Abstract
The adult mammalian central nervous system (CNS) lacks the capacity for regeneration, making it a highly sought-after topic for researchers. The identification of neural stem cells (NSCs) in the adult CNS wiped out a long-held dogma that the adult brain contains a set number of neurons and is incapable of replacing them. The discovery of adult NSCs (aNSCs) stoked the fire for researchers who dream of brain self-repair. Unfortunately, the quiescent nature and limited plasticity of aNSCs diminish their regenerative potential. Recent studies evaluating aNSC plasticity under pathological conditions indicate that a switch from quiescent to active aNSCs in neurogenic regions plays an important role in both repairing the damaged tissue and preserving progenitor pools. Here, we summarize the most recent findings and present questions about characterizing the active and quiescent aNSCs in major neurogenic regions, and factors for maintaining their active and quiescent states, hoping to outline an emerging view for promoting the endogenous aNSC-based regeneration.
Keywords: Quiescent neural stem cell, Active neural stem cell, Proliferation, Differentiation, Niche, Regeneration
Introduction
The mammalian central nervous system (CNS) has long been known for its poor capacity of self-regeneration, likely owing to the lack of adult neural stem cells (aNSCs) and neurogenesis. In the past few decades, extensive studies have identified aNSCs in two brain areas, the subependymal zone (SEZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus, which inspired researchers to explore the possibility of the CNS repairing itself. Unfortunately, brain aNSCs are largely quiescent, maintain a low metabolic rate, and undergo self-renewal coupled with a very long cell cycle under physiological conditions. Upon injury or pathological challenge, although these aNSCs respond by increasing proliferation, reactivating the differentiation program and migrating toward lesion sites, only a very limited number of new neurons mature and integrate into the preexisting circuits. Most of the newborn cells die, and others become astrocytes or oligodendrocytes [1], leaving a slim hope for CNS self-regeneration. To achieve endogenous NSC-based CNS regeneration, the successful strategy reported thus far is to expand NSCs with an external stimulus. For example, post-ischemic administration of epidermal growth factor (EGF) and fibroblast growth factor (FGF), two commonly used growth factors in NSC culture, can amplify SGZ neural progenitors, generate more projection neurons that integrate into the damaged circuit, and thereby ameliorate neurological deficits [2]. Therefore, a “competent” stem cell pool may be particularly important for regeneration.
Some mammalian tissues, including intestine, skin, and blood system, are capable of regeneration. A recent proposal suggested that an important reason for the quick response and successful repair after injury in these regions outside of the CNS is the coexistence of quiescent and active stem cells within the tissue [3]. These active stem cells are multipotential, undergo self-renewal with a short cell cycle, are derived from quiescent stem cells, and give rise to transit amplifying progenitors (TAPs). In comparison with quiescent stem cells, active stem cells respond more rapidly to damage signals. In these tissues, the active and quiescent stem cells are maintained by Wnt and bone morphogenetic protein (BMP) signals, respectively, which indicates distinct supporting microenvironment [3]. In the salamander, a prominent phenomenon that occurs after spinal cord injury is that the local aNSCs proliferate quickly to form blastema, a structure essential for reestablishing the lost tissue [4]. One reason why salamanders, but not mammals, form a blastema after injury is that aNSCs in the salamander are constantly cycling, whereas mammalian aNSCs remain largely quiescent. In the olfactory epithelium (OE), a well known regenerative region in the adult mammalian peripheral nervous system, proliferative aNSCs can easily be detected and new neurons are consistently produced throughout the life span of the animal.
The existence of highly proliferative aNSCs (active aNSCs) or activation of quiescent aNSCs in nonmammalian species and non-CNS mammalian tissue suggests that these two components are crucial for endogenous NSC-based CNS regeneration. This review summarizes recent progress and proposed questions about the identification of active NSCs in three major neurogenic regions (SEZ, SGZ, and OE) of the adult mammalian nervous system and the factors that regulate the balance between the active and quiescent state of aNSCs, hoping to gain insight for future studies of CNS regeneration.
Quiescent and Active NSCS in the Adult Nervous System
Quiescent and Active NSCs in the Adult Hippocampus
In the SGZ, two types of astroglial-like cells are thought to be aNSCs based on their markers expression, proliferation kinetics, and differentiation abilities [5]. Type 1 cells express nestin (Nes), glial fibrillary acidic protein (Gfap), brain lipid-binding protein (Blbp), glutamate transporter (Glast), and Sox2 and are generally quiescent. Type 2 cells, which display a nonradial or horizontal morphology (have no processes or bear a very short process), express Nes and Sox2, are derived from type 1 cells, and are highly proliferative [5]. Type 2 cells can be subgrouped based on their proneuronal transcription factor expression; type 2a cells express Mash1 and type 2b cells (the TAPs) express prospero-related homeobox 1 gene (Prox1) and neurogenic differentiation 1 (Neurod1) [6].
A recent study using the canonical Notch signaling reporter, Hes5, to label aNSCs in the SGZ revealed the presence of two groups of aNSCs: (a) proliferating horizontal type 1 cells or active NSCs that express proliferating cell nuclear antigen (Pcna) and can be identified by a 1-day BrdU pulse; and (b) BrdU-retaining quiescent aNSCs. External stimuli activate the quiescent aNSCs. For example, running specifically recruits quiescent NSCs into the active NSC pool. Depletion of active NSCs rather than quiescent NSCs leads to impaired neurogenesis in aged mice. Furthermore, if stimulated by seizure, the quiescent NSCs in aged mice can be reactivated [6]. These data indicate that active and quiescent stem cells of functionally different groups coexist in the hippocampus. Another study further demonstrated that the quiescence of SGZ NSCs is specifically maintained by BMP signaling; inhibition or ablation of BMP signaling results in a temporal increase in progenitor proliferation and subsequent decrease of neurogenesis due to exhaustion of the stem cell pool [7]. This raises the question as to whether the Wnt signaling pathway, like in the regenerative tissues (skin, intestine, and blood system), is involved in the regulation of active aNSCs in the SGZ? Previous studies reported that Wnt signaling was activated in the SGZ progenitors and in coordination with Sox2 promoted the proliferation and neuronal differentiation of hippocampal progenitors through inducing the expression of Neurod1 [8]. These results indicate that Wnts may act as a key signal to activate NSCs in the hippocampus.
Quiescent and Active NSCs in the Adult SEZ
Similar to the SGZ, two types of astroglial-like cells have been proposed to be aNSCs in the SEZ. One is the type B cells, which are Nes negative and Gfap positive, and lie between migrating neuroblasts and the underlying striatum as well as between migrating neuroblasts and the ependymal cells. Type B cells give rise to the most actively proliferating cells called type C cells (the TAPs), which serve to increase the neuroblast (type A cells) pool. Type B stem cells are further subgrouped as type B1 and type B2 cells. Type B1 cells physically separate type A cells from the ependymal layer. Type B2 cells separate type A cells from the surrounding striatal parenchyma. A recent cell sorting and genetic lineage tracing study showed that Id1high type B1 astrocytes were the quiescent NSCs [9], whereas type B2 cells were more proliferative. After a 10-year debate, CD133/prominin1 positive ependymal cells, found in the ependymal zone, were demonstrated to be multipotent [10]. Furthermore, ischemia activates ependymal cells leading them to generate neuroblasts and astrocytes and suggesting the quiescent stem cell identity of ependymal cells [11]. Recently, a study using the split-Cre technology showed that CD133/prominin1 and Gfap double positive radial glial-like cells in the SEZ, which extend processes basally between ependymal cells to the ventricle and apically through TAPs and neuroblasts to blood vessels, were quiescent NSCs [12]. It is not clear whether the previously identified CD133/prominin1 positive ependymal stem cells might be contaminated by CD133/prominin1 and Gfap double-positive radial glial-like cells in the SEZ. If this is true, the identity of SEZ quiescent stem cells can be more unambiguously defined.
Although the injury-induced SEZ cell response has been widely acknowledged, the characterization of active NSCs has not been clearly described, except in one study indicating that EGF-responsive type C cells are likely the active stem cells because of their active proliferation, neurosphere formation, and multipotency [13]. In demyelination models, polysialylated neural cell adhesion molecule/Sox9 double positive progenitors or type C cells have also been demonstrated to exhibit properties of short cell cycle and multipotency [14, 15]. In addition, physiological stimulation such as olfactory enrichment promotes neurogenesis by increasing the proliferation of type C cells [16]. Pharmacological depletion of type C cells using AraC-treatment induces the proliferation of quiescent aNSCs. Stroke also activates ependymal cells, prompting them to re-enter the cell cycle [11]. Another question concerning active versus quiescent NSCs in the SEZ is whether these two subtypes are differentially regulated. Emerging evidence indicates that quiescent and active NSCs in the SEZ are regulated by BMP and Wnt signaling, respectively. Noggin is expressed by ependymal cells, whereas BMP2, BMP4, and their receptors are expressed by SEZ cells. Activation of BMP signaling blocks adult neurogenesis and promotes astrocyte differentiation [17]. Given the glial-like identity of quiescent NSCs, these data implicate an important role of BMP signaling in maintaining quiescent NSCs. Using Axin2 as a reporter of Wnt activation, Wnt signaling was shown to be activated in Mash1-positive type C and Gfap-positive type B cells [18]. Activation of Wnt signaling promotes the proliferation of Mash1-positive cells, and thus increases neurogenesis. Overactivation of Wnt signaling can lead to exhaustion of stem cells in the SEZ [18]. Considering that type B2 cells are also proliferative and give rise to type C cells, whether they could be the active NSCs in the SEZ remains to be determined.
Quiescent and Active NSCs in the OE
The OE is another well-known neurogenic niche in the adult mammalian nervous system. Two groups of progenitors or stem cells with different morphology and proliferation properties have been identified in the OE, namely the horizontal basal cells (HBCs) and globose basal cells (GBCs). Both cells express Sox2 and have been suggested to be multipotent [19–21]. GBCs express GBC1/GBC2 and the neural progenitor marker Mash1 and are more proliferative. HBCs express K5/K14 and are more quiescent. GBCs respond to most injuries while HBCs are only activated in response to severe injury that depletes GBCs [22]. In the OE, BMP acts on the early phase of neural progenitors, reduces the expression of Mash1 and proliferating cells, and thus strongly inhibits neurogenesis [23]. During development and chemically-induced OE regeneration, Wnt signaling is activated (and reactivated) in and can promote both proliferation and neuronal differentiation of proliferating Sox2-positive NSCs. These Wnt responsive, proliferative cells largely overlap with GBC markers and partly with HBC markers, suggesting a transit status between the quiescent stem cells and TAPs [24]. Therefore, BMP and Wnt also regulate the different states or groups of aNSCs in the OE. Whether the previously reported BMP signaling is specifically activated in HBCs, which can be easily identified by its unique morphology and K14/K5 expression, remains to be determined.
Active NSCs, Quiescent NSCs, and TAPs
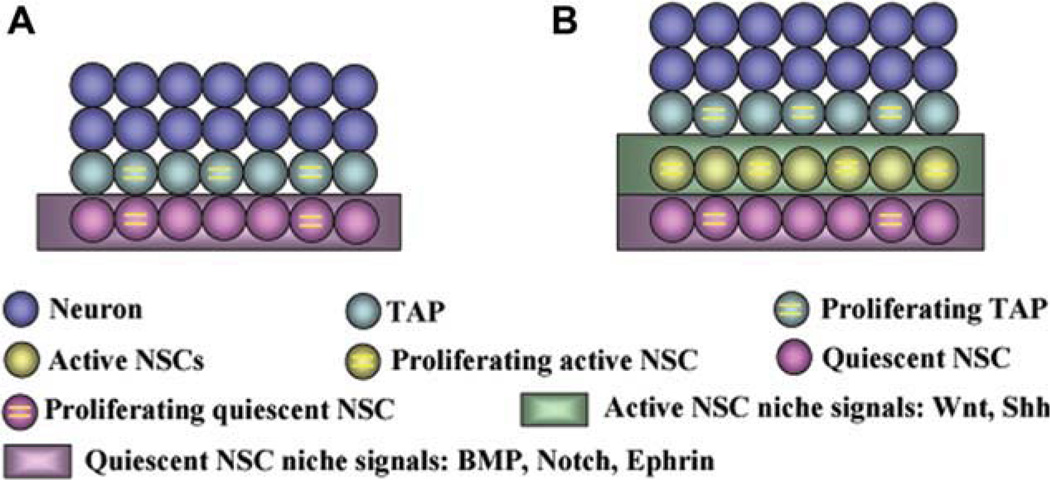
In general, the progression of aNSCs flows from quiescent NSCs to TAPs and then to neuroblasts. As discussed above, emerging data suggest that in the neurogenic regions of the adult nervous system, distinct populations of NSCs exist with different cell cycles, respond differently to extrinsic stimuli and are regulated by different signals. In other words, it is likely that a group of active NSCs exists in neurogenic regions to function as an intermediate between quiescent NSCs and TAPs (Fig. 1). As defined by the term “stem,” active NSCs are multipotent and self-renewing and “active” NSCs are actively cycling cells. In comparison with quiescent NSCs, candidate active NSCs: (a) are highly proliferative (can not retain DNA labeling [3]), (b) are more responsive to extrinsic, and in particular, pathological stimulation, and (c) may be sustained by different niche signals (e.g., Wnt and BMP). In comparison with TAPs, which are usually thought to be lineage restricted, active NSCs: (a) are multipotent and (b) can be clonally propagated for long-term in vitro if the cells were initially selected using the proper markers (e.g., Lgr5 for active stem cells in the intestine) and cultured in proper conditions. The advantage of this “coexistence” is that quiescent NSCs act as a reservoir of stem cells while active NSCs as the competent stem cells for CNS homeostasis and regeneration. Upon injury, the active stem cell pool enlarges quickly to replenish the damaged tissue and shrinks after regeneration. The response of Wnt reactivated progenitors has been shown to be essential for retina regeneration [25]. Therefore, full characterization of active NSCs and understanding the mechanisms that control the mutual shift between these two groups or their status may provide insight for future studies of CNS regeneration.
Figure 1.
A simplified model of adult neural stem cells (aNSCs) lineages. (A): In the traditional model, aNSCs are viewed as quiescent and directly give rise to transit amplifying progenitors (TAPs). (B): In the newly proposed coexistence model, active NSCs act as an intermediate between quiescent NSCs and TAPs. Active and quiescent NSCs are maintained by distinct niche signals. Abbreviations: BMP, bone morphogenetic protein; NSCs, neural stem cells; Shh, Sonic hedgehog; TAP, transit amplifying progenitors.
Factors that Regulate the Balance Between Active and Quiescent NSC Status
Tumor Suppressive and Oncogenic Cell Cycle Regulators
The major difference between active and quiescent NSCs is the length of cell cycle. Therefore, cell cycle regulators, particularly oncogenic and tumor suppressing genes, may be involved in maintaining the active and quiescent states of aNSCs. The cell cycle progression inhibitor p21 is reportedly essential for maintaining aNSCs in a quiescent state [26]. Deletion of p21 results in increased proliferation and ultimately exhaustion of aNSCs [26]. After ischemia, removal of p21 enhances proliferation and neuroblast migration [27]. The phosphatase and tensin homologue deleted on chromosome 10 (Pten), an upstream factor of p21, suppresses NSC self-renewal by modulating G0–G1 entry [28]. Deletion of Pten in aNSCs enhances constitutive neurogenesis in the SEZ [29]. Bmi-1, a polycomb group gene and tumor suppressor, regulates proliferation and self-renewal of NSCs in different developmental stages by targeting different cell cycle regulators. Cell cycle suppressors, p16ink4a and p19arf, mediate the effects of Bmi-1 in postnatal NSCs [30], whereas p21 mediates its function in embryonic and adult NSCs [31]. p53, a well-known tumor suppressor, is expressed in SEZ progenitors. Loss of p53 increases progenitor proliferation, suggesting a role for p53 in maintaining aNSC quiescence [32]. p63 and p73 also regulate the proliferation of NSCs. p73, in particular, maintains the neurogenic pool by promoting self-renewal and proliferation and inhibiting premature senescence of NSCs [33]. These data suggest that cell cycle inhibitors may be crucial for maintaining the quiescent status of aNSCs. Similarly, oncogenes or oncogenic signals may play roles in NSC activation. For example, depletion of proto-oncogene c-myb in neural progenitors reduced proliferative capacity and expression of Sox2 and Pax6 [34]. Future research needs to address how cell cycle modulators can be manipulated to activate quiescent NSCs in the context of injury.
Intrinsic Self-Renewal and Differentiation Modulators
To activate quiescent NSCs, the balance between self-renewal and differentiation must be adjusted. Active NSCs are programmed for differentiation, whereas quiescent NSCs are kept under tight control to maintain the self-renewal process. Intracellular modulators, especially transcription factors and epigenetic regulators that stabilize the gene expression profile of a cell, are essential for maintaining the status of self-renewal versus differentiation. How is NSC self-renewal sustained intrinsically? Nuclear orphan receptor NR2E1 (Tlx) maintains the undifferentiated state of NSCs by recruiting histone deacetylases (HDACs) to its downstream target genes, such as p21 (cip1/waf1) and Pten [35]. Bmi-1 regulates NSC self-renewal as discussed above [30]. Sox2, highly expressed in adult neural progenitors, regulates numerous downstream genes and forms regulatory loops with other important pathways such as Sonic hedgehog (Shh) and epidermal growth factor receptor to maintain NSC stemness [36,37]. Foxo3, a member of the Foxo transcription factor family associated with longevity, has recently been shown to be important for maintaining the aNSC pool by inducing a program of genes that preserves quiescence, prevents premature differentiation, and controls oxygen metabolism [38]. It is unknown whether interactions occur among Tlx, Bmi1, and Foxo3. This begs the question of whether there is a central molecule that unites multiple pathways. A recent study shows that Gsk3 may be an important molecule that is downstream of Wnt, Shh, Notch, and FGF signaling and maintains NSC homeostasis. Deletion of Gsk3 leads to massive hyperproliferation of neural progenitors while reducing the intermediate progenitor and postmitotic neuron populations [39].
To initiate differentiation, global gene expression in NSCs is epigenetically modified to either promote the expression of neuronal genes or suppress the expression of glial genes or vice versa. DNA methyltransferase 1 (Dnmt1) is highly expressed in NSCs. Dnmt1 deficiency creates a hypomethylation in progeny and results in precocious astrocyte differentiation [40], suggesting a role for DNA methylation of astrocytic genes in neuronal differentiation. As for neuronal genes, HDACs inhibit the expression of key neuronal genes such as Neurod1, whereas deletion of HDAC2 disrupts neuronal differentiation specifically in the adult but not in embryonic stages [41]. Emerging data indicate that microRNAs are also important in NSC regulation. MiR-let7b regulates NSC proliferation while miR-9 regulates NSC differentiation, both by targeting and forming a feedback loop with Tlx [42, 43]. MiR-184 and miR-137 act as the downstream targets of Methyl-CpG binding protein 1 (Mbd1) and Sox2, respectively [44]. In addition, miR-137 suppresses expression of the polycomb group protein Ezh2, thereby leading to a global reduction of H3K27 methylation in aNSCs in the SGZ [44]. One of the most abundant microRNAs in the brain, miR-124, is essential for neuronal differentiation of aNSCs in the SEZ, as it is a downstream target of Sox9 [45]. It is not known whether these epigenetic modifications are specifically related to distinct groups of aNSCs or can be regulated differently by the active and quiescent niche signals.
Niche Signals for the Active and Quiescent NSCs
aNSCs reside exclusively in their special niches. In the brain, NSC niches are composed mainly of the surrounding astrocytes, vascular cells, and extracellular matrix. As suggested, quiescent and active NSCs are regulated differently by different signals, such as BMP and Wnt. Therefore, niche signals may play an important role in regulating the active and quiescent states of aNSCs. In Drosophila, a group of nutrition responsive glia release insulin-like peptides to trigger the cell cycle reentry of quiescent NSCs [46]. In mice, astrocytes in the SGZ release Wnt3, and in the SEZ release Wnt7a to stimulate the proliferation and neuronal differentiation of aNSCs [8]. Astrocytes in both regions express Shh to induce neurogenesis [47]. Endothelial cells in the SEZ release vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF) to modulate NSC proliferation [48], which is consistent with the observation that aNSCs closely opposed to vascular endothelial cells are more proliferative [49, 50].
How are the quiescent aNSCs maintained by niche signals? In addition to BMP, Notch signaling is reportedly essential for maintaining the quiescence of aNSCs in both the SEZ and the SGZ [51]. Stroke can disrupt notch signaling in the ependymal cells, thereby mobilizing ependymal cells to generate neuroblasts and astrocytes in the SEZ [11]. Whether Notch signaling is required for HBC quiescence in the OE is not known. Other candidate signals come from the Eph/Ephrins family. Astrocytes in areas outside of the SEZ and sub-ventricular zone express high levels of ephrin-A2 and ephrin-A3, which negatively regulate the NSC growth in these regions [52]. Ephrin-B2/ephrin-B3 is expressed by ependymal cells and astrocytes in the SEZ. Activation of this pathway is required for ependymal cell maintenance, whereas disruption of this pathway increases SEZ cell proliferation [53]. Further studies are needed to elucidate whether these signals are specifically associated with the active versus quiescent NSC groups, and how the niche signals are changed upon injury to active quiescent NSCs.
An important question is that how these niche signals and the above mentioned intracellular modulators work together to regulate the behavior of aNSCs? Wnt signaling can promote the symmetric division [54] and shorten the cell cycle of aNSCs [55], thereby increasing the cell proliferation. Cycling D1 has been demonstrated as a target gene of Wnt signaling during this process. Simultaneously, Wnt activation induces the expression of Neurod1 and Prox1 [8, 56] and thus promoting neuronal differentiation. BMP signaling promotes the cell cycle exit of SEZ NSCs by upregulation of Pten [57]. For other niche signals, such as Notch, VEGF, PEDF, Shh, and Eph/Ephrins, the detailed underlying mechanisms of their regulation of and coordinative effects on the two populations of aNSCs are still largely unknown.
Conclusion
Both development and regeneration are self-organizing processes, in which the starting point paves the way for the final output. The coexistence of active NSCs in neurogenic regions of the adult nervous system and the reactive response of quiescent NSCs to injury suggests an important role for active NSCs in regeneration. Understanding the mechanisms that balance the active versus quiescent states of aNSCs will have important implications in developing novel endogenous stem cell-based regenerative therapies. One thing to note is that most, if not all, of the studies summarized here are from mice. To what extent these lessons can be expanded to humans requires future investigations. With the progress of NSC biology and the convergence of new knowledge in related fields, it is our hope to make the dream of CNS regeneration a reality in the not so distant future.
Acknowledgments
We apologize to those authors whose excellent work in the field could not be cited here due to space limitations. This work was supported in part by NIH grants R01NS059043 and R01ES015988 (to W.D.) and R01DE021696 (to C.J.Z.) and a grant from the Natural Science Foundation of China (30800549) (to Y.-Z.W.), Shriners Postdoctoral Fellowships (to Y.-Z.W. and P.J.), and a postdoctoral training grant from the NIH (to J.M.P).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interests.
Author contributions: Y.-Z.W.: conception, manuscript writing, financial support; J.M.P. and P.J.: conception, manuscript writing; C.J.Z.: conception, manuscript writing, financial support; W.D.: conception, manuscript writing, financial support, final approval of manuscript.
References
- 1.Li L, Harms KM, Ventura PB, et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka EM. Regeneration: If they can do it, why can’t we? Cell. 2003;113:559–562. doi: 10.1016/s0092-8674(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 5.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 6.Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Mira H, Andreu Z, Suh H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coskun V, Wu H, Blanchi B, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlen M, Meletis K, Goritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 12.Beckervordersandforth R, Tripathi P, Ninkovic J, et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Doetsch F, Petreanu L, Caille I, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 14.Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci USA. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonska B, Aguirre A, Raymond M, et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso M, Ortega-Perez I, Grubb MS, et al. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 2008;28:11089–11102. doi: 10.1523/JNEUROSCI.3713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 18.Adachi K, Mirzadeh Z, Sakaguchi M, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 19.Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, Packard A, Krolewski RC, et al. Expression of pax6 and sox2 in adult olfactory epithelium. J Comp Neurol. 2010;518:4395–4418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 23.Shou J, Rim PC, Calof AL. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nat Neurosci. 1999;2:339–345. doi: 10.1038/7251. [DOI] [PubMed] [Google Scholar]
- 24.Wang YZ, Yamagami T, Gan Q, et al. Canonical Wnt signaling promotes proliferation and neurogenesis of peripheral olfactory stem cells/progenitors during postnatal development and adult regeneration. J Cell Sci. 2011;124:1553–1563. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osakada F, Ooto S, Akagi T, et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Takagi Y, Harada J, et al. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0–G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregorian C, Nakashima J, Le Belle J, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molofsky AV, He S, Bydon M, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasano CA, Dimos JT, Ivanova NB, et al. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Perotin S, Marin-Husstege M, Li J, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: Implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talos F, Abraham A, Vaseva AV, et al. p73 is an essential regulator of neural stem cell maintenance in embryonal and adult CNS neurogenesis. Cell Death Differ. 2010;17:1816–1829. doi: 10.1038/cdd.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malaterre J, Mantamadiotis T, Dworkin S, et al. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells. 2008;26:173–181. doi: 10.1634/stemcells.2007-0293. [DOI] [PubMed] [Google Scholar]
- 35.Sun G, Yu RT, Evans RM, et al. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci USA. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Favaro R, Valotta M, Ferri AL, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 37.Hu Q, Zhang L, Wen J, et al. The EGF receptor-sox2-EGF receptor feedback loop positively regulates the self-renewal of neural precursor cells. Stem Cells. 2010;28:279–286. doi: 10.1002/stem.246. [DOI] [PubMed] [Google Scholar]
- 38.Renault VM, Rafalski VA, Morgan AA, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim WY, Wang X, Wu Y, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan G, Martinowich K, Chin MH, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery RL, Hsieh J, Barbosa AC, et al. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci USA. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao C, Sun G, Li S, et al. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C, Sun G, Li S, et al. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szulwach KE, Li X, Smrt RD, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng LC, Pastrana E, Tavazoie M, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chell JM, Brand AH. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, et al. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 49.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapouton P, Skupien P, Hesl B, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci USA. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conover JC, Doetsch F, Garcia-Verdugo JM, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 54.Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29:528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- 55.Yoshinaga Y, Kagawa T, Shimizu T, et al. Wnt3a promotes hippocampal neurogenesis by shortening cell cycle duration of neural progenitor cells. Cell Mol Neurobiol. 2010;30:1049–1058. doi: 10.1007/s10571-010-9536-6. [DOI] [PubMed] [Google Scholar]
- 56.Karalay O, Doberauer K, Vadodaria KC, et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathieu C, Sii-Felice K, Fouchet P, et al. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol Cell Neurosci. 2008;38:569–577. doi: 10.1016/j.mcn.2008.05.005. [DOI] [PubMed] [Google Scholar]