Abstract
External wetting poses problems of immediate heat loss and long-term pathogen growth for vertebrates. Beyond these risks, the locomotor ability of smaller animals, and particularly of fliers, may be impaired by water adhering to the body. Here, we report on the remarkable ability of hummingbirds to perform rapid shakes in order to expel water from their plumage even while in flight. Kinematic performance of aerial versus non-aerial shakes (i.e. those performed while perching) was compared. Oscillation frequencies of the head, body and tail were lower in aerial shakes. Tangential speeds and accelerations of the trunk and tail were roughly similar in aerial and non-aerial shakes, but values for head motions in air were twice as high when compared with shakes while perching. Azimuthal angular amplitudes for both aerial and non-aerial shakes reached values greater than 180° for the head, greater than 45° for the body trunk and slightly greater than 90° for the tail and wings. Using a feather on an oscillating disc to mimic shaking motions, we found that bending increased average speeds by up to 36 per cent and accelerations of the feather tip up to fourfold relative to a hypothetical rigid feather. Feather flexibility may help to enhance shedding of water and reduce body oscillations during shaking.
Keywords: feather, flexibility, flight, hovering, manoeuvrability, oscillation
1. Introduction
Shaking bursts are commonly exhibited by mammals to expel water accumulated within the fur. As the thermal conductivity of water is some 25 times higher than that of air, such shaking may help avoid excessive heat loss, as well as to impede pathogen growth on the skin [1–3]. In smaller species with higher surface area : volume ratios, water load on the body may also reduce locomotor performance [4], and may be particularly relevant for volant taxa given the much higher energetic costs of flapping flight relative to cursorial locomotion [5]. Superficially, wetted feathers can also impede airflow through the structure, similar to the action of a windproof cloth [6]. For birds, shaking behaviour has been characterized in the course of water bathing [7,8], but has not yet been described either during free flight or in direct response to rain. Hummingbirds are of particular interest in this regard because they include some of the smallest bird species, are most abundant at mid-montane elevations characterized by substantial cloud cover and rain [9] and remain active even during heavy rainfall [10]. The rapid removal of adhered water either before take-off or while in flight would probably be beneficial for hummingbirds to mitigate any negative consequences of plumage wetting for flight energetics and manoeuvrability.
Water-shaking frequencies of different terrestrial mammals were recently shown to vary inversely with the 0.76 power of the shoulder width [11], but were also lower than those frequencies used for water ejection from continuously spinning discs of similar size [12]. This result may derive from greater capillary forces on clusters of wet hairs relative to those on a smooth surface [13], as well as from the mechanical difference between continuous rotation and the reversing oscillations that characterize shaking. Given the broad range of fur and feather morphologies evident in terrestrial vertebrates, a variety of kinematic mechanisms may in fact be used to affect water-shedding. Here, we report for the first time the notable aerial shake used by Anna's hummingbirds (Calypte anna) to expel water from plumage. Using an oscillating disc, we also evaluate the role of feather flexibility in enhancing speeds and accelerations during rapid reversing oscillations.
2. Material and methods
We studied water-shaking in three adult male Anna's hummingbird (mean mass ± s.e.: 4.50 ± 0.04 g). Birds were placed individually in a Plexiglas cube (0.6 × 0.6 × 0.6 m) which contained a perch and a feeder. A water spray nozzle was placed 40 cm above the feeder to simulate light rain, and was manually activated when the bird was hover-feeding. Water pressure in the spray nozzle was 10 psi (69 kPa). Aerial shaking was typically performed by the hummingbird immediately upon the cessation of feeding and while the bird was moving backwards at slow velocities. A second set of experiments involved placing the bird's perch 40 cm directly beneath the same nozzle, which was activated when the bird was at rest. In this configuration, hummingbirds generally performed a shaking response, while remaining perched, within 3 s of exposure to the artificial rain. Shaking movements for both free-flying and perched hummingbirds were filmed with two synchronized high-speed video cameras (AOS Technologies) operated at 500 frames s−1. Cameras were orthogonally oriented above and lateral to the filmed bird.
Physical characteristics of artificial rain drops were determined from high-speed video recordings at 500 frames s−1. Average drop diameter, descent speed and intensity were 0.6 mm (range: 0.4–1 mm), 1.82 m s−1 (range: 1.3–2.8 m s−1) and 6.4 mm 5 min–1, respectively. These values correspond to those of drizzle-to-light rain conditions [14]. Air temperature in the cube averaged 25.3°C. Body mass increments owing to plumage wetting were measured using a perch positioned in the cage and connected to a digital balance (accuracy ± 0.01 g). The average added mass of adhered water for a perched bird (n = 5) was 0.15 ± 0.04 g (mean ± s.e.), corresponding to an average 3.3 per cent of unwetted body mass. After perched shakes, the remaining water mass on the body then averaged to 0.01 ± 0.002 g.
One aerial and one perching shake were evaluated for each hummingbird. High-speed video recordings were used to determine rotational and translational displacements of the wings and body during shaking. Camera calibration and digitization were carried out using a custom Matlab routine [15]. The positions of both eyes, both shoulders, the left and right tips of the outer tail feathers and both wing tips were digitized for each frame of a shaking sequence. Intermittent gaps in recorded camera coverage were filled manually with single points using an extended Kalman predictor as employed by Hedrick [15]. Derived Cartesian coordinates for all aforementioned landmarks were smoothed to reduce digitization bias using a mean square error quintic spline, as implemented in QuickSAND [16]. Digitization error variances were obtained from five repeated measurements of the same sequence. First and second temporal derivatives of positional data were used to calculate the three-dimensional vector components of speed and acceleration for each landmark. The backward translational velocity vector of flying hummingbirds (mean ± s.e.: 0.38 ± 0.1 m s−1) was subtracted from all derived velocities calculated for aerial shake-offs. Translational speeds were calculated from the distance travelled by the mean shoulder positions divided by the entire shake-off period.
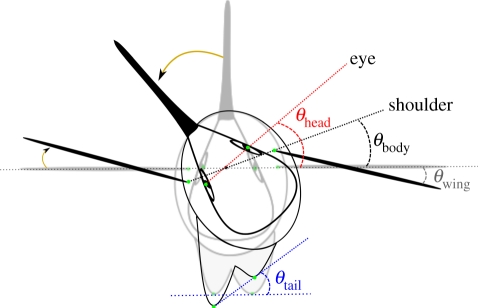
Rotational angles (θ) of the head, body trunk, tail and wings were calculated as the arccosine of [(A1 An+1)/(|A1‖An+1|)], where A1 is the vector formed by the first digitized point and the relevant rotational axis, and An+1 is the vector formed by the (n + 1) digitized point and the rotational axis. Rotational axes of the eye, shoulders and tail were assumed to be the line segments connecting the two eyes, the two shoulder points and two tail tips, respectively. For wings, the rotational axis is defined by the two shoulder points (figure 1). The initial starting angle for each wing was assumed to be the semi-angle defined by the initial position of the two longitudinal wing axes and their intersection with the sagittal plane of the body. Angular speeds were calculated as 2θf, where θ is the average angular amplitude of displacement (in radians) and f is the average oscillation frequency for an entire cycle (in hertz).
Figure 1.
Dorsal diagram of a shaking hummingbird and designation of turning angles: θwing (grey), θhead (red), θtail (blue) and θbody (black). Digitized points are shown as green dots. Yellow arrows indicate the relative turning direction.
Effects of feather flexibility on speeds and accelerations during shaking were evaluated using a physical device consisting of an electric motor, a metal arm with a circular and an elliptical hole at each end, and two plastic discs (with radii of 7 and 15 mm). The larger disc was mounted horizontally on the motor axis and was connected with the metal arm to the smaller disc such that angular rotation of the former induced reciprocating oscillation of the latter (electronic supplementary material, video M1). Five feathers (mean length ± s.e.: 4.4 ± 0.3 mm) obtained from the head of a male Anna's hummingbird were then glued (in separate experiments) to the base of the smaller disc, and were wetted using a syringe. A high-speed video camera (AOS Technologies) operated at 2000 frames s−1 was used to film the top view of the reciprocating disc. Positions of the feather base and tip, the disc centre, and of water drops at the moment of ejection were digitized, and corresponding feather angles, speeds and accelerations were calculated using quintic splines as mentioned above. Accelerations were normalized with respect to gravitational acceleration (9.81 m s−2). Five video recordings were made for each rotational frequency, which was varied from 28.4 to 31.7 Hz. We calculated the average transitional speed of the single feather (at both the base and the tip) as the product of the amplitude (2θr, where r is the radius and θ is the angular displacement in radians) and the oscillation frequency. These calculations assume the feather to be rigid. The radii used for the feather base and the tip were the disc radius and the sum of the feather length and the disc radius, respectively. Centrifugal acceleration of the feather tip was calculated as the translational speed squared divided by the sum of the assumed rigid feather length and the disc radius. The mean value of θ (±s.e.) was 97.3° ± 3.2°, corresponding to the rotational angle attained between the head and shoulders during shaking (table 1).
Table 1.
Kinematic variables for perched and aerial shakes of Anna's hummingbirds. Data shown indicate the mean value ± 1 s.e. (n = 3).
| variable | perched | aerial | t-value | p |
|---|---|---|---|---|
| shake duration (s) | 0.2 ± 0.02 | 0.11 ± 0.01 | 7.36 | 0.02 |
| frequency (Hz) | ||||
| body | 30.25 ± 1.54 | 18.85 ± 0.99 | 5.08 | 0.04 |
| wings | 30.04 ± 0.78 | 24.84 ± 1.17 | 2.67 | 0.12 |
| angular displacement (°) | ||||
| head | 204.89 ± 10.15 | 202.24 ± 14.25 | 0.64 | 0.59 |
| body | 40.77 ± 3.88 | 46.14 ± 8.40 | 0.68 | 0.57 |
| tail | 95.74 ± 22.48 | 101.04 ± 9.56 | 0.22 | 0.85 |
| wings | 96.57 ± 9.89 | 107.96 ± 3.82 | 0.9 | 0.46 |
| angular speed (rad s–1) | ||||
| head | 216.3 ± 14.9 | 132.22 ± 4.84 | 5.42 | 0.03 |
| body | 43.42 ± 6.31 | 30.88 ± 7.20 | 1.33 | 0.31 |
| tail | 103.46 ± 29.86 | 66.02 ± 4.42 | 1.15 | 0.37 |
| wings | 101.51 ± 12.12 | 93.69 ± 5.99 | 0.44 | 0.7 |
| speed (m s–1) | ||||
| head | 0.98 ± 0.08 | 0.67 ± 0.10 | 3 | 0.1 |
| body | 0.44 ± 0.11 | 0.44 ± 0.06 | 0.01 | 1 |
| tail | 1.19 ± 0.11 | 0.93 ± 0.15 | 1.24 | 0.34 |
| wings | 5.33 ± 0.40 | 5.62 ± 0.42 | 0.39 | 0.73 |
| acceleration (g) | ||||
| head | 34.40 ± 3.86 | 14.12 ± 3.18 | 5.39 | 0.03 |
| body | 10.56 ± 2.67 | 6.39 ± 1.03 | 1.17 | 0.36 |
| tail | 26.03 ± 4.08 | 14.56 ± 3.82 | 1.8 | 0.21 |
| wings | 147.30 ± 11.50 | 194.12 ± 37.20 | 0.98 | 0.43 |
Kinematic results for aerial and perching shakes (i.e. frequency, angle, tangential speed, angular speed and tangential acceleration) were compared statistically for the points on the head, body, tail and wings using paired t-tests. Mean differences between average speeds and accelerations of the feather base in the rotational apparatus and those calculated using the quintic spline were compared. For individual water droplets consecutively ejected from wet feathers oscillating on the physical model, a linear regression was fitted to translational speed of consecutive droplets as a function of time since ejection of the first water droplet. Finally, t-tests were used to determine differences between feather tip speeds and accelerations for assumed rigid feathers and for the empirically assayed flexible feathers. Normality and homogeneity of variance were assessed for the variables of frequency, angle, speed and acceleration. Accelerations of tip feathers were log-transformed to obtain normality and equality of variance. All statistical analyses were conducted with R v. 2.10.1 [17] using a fiducial level of significance of 5 per cent.
3. Results
Water shaking typically started with neck elongation, which was more pronounced in flight (electronic supplementary material, videos P1, A1 and A2). Both body and tail motions were then synchronized with head motions, yielding rotations in the same direction. Head oscillation frequencies while perched were significantly higher (by 39%) when compared with those in flight (paired t-test, p = 0.04). The total number of oscillatory cycles completed during perching shake-offs was 4.7 ± 0.3, and during aerial shake-offs averaged to 1.7 ± 0.3. Perching birds unfolded the wings immediately after the first head movement, but then synchronized their wing motions relative to head oscillations, albeit in the opposite direction. By contrast, flying birds synchronized wing motions opposite to the direction of head rotation after one complete wingbeat (electronic supplementary material, videos P1 and A1). Wingbeat frequencies during perching and aerial shake-offs were not significantly different (table 1).
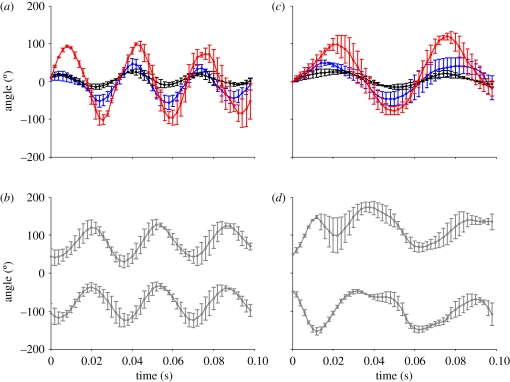
Rotational amplitudes of the head typically exceeded 180° for both aerial and non-aerial shaking, whereas those of the body were about 45°, and those of the tail and wings near 90° (figure 2 and table 1). The mean speeds (both angular and tangential) and accelerations of the head were about two times greater for perched shaking compared with in flight values, but tangential speeds did not significantly differ. Mean speeds (angular and tangential) and accelerations of the body, tail and wings in perched shake-offs were slightly but non-significantly greater than those in aerial shake-offs (table 1).
Figure 2.
Rotational angles through time for (a,b) aerial and (c,d) perched shakes performed by three Anna's hummingbirds. Values and error bars correspond to means and 1 s.e., respectively, of the angle of the head (red), body (black), tail (blue) and wings (grey).
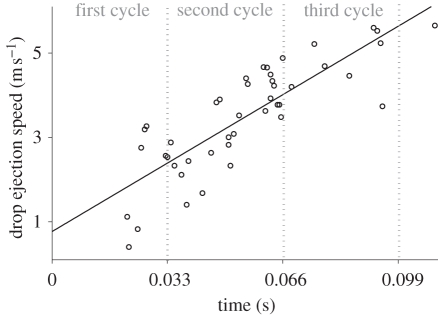
Oscillation frequencies employed for the physical shaking model (30.21 ± 0.67 Hz) were not significantly different from those employed in perched shakes (table 1; t6 = 0.0247, p = 0.981). Comparison of translational speeds of the feather base calculated using a spline function (i.e. 0.68 ± 0.02 m s−1) and those calculated as the product of oscillation amplitude and frequency (0.72 ± 0.01 m s−1) also were not significantly different (t8 = 1.39, p = 0.20). Average speeds and accelerations of the tip of the flexible feather (1.59 ± 0.05 m s−1 and 63.97 ± 2.78g, respectively) were significantly higher (by 36% and 440%, respectively) than those for an assumed rigid feather (1.17 ± 0.02 m s−1 and 12.19 ± 0.38g, respectively; speed comparison: t8 = −9.5, p < 0.001, acceleration comparison: t8 = 34.3, p < 0.001). Maximal calculated acceleration of the flexible feather tip was 159.8 ± 16.1g (see electronic supplementary material, video M1). The speed of water drops at the moment of ejection increased linearly with time (figure 3). This result suggests that progressively higher initial forces were required to accelerate consecutively ejected drops.
Figure 3.
Speed of water drops (n = 44) consecutively ejected from a feather mounted on an oscillating disc. The linear equation is given by speed = 49.16 time + 0.77 (r2 = 0.691, p < 0.05).
4. Discussion
In general, aerial and non-aerial shakes performed by hummingbirds resemble those of mammals, albeit with some important differences. Oscillating frequencies of non-aerial and aerial shakes of hummingbirds are some 25 per cent and 50 per cent lower, respectively, than those predicted for animals of similar size (i.e. with a typical inter-shoulder distance of approx. 0.75 cm in Anna's hummingbirds; see [11]). The somewhat lower shaking frequency exhibited by hummingbirds may be in part offset by greater hydrophobicity of feathers compared with fur, mainly deriving from microstructural differences. Pairs of hairs tend to clump together in contact with a wetting liquid as a result of capillary forces and hair elasticity [13]. In contrast, the arrangement of barbs and barbules in feathers permits trapping of some air interstitially, thereby enhancing higher contact angles of sitting drops [18]. Angular accelerations for birds might thus be somewhat lower to overcome capillary forces, because only superficial water adhering to the plumage needs to be expelled. However, the force of capillarity increases inversely with drop radius [19], and tiny water drops on the feather surface may penetrate the interstitial spaces formed by the barb/barbule matrix [18]. Surprisingly, recent evidence indicates that preen oil has no effect on drop contact angles for contour feathers [20,21]. Removal of such intersitial water would require increased accelerations to overcome capillary forces and to eject small droplets, to which end the enhanced accelerations associated with feather flexibility might be advantageous (see below).
A notable difference between the shake performed by hummingbirds and dogs is the use by the latter of the forelimbs and hindlimbs to counteract the angular momentum generated by the head, trunk and tail [11]. In general, smaller mammals are known to shake with their forelimbs off the ground, whereas larger species oscillate the body with all legs fixed on the ground. Mice and rats shake their bodies at oscillation frequencies of 27 Hz and 18 Hz, respectively [11]. For hummingbirds, in contrast, the wings rotate opposite to body motions during shaking, apparently in accordance with conservation of angular momentum. Aerodynamic stability can thus be enhanced given that cessation of opposite rotational motions can return the system to an original positional state; the head motions observed here are presumably under active control. The remarkable capacity of hummingbirds to independently alter their head, body, tail and wing postures during shaking may enhance flight control and manoeuvrability compared with other small fliers such as insects. Feet of hummingbirds and of the Apodiformes more generally are diminutive and weak, so that the wing counteroscillations performed by hummingbirds during non-aerial shakes may reduce torque on the feet and any ensuing risk of bone fracture. Nevertheless, feet reaction forces of hummingbirds could be sufficiently high so as to permit the higher oscillation frequencies found in perching than during aerial shakes (table 1). Moreover, birds carrying out aerial manoeuvres face substantial fluctuations not only in aerodynamic but also in substantial inertial forces while simultaneously maintaining flight control [22].
The reciprocal head and body oscillations exhibited by hummingbirds during aerial shakes are remarkable given that pigeons hold their heads stationary during aerial manoeuvres [23]. Head accelerations during the aerial and perched shakes of hummingbirds are as high as 14g and 34g, respectively, and well exceed the whole-body accelerations of 10g experienced by male Anna's hummingbirds during courtships dives [24]. These accelerations are nonetheless imposed over very short intervals (table 1). As in the courtship dive at pullout, these periods of intense accelerations are apparently sufficiently short so as to preclude adverse effects of cerebral hypoxia (i.e. blackout) or permanent tissue damage [25]. The sensory means by which hummingbirds maintain stability in hovering flight while simultaneously engaging in rapid head motions clearly merit further attention.
From recorded videos, it can be appreciated that oscillations of the bird's head expel a higher number of drops than the body and tail (electronic supplementary material, videos A1 and A2). Water volumes adhered to the head may accordingly be greater, and head feathers (either singly or in aggregate) may be less hydrophobic than elsewhere on the body. Drops impacting on the head may also penetrate further into the interstitial regions of head feathers, depending on the initial angle of incidence and the peak pressure reached during impacts (see [26] for drop impact forces attained on a solid surface). Oscillating frequencies in non-aerial shakes are higher than those in free flight, and although the rotational amplitude of the head is also higher, the precession angle of the beak is much smaller (see electronic supplementary material, video P1). Heads of perching birds are oriented much more vertically than during flight. As in spinning tops, the associated gravitational torque may be much lower and the precession angle of the head correspondingly reduced.
The smaller number of oscillatory cycles during aerial shaking may associate with reduced vertical force production during the behaviour (see electronic supplementary material, videos A1 and A2, in which height loss and subsequent compensation are evident). Otherwise, there is no indication that hummingbirds experience a loss of control during shakes; hovering remains remarkably stable in spite of the rapid head motions and presumably the associated disruption of optomotor responses otherwise used in stabilization.
Kinematic means of water expulsion from feathers are relevant to a wide diversity of aquatic birds. For example, cormorants [8] and shags [7] rapidly flap their wings to expel water adhered to the plumage. Although feathers are structurally complicated, the use of a simplified physical model to study water expulsion is informative. Liquid drops on the smooth horizontal surface of a spinning disc are ejected when the ratio between centrifugal forces and forces owing to surface tension exceeds approximately unity [27]. This ratio predicts that the angular speed at ejection scales as Rdisc−1/2 Rdrop−1, where Rdisc is the rotating disc radius and Rdrop is the radius of the drop [12]. Dickerson et al. [11] showed that the oscillation frequency of terrestrial mammals during wet shakes is proportional to 33Ras−0.76, where Ras is the body radius at the shoulder. If Rdrop, and the angular amplitude of oscillation are assumed to be constant, then body shaking by mammals larger than guinea pigs would at lower angular velocities than predicted for rotating plates of similar dimensions, albeit given the use in latter experiments of a constant fluid flux. Because lower oscillation frequencies imply reduced rates of drop ejection, it can be hypothesized that other mechanisms may serve to increase droplet acceleration. Dickerson et al. [11] pointed out that the relative looseness of dog skin significantly increases displacement amplitudes (by a factor of three) and consequently angular speeds (by a factor of nine) during shaking. Thus, resonant frequencies in mammal shaking will be strongly influenced by the relatively loose thick skin, whereas the dermis is reduced in birds and inertial effects will derive primarily from the much lower mass of the feathers alone.
In hummingbirds, analogous effects may be attained by feather bending. The energy stored in a feather during bending is inversely related to the Young modulus for any given stress, and avian contour feathers typically exhibit values of this modulus lower than those for hair [28,29]. Results with the oscillating disc indicate that bending, and thus elastic enhancement of droplet shedding, dramatically increase the speed and acceleration of the feather tip relative to a rigid feather. Moreover, our calculations of the drop speed at the moment of ejection suggest that acceleration (and by implication the underlying force) required to eject a water drop increases with time. Use of a disc in continuous rotation with uniform centripetal acceleration may also underestimate actual shedding via reciprocating oscillation.
Using videos of the reciprocating discs, we determined that the average number of water drops ejected per second (nd) was 66.7; average drop diameter was 0.4 mm. Assuming a spherical shape, the mass (md) of each drop then corresponds to 3.4 × 10−8 kg, and the corresponding rate of water loss (i.e. md × nd) is approximately 2.2 mg s–1. Accordingly, a single feather from a bird shaking at this frequency either aerially or while perched would eject 0.25 mg and 0.45 mg, respectively, if this rate is assumed to pertain to the entire shake duration. Thus, perched birds may dry more effectively by shaking the body for almost twice as long as do flying birds. In support of this possibility, we sometimes observed a free-flying bird making two consecutive aerial shakes (with a number of intervening wingbeats) following prolonged exposure to heavy precipitation.
Our rotating disc data suggest that flexibility of feathers and hairs, their reciprocating motions, and also loose skin in mammals (the last two factors as suggested by Dickerson et al. [11]) are important factors that can influence the scaling of shaking frequency with body size. We present here the first relevant kinematic data for shaking-off by birds, and suggest that a broader comparative study across a wide range of avian body sizes would now be relevant for comparison with existing mammalian data. Furthermore, both the areal density and length of feathers may influence the effectiveness of shaking, particularly given the likelihood of mechanical interactions between adjacent structures. High feather density may increase capillary forces and thus the forces required to eject drops during shaking, whereas increased length may create whip-like motions that enhance shedding, either by individual feathers or by collective groups.
Nevertheless, our disc results, must be taken with caution, because an isolated oscillating head feather might experience higher aerodynamic drag and acceleration reaction forces than would an assemblage of feathers in the plumage. Direct kinematic measurements on the tip and the base of contour feathers during hummingbird shaking would be required to verify the potential role of feather bending in water-shedding.
In conclusion, hummingbirds exhibit a remarkable ability to perform both aerial and perched shaking to expel water from the feathers. Motions of the head, body, tail and wings are synchronized but the wings oscillate in a direction opposite to that of the head. Oscillation frequencies of the head, body and tail of aerial shakes were higher than for non-aerial shakes. Speed and acceleration of the body, tail and wings were roughly similar between shake types, but for non-aerial shakes, the speed and acceleration of the head were higher. Finally, using a head feather mounted on a oscillating disc, we found that feather flexibility increases the average speed and acceleration of the feather tip up to 36 per cent and 440 per cent more, respectively, than values calculated for an assumed rigid feather during uniform oscillation. The capacity of hummingbirds to perform high-amplitude head and body shakes while flying represents an outstanding example of aerodynamic control. Given resurgent interest in aerial righting reflexes that nonetheless obey conservation of angular momentum [30], these aerial shakes illustrate an extreme of rapid head, body and appendage motions that are coordinated while simultaneously maintaining stable flight.
Acknowledgements
We thank members of the Dudley laboratory, especially Nir Sapir, Marta Wolf and Dennis Evangelista, for comments and suggestions on the manuscript, and David Hu for insightful critique. We also thank Sarahi Arriaga-Ramirez for sharing her Matlab skills with us. We also thank the two anonymous reviewers for their comments. V.M.O.-J. was supported by UC University of California MEXUS-CONACYT, and dedicates this paper to his parents (a mis padres). Hummingbird care and experimental procedures were approved by the IACUC of the University of California, Berkeley.
References
- 1.Humphreys P. N. 1975. Wet-feather associated with Holomenopon leucoxanthum in a duck. Vet. Rec. 97, 96–97 10.1136/vr.97.5.96 (doi:10.1136/vr.97.5.96) [DOI] [PubMed] [Google Scholar]
- 2.Webb D. R., King J. R. 1984. Effects of wetting of insulation of birds and mammal coats. J. Therm. Biol. 9, 189–191 10.1016/0306-4565(84)90020-2 (doi:10.1016/0306-4565(84)90020-2) [DOI] [Google Scholar]
- 3.White S. D., Bourdeau P. J., Meredith A. 2002. Dermatologic problems of rabbits. Semin. Avian Exotic Pet Med. 11, 141–151 10.1053/saep.2002.123982 (doi:10.1053/saep.2002.123982) [DOI] [Google Scholar]
- 4.Ortega-Jiménez V. M., Álvarez-Borrego S., Arriaga-Ramírez S., Renner M., Bridge E. S. 2010. Takeoff flight performance and plumage wettability in Cassin's auklet Ptychoramphus aleuticus, Xantus's murrelet Synthliboramphus hypoleucus and Leach's storm-petrel Oceanodroma leucorhoa. J. Ornithol. 151, 169–177 10.1007/s10336-009-0441-z (doi:10.1007/s10336-009-0441-z) [DOI] [Google Scholar]
- 5.Schmidt-Nielsen K. 1972. Locomotion: energy cost of swimming, running and flying. Science 177, 222–228 10.1126/science.177.4045.222 (doi:10.1126/science.177.4045.222) [DOI] [PubMed] [Google Scholar]
- 6.Ortega-Jiménez V. M., Álvarez-Borrego S. 2010. Alcid feathers wet on one side impede air outflow without compromising resistance to water penetration. Condor 112, 172–176 10.1525/cond.2010.090137 (doi:10.1525/cond.2010.090137) [DOI] [Google Scholar]
- 7.Cook T. R., Leblanc G. 2007. Why is wing-spreading behaviour absent in blue-eyed shags? Anim. Behav. 74, 649–652 10.1016/j.anbehav.2006.11.024 (doi:10.1016/j.anbehav.2006.11.024) [DOI] [Google Scholar]
- 8.Sellers R. M. 1995. Wing-spreading behavior of the cormorant Phalacrocorax carbo. Ardea 83, 27–36 [Google Scholar]
- 9.Altshuler D. L., Dudley R. 2002. The ecological and evolutionary interface of hummingbird flight physiology. J. Exp. Biol. 205, 2325–2336 [DOI] [PubMed] [Google Scholar]
- 10.Aizen M. A. 2003. Down-facing flowers, hummingbirds and rain. Taxon 52, 675–680 10.2307/3647342 (doi:10.2307/3647342) [DOI] [Google Scholar]
- 11.Dickerson A., Mills G., Bauman J., Chang Y.-H., Hu D. 2010. The wet-dog shake. (http://arxiv.org/abs/1010.3279)
- 12.Walton W., Prewett W. 1949. The production of sprays and mists of uniform drop size by means of spinning disc type sprayers. Proc. R. Soc. B 62, 341–350 10.1088/0370-1301/62/6/301 (doi:10.1088/0370-1301/62/6/301) [DOI] [Google Scholar]
- 13.Bico J., Roman B., Moulin L., Boudaoud A. 2004. Adhesion: elastocapillary coalescence in wet hair. Nature 432, 690. 10.1038/432690a (doi:10.1038/432690a) [DOI] [PubMed] [Google Scholar]
- 14.Huschke, R. E. (ed.) 1959. Glossary of meteorology. Boston, MA: American Meteorological Society [Google Scholar]
- 15.Hedrick T. L. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001. 10.1088/1748-3182/3/3/034001 (doi:10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 16.Walker J. A. 1997. QuickSAND: quick smoothing and numerical differentiation for the power MacIntosh. See http://www.usm.maine.edu/~walker/software.html
- 17.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 18.Bormashenko E., Bormashenko Y., Stein T., Whyman G., Bormashenko E. 2007. Why do pigeon feathers repel water? Hydrophobicity of pennae, Cassie–Baxter wetting hypothesis and Cassie–Wenzel capillarity-induced wetting transition. J. Colloid Interface Sci. 311, 212–216 10.1016/j.jcis.2007.02.049 (doi:10.1016/j.jcis.2007.02.049) [DOI] [PubMed] [Google Scholar]
- 19.Quéré D. 2008. Wetting and roughness. Ann. Rev. Mater. Res. 38, 71–99 10.1146/annurev.matsci.38.060407.132434 (doi:10.1146/annurev.matsci.38.060407.132434) [DOI] [Google Scholar]
- 20.Eliason C. M., Shawkey M. D. 2011. Decreased hydrophobicity of iridescent feathers: a potential cost of shiny plumage. J. Exp. Biol. 214, 2157–2163 10.1242/jeb.055822 (doi:10.1242/jeb.055822) [DOI] [PubMed] [Google Scholar]
- 21.Rijke A. M. 1970. Wettability and phylogenetic development of feather structure in water birds. J. Exp. Biol. 52, 469–479 [Google Scholar]
- 22.Hedrick T. L., Usherwood J. R., Biewener A. A. 2007. Low speed maneuvering flight of the rose-breasted cockatoo (Eolophus roseicapillus). II. Inertial and aerodynamic reorientation. J. Exp. Biol. 210, 1912–1924 10.1242/jeb.002063 (doi:10.1242/jeb.002063) [DOI] [PubMed] [Google Scholar]
- 23.Warrick D. R., Bundle M., Dial K. P. 2002. Avian maneuverability and stability: blurred bodies, clear heads. Integr. Comp. Biol. 42, 141–148 10.1093/icb/42.1.141 (doi:10.1093/icb/42.1.141) [DOI] [PubMed] [Google Scholar]
- 24.Clark C. J. 2009. Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proc. R. Soc. B 276, 3047–3052 10.1098/rspb.2009.0508 (doi:10.1098/rspb.2009.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston P. C. 1939. The problem of ‘black out’ in aviation (Amaurosis fugax). Brit. J. Sur. 26, 749–756 10.1002/bjs.18002610408 (doi:10.1002/bjs.18002610408) [DOI] [Google Scholar]
- 26.Sahaya Grinspan A., Gnanamoorthy R. 2010. Impact force of low velocity liquid droplets measured using piezoelectric PVDF film. Colloids Surf. A 356, 162–168 10.1016/j.colsurfa.2010.01.005 (doi:10.1016/j.colsurfa.2010.01.005) [DOI] [Google Scholar]
- 27.Goodwin R., Rice D., Middleman S. 1988. A model of the onset of motion of a sessile liquid drop on a rotating disk. J. Colloid Interface Sci. 125, 162–169 10.1016/0021-9797(88)90065-3 (doi:10.1016/0021-9797(88)90065-3) [DOI] [Google Scholar]
- 28.Bonser R. H. C., Purslow P. P. 1995. The Young's modulus of feather keratin. J. Exp. Biol. 198, 1029–1033 [DOI] [PubMed] [Google Scholar]
- 29.Macleod G. D. 1980. Mechanical properties of contour feathers. J. Exp. Biol. 87, 65–71 [Google Scholar]
- 30.Jusufi A., Kawano D. T., Libby T., Full R. J. 2010. Righting and turning in mid-air using appendage inertia: reptile tails, analytical models and bio-inspired robots. Bioinspir. Biomim. 5, 1–12 10.1088/1748-3182/5/4/045001 (doi:10.1088/1748-3182/5/4/045001) [DOI] [PubMed] [Google Scholar]