Abstract
Signatories of the Kyoto Protocol are obliged to submit annual accounts of their anthropogenic greenhouse gas emissions, which include nitrous oxide (N2O). Emissions from the sectors industry (3.8 Gg), energy (14.4 Gg), agriculture (86.8 Gg), wastewater (4.4 Gg), land use, land-use change and forestry (2.1 Gg) can be calculated by multiplying activity data (i.e. amount of fertilizer applied, animal numbers) with simple emission factors (Tier 1 approach), which are generally applied across wide geographical regions. The agricultural sector is the largest anthropogenic source of N2O in many countries and responsible for 75 per cent of UK N2O emissions. Microbial N2O production in nitrogen-fertilized soils (27.6 Gg), nitrogen-enriched waters (24.2 Gg) and manure storage systems (6.4 Gg) dominate agricultural emission budgets. For the agricultural sector, the Tier 1 emission factor approach is too simplistic to reflect local variations in climate, ecosystems and management, and is unable to take into account some of the mitigation strategies applied. This paper reviews deviations of observed emissions from those calculated using the simple emission factor approach for all anthropogenic sectors, briefly discusses the need to adopt specific emission factors that reflect regional variability in climate, soil type and management, and explains how bottom-up emission inventories can be verified by top-down modelling.
Keywords: emission factors, nitrogen fertilizer, manure, atmospheric deposition, leaching, land-use change
1. Introduction
To keep the projected global average temperature increases within 2°C of preindustrial levels, developed countries need to reduce their greenhouse gas emissions by 25–40% below 1990 levels by 2020 [1]. The EU-27, responsible for about 10 per cent of the global annual greenhouse gas emissions, has agreed to reduce emissions by 20 per cent by 2020 and by 80 per cent by 2050. They predict that this reduction can be achieved by improved efficiency, new low carbon technologies, renewable energy and abatement strategies [2]. To monitor progress, greenhouse gas emissions are reported annually and are submitted to the United Nations Framework Convention on Climate Change (UNFCCC) by individual countries and the EU-27. Emissions of carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O) and the fluorinated greenhouse gases are reported for all anthropogenic sources, which fall into the categories (i) energy, (ii) industrial processes and product use, (iii) agriculture, (iv) forestry and other land use, (v) waste, and (vi) other sources (this includes indirect emissions as a result of atmospheric N deposition). The reporting follows internationally agreed protocols often using simple equations, represented by a Tier 1 methodology [1,3]. The UK, in common with most Annex I countries, uses the Intergovernmental Panel on Climate Change (IPCC) 1996 reporting guidelines [3,4] to prepare emission inventories for the first Kyoto commitment period (2008–2012). It is likely that there will be a general move to the 2006 guidelines [5], which are slight modifications of the 1996 guidelines, after the 2012 inventory reporting.
The basic Tier 1 approach is useful to compare anthropogenic emissions from different countries, but does not capture the well-documented variations across climate regions, agricultural management or combustion technologies and the potential effects of mitigation practices [6]. Specific methodologies, country/regional, technology-specific emission factors (Tier 2) and a range of simple to complex process-based models (Tier 3) have been developed to address this problem [7]. The IPCC recommends such improved methods to be used alongside the Tier 1 methodologies, as long as these methods are transparent and documented. Tier 2 methodology is applied to some of the IPCC categories by some EU countries. For example, the Netherlands apply a country-specific emission factor for CH4 emissions from animal production; UK CH4 emission factors are related to animal live weight, milk production and milk fat content, and Sweden uses a mixture of default and national emission factors for different sub-sources of direct N2O emissions.
Nitrous oxide is one of the main greenhouse gases and contributes 10 per cent (0.16 W m−2) of the total global anthropogenic radiative forcing [1]. Owing to the decline in emissions of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), N2O is now the most important ozone-depleting molecule in the stratosphere [8]. The annual global N2O budget is currently estimated at 27.8 Tg N2O yr−1 (range 13.4–43.5); microbial processes in soils and aquatic systems are responsible for 89 per cent of the annual global N2O emissions. Of this total, natural soils and oceans make up the largest components (37% and 21%, respectively) [1]. Various estimates suggest that tropical forest soils are globally the largest natural soil source of N2O (2.11 Tg N2O yr−1 (range 1.38–3.72) [9], owing to high turnover rates and the wet, warm environment conducive to N2O production. The anthropogenic emissions from agricultural soils are the third most important source (15.8%, or 4.4 Tg N2O yr−1; range 2.7–7.5) and those from rivers, estuaries and coastal zones next (9.6%, or 2.7 Tg N2O–N yr−1; range 0.8–4.6).
In the UK and similar densely populated countries in temperate climates, N2O emissions are dominated by anthropogenic sources. Biological emissions from the agriculture sector are the main anthropogenic source of N2O and in 2008 accounted for approximately 75 per cent of the annual N2O emissions, both in the EU-27 (877.0 Gg) and in the UK (87.6 Gg) [10]. The remaining source categories: energy, industry, land use, land-use change and forestry (LULUCF) and waste are similarly proportioned in the UK (13.2, 6.6, 1.8 and 3.9%) and the EU-27 (9.5, 10.9, 0.9 and 3.8%) [10]. Overall, the UK emits about one-tenth of the EU-27 annual N2O emissions.
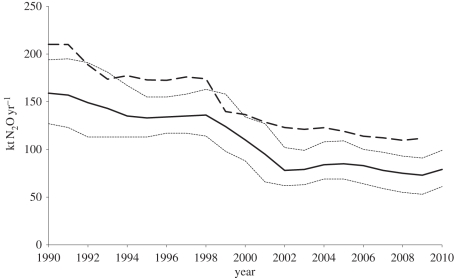
Total UK emissions have steadily declined from 218 Gg N2O in 1990 to 112 Gg N2O in 2009 (figure 1). This is mainly due to a sharp decline in industrial emissions by 90 per cent, and a decline in agricultural emissions by 23 per cent (table 1). Later, we discuss the anthropogenic emission inventory in detail, using the UK situation as an example.
Figure 1.
Nitrous oxide emission estimates for the UK, calculated by the NAME-inversion model from atmospheric concentration measurements at Mace Head on the west coast of Ireland (bold solid line, dotted lines represent the 5% and 95% uncertainty estimates) and by the bottom-up approach using IPCC Tier 1 emission factors (bold dashed line; [11] annex 10; [12]).
Table 1.
The UK nitrous oxide emission inventory in 1990 and 2009 (Gg N2O) [11].
| source | 1990 | 2009 |
|---|---|---|
| non-biological sources | ||
| industrial processes | 79.52 | 3.82 |
| fuel combustion including waste incinerationa | 19.89 | 14.35 |
| biological sources | ||
| wastewater handling | 4.04 | 4.44 |
| agriculture: direct emissions | ||
| manure storage | 8.67 | 6.44 |
| agricultural soils | ||
| synthetic fertilizers | 28.89 | 18.98 |
| animal manure and sewage sludge applied to soil | 9.41 | 8.65 |
| pasture range and paddock manure | 16.07 | 13.29 |
| crop residue | 7.17 | 8.46 |
| N-fixing crops | 0.85 | 0.92 |
| cultivation of histosols | 0.49 | 0.49 |
| improved grassland | 0.54 | 0.58 |
| agriculture: indirect emissions | ||
| atmospheric deposition | 6.42 | 4.77 |
| N leaching and runoff | 33.69 | 24.21 |
| LULUCF | ||
| forests | 0.02 | 0.01 |
| land-use change to croplandb | 2.54 | 2.05 |
| total | 218c | 112c |
aWaste incineration = 0.14 and 0.15 Gt in 2009 and 1990.
bFor 1990 this included 0.02 Gt land-use change to wetland and settlement.
cThe overall estimate has been rounded to avoid unjustifiable precision.
2. Industrial processes
Industrial production of nitric acid and adipic acid contributed 37 per cent (79 Gg) to the UK's annual N2O emissions in 1990, but since then have declined by 90 per cent and now only contribute 3 per cent (3.8 Gg) of the total UK annual N2O emission. This reduction was achieved by lowering production rates and removing N2O from adipic acid production by thermal decomposition, and by lowering the temperature during nitric acid production. Similar reductions were achieved by other EU countries [13]. Currently, nitric acid is manufactured at two locations in the UK and adipic acid production in the UK stopped in April 2009 [11]. Industrial emissions are monitored by the producers and Environment Agency before being reported to the Pollution Inventory. The emission factor uncertainty for adipic acid production is 15 per cent and for nitric acid production 230 per cent (annex 7 of MacCarthy et al. [11]).
3. Fuel combustion
The energy sector (stationary and mobile fuel combustion) contributed 13 per cent of the total UK N2O emissions in 2009, and has declined by 28 per cent since 1990 owing to effective abatement measures and improved combustion efficiencies. Emissions are calculated from fuel combustion statistics and emission factors. The emission factor uncertainty ranges from 33 to 170 per cent for fuel combustion processes and 195 per cent for other combustion processes (annex 7 of MacCarthy et al. [11]).
4. Biological sources
Microbial production in soils and aquatic systems (including wastewaters), primarily via nitrification and denitrification processes, is the largest source of N2O. These processes are ubiquitous and many microbial species have the necessary suite of enzymes to produce N2O [14]. The rate of N2O production and emission to the atmosphere depends on climate (rainfall and temperature), soil type, availability of mineral nitrogen, the redox potential and pH of the soil/aquatic environment and for denitrifiers and heterotrophic nitrifiers the availability of simple carbon compounds [15–17]. The combined requirements of the above variables result in highly variable emissions, both in space and time. Consequently, biological emissions calculated by the IPCC Tier 1 methodology are very uncertain. The combined uncertainty range as a percentage of source category is 424 per cent for agricultural soils, 414 per cent for manure handling and 401 per cent for wastewater handling (annex 8 of MacCarthy et al. [11]).
(a). Wastewater handling
Wastewater handling (i.e. sewage treatment plants) contributes 4 per cent of the UK N2O emissions, produced in activated sludge by nitrification and denitrification processes as discussed in detail by Foley et al. [18]. Emissions are calculated from per capita protein consumption, using the assumption that 1 per cent of sewage N produced is emitted as N2O [3], but measurements from treatment plants have shown that emission factors range from 0 to 15 per cent [15]. Nitrous oxide emissions can be minimized by maintaining high oxygen (O2) and small nitrite concentrations during the aerobic part of the wastewater treatment, and by maintaining a high C–N ratio during the anaerobic treatment stage [15]. N2O emissions also occur when sewage sludge is applied to land [11]. The emission factor uncertainty for wastewater handling is 401 per cent (annex 7 of MacCarthy et al. [11]).
(b). Agricultural activities
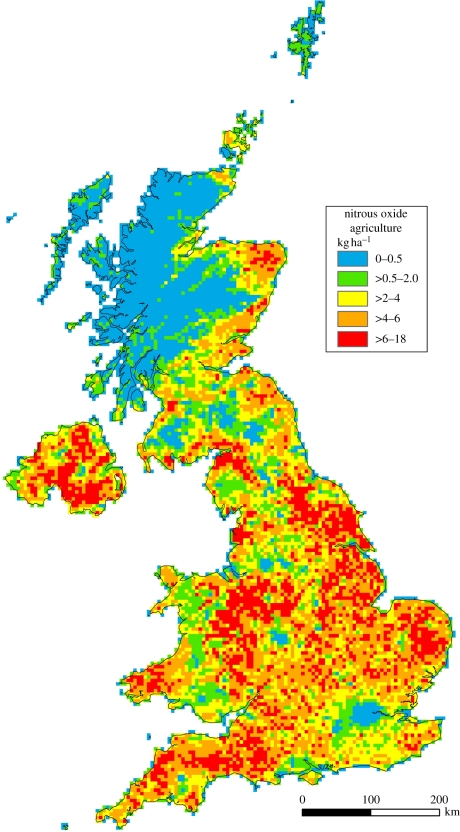
Since the decline in industrial emissions the agricultural sector has been the largest source of N2O in the UK and is responsible for more than three-quarters of the total annual emissions (78% in 2009) [11]. The main agricultural sources are fertilizer and manure application to soils (23%) and indirect emissions of NH3 and NOx to the atmosphere, and of NO3− to waters, which downstream or when re-deposited can be nitrified or denitrified and produce N2O (26%). Nitrogen excretion onto pasture range and paddocks accounted for 12 per cent, crop residue incorporation for 8 per cent and manure storage systems for 6 per cent of the UK's N2O emissions in 2009 (table 1). The spatial distribution of N2O emissions across the UK clearly shows largest emissions in regions dominated by livestock production, the grazed grassland regions in the high rainfall areas in the west of Great Britain and in Ireland, beef production in northeast Scotland and intensive poultry and pig production in eastern England (Yorkshire, Lincolnshire and East Anglia; figure 2). Overall, agricultural emissions have decreased steadily by approximately 23 per cent since 1990, mainly owing to reduced N fertilizer application rates and a decrease in livestock numbers [20].
Figure 2.
UK nitrous oxide emissions from agriculture (soils, direct and indirect emissions, and manure management), calculated for every 5 km2 using the IPCC Tier 1 methodology and using 2009 activity data [19].
(i). Agricultural soils: direct emissions
Nitrous oxide emissions as a result of mineral fertilizer, animal manure and sewage sludge applied to soils, incorporated crop residues, biological N fixation in legumes and improved grassland are calculated assuming that 1 per cent of the N applied, incorporated or fixed is emitted as N2O [3]. Only the organic rich histosols have a much larger emission factor of 8 per cent. In these, high mineralization rates provide a constant supply of mineral N and carbon ideal for denitrification to occur. However, only a small area of histosols is farmed in the UK and emissions from these accounted for less than 1 per cent of the total N2O emissions in 2009.
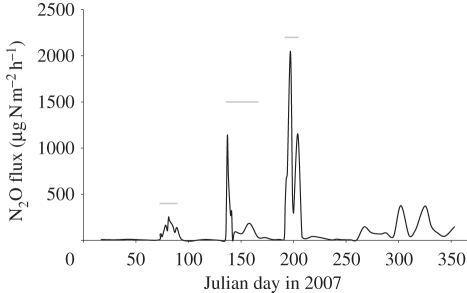
Under optimal conditions, fertilizer application stimulates a rapid rise in N2O emissions, which usually only lasts for one to three weeks [21] (figure 3). However, in addition to fertilizer rate, which is the only factor accounted for by the Tier 1 emission inventory, the onset, magnitude and length of fertilizer-induced emissions depend on rainfall, particularly the timing of fertilizer application in relation to rainfall and temperature, soil type, organic matter content in mineral soils, drainage and fertilizer type [16,22,23]. Consequently, N2O emissions show large seasonal and inter-annual variations [6,24]. An example of such variations is reported here from long-term monitoring of greenhouse gas fluxes from an intensively managed, mostly sheep grazed, grassland [25,26]. The grassland was fertilized three times during spring/early summer, each time with 50–70 kg NH4NO3–N ha yr−1. Weekly N2O fluxes were measured using static chambers throughout the year, with daily measurements immediately after fertilizer application, between August 2006 and November 2010 [26]. Results for 2007 are shown in figure 3.
Figure 3.
Nitrous oxide fluxes from a sheep grazed grassland in southeast Scotland. Data are median emissions from eight chambers. The fertilizer-induced emission peaks are identified by grey bars; the start of these bars marks the addition of NH4NO3 (69, 52, 52 kg N ha−1 yr−1 on Julian days 73 (14 March), 136 (16 May) and 192 (11 July), respectively; S. K. Jones & U. Skiba 2007, unpublished data).
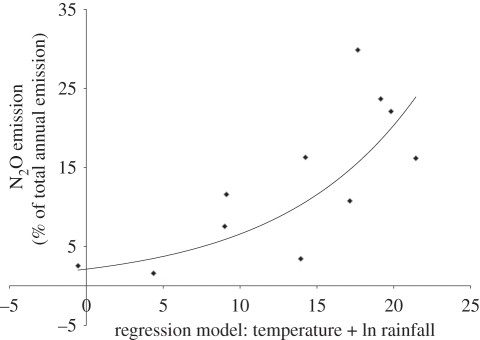
Fertilizer-induced emissions were short-lived (less than 15 days) and varied in magnitude. Collectively, the three emission events were responsible for 52 per cent of the total annual emission (7.5% (14–30 March) + 16.3% (16–31 May) + 30% (11–23 July)) in 2007. In 2008, fertilizer-induced emission peaks accounted for 65 per cent and in 2009 for 27 per cent of the annual flux. For 2007–2009, these seasonal and inter-annual variations could be modelled reasonable well by variations in the rainfall amount from 5 days before fertilization until the end of the fertilizer-induced emission peak plus the average air temperature during the emission period (figure 4).
Figure 4.
The dependence of the magnitude of the nitrous oxide (N2O) emission peaks induced by N fertilizer application on the synergistic influence of rainfall (cumulative rainfall (mm) 5 days before and during the fertilized-induced N2O emission peaks) and temperature (average air temperature during the fertilizer-induced N2O emission peaks), calculated using a multi-linear regression model (% of total annual flux = −8.81 + 3.18 ln rain + 0.983 temp, r2 = 55.9%, p < 0.05; x-axis). The data are from weekly flux measurements, 2007–2009, at the sheep grazed grassland (figure 3; S. K. Jones & U. Skiba 2007, unpublished data).
The IPCC Tier 1 emission factor approach assumes a linear relationship between N fertilizer application rate and emissions, but there is good evidence to suggest that this is not the case, with emissions rising more steeply beyond optimum fertilizer application rates [22,27,28]. A better understanding of this relationship will allow us to determine the difference between the currently applied economic optimum fertilizer rate and environmentally optimum rates. In some circumstances, it may be more appropriate to report emissions on a unit yield basis rather than unit area [29]. It is also apparent that the chemical form of fertilizer N [28] and crop type [7] can influence emissions. The latter study applied smaller emission factors for NH4+ compared with NO3−-based fertilizers, and smaller emission factors for arable land compared with grassland to calculate N2O emissions for Europe's agricultural land.
(ii). Agricultural soils: application of slurry, sewage sludge and manure
Most of the N2O generated from the manure management continuum (livestock buildings, manure stores, manure treatment and land spreading) occurs after it has been spread onto soils [30]. Resulting emissions depend on the soil and environmental factors discussed previously, but also on the C and N content and N forms of the manure, especially the decomposability of the organic material and the ratio of NH4+ to total N. These factors vary, depending on the origin of the manure, storage conditions, treatment process to which the manure may be subjected, and the climate conditions during storage [31]. The Tier 1 IPCC methodology uses the same N2O emission factor for manure spreading as for mineral fertilizers: 1 per cent of the N content of manure is emitted as N2O [3]. This approach does not reflect the large differences in the manure's chemical composition, its C–N ratio and method of application. For example, the emission factor from pig slurry (7–14%) was larger than from cattle slurry (2–3%) [32]. This was a result of the larger content of ammoniacal N in the pig manure. Comparing N2O emission factors from mineral fertilizers with those from animal manures has provided contrasting results. For example, Vallejo et al. [33] have shown that N2O emissions from a clay loam soil cropped with potatoes were significantly smaller (by 23%) when fertilized with pig manure than with urea. However, Schils et al. [34] reported no conclusive differences when applications of cattle slurry with and without calcium nitrate to grasslands on sandy soils were compared.
(iii). Manure management: storage of animal waste
Part of the livestock manures require containment and storage prior to spreading, to ensure they can be spread when there is a crop demand for the nutrients they contain. This is especially important in those areas designated in the UK as nitrate vulnerable zones (NVZs). Liquid livestock manure, i.e. slurries, is stored in lagoons or tanks, while solid manures are stacked in heaps prior to spreading. The anaerobic conditions and high C and N concentrations of lagoons, slurry tanks and dung heaps provide perfect conditions for methanogenesis and denitrification [35,36]. In the UK, manure storage systems are responsible for 15 per cent of the total agricultural CH4 emissions, but only for 6 per cent (6.4 Gg yr−1) of agricultural N2O emissions [11]. Nitrous oxide emissions are very variable [37] and depend on the C and N composition of the manure (which itself depends on the animal species and diet), the temperature and storage method and length of storage. The IPCC emission inventory assumes no N2O emissions from slurry-based livestock buildings and slurry stores, because the slurry remains in an anaerobic state and there is little opportunity for NH4+ to be nitrified (untreated slurry contains no or very little NO3−) [30]. However, as reviewed by Chadwick et al. [30], crust development can provide a zone of nitrification and hence a source of NO3− which can subsequently be denitrified, so the crust may be a source of N2O [38]. Amon et al. [35] reported the effects of cattle slurry treatment on N2O emissions during storage. Untreated slurry emitted 24 g N2O m−3 slurry, anaerobically digested slurry emitted 31.2 g N2O m−3 and aerated slurry 54.2 g N2O m−3.
The IPCC emission factor for solid storage systems is 0.02 kg N2O kg−1 N in the manure [3]. In reality, emissions from solid manure stores are very variable. Typical emissions in Europe range between 1 per cent and 4.3 per cent of the total N stored in cattle and pig farmyard manure heaps [30]. Emissions depend on the C–N ratio, dry matter content at the start of storage [39] and storage conditions [31,40,41]. Although solid manure stores are only a small component of the total agricultural N2O emission budget, they are N2O hotspots in the landscape, easily detectable by fast response high-resolution laser systems [42].
5. Indirect nitrous oxide emissions: atmospheric deposition
The rise in rates of atmospheric N deposition is directly linked to the rise in population growth and demand for food and industrialization [43]. Over the past century, a total of 29 Mt of N was deposited to the UK, which is an equivalent of 1.2 t N deposited to each ha in the UK [44]. Recent deposition rates were around 400 Gg N yr−1 in 2006 with approximately 50 per cent each of reduced (NHx) and oxidized (NHx) N [45]. Agriculture is by far the largest source of NHx, predominately as NH3 and NH4+ emitted from livestock, manure storage and N fertilizers. Combustion processes are the main source of NOx and microbial nitrification in soils account for 10–20% of the global total NOx emission [46]. These gases are deposited downwind of the original source as a gas, aerosol (dry deposition) or through precipitation (wet deposition) and unintentionally fertilize ‘natural’ ecosystems. Consequences are changes in species composition [47] and increased rates of NO and N2O emissions [48,49].
Agriculture-related indirect N2O emissions from atmospheric deposition are calculated by the IPCC Tier 1 methodology assuming that 10 per cent (range 3–30%) of mineral fertilizer and 20 per cent (range 5–50%) of the organic N fertilizer or excreta from grazing livestock are volatilized and deposited. One per cent (range 0.2–5%) of the deposited N is emitted as N2O [3]. In the UK, this sector accounted for 4.8 Gg N2O in 2009 [11]. Indirect emissions owing to NOx and NH3 emissions from non-agricultural sources do not need to be included in national inventories, but can be calculated assuming that 1 per cent of the NOx–N and NH3–N emitted is deposited as N2O [3]. This is likely to overestimate this source as only a third of the UK's NOy emissions (580 Gg in 2005) are deposited to the UK [45]; the rest is deposited to other countries and the seas. A more accurate approach would be to calculate N deposition-induced N2O emissions directly from measured and modelled atmospheric N deposition rates.
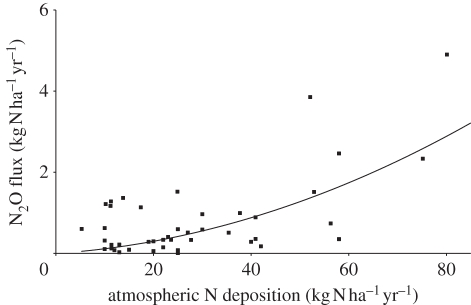
Soil microbes do not distinguish between N applied via fertilization or atmospheric deposition so one can assume that the emission factor applied to calculate fertilizer-induced emissions is applicable also to atmospheric deposited N. Thus, 1 per cent of the 400 Gg N annually deposited (4 Gg N) is emitted as N2O–N (6.3 Gg N2O). Similar to fertilized soils, there are large variations in emissions in relation to atmospheric deposition owing to variations in soil type, vegetation, climate and rate of N deposition; the relationship may not be linear (figure 5), and should be further investigated.
Figure 5.
The relationship between N2O and N deposition from forests, heather and grass moorlands on mineral soil in Great Britain. Locations with N deposition rates greater than 40 kg N ha−1 yr−1 were close to NH3 emitting livestock farms. These measurements were made by the Centre for Ecology and Hydrology between 1991 and 1997 [48,49].
6. Indirect nitrous oxide emissions: NO3− leaching and runoff
A proportion of fertilizer, manure, sewage sludge and excreta N is lost through leaching and surface runoff to groundwater, rivers and estuaries, where N2O is produced via nitrification and denitrification in sediments and the water columns [50,51]. Nitrous oxide is highly soluble in water (0.15 g 100 ml−1 at 15°C), and often supersaturated by several orders of magnitude of ambient atmospheric N2O concentrations. Emissions to the atmosphere are highly variable and depend on the solubility, the water-to-air pressure difference and transfer velocity (which increases with wind speed and turbulence). For example, maximum N2O emissions were measured where field drains feed into a river [52]. In general, N2O emissions from aqueous systems are directly related to N concentrations in the water [53]. The Tier 1 IPCC approach assumes that 30 per cent (range 10–80%) of the fertilizer or manure N applied to soils is lost to the water bodies when rainfall is greater than 0.5*pan evaporation (616 Gg in 2009) and 0.75 per cent (range 0.05–2.5%) of the leached N is emitted as N2O, 0.25 per cent each from groundwater and surface drainage, rivers and estuaries [3]. The IPCC has reduced the emission factor for these sources from 2.5 per cent, based on experimental evidence [50]. However, a study in the USA [54] estimated that 0.75 per cent (range 0–0.9%) of the dissolved inorganic nitrogen input to rivers is emitted as N2O. The UK still uses the IPCC 1996 default value of 2.5 per cent [3] and N2O emissions resulting from leaching and runoff amount to 24.2 Gg yr−1 (table 1). The new IPCC 2006 emission factor (0.75%) [5] calculates indirect N2O emissions of aqueous bodies as only 4.6 Gg yr−1 (2009), with 1.5 Gg emitted each from groundwaters, rivers and estuaries. Studies are needed to identify the most appropriate emission factor for the UK. An annual emission of 1.5 Gg N2O from estuaries, calculated with the newer 0.75 per cent emission factor is in good agreement with a recent measurement-based estimate by Barnes & Upstill-Goddard [51] (1.9 ± 1.2 Gg N2O yr−1; the uncertainty is 64%).
7. Land use and land-use change and forestry
Nitrous oxide and CH4 emissions from forests, and land-use change from and to forests, have only very recently been included in the LULUCF inventory. Annual N2O emissions from the LULUCF sector are very small (less than 2% of UK 2009 emissions). Anthropogenic activities leading to N2O emissions considered are N fertilization to newly planted forest and emissions from soil drainage, but reporting on this latter activity is currently not mandatory. Direct fertilizer-induced N2O emissions from forests are calculated using the same emission factor as used for fertilized agricultural soils. Fertilizer is rarely applied to forests, and so far the annual inventory has been set at zero. Emissions resulting from forest management, such as felling and thinning are not considered, although these activities could potentially change N2O emission rates by altering the soil water content owing to the absence of trees (felling) or reduction of shading (thinning). Indirect emissions owing to atmospheric N deposition is not included either, in spite of observations that deposition rates to forests can be two to threefold larger than to shorter vegetation [44] and can be especially large to small forests in intensively managed agricultural areas [48,49].
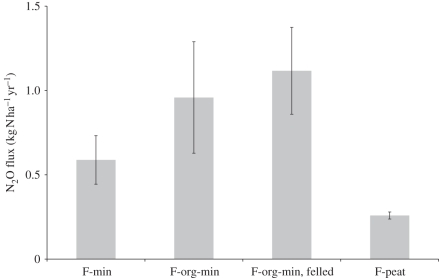
Conversion from one land use to another invariably requires ploughing and perhaps drainage. Forests converted to cropland or grassland need to be clear-felled. These activities stimulate soil organic matter mineralization and increase soil mineral N concentrations. In the absence of plants, there is no competition for this newly available N, thereby maximizing substrate availability for microbial nitrification and denitrification and release of N2O. The few studies that investigated effects of clear-felling on greenhouse gas fluxes revealed that clear-felling resulted in a pulse of N2O, NO and CO2 emissions [55–57]. Morison et al. [58] have compiled the data relevant to the UK, which demonstrate that N2O emissions from forests are influenced by soil type and that clear-felling may increase N2O emissions in the short-term (figure 6).
Figure 6.
Comparison of nitrous oxide emissions from British forests (F) grown on different soil types. Average and standard error of the mean for F-min, seven forests/woodland plantations on mineral soil; F-org-min, five forests on peaty podzols and peaty gleys; F-org-min, felled, two clear-felled plantations; F-peat, three plantations on peat [58].
Growing interest in perennial bioenergy crops is likely to increase areas undergoing land-use change. Perennial bioenergy crops are favoured over annual crops because they sequester carbon over 15–20 year periods and have very low N fertilizer requirements, and thus much lower N2O emissions [59,60]. However, they are economically productive for a finite period only, at the end of which they will be removed. Removal by clear-felling is one option, but as shown in figure 6, there are too few studies to predict the rate and length of increased emissions of N2O and other greenhouse gases triggered by clear-felling. There is urgent need to understand clear-felling effects on greenhouse gas emissions, and account for these in life cycle analyses [60].
The total area in the UK converted to wetlands is restricted to small insignificant areas of newly created riparian zones, along rivers in NVZs, or reinstating peat wetlands. This activity has a potential to decrease N2O emissions if the soil water-filled pore space can be maintained above 90 per cent [16]. Under such conditions, anaerobic conditions and accumulation of soil organic matter content will favour denitrification to proceed to N2 rather than stop at N2O production, which is generally the case in more aerobic soils. The uncertainty of this source is very high, and depends on maintaining a high water table. In spite of lack of data to calculate N2O emissions from the LULUCF sector, surprisingly small levels of uncertainty of 20 per cent were calculated for all LULUCF categories, except for the conversion to croplands, which have an uncertainty of 50 per cent (annex 7 of MacCarthy et al. [11]).
8. Natural emissions
Nitrous oxide emissions from ‘natural’ ecosystems are not included in the UK's anthropogenic emission inventory. Nitrogen inputs and losses, including N2O to and from ‘natural’ environments tend to be small [26]. For a country like the UK, where only about 20 per cent of the land area is not used for agriculture or settlements (12% is in forest, the rest are heaths, moorlands, bogs and montane ecosystems), it is difficult to separate natural emissions from those resulting from enhanced N deposition rates. In a review of greenhouse gas fluxes from natural ecosystems, Dalal & Allen [61] calculated average emissions of 1.57 kg N2O ha−1 yr−1 for temperate forests and 0.55 kg N2O ha−1 yr−1 for temperate grasslands. Based on the above-mentioned emission rates natural emissions in the UK may contribute an extra 5.8 Gg of N2O to the anthropogenic emission inventory.
9. Developing Tier 2 emission factors for the UK for agriculture
The current largely Tier 1-based N2O inventory methodology used in the UK is a fairly blunt tool. For the main N2O source, the agricultural sector, there is now a requirement to produce a reporting tool that better reflects the climatic and soil variability and production systems (including N management) throughout the UK. Importantly, any new approach should explicitly account for mitigation practices so that their uptake is fully reflected in emission estimates. An improved inventory approach will not only be used for reporting UK agricultural N2O emissions to the UNFCCC, but will also allow the UK government to track progress against the challenging targets it has published in its low carbon transition plan, i.e. an 80 per cent reduction in greenhouse gas emissions (CO2 equivalents) by 2050 [2], and assist the agricultural industry to monitor progress against the sector roadmaps. Tier 2, country-specific emission factors will need to be generated through a carefully coordinated approach to modelling, reviews of existing literature and experiments. These activities will provide temporally and spatially disaggregated direct and indirect N2O emissions factors for the major sources of N (fertilizer, dung and urine, and livestock manure applications to land). The efficacy of key potential mitigation methods will need to be tested and introduced into the new inventory structure, e.g. use of nitrification inhibitors. The complexity of the new inventory will, by necessity, be increased, not only through the increased level of disaggregation, but also through our growing level of understanding of the controls on N2O emissions, and how these are influenced by on-farm management decisions.
The development of Tier 2 emission factors is an interesting scientific endeavour per se, but represents only one part of the inventory calculation. Activity data, e.g. livestock numbers, annual N excretion by different livestock, knowledge of the grazing season length and application rates of different N fertilizer forms, are just as important in determining the total source of N2O from any region and at any point in time. Thus, a hugely important component of the development of a Tier 2 approach is to ensure that this information is available at the most suitable level of disaggregation (in both time and space). Indeed, the spatial and temporal availability of appropriate activity data is the limiting factors in the ability to generate robust estimates of N2O emissions at a given spatial scale.
Given the increased complexity of producing a reporting tool that better reflects the range of soil, climate and farming systems in the UK (that a Tier 2 approach requires), it is not necessarily the case that the overall uncertainty value for the annual UK agricultural N2O emission total will be less than the current Tier 1 estimate, i.e. ±250 per cent, although it is very much hoped that it will. What is of key importance, is that the revised approach will allow the uncertainty to be apportioned to the different steps and parts of the inventory, allowing us to focus future resources on research (perhaps improved emission factors or/and improved spatial and temporal activity data) to reduce those uncertainties. Section 10 describes how the new (bottom-up) inventory could be verified by a top-down modelling approach.
10. Verification of the UK nitrous oxide emission inventory
In order to provide verification of the UK greenhouse gas inventory, the UK government maintains a high-quality remote observation station at Mace Head on the west coast of Ireland. Mace Head reports high-frequency concentrations of the key greenhouse gases [62]. A Lagrangian dispersion model numerical atmospheric dispersion modelling environment (NAME) [63,64] driven by three-dimensional modelled meteorology is used to interpret the observations. NAME determines the history of the air arriving at Mace Head at the time of each observation. Deviation from the baseline is used to estimate the N2O source strength of the UK and regions of northwestern Europe. This NAME-inversion methodology uses an iterative best-fit technique that searches a set of random emission maps to determine the one that most accurately mimics the Mace Head observations [65]. The ‘top-down’ NAME-inversion estimates of UK emissions 1990–2010 are compared with the ‘bottom-up’ greenhouse gas inventory estimates and are shown in figure 1. The median NAME-inversion estimates are approximately 30–40 Gg lower than the greenhouse gas inventory estimates throughout the whole time period. The trends in the time-series are in good agreement, with both showing declining UK totals. The greenhouse gas inventory estimates show a sharp decline (40 Gg) between 1998 and 1999 in line with the introduction of the clean technology at an adipic acid plant in Wilton, northeast UK. The NAME-inversion estimates, with a longer averaging period, show a more gradual decline from 1998 to 2003 but the overall reduction is similar.
More direct measurements of N2O and other greenhouse gas emissions from aircraft have also used inversion methods to deduce the UK source strength and its spatial distribution [66]. Improved validation of the UK greenhouse gas emission inventories could be provided by a network of tall towers monitoring greenhouse gas concentrations across the UK and aircraft measurements.
11. Conclusions
The above account of the deviations of observed N2O emissions from those calculated using the Tier 1 emission factor approach clearly shows that this methodology is too simplistic to reflect regional variations of biologically produced N2O emissions or provide the best estimate of the UK source strengths. The reasonably good agreement of the bottom-up emission factor and top-down inverse modelling approaches imply that total UK N2O emissions may be accounted for adequately, but the attribution to individual sources using the bottom-up methodology within the agricultural sector needs to be refined taking into consideration regional variations in climate, soil properties and seasonal agricultural management. Developing a methodology that can account for such variations is not a trivial task. In spite of the wealth of N2O emission measurements made in the past 20 years, there are still not enough long-term datasets to provide the information needed to develop emission factors for the range of combinations of different climate zones or soil types and N sources. Modelling is required to aid the interpolation between measured scenarios. Adopting Tier 2 methodologies requires detailed knowledge of variations of emissions in relation to easily measureable activity data. All sectors (energy, industry, wastewater, agriculture and LULUCF) would benefit from adopting Tier 2 emission accounting. However, in the first instance, Tier 2 methods need to be developed for the largest N2O emitter, the agricultural sector. A Tier 2 approach should provide a more transparent and accurate picture of N2O emissions, capable of reflecting changes in soil and N management, and take explicit account of mitigation strategies. Improved verification of the new approach to inventory reporting could be obtained by monitoring atmospheric N2O concentrations from a more extensive tower network across the UK.
Acknowledgements
We wish to thank Tom Misselbrook and Sarah Gilhespy at Rothamsted Research, North Wyke for their contribution to the N2O agriculture inventory, and BBSRC and NERC for supporting Rothamsted Research (North Wyke) and CEH, respectively. We also thank DEFRA and the Devolved Administrations for funding the projects on ‘Improving the UK's agricultural greenhouse gas emission inventories’ which inspired this paper and will enable the UK adopting the Tier 2 methodology to account for agricultural greenhouse gas emissions (both N2O and CH4).
References
- 1.IPCC 2007. Climate change 2007: the physical science basis. Contribution of the Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.European Commission 2011. A roadmap for moving to a competitive low carbon economy in 2050. Report no. COM(2011) 112 Brussels, Belgium: EU. [Google Scholar]
- 3.IPCC 1996. Revised 1996 IPCC guidelines for national greenhouse gas inventories (eds Houghton J. T., Meira Filho L. G., Lim B., Treanton K., Mamaty I., Bonduki Y., Griggs D. J., Callender B. A.), IPCC/OECD/IEA. Bracknell, UK: Meteorological Office [Google Scholar]
- 4.IPCC 2000. Good practice guidance and uncertainty management in national greenhouse gas inventories (eds Penman J., Kruger D., Galbally I., Hiraishi T., Nyenzi B., Emmanul S., Buendia L., Hoppaus T., Martinsen J., Meijer J., Miwa K., Tanabe K.). Hayama, Japan: Institute for Global Environmental Strategies (IGES) [Google Scholar]
- 5.IPCC 2006. IPCC guidelines for national greenhouse gas inventories, prepared by the national greenhouse gas inventories programme (eds Eggleston H. S., Buendia L., Miwa K., Ngara T., Tanabe K.). Hayama, Japan: IGES [Google Scholar]
- 6.Dobbie K. E., Smith K. A. 2003. Impact of different forms of N fertilizer on N2O emissions from intensive grassland. Nutrient Cycling Agroecosyst. 67, 37–46 10.1023/A:1025119512447 (doi:10.1023/A:1025119512447) [DOI] [Google Scholar]
- 7.Lesschen J., van den Berg M., Westhoek H., Witzke H., Oenema O. 2011. Greenhouse gas emission profiles of European livestock sectors. Anim. Feed Sci. Technol. 166–167, 16–28 10.1016/j.anifeedsci.2011.04.058 (doi:10.1016/j.anifeedsci.2011.04.058) [DOI] [Google Scholar]
- 8.Ravishankara A., Daniel J. S., Portmann R. W. 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 10.1126/science.1176985 (doi:10.1126/science.1176985) [DOI] [PubMed] [Google Scholar]
- 9.Werner C., Butterbach-Bahl K., Haas E., Hickler T., Kiese R. 2007. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Glob. Biogeochem. Cycles 21, 3010. 10.1029/2006GB002909 (doi:10.1029/2006GB002909) [DOI] [Google Scholar]
- 10.EEA 2010. Annual European Union greenhouse gas inventory 1990–2008 and inventory report 2010. EEA technical report no. 6/2010. Copenhagen, Denmark: EEA
- 11.Thomas J., et al. 2011. Greenhouse gas inventories for England, Scotland, Wales and Northern Ireland: 1990–2009. Report no. AEAT/ENV/R/3222, Issue 1. Didcot, UK: AEA
- 12.Manning A., O'Doherty D. B., Jones A. R., Simmonds P. G., Derwent R. G. 2011. Estimating UK methane and nitrous oxide emissions from 1990 to 2007 using an inversion modeling approach . J. Geophys. Res. 116, D02305. 10.1029/2010JD014763 (doi:10.1029/2010JD014763) [DOI] [Google Scholar]
- 13.UNFCC 2010. National Inventory Submissions 2010 from Annex-I parties. See http://unfccc.int/national_reports/annex_i_ghg_inventories/national_inventories_submissions/items/4771.php.
- 14.Bakken L. R., Bergaust L., Liu B., Frostegård Å. 2012. Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Phil. Trans. R. Soc. B 367, 1226–1234 10.1098/rstb.2011.0321 (doi:10.1098/rstb.2011.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kampschreur M. J., Temmink H., Kleerebezem R., Jetten M. S., van Loosdrecht M. C. 2009. Nitrous oxide emission during wastewater treatment. Water Res. 43, 4093–4103 10.1016/j.watres.2009.03.001 (doi:10.1016/j.watres.2009.03.001) [DOI] [PubMed] [Google Scholar]
- 16.Skiba U. M., Smith K. A. 2000. The control of nitrous oxide emissions from agricultural and natural soils. Chemosphere 2, 379–386 [Google Scholar]
- 17.Seitzinger S. P., Kroeze C. 1998. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Glob. Biogeochem. Cycles 12, 93–113 10.1029/97GB03657 (doi:10.1029/97GB03657) [DOI] [Google Scholar]
- 18.Foley J., de Haas D., Yuan Z., Lant P. 2010. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res. 44, 831–844 10.1016/j.watres.2009.10.033 (doi:10.1016/j.watres.2009.10.033) [DOI] [PubMed] [Google Scholar]
- 19.Dragosits U., Sutton M. A. 2011. The spatial distribution of ammonia, methane and nitrous oxide emissions from agriculture in the UK 2009. Report no. ACO112 London, UK: DEFRA [Google Scholar]
- 20.DEFRA 2011. Agricultural statistics and climate change, 1st edn London, UK: DEFRA. See http://www.defra.gov.uk/statistics/foodfarm/enviro/climate/. [Google Scholar]
- 21.Bouwman A. F. 1996. Direct emission of nitrous oxide from agricultural soils. Nutrient Cycling Agroecosyst. 46, 53–70 10.1007/BF00210224 (doi:10.1007/BF00210224) [DOI] [Google Scholar]
- 22.Cardenas L. M., et al. 2010. Quantifying annual N2O emission fluxes from grazed grassland under a range of inorganic fertiliser nitrogen inputs. Agric. Ecosyst. Environ. 136, 218–226 10.1016/j.agee.2009.12.006 (doi:10.1016/j.agee.2009.12.006) [DOI] [Google Scholar]
- 23.Dobbie K. E., McTaggart I. P., Smith K. A. 1999. Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 104, 26 891–26 899 [Google Scholar]
- 24.Flechard C. R., et al. 2007. Effects of climate and management intensity on nitrous oxide emissions in grassland systems across Europe. Agric. Ecosyst. Environ. 121, 135–152 10.1016/j.agee.2006.12.024 (doi:10.1016/j.agee.2006.12.024) [DOI] [Google Scholar]
- 25.Jones S. K., Famulari D., Di Marco C., Nemitz E., Skiba U. M., Rees R. M., Sutton M. A. 2011. Nitrous oxide emissions from a managed grassland: a comparison of eddy covariance and static chamber measurements. Atmos. Meas. Tech. Discuss 4, 1079–1112 10.5194/amtd-4-1079-2011 (doi:10.5194/amtd-4-1079-2011) [DOI] [Google Scholar]
- 26.Skiba U., et al. 2009. Biosphere–atmosphere exchange of reactive nitrogen and greenhouse gases at the NitroEurope core flux measurement sites: measurement strategy and first data sets. Agric. Ecosyst. Environ. 133, 139–149 10.1016/j.agee.2009.05.018 (doi:10.1016/j.agee.2009.05.018) [DOI] [Google Scholar]
- 27.Hoben J. P., Gehl R. J., Millar N., Grace P. R., Robertson G. P. 2011. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Glob. Change Biol. 17, 1140–1152 10.1111/j.1365-2486.2010.02349.x (doi:10.1111/j.1365-2486.2010.02349.x) [DOI] [Google Scholar]
- 28.Bouwman A. F., Boumans L. J. M., Batjes N. H. 2002. Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob. Biogeochem. Cycles 16, 1058. 10.1029/2001GB001811 (doi:10.1029/2001GB001811) [DOI] [Google Scholar]
- 29.Pappa V. A., Thorman R., Rees R. M., Sylvester-Bradley R. 2011. Effects of nitrogen fertiliser use on N2O intensities of arable crop products. EGU General Assembly 2011. Geophys. Res. Abstr.13, EGU2011-11866. [Google Scholar]
- 30.Chadwick D., Sommer S., Thorman R., Fangueiro D., Cardenas L., Amon B., Misselbrook T. 2011. Manure management: implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 166–167, 514–531 10.1016/j.anifeedsci.2011.04.036 (doi:10.1016/j.anifeedsci.2011.04.036) [DOI] [Google Scholar]
- 31.Chadwick D. R. 2005. Emissions of ammonia, nitrous oxide and methane from cattle manure heaps: effect of compaction and covering. Atmos. Environ. 39, 787–799 10.1016/j.atmosenv.2004.10.012 (doi:10.1016/j.atmosenv.2004.10.012) [DOI] [Google Scholar]
- 32.Velthof G. L., Kuikman P. J., Oenema O. 2003. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 37, 221–230 [Google Scholar]
- 33.Vallejo A., Skiba U. M., Garcia-Torres L., Arce A., Lopez-Fernandez S., Sanchez-Martin L. 2006. Nitrogen oxides emission from soils bearing a potato crop as influenced by fertilization with treated pig slurries and composts. Soil Biol. Biochem. 38, 2782–2793 10.1016/j.soilbio.2006.04.040 (doi:10.1016/j.soilbio.2006.04.040) [DOI] [Google Scholar]
- 34.Schils R. L. M., van Groenigen J. W., Velthof G. L., Kuikman P. J. 2008. Nitrous oxide emissions from multiple combined applications of fertiliser and cattle slurry to grassland. Plant Soil 310, 89–101 10.1007/s11104-008-9632-2 (doi:10.1007/s11104-008-9632-2) [DOI] [Google Scholar]
- 35.Amon B., Kryvoruchko V., Amon T., Zechmeister-Boltenstern S. 2006. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 112, 153–162 10.1016/j.agee.2005.08.030 (doi:10.1016/j.agee.2005.08.030) [DOI] [Google Scholar]
- 36.Monteny G. J., Bannink A., Chadwick D. 2006. Greenhouse gas abatement strategies for animal husbandry. Agric. Ecosyst. Environ. 112, 163–170 10.1016/j.agee.2005.08.015 (doi:10.1016/j.agee.2005.08.015) [DOI] [Google Scholar]
- 37.Amon B., Amon T., Boxberger J., Alt C. 2001. Emissions of NH3, N2O and CH4 from dairy cows housed in a farmyard manure tying stall (housing, manure storage, manure spreading). Nutrient Cycling Agroecosyst. 60, 103–113 10.1023/A:1012649028772 (doi:10.1023/A:1012649028772) [DOI] [Google Scholar]
- 38.Sommer S. G., Petersen S. O., Sogaard H. T. 2000. Greenhouse gas emission from stored livestock slurry. J. Environ. Qual. 29, 744–751 10.2134/jeq2000.00472425002900030009x (doi:10.2134/jeq2000.00472425002900030009x) [DOI] [Google Scholar]
- 39.Yamulki S. 2006. Effect of straw addition on nitrous oxide and methane emissions from stored farmyard manures. Agric. Ecosyst. Environ. 112, 140–145 10.1016/j.agee.2005.08.013 (doi:10.1016/j.agee.2005.08.013) [DOI] [Google Scholar]
- 40.Parkinson R., Gibbs P., Burchett S., Misselbrook T. 2004. Effect of turning regime and seasonal weather conditions on nitrogen and phosphorus losses during aerobic composting of cattle manure. Bioresour. Technol. 91, 171–178 10.1016/S0960-8524(03)00174-3 (doi:10.1016/S0960-8524(03)00174-3) [DOI] [PubMed] [Google Scholar]
- 41.Szanto G., Hamelers H., Rulkens W., Veeken A. 2007. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 98, 2659–2670 10.1016/j.biortech.2006.09.021 (doi:10.1016/j.biortech.2006.09.021) [DOI] [PubMed] [Google Scholar]
- 42.Skiba U., DiMarco C., Hargreaves K., Sneath R., McCartney L. 2006. Nitrous oxide emissions from a dung heap measured by chambers and plume methods. Agric. Ecosyst. Environ. 112, 135–139 10.1016/j.agee.2005.08.012 (doi:10.1016/j.agee.2005.08.012) [DOI] [Google Scholar]
- 43.Galloway J. N., Townsend A. R., Erisman J. W., Bekunda M., Cai Z. C., Freney J. R., Martinelli L. A., Seitzinger S. P., Sutton M. A. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 10.1126/science.1136674 (doi:10.1126/science.1136674) [DOI] [PubMed] [Google Scholar]
- 44.Fowler D., O'Donoghue M., Muller J. B., Smith R. I., Dragosits U., Skiba U., Sutton M. A. 2004. A chronology of nitrogen deposition in the UK between 1900 and 2000. Water Air Soil Pollut. Focus 4, 9–23 10.1007/s11267-004-3009-1 (doi:10.1007/s11267-004-3009-1) [DOI] [Google Scholar]
- 45.RoTAP 2011 Review of transboundary air pollution Penicuik, UK: Centre for Ecology and Hydrology [Google Scholar]
- 46.Simpson D., et al. 1999. Inventorying emissions from nature in Europe. J. Geophys. Res. Atmos. 104, 8113–8152 10.1029/98JD02747 (doi:10.1029/98JD02747) [DOI] [Google Scholar]
- 47.McClean C. J., van den Berg L., Ashmore M. R., Preston C. D. 2011. Atmospheric nitrogen deposition explains patterns of plant species loss. Glob. Change Biol. 17, 2882–2892 10.1111/j.1365-2486.2011.02462.x (doi:10.1111/j.1365-2486.2011.02462.x) [DOI] [Google Scholar]
- 48.Skiba U., Sheppard L., Pitcairn C. E. R., Leith I., Crossley A., van Dijk S., Kennedy V. H., Fowler D. 1998. Soil nitrous oxide and nitric oxide emissions as indicators of elevated atmospheric N deposition rates in seminatural ecosystems. Environ. Pollut. 102, 457–461 10.1016/S0269-7491(98)80069-9 (doi:10.1016/S0269-7491(98)80069-9) [DOI] [Google Scholar]
- 49.Skiba U. M., Sheppard L. J., Pitcairn C. E. R., van Dijk S., Rossall M. J. 1999. The effect of N deposition on nitrous oxide and nitric oxide emissions from temperate forest soils. Water Air Soil Pollut. 116, 89–98 10.1023/A:1005246625038 (doi:10.1023/A:1005246625038) [DOI] [Google Scholar]
- 50.Reay D. S., Smith K. A., Edwards A. C., Hiscock K. M., Dong L. F., Nedwell D. B. 2005. Indirect nitrous oxide emissions: revised emission factors. Environ. Sci. 2, 153–158 10.1080/15693430500415525 (doi:10.1080/15693430500415525) [DOI] [Google Scholar]
- 51.Barnes J., Upstill-Goddard R. 2011. N2O seasonal distributions and air-sea exchange in UK estuaries: implications for the tropospheric N2O source from European coastal waters. J. Geophys. Res. Biogeosci. 116, G01006. 10.1029/2009JG001156 (doi:10.1029/2009JG001156) [DOI] [Google Scholar]
- 52.Reay D. S., Smith K. A., Edwards A. C. 2004. Nitrous oxide in agricultural drainage waters following field fertilisation. Water, Air Soil Pollut. Focus 4, 437–451 10.1023/B:WAFO.0000028370.68472.d2 (doi:10.1023/B:WAFO.0000028370.68472.d2) [DOI] [Google Scholar]
- 53.Kroeze C., Dumont E., Seitzinger S. P. 2010. Future trends in emissions of N2O from rivers and estuaries. J. Integr. Environ. Sci. 7, 71–78 10.1080/1943815X.2010.496789 (doi:10.1080/1943815X.2010.496789) [DOI] [Google Scholar]
- 54.Beaulieu J. J., et al. 2011. Nitrous oxide emission from denitrification in stream and river networks. Proc. Natl Acad. Sci. USA 108, 214–219 10.1073/pnas.1011464108 (doi:10.1073/pnas.1011464108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emmett B. A., Quarmby C. 1991. The effect of harvesting intensity on the fate of applied N-15-ammonium to the organic horizons of a coniferous forest in N Wales. Biogeochemistry 15, 47–63 [Google Scholar]
- 56.Keller M., Kaplan W. A., Wofsy S. C. 1986. Emissions of N2O, CH4 and CO2 from tropical forest soils. J. Geophys. Res. 91, 11 791–11 802 [Google Scholar]
- 57.Zerva A., Mencuccini M. 2005. Short-term effects of clearfelling on soil CO2, CH4, and N2O fluxes in a Sitka spruce plantation. Soil Biol. Biochem. 37, 2025–2036 10.1016/j.soilbio.2005.03.004 (doi:10.1016/j.soilbio.2005.03.004) [DOI] [Google Scholar]
- 58.Morison J. I. L., Matthews R., Miller G., Perks M., Randle T., Vanguelova E., White M., Yamulki S. 2011. Understanding the carbon and greenhouse gas balance of UK forests. Report for Forestry Commission, April 2011. Farnham, UK: Forest Research
- 59.Drewer J., Finch J. W., Lloyd C. R., Baggs E. M., Skiba U. M. In press How do soil emissions of N2O, CH4 and CO2 from perennial bioenergy crops differ from arable annual crops? Glob. Change Biol. Bioenergy. 10.1111/j.1757-1707.2011.01136.x (doi:10.1111/j.1757-1707.2011.01136.x) [DOI] [Google Scholar]
- 60.Don A., et al. In press Land-use change to bioenergy production in Europe: implications for the greenhouse gas balance and soil carbon. Glob. Change Biol. Bioenergy (doi:101111/j.1757–1707.2011.01116.x) [Google Scholar]
- 61.Dalal R. C., Allen D. E. 2008. Greenhouse gas fluxes from natural ecosystems. Aust. J. Botany 56, 369–407 10.1071/BT07128 (doi:10.1071/BT07128) [DOI] [Google Scholar]
- 62.O'Doherty S., et al. 2004. Rapid growth of hydrofluorocarbon 134a and hydrochlorofluorocarbons 141b, 142b, and 22 from advanced global atmospheric gases experiment (AGAGE) observations at Cape Grim, Tasmania, and Mace Head, Ireland. J. Geophys. Res. Atmos. 109, D06310. 10.1029/2003JD004277 (doi:10.1029/2003JD004277) [DOI] [Google Scholar]
- 63.Ryall D. B., Maryon R. H. 1998. Validation of the UK Met. Office's name model against the ETEX dataset. Atmos. Environ. 32, 4265–4276 10.1016/S1352-2310(98)00177-0 (doi:10.1016/S1352-2310(98)00177-0) [DOI] [Google Scholar]
- 64.Jones A., Thomson D., Hort M., Devenish B. 2007. The UK Met Office's next-generation atmospheric dispersion model, NAME III. Air Pollut. Model. Appl. 17, 580–589 [Google Scholar]
- 65.Manning A. J., Ryall D. B., Derwent R. G., Simmonds P. G., O'Doherty S. 2003. Estimating European emissions of ozone-depleting and greenhouse gases using observations and a modeling back-attribution technique. J. Geophys. Res. Atmos. 108, 4405. 10.1029/2002JD002312 (doi:10.1029/2002JD002312) [DOI] [Google Scholar]
- 66.Polson D., et al. 2010. Estimation of spatial apportionment of greenhouse gas emissions for the UK using boundary layer measurements and inverse modelling technique. Atmos. Environ. 45, 1042–1049 10.1016/j.atmosenv.2010.10.011 (doi:10.1016/j.atmosenv.2010.10.011) [DOI] [Google Scholar]