74]. Therefore, transient and dynamic aerobic and anaerobic conditions will likely increase N2O emission from heterotrophic denitrification. Oxygen inhibits both the synthesis and activity of denitrifying enzymes of Alcaligenes faecalis, in particular the N2O reductase [18]. The synthesis of the N2O reductase has a longer lag phase compared with the NO2− reductase synthesis after the transition from aerobic to anaerobic conditions. In addition, N2O reductase activity stops immediately during the transition from anaerobic to aerobic conditions, while the activity of NO2− reductase continues at a lower rate for several hours.
(c). The effect of nitrite, free nitrous acid and pH
(i). Nitrifying bacteria
Hynes & Knowles [75] demonstrate that addition of exogenous NO2− does not cause an increase in N2O production from a fully aerobic N. europaea culture. In addition, the optimum pH for the production of NO2− and N2O is approximately 8.5, in the investigated pH range of 5.4–9.5, further suggesting that a high free nitrous acid (HNO2) concentration, the true substrate for NO2− reduction [76], is not required for higher N2O production. As the aerobic N2O production is completely inhibited by acetylene (C2H2), the authors suggest that N2O is predominantly produced through degradation of NOH under aerobic conditions [75]. Increased N2O production rate of an enriched AOB culture at pH 8.0 when compared with pH 6.0 is also reported by Law et al. [49].
However, contradictory evidence is produced in some recent studies that report elevated N2O production rates by AOB in the presence of NO2−. Correlation between N2O production and high NO2− concentration by AOB is reported in several full-scale studies [15,25,63,67,77]. In laboratory scale studies, NO2− pulses of 10 mg NO2−-N l−1 are shown to increase N2O production by a nitrifying mixed culture especially at higher DO concentrations, with eightfold and fourfold increases occurring at DO concentrations of 1.0 and 0.1 mg O2 l−1, respectively [42]. Kampschreur et al. [68] also reveal that NO2− pulsing increases N2O production by an enriched AOB culture under aerobic conditions. The contrasting observations on the effect of NO2− on N2O production by AOB are yet to be resolved.
(ii). Denitrifying bacteria
The presence of NO2− has been shown to affect the activity of N2O reductase in a denitrifying bacterial culture leading to increased N2O emission. NO2− accumulation of up to 10 mg NO2−-N l−1 was identified as a possible cause of N2O production in denitrifying sludge [78]. However, the effect of NO2− addition on N2O accumulation is seen to be highly inconsistent [79]. Schulthess et al. [80] suggest that NO rather than NO2−, which accumulates upon NO2− addition, is the true inhibitor of N2O reductase.
Zhou et al. [81] show that HNO2 rather than NO2− is responsible for inhibiting the N2O reductase in an enriched denitrifying biological phosphorus removal system. N2O reductase activity was inhibited by 50 per cent at a HNO2 concentration of 0.0007–0.001 mg HNO2-N l−1 (equivalent to 3–4 mg NO2− N l−1 at pH 7). However, an internal storage polymer was the sole carbon source available as shown in the study of Zhou et al. [81], which is suggested to be a factor affecting N2O production (further discussed in §6d(iii)). Since the concentration of NO in the study was not reported, it is unclear whether HNO2 could have triggered transient NO accumulation to affect the N2O reductase activity. The high sensitivity of N2O reductase to low pH (<6.5) [82] also renders it difficult to distinguish the effect of pH and HNO2 in denitrifying cultures.
(d). Effect of carbon sources
(i). Availability of carbon source
The lack of biodegradable organic carbon is an important factor governing N2O production during denitrification [83,84]. The availability of organic carbon is typically measured as chemical oxygen demand (COD). For complete denitrification, a COD to N ratio above 4 is required. Under conditions of limited carbon sources, the various denitrification enzymes (NO3− reductase, NO2− reductase, NO reductase and N2O reductase) compete for electrons, potentially resulting in incomplete denitrification.
In an intermittently aerated laboratory scale reactor, approximately 20–30% of influent N was emitted as N2O when the COD to N ratio was less than 3.5 [78]. Similar observations have also been reported by Kishida et al. [85]. A pure culture study with A. faecalis shows that when carbon sources are limiting, N2O formation increases by 32–64%, while N2 production decreases significantly [84]. When excess carbon was supplied to remove electron competition, N2O formation decreased immediately. On the contrary, it is reported in full-scale studies that only little N2O generation and emission is observed regardless of carbon deficiency or sufficiency in anoxic zones or aerobic zones [14]. The nitrogen or helium sparging used to induce anoxia in the laboratory scale studies [84,85] may have contributed to the discrepancy between the observation in laboratory scale and full-scale studies. The continuous sparging may have stripped off the dissolved N2O to render it unavailable for further reduction to N2. This requires further investigation and verification.
In theory, N2O and NO are expected to accumulate during COD-limited denitrification as the NO3− and NO2− reductases have relatively higher affinity for electrons than the NO and N2O reductases [86]. However, this may not be generalized for all types of carbon sources as different metabolic pathways are employed for different carbon sources.
(ii). Types of carbon sources
The availability of different types of carbon sources may enrich different groups of bacteria and have different impacts on denitrification efficiency [87,88]. Methanol, ethanol and acetate, and to a lesser extent glycerol or sludge fermentates, are widely used as supplemented carbon sources for enhancing denitrification in BNR plants. While a COD/N of lower than 1.5 resulted in N2O production in a denitrifying culture fed with acetate and yeast extract [89,90], a COD limitation did not have an apparent impact on ethanol- and methanol-fed denitrifying cultures [19]. In addition, the methanol-fed denitrifiers are shown to have higher susceptibility to oxygen inhibition when compared with the ethanol-fed denitrifiers [19].
On the contrary, pure culture studies with A. faecalis indicate that N2O production is independent of the energetics of the substrate or the turnover rates of the enzymes. The type of supplemented carbon source (acetate versus butyrate) and the growth rate of the bacteria do not have any impact on overall N2O production [84]. The discrepancy between different studies may be attributed to the enrichment of different denitrifying populations. Therefore, the types of carbon sources used would affect the types of denitrifiers enriched which potentially have different susceptibility to other operational variables (e.g. NO2− and O2 inhibition).
(iii). Consumption of internal storage compound
Systems operated to achieve simultaneous nitrification, denitrification and phosphorus removal can encourage the growth of denitrifiers, such as polyphosphate-accumulating organisms and glycogen-accumulating organisms that are capable of storing organic carbon in the form of polyhydroxybutyrate (PHB). Laboratory scale studies on such systems show that denitrification by glycogen-accumulating organisms leads to increased N2O emission [17,91,92]. During anaerobic periods, these micro-organisms take up organic carbon for storage and subsequently degrade the PHB stored during aerobic/anoxic periods. Since PHB consumption is the rate-limiting step in these organisms [93], high N2O emission is possible by organisms growing on storage compounds owing to a slow supply of electrons, resulting in competition for electrons between denitrifying enzymes. Schalk-Otte et al. [84] observed that N2O accumulation coincides with the onset of storage compound usage upon COD depletion.
On the contrary, in a PHB-degrading denitrifying pure culture, no accumulation of N2O or nitrite was detected when PHB was used as the sole carbon source [94]. Further confirmation on the relationship between internal storage compounds and N2O production is essential as the dynamic conditions employed in treatment plants, such as in P-removal processes and bioselectors, are operated to select for organisms that are able to store carbon sources.
(e). Availability of copper ions
Copper is essential for the biosynthesis of N2O reductase and its availability affects N2O production in soil and marine environments [62]. Deficiency in copper supply is found to shift the endproduct of heterotrophic denitrification from N2 to N2O, whereas replenishing the copper supply reduces N2O production and increases N2 production [95,96]. Although copper is demonstrated to increase the N2O reductase activity and to reduce N2O production in an activated sludge [97], the availability of copper in wastewater systems and its subsequent effect on N2O production has thus far not been investigated.
7. Possible mitigation strategies
Although the exact triggers for N2O production by nitrifying and denitrifying sludge are yet to be fully revealed, and the predominant pathway relating to N2O production by AOB remains to be elucidated, it is generally observed that sudden process perturbations such as rapid shifts in reactor pH, DO and NH4+ or NO2− spikes lead to immediate increases in N2O emissions [68,90,98]. In fact, N2O emission has been recommended to be used as an indication of biological nitrification failure owing to toxic shock loads or insufficient aeration [13].
It is postulated that full-scale plants that are designed and configured to operate under more stable process conditions, such as oxidation ditches with uniform DO concentrations, produce less N2O when compared with those that are subject to frequent transitions (such as a modified Ludzack–Ettinger plant; figure 1) [70]. Full-scale studies also report that treatment plants designed and operated to achieve low total nitrogen in their effluents are equipped with design features that result in relatively low N2O emission levels [67]. These design features include influent flow balancing, high recycle rates, large bioreactor volumes and long solids’ retention time. Large bioreactor volumes and influent flow-balancing facilities equip the system with the ability to buffer loadings and reduce the risk of transient oxygen depletion. High recycle rates also tend to dilute the concentrations of NH4+ and nitrogen intermediates which lessens the effects of nitrification and denitrification, preventing the buildup of NO2− and NH4+ to levels that may increase N2O production [67,99,100].
Several mitigation strategies have been trialled in laboratory scale studies to minimize N2O emissions. Yang et al. [21] demonstrate that NH4+ and NO2− concentrations in the reactor can be maintained at low concentrations through step feeding, resulting in a 50 per cent reduction in N2O production. Avoiding transient pH changes under aerobic conditions by slow feeding rather than pulse feeding is shown to significantly reduce N2O production by an enriched AOB culture [49]. Applying longer solids retention time to increase the AOB biomass concentration (greater than 5 days) and higher DO (>0.5 mg O2 l−1) is also proposed to minimize N2O production from nitrification [71]. Pellicer-Nàcher et al. [101] demonstrate the possibility of minimizing N2O emission through sequential aeration in a membrane-aerated biofilm reactor. Here, the N2O produced by AOB within the membrane bundle is consumed by heterotrophic bacteria outside the bundle. To minimize N2O production during denitrification, methanol addition prevented N2O accumulation by eliminating electron competition from other denitrifying enzymes [90].
These mitigation strategies have so far only been demonstrated in laboratory scale systems. Their effectiveness is yet to be verified through full-scale trials. The research community is making steady progress in gaining understanding of the mechanisms involved in N2O emission in wastewater treatment systems, which will enable the development of effective mitigation strategies.
8. Conclusions
Despite their relatively small contribution to the overall global greenhouse gas emissions, N2O emissions from BNR wastewater treatment plants can be very significant in terms of their contribution to the overall carbon footprint of wastewater treatment systems, and should be understood, accounted for and mitigated.
N2O emissions from wastewater treatment processes vary substantially between plants depending on the design and operation of the plants, and the flow and characteristics of wastewater. These variations indicate that N2O may be mitigated through proper process design and operation. Indeed, preliminary strategies have been developed but remain to be verified through full-scale applications.
In contrast with many other systems (e.g. soil), where denitrification is revealed to be the primary source of N2O, autotrophic NH3 oxidation is found to make relatively more contributions than heterotrophic denitrification in most wastewater treatment plants. This is probably related to the fact that AOB produce N2O under aerated conditions; and most of the N2O produced is stripped instantly by aeration. In contrast, denitrifiers produce N2O primarily under non-aerated conditions. N2O can remain dissolved in the absence of stripping, giving time for its subsequent reduction to N2. However, N2O carried over from non-aerated zones/periods to the aerated zones/periods will probably be stripped there.
The detailed mechanisms involved in N2O production by AOB remain to be fully elucidated. Both nitrifier denitrification and the breakdown or degradation of nitrification intermediates probably contribute to the overall N2O production. However, the level of contribution by each of these two processes is unclear and contradictory evidence has been produced. Indeed, their relative contributions could be dependent on process conditions.
Various factors have been reported in the wastewater literature to induce N2O emissions by AOB and denitrifiers. A detailed understanding of the factors is currently missing.
Future research in the field will focus on both the quantification and reduction of N2O emissions from various full-scale wastewater treatment plants. Additionally, future studies will reveal the fundamental processes involved in N2O production by both nitrification and denitrification.
Acknowledgements
The authors would like to thank the Australian Research Council (ARC) for funding this work through projects LP0991765 and DPO0987204. The Western Australian Water Corporation also supported the research through project LP0991765. Y.L. is an Australian Postgraduate Award recipient. Y.P. acknowledges the scholarship support by the University of Queensland.
References
- 1.IPCC 2007. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Team C. W., Pachauri R. K., Reisinger A.). Geneva, Switzerland: IPCC [Google Scholar]
- 2.Ravishankara A. R., Daniel J. S., Portmann R. W. 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 10.1126/science.1176985 (doi:10.1126/science.1176985) [DOI] [PubMed] [Google Scholar]
- 3.Dalal Ram C., Robertson W. W., Philip G., Parton W. J. 2003. Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust. J. Soil Res. 41, 165–195 10.1071/SR02064 (doi:10.1071/SR02064) [DOI] [Google Scholar]
- 4.Czepiel P., Crill P., Harriss R. 1995. Nitrous oxide emissions from municipal wastewater treatment. Environ. Sci. Technol. 29, 2352–2356 10.1021/es00009a030 (doi:10.1021/es00009a030) [DOI] [PubMed] [Google Scholar]
- 5.United States Environmental Protection Agency 2006. Global anthropogenic non-CO2 greenhouse gas emissions: 1990 to 2020. Washington, DC: US-EPA [Google Scholar]
- 6.Kampschreur M. J., Tan N. C. G., Kleerebezem R., Picioreanu C., Jetten M. S. M., Van Loosdrecht M. C. M. 2008. Effect of dynamic process conditions on nitrogen oxide emission from a nitrifying culture. Environ. Sci. Technol. 42, 429–435 10.1021/es071667p (doi:10.1021/es071667p) [DOI] [PubMed] [Google Scholar]
- 7.Tchobanoglous G., Burton F., Stensel H. D. 2002. Wastewater engineering treatment and reuse, 4th edn New York: McGraw-Hill [Google Scholar]
- 8.Seviour R. J., Lindrea K. C., Griffiths P. C., Blackall L. L. 1999. The activated sludge process. In The microbiology of activated sludge (eds Seviour R. J., Blackall L. L.). Boston, MA: Kluwer Publishing [Google Scholar]
- 9.Münch E. V., Lant P., Keller J. 1996. Simultaneous nitrification and denitrification in bench-scale sequencing batch reactors. Water Res. 30, 277–284 10.1016/0043-1354(95)00174-3 (doi:10.1016/0043-1354(95)00174-3) [DOI] [Google Scholar]
- 10.Irvine R. L., Ketchum L. H., Asano T. 1989. Sequencing batch reactors for biological wastewater treatment. Crit. Rev. Environ. Sci. Technol. 18, 255–294 10.1080/10643388909388350 (doi:10.1080/10643388909388350) [DOI] [Google Scholar]
- 11.Kimochi Y., Inamori Y., Mizuochi M., Xu K.-Q., Matsumura M. 1998. Nitrogen removal and N2O emission in a full-scale domestic wastewater treatment plant with intermittent aeration. J. Ferment. Bioeng. 86, 202–206 10.1016/S0922-338X(98)80114-1 (doi:10.1016/S0922-338X(98)80114-1) [DOI] [Google Scholar]
- 12.Burgess J. E., Colliver B. B., Stuetz R. M., Stephenson T. 2002. Dinitrogen oxide production by a mixed culture of nitrifying bacteria during ammonia shock loading and aeration failure. J. Indust. Microbiol. Biotechnol. 29, 309–313 10.1038/sj.jim.7000286 (doi:10.1038/sj.jim.7000286) [DOI] [PubMed] [Google Scholar]
- 13.Butler M. D., Wang Y. Y., Cartmell E., Stephenson T. 2009. Nitrous oxide emissions for early warning of biological nitrification failure in activated sludge. Water Res. 43, 1265–1272 10.1016/j.watres.2008.12.027 (doi:10.1016/j.watres.2008.12.027) [DOI] [PubMed] [Google Scholar]
- 14.Ahn J. H., Kim S. P., Park H. K., Rahm B., Pagilla K., Chandran K. 2010. N2O Emissions from activated sludge processes, 2008–2009: results of a national monitoring survey in the United States. Environ. Sci. Technol. 44, 4505–4511 10.1021/es903845y (doi:10.1021/es903845y) [DOI] [PubMed] [Google Scholar]
- 15.Desloover J., De Clippeleir H., Boeckx P., Du Laing G., Colsen J., Verstraete W., Vlaeminck S.E. 2011. Floc-based sequential partial nitration and anammox at full scale with contrasting N2O emissions. Water Res. 45, 2811–2821 10.1016/j.watres.2011.02.028 (doi:10.1016/j.watres.2011.02.028) [DOI] [PubMed] [Google Scholar]
- 16.Joss A., et al. 2009. Full-scale nitrogen removal from digester liquid with partial nitration and anammox in one SBR. Environ. Sci. Technol. 43, 5301–5306 10.1021/es900107w (doi:10.1021/es900107w) [DOI] [PubMed] [Google Scholar]
- 17.Zeng R. J., Lemaire R., Yuan Z., Keller J. 2003. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 84, 170–178 10.1002/bit.10744 (doi:10.1002/bit.10744) [DOI] [PubMed] [Google Scholar]
- 18.Otte S., Grobben N., Robertson L., Jetten M., Kuenen J. 1996. Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl. Environ. Microbiol. 62, 2421–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H., Chandran K. 2010. Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol. Bioeng. 106, 390–398 10.1002/bit.22704 (doi:10.1002/bit.22704) [DOI] [PubMed] [Google Scholar]
- 20.Garrido J. M., Moreno J., Mendez-Pampn R., Lema J. M. 1998. Nitrous oxide production under toxic conditions in a denitrifying anoxic filter. Water Res. 32, 2550–2552 10.1016/S0043-1354(97)00433-8 (doi:10.1016/S0043-1354(97)00433-8) [DOI] [Google Scholar]
- 21.Yang Q., Liu X., Peng C., Wang S., Sun H., Peng Y. 2009. N2O production during nitrogen removal via nitrite from domestic wastewater: main sources and control method. Environ. Sci. Technol. 43, 9400–9406 10.1021/es9019113 (doi:10.1021/es9019113) [DOI] [PubMed] [Google Scholar]
- 22.Kampschreur M. J., van der Star W. R. L., Wielders H. A., Mulder J. W., Jetten M. S. M., van Loosdretch M. C. M. 2008. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 42, 812–826 10.1016/j.watres.2007.08.022 (doi:10.1016/j.watres.2007.08.022) [DOI] [PubMed] [Google Scholar]
- 23.Foley J., De Haas D., Yuan Z., Lant P. 2009. Nitrous oxide generation in full scale BNR wastewater treatment plants. Water Res. 44, 831–844 10.1016/j.watres.2009.10.033 (doi:10.1016/j.watres.2009.10.033) [DOI] [PubMed] [Google Scholar]
- 24.Andersen K., Kjaer T., Revsbech N. P. 2001. An oxygen insensitive microsensor for nitrous oxide. Sens. Actuat. B-Chem. 81, 42–48 10.1016/S0925-4005(01)00924-8 (doi:10.1016/S0925-4005(01)00924-8) [DOI] [Google Scholar]
- 25.Sümer E., Weiske A., Benckiser G., Ottow J. C. G. 1995. Influence of environmental conditions on the amount of N2O released from activated sludge in a domestic waste water treatment plant. Cell. Mol. Life Sci. 51, 419–422 10.1007/BF01928908 (doi:10.1007/BF01928908) [DOI] [Google Scholar]
- 26.Benckiser G., Eilts R., Linn A., Lorch H. J., Sümer E., Weiske A., Wenzhöfer F. 1996. N2O emissions from different cropping systems and from aerated, nitrifying and denitrifying tanks of a municipal waste water treatment plant. Biol. Fertility Soils 23, 257–265 10.1007/BF00335953 (doi:10.1007/BF00335953) [DOI] [Google Scholar]
- 27.Peu P., Beline F., Picard S., Heduit A. 2006. Measurement and quantification of nitrous oxide emissions from municipal activated sludge plants in France. In Proceedings of the 5th IWA World Water Congress, 10–14 September 2006, Beijing, China International Water Association [Google Scholar]
- 28.Foley J., Yuan Z., Keller J., Senante E., Chandran K., Willis J., Shah A., van Loosdrecht M. 2011. N2O and CH4 emission from wastewater collection and treatment systems. Technical report, Global Water Research Coalition, London, UK [Google Scholar]
- 29.de Haas D., Hartley K.Greenhouse gas emission from BNR plants: do we have the right focus?. Proceeedings of EPA Workshop: Sewage Management: Risk Assessment and Triple Bottom Line, 5–7 April 2004, Cairns, Australia.2004. [Google Scholar]
- 30.Casciotti K. L., Ward B. B. 2001. Dissimilatory nitrite reductase genes from autotrophic ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 67, 2213–2221 10.1128/AEM.67.5.2213-2221.2001 (doi:10.1128/AEM.67.5.2213-2221.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casciotti K. L., Ward B. B. 2005. Phylogenetic analysis of nitric oxide reductase gene homologues from aerobic ammonia-oxidizing bacteria. FEMS Microbiol. Ecol. 52, 197–205 10.1016/j.femsec.2004.11.002 (doi:10.1016/j.femsec.2004.11.002) [DOI] [PubMed] [Google Scholar]
- 32.Beaumont H., Lens S., Reijinders W., Westerhoff H., van Spanning R. 2004. Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol. Microbiol. 54, 148–158 [DOI] [PubMed] [Google Scholar]
- 33.Beaumont H. J. E., Lens S. I., Westerhoff H. V., van Spanning R. J. M. 2005. Novel nirK cluster genes in Nitrosomonas europaea are required for NirK-dependent tolerance to nitrite. J. Bacteriol. 187, 6849–6851 10.1128/JB.187.19.6849-6851.2005 (doi:10.1128/JB.187.19.6849-6851.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantera J. J. L., Stein L. Y. 2007. Molecular diversity of nitrite reductase genes (nirK) in nitrifying bacteria. Environ. Microbiol. 9, 765–776 10.1111/j.1462-2920.2006.01198.x (doi:10.1111/j.1462-2920.2006.01198.x) [DOI] [PubMed] [Google Scholar]
- 35.Shaw L. J., Nicol G. W., Smith Z., Fear J., Prosser J. I., Baggs E. M. 2006. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ. Microbiol. 8, 214–222 10.1111/j.1462-2920.2005.00882.x (doi:10.1111/j.1462-2920.2005.00882.x) [DOI] [PubMed] [Google Scholar]
- 36.Garbeva P., Baggs E. M., Prosser J. I. 2007. Phylogeny of nitrite reductase (nirK) and nitric oxide reductase (norB) genes from Nitrosospira species isolated from soil. FEMS Microbiol. Lett. 266, 83–89 10.1111/j.1574-6968.2006.00517.x (doi:10.1111/j.1574-6968.2006.00517.x) [DOI] [PubMed] [Google Scholar]
- 37.Kim S.-W., Miyahara M., Fushinobu S., Wakagi T., Shoun H. 2010. Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresource Technol. 101, 3958–3963 10.1016/j.biortech.2010.01.030 (doi:10.1016/j.biortech.2010.01.030) [DOI] [PubMed] [Google Scholar]
- 38.Bock E., Schmidt I., Stüven R., Zart D. 1995. Nitrogen loss caused by denitrifying Nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch. Microbiol. 163, 16–20 10.1007/BF00262198 (doi:10.1007/BF00262198)7646315 [DOI] [Google Scholar]
- 39.Poth M., Focht D. 1985. 15N Kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl. Environ. Microbiol. 49, 1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritchie G. A. F., Nicholas D. J. D. 1972. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem. J. 126, 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. 1980. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40, 526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tallec G., Garnier J., Billen G., Gousailles M. 2006. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: effect of oxygenation level. Water Res. 40, 2972–2980 10.1016/j.watres.2006.05.037 (doi:10.1016/j.watres.2006.05.037) [DOI] [PubMed] [Google Scholar]
- 43.Suzuki I., Dular U., Kwok S. C. 1974. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 120, 556–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson K. K., Hooper A. B. 1983. O2 and H2O are each the source of one O in NO−2 produced from NH3 by Nitrosomonas: 15N-NMR evidence. FEBS Lett. 164, 236–240 10.1016/0014-5793(83)80292-0 (doi:10.1016/0014-5793(83)80292-0) [DOI] [Google Scholar]
- 45.Igarashi N., Moriyama H., Fujiwara T., Fukumori Y., Tanaka N. 1997. The 2.8 A structure of hydroxylamine oxidoreductase from a nitrifying chemoautotrophic bacterium, Nitrosomonas europaea. Nat. Struct. Mol. Biol. 4, 276–284 10.1038/nsb0497-276 (doi:10.1038/nsb0497-276) [DOI] [PubMed] [Google Scholar]
- 46.Poughon L., Dussap C. G., Gros J. B. 2001. Energy model and metabolic flux analysis for autotrophic nitrifiers. Biotechnol. Bioeng. 72, 416–433 (doi:10.1002/1097-0290(20000220)72:4<416::AID-BIT1004>3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- 47.Bock E., Koop H. P., Harms H., Ahlers B. 1991. The biochemistry of nitrifying organisms. In Variations in autotrophic life (eds Shively J. M., Barton L. L.), pp. 171–199 New York, NY: Academic Press [Google Scholar]
- 48.Yu R., Kampschreur M. J., Loosdrecht M. C. M. V., Chandran K. 2010. Molecular mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ. Sci. Technol. 44, 1313–1319 10.1021/es902794a (doi:10.1021/es902794a) [DOI] [PubMed] [Google Scholar]
- 49.Law Y., lant P., Yuan Z. 2012. The effect of pH on N2O production under aerobic conditions in a partial nitration system. Water Res. 45, 5934–5944 10.1016/j.watres.2011.08.055 (doi:10.1016/j.watres.2011.08.055) [DOI] [PubMed] [Google Scholar]
- 50.Law Y., Ni B.-J., lant P., Yuan Z. Submitted Nitrous oxide (N2O) production rate of an enriched culture of ammonia oxidising bacteria exponentially correlated to its ammonia oxidation rate. [DOI] [PubMed] [Google Scholar]
- 51.Arp D., Chain P., Klotz M. 2007. The impact of genome analyses on our understanding of ammonia-oxidizing bacteria. Annu. Rev. Microbiol. 61, 503–528 10.1146/annurev.micro.61.080706.093449 (doi:10.1146/annurev.micro.61.080706.093449) [DOI] [PubMed] [Google Scholar]
- 52.Upadhyay A. K., Hooper A. B., Hendrich M. P. 2006. NO reductase activity of the tetraheme cytochrome c554 of Nitrosomonas europaea. J. Am. Chem. Soc. 128, 4330–4337 10.1021/ja055183+ (doi:10.1021/ja055183+) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein L. 2011. Surveying N2O-producing pathways in bacteria. Methods Enzymol. 486, 131–152 10.1016/B978-0-12-381294-0.00006-7 (doi:10.1016/B978-0-12-381294-0.00006-7) [DOI] [PubMed] [Google Scholar]
- 54.Schmidt I., Jetten M. S. M. 2004. Anaerobic oxidation of inorganic nitrogen compounds. In Strict and facultative anaerobes: medical and environmental aspects (eds Nakano M. M., Zube P.), pp. 283–303 Berlin, Germany/UK: Springer/Horizon Scientific Press [Google Scholar]
- 55.Schmidt I., Bock E. 1998. Anaerobic ammonia oxidation by cell-free extracts of Nitrosomonas eutropha. Antonie Van Leeuwenhoek 73, 271–278 10.1023/A:1001572121053 (doi:10.1023/A:1001572121053) [DOI] [PubMed] [Google Scholar]
- 56.Schmidt I., Zart D., Bock E. 2001. Gaseous NO2 as a regulator for ammonia oxidation of Nitrosomonas eutropha. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 79, 311–318 10.1023/A:1012038314206 (doi:10.1023/A:1012038314206) [DOI] [PubMed] [Google Scholar]
- 57.Schmidt I., Enrique C., Lester P. 2008. Nitric oxide: interaction with the ammonia monooxygenase and regulation of metabolic activities in ammonia oxidizers. Methods Enzymol. 440, 121–135 10.1016/S0076-6879(07)00807-5 (doi:10.1016/S0076-6879(07)00807-5) [DOI] [PubMed] [Google Scholar]
- 58.Von Schulthess R., Wild D., Gujer W. 1994. Nitric and nitrous oxide from denitrifying activated sludge at low oxygen concentrations. Water Sci. Technol. 30, 123–132 [Google Scholar]
- 59.Wicht H. 1996. A model for predicting nitrous oxide production during denitrification in activated sludge. Water Sci. Technol. 34, 99–106 [Google Scholar]
- 60.Holtan-Hartwig L., Dörsch P., Bakken L. R. 2000. Comparison of denitrifying communities in organic soils: kinetics of NO3− and N2O reduction. Soil Biol. Biochem. 32, 833–843 10.1016/S0038-0717(99)00213-8 (doi:10.1016/S0038-0717(99)00213-8) [DOI] [Google Scholar]
- 61.Brettar I., Hofle M. G. 1993. Nitrous oxide producing heterotrophic bacteria from a water column of the central Baltic: abundance and molecular identification. Mar. Ecol. Prog. Ser. 94, 253–265 10.3354/meps094253 (doi:10.3354/meps094253) [DOI] [Google Scholar]
- 62.Richardson D., Felgate H., Watmough N., Thomson A., Baggs E. 2009. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—could enzymic regulation hold the key. Trends Biotechnol. 27, 388–397 10.1016/j.tibtech.2009.03.009 (doi:10.1016/j.tibtech.2009.03.009) [DOI] [PubMed] [Google Scholar]
- 63.Kampschreur M. J., Temmink H., Kleerebezem R., Jetten M. S. M., van Loosdrecht M. C. M. 2009. Nitrous oxide emission during wastewater treatment. Water Res. 43, 4093–4103 10.1016/j.watres.2009.03.001 (doi:10.1016/j.watres.2009.03.001) [DOI] [PubMed] [Google Scholar]
- 64.Weiss R. F., Price B. A. 1980. Nitrous oxide solubility in water and seawater. Mar. Chem. 8, 347–359 10.1016/0304-4203(80)90024-9 (doi:10.1016/0304-4203(80)90024-9) [DOI] [Google Scholar]
- 65.Dean J. A. 1992. Lange's handbook of chemistry. New York: McGraw-Hill, Inc [Google Scholar]
- 66.Ahn J. H., Kim S., Park H., Rahm B., Pagilla K., Chandran K. 2010. N2O Emissions from activated sludge processes, 2008–2009: results of a national monitoring survey in the United States. Environ. Sci. Technol. 44, 4505–4511 10.1021/es903845y (doi:10.1021/es903845y) [DOI] [PubMed] [Google Scholar]
- 67.Foley J., de Haas D., Yuan Z., Lant P. 2009. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res. 44, 831–844 10.1016/j.watres.2009.10.033 (doi:10.1016/j.watres.2009.10.033) [DOI] [PubMed] [Google Scholar]
- 68.Kampschreur M. J., Tan N. C. G., Kleerebezem R., Picioreanu C., Jetten M. S. M., van Loosdrecht M. C. M. 2008. Effect of dynamic process conditions on nitrogen oxides emission from a nitrifying culture. Environ. Sci. Technol. 42, 429–435 10.1021/es071667p (doi:10.1021/es071667p) [DOI] [PubMed] [Google Scholar]
- 69.Kester R. A., De Boer W., Laanbroek H. J. 1997. Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl. Environ. Microbiol. 63, 3872–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu R., Kampschreur M. J., Loosdrecht M. C. M. V., Chandran K. 2010. Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ. Sci. Technol. 44, 1313–1319 10.1021/es902794a (doi:10.1021/es902794a) [DOI] [PubMed] [Google Scholar]
- 71.Zheng H., Hanaki K., Matsuo T. 1994. Production of nitrous oxide gas during nitrification of wastewater. Water Sci. Technol. 30, 133–141 [Google Scholar]
- 72.Chuang H.-P., Ohashi A., Imachi H., Tandukar M., Harada H. 2007. Effective partial nitrification to nitrite by down-flow hanging sponge reactor under limited oxygen condition. Water Res. 41, 295–302 10.1016/j.watres.2006.10.019 (doi:10.1016/j.watres.2006.10.019) [DOI] [PubMed] [Google Scholar]
- 73.Yu R., Chandran K. 2010. Strategies of Nitrosomonas europaea 19718 to counter low dissolved oxygen and high nitrite concentrations. BMC Microbiol. 10, 70. 10.1186/1471-2180-10-70 (doi:10.1186/1471-2180-10-70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tallec G., Garnier J., Billen G., Gousailles M. 2008. Nitrous oxide emissions from denitrifying activated sludge of urban wastewater treatment plants, under anoxia and low oxygenation. Bioresour. Technol. 99, 2200–2209 10.1016/j.biortech.2007.05.025 (doi:10.1016/j.biortech.2007.05.025) [DOI] [PubMed] [Google Scholar]
- 75.Hynes R. K., Knowles R. 1984. Production of nitrous oxide by Nitrosomonas europaea: effects of acetylene, pH, and oxygen. Can. J. Microbiol. 30, 1397–1404 10.1139/m84-222 (doi:10.1139/m84-222) [DOI] [Google Scholar]
- 76.Shiskowski D. M., Mavinic D. S. 2006. The influence of nitrite and pH (nitrous acid) on aerobic-phase, autotrophic N2O generation in a wastewater treatment bioreactor. J. Environ. Eng. Sci. 5, 273–283 10.1139/s05-034 (doi:10.1139/s05-034) [DOI] [Google Scholar]
- 77.Kampschreur M. J., van der Star W. R. L., Wielders H. A., Mulder J. W., Jetten M. S. M., van Loosdrecht M. C. M. 2008. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 42, 812–826 10.1016/j.watres.2007.08.022 (doi:10.1016/j.watres.2007.08.022) [DOI] [PubMed] [Google Scholar]
- 78.Itokawa H., Hanaki K., Matsuo T. 2001. Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res. 35, 657–664 10.1016/S0043-1354(00)00309-2 (doi:10.1016/S0043-1354(00)00309-2) [DOI] [PubMed] [Google Scholar]
- 79.Betlach M. R., Tiedje J. M. 1981. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl. Environ. Microbiol. 42, 1074–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulthess R. V., Kühni M., Gujer W. 1995. Release of nitric and nitrous oxides from denitrifying activated sludge. Water Res. 29, 215–226 10.1016/0043-1354(94)E0108-I (doi:10.1016/0043-1354(94)E0108-I) [DOI] [Google Scholar]
- 81.Zhou Y., Pijuan M., Zeng R. J., Yuan Z. 2008. Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ. Sci. Technol. 42, 8260–8265 10.1021/es800650j (doi:10.1021/es800650j) [DOI] [PubMed] [Google Scholar]
- 82.Ghosh S., Gorelsky S. I., George S. D., Chan J. M., Cabrito I., Dooley D. M., Moura J. J., Moura I., Solomon E. I. 2007. Spectroscopic, computational, and kinetic studies of the µ4-sulfide-bridged tetranuclear CuZ cluster in N2O reductase: pH effect on the edge ligand and its contribution to reactivity. J. Am. Chem. Soc. 129, 3955–3965 10.1021/ja068059e (doi:10.1021/ja068059e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung Y. C., Chung M. S. 2000. BNP test to evaluate the influence of C/N ratio on N2O production in biological denitrification. Water Sci. Technol. 42, 23–27 [Google Scholar]
- 84.Schalk-Otte S., Seviour R. J., Kuenen J. G., Jetten M. S. M. 2000. Nitrous oxide (N2O) production by Alcaligenes faecalis during feast and famine regimes. Water Res. 34, 2080–2088 10.1016/S0043-1354(99)00374-7 (doi:10.1016/S0043-1354(99)00374-7) [DOI] [Google Scholar]
- 85.Kishida N., Kim J. H., Kimochi Y., Nishimura O., Sasaki S., Sudo R. 2004. Effect of C/N ratio on nitrous oxide emission from swine wastewater treatment process. Water Sci. Technol. 49, 359–371 [PubMed] [Google Scholar]
- 86.Knowles R. 1982. Denitrification. Microbiol. Rev. 46, 43–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christensson M., Lie E., Welander T. 1994. A comparison between ethanol and methanol as carbon sources for denitrification. Water Sci. Technol. 30, 83–90 [Google Scholar]
- 88.Hallin S., Pell M. 1998. Metabolic properties of denitrifying bacteria adapting to methanol and ethanol in activated sludge. Water Res. 32, 13–18 10.1016/S0043-1354(97)00199-1 (doi:10.1016/S0043-1354(97)00199-1) [DOI] [Google Scholar]
- 89.Hanaki K., Hong Z., Matsuo T. 1992. Production of nitrous oxide gas during denitrification of wastewater. Water Sci. Technol. 26, 1027–1036 [Google Scholar]
- 90.Park K. Y., Inamori Y., Mizuochi M., Ahn K. H. 2000. Emission and control of nitrous oxide from a biological wastewater treatment system with intermittent aeration. J. Biosci. Bioeng. 90, 247–252 [PubMed] [Google Scholar]
- 91.Lemaire R., Meyer R., Taske A., Crocetti G. R., Keller J., Yuan Z. 2006. Identifying causes for N2O accumulation in a lab-scale sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal. J. Biotechnol. 122, 62–72 10.1016/j.jbiotec.2005.08.024 (doi:10.1016/j.jbiotec.2005.08.024) [DOI] [PubMed] [Google Scholar]
- 92.Zeng R. J., Yuan Z., Keller J. 2003. Enrichment of denitrifying glycogen-accumulating organisms in anaerobic/anoxic activated sludge system. Biotechnol. Bioeng. 81, 397–404 10.1002/bit.10484 (doi:10.1002/bit.10484) [DOI] [PubMed] [Google Scholar]
- 93.Murnleitner E., Kuba T., Loosdrecht M. C. M. V., Heijnen J. J. 1997. An integrated metabolic model for the aerobic and denitrifying biological phosphorus removal. Biotechnol. Bioeng. 54, 434–450 (doi:10.1002/(SICI)1097-0290(19970605)54:5<434::AID-BIT4>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 94.Tabrez Khan S., Hiraishi A. 2001. Isolation and characterization of a new poly(3-hydroxybutyrate)-degrading, denitrifying bacterium from activated sludge. FEMS Microbiol. Lett. 205, 253–257 10.1111/j.1574-6968.2001.tb10957.x (doi:10.1111/j.1574-6968.2001.tb10957.x) [DOI] [PubMed] [Google Scholar]
- 95.Matsubara T., Frunzke K., Zumft W. 1982. Modulation by copper of the products of nitrite respiration in Pseudomonas perfectomarinus. J. Bacteriol. 149, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Granger J., Ward B. B. 2003. Accumulation of nitrogen oxides in copper-limited cultures of denitrifying bacteria. Limnol. Oceanogr. 48, 313–318 10.4319/lo.2003.48.1.0313 (doi:10.4319/lo.2003.48.1.0313) [DOI] [Google Scholar]
- 97.Zhu X., Chen Y. 2011. Reduction of N2O and NO generation in anaerobic–aerobic (low dissolved oxygen) biological wastewater treatment process by using sludge alkaline fermentation liquid. Environ. Sci. Technol. 45, 2137–2143 10.1021/es102900h (doi:10.1021/es102900h) [DOI] [PubMed] [Google Scholar]
- 98.Sinha B., Annachhatre A. 2007. Partial nitrification—operational parameters and microorganisms involved. Rev. Environ. Sci. Biotechnol. 6, 285–313 10.1007/s11157-006-9116-x (doi:10.1007/s11157-006-9116-x) [DOI] [Google Scholar]
- 99.Casey T. G., Wentzel M. C., Ekama G. A. 1999. Filamentous organism bulking in nutrient removal activated sludge systems. Paper 9: Review of biochemistry of heterotrophic respiratory metabolism. Water S.A. 25, 409–424 [Google Scholar]
- 100.Casey T. G., Wentzel M. C., Ekama G. A. 1999. Filamentous organism bulking in nutrient removal activated sludge systems. Paper 10: Metabolic behaviour of heterotrophic facultative aerobic organisms under aerated/unaerated conditions. Water S.A. 25, 425–442 [Google Scholar]
- 101.Pellicer-Nàcher C., Sun S., Lackner S., Terada A., Schreiber F., Zhou Q., Smets B. F. 2010. Sequential aeration of membrane-aerated biofilm reactors for high-rate autotrophic nitrogen removal: experimental demonstration. Environ. Sci. Technol. 44, 7628–7634 10.1021/es1013467 (doi:10.1021/es1013467) [DOI] [PubMed] [Google Scholar]