Abstract
Point-light biological motions, conveying various different attributes of biological entities, have particular spatiotemporal properties that enable them to be processed with remarkable efficiency in the human visual system. Here we demonstrate that such signals automatically lengthen their perceived temporal duration independent of global configuration and without observers’ subjective awareness of their biological nature. By using a duration discrimination paradigm, we showed that an upright biological motion sequence was perceived significantly longer than an inverted but otherwise identical sequence of the same duration. Furthermore, this temporal dilation effect could be extended to spatially scrambled biological motion signals, whose global configurations were completely disrupted, regardless of whether observers were aware of the nature of the stimuli. However, such an effect completely disappeared when critical biological characteristics were removed. Taken together, our findings suggest a special mechanism of time perception tuned to life motion signals and shed new light on the temporal encoding of biological motion.
Keywords: point-light walker, temporal expansion, psychometric function
Our senses not only enable our brain to perceive images and sounds, but they also inform us about the passage of time. Time perception over fine scales is fundamental to a range of everyday human activities (for example, to estimate how fast you need to run to catch a ball), and we seem to be able to accurately estimate time as if there exists a specific mechanism that can measure time (e.g., an internal clock; refs. 1, 2). Although it seems quite natural, perceiving time is an extremely complex phenomenon that involves a number of unresolved issues in psychology, and we are far from completely understanding its underlying mechanisms. It has been shown that there is not a specific sensory organ designed to tell time and our representations of subjective temporal duration vary in response to variations of external sensory inputs (reviewed in ref. 3).
Stimulus motion is thought of as a crucial property that can modulate and alter our perception of time. Several studies have shown that a moving stimulus is perceived to last longer in duration than a slower or stationary stimulus of the same physical duration, a phenomenon referred to as subjective time dilation (4–9). Some researchers proposed that this subjective time dilation effect might reflect a result of long-term evolutionary adaptation (6, 10). By lengthening the subjective duration of those moving and important stimuli and thus enhancing their temporal resolution, observers may be able to process such signals in greater depth per unit of objective time (11), which potentially provides an ecological advantage allowing living organisms to anticipate events or actions and to better adapt themselves to the environment (6). Although the underlying mechanism of the time distortion induced by motion has often been speculated from a point of evolutionary view, all these studies used highly simplified and artificial stimuli, such as Gabor patches (6) and textured spheres (7), and little is known regarding the temporal encoding of animate motions in real life.
Different from inanimate object motions, humans have evolved to be highly sensitive to other biological entities’ movements, quickly understanding the intentions behind their movements and anticipating their next move (12–14), even when the motions are portrayed by only a handful of point lights attached to the head and major joints (15). The evolutionary importance of biological motion processing makes it special and distinguishes it from other forms of motion (16). Previous studies have shown that the imitation of biological movements is faster than kinematically identical motion of nonbiological stimuli (17–20), and the effects of temporal modulations on biological motion processing also differ from nonbiological motion (21, 22). More importantly, there is ample evidence showing that the neural substrates associated with human motion processing are at least in part distinct from those associated with inanimate motion (12, 23, 24). Hence, it raises a fundamental question whether the temporal encoding of biological motion is distinct from that of inanimate motion. Although the specialized processing of the spatial configuration of biological motion has been extensively examined in most previous studies (25–30), the encoding of temporal information has received much less attention.
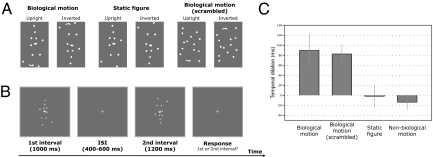
The present study aimed to probe this issue by adopting a type of biological motion stimuli, point-light walkers, for use in a duration discrimination task (Fig. 1). We tested both intact and scrambled biological motion sequences, as the scrambled ones shared the same local motion components as the intact walkers but without the gestalt of a global figure. Some more recent studies have found that these scrambled biological motion sequences still convey specific biological information (31–33). In the present study, the animate motions (intact and scrambled) were contrasted with inanimate motions that were created by inverting the upright sequences (34–36) or disrupting critical biological information (e.g., motion acceleration and motion phase) (37).
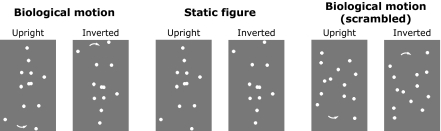
Fig. 1.
Static frames of sample stimuli. Intact point-light walkers, static frames of point-light walkers, and scrambled point-light walkers were used in the present study, including upright and inverted stimuli. Arrows indicate the motion direction and were not presented in the actual experiments.
Results
Experiment 1: Temporal Dilation of Biological Motion Signals.
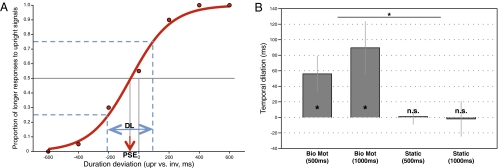
The statistical analyses were conducted on the point of subjective equality (PSE) and different limen (DL) obtained through fitting a psychometric function to the data of each individual observer (Fig. 2A and Methods). In the biological motion condition, one-sample t tests revealed a significant negative PSE in the 500-ms standard-duration condition [t(7) = −2.44; P < 0.05] and in the 1,000-ms standard-duration condition [t(7) = −2.65; P < 0.05], suggesting a temporal dilation (i.e., expansion) effect of the upright biological motion sequences compared with the inverted ones of identical physical duration. In the static figure condition, the PSE was not different from zero in both the 500-ms [t(7) = −0.06; P > 0.1] and 1,000-ms [t(7) = 0.09; P > 0.1] standard-duration conditions, indicating that upright static frames with a discernable human figure could not elicit the temporal dilation effect compared with inverted static frames. Two-way mixed-design ANOVA analyses revealed that the main effect of the stimulus condition (biological motion vs. static figure) for the PSE was significant [F(1, 14) = 7.35; P < 0.02], indicating that the temporal dilation effect (i.e., minus PSE) of the biological motion stimuli was significantly larger than that of the static figures (Fig. 2B). Neither the main effect of the standard duration [500 ms vs. 1,000 ms, F(1, 14) = 0.62; P > 0.1] nor its interaction with stimulus condition [F(1, 14) = 0.84; P > 0.1] was significant. Moreover, the observers’ temporal discrimination sensitivities (i.e., DL) were not significantly different between the biological motion and the static figure conditions [F(1, 14) = 0.46; P > 0.1]. Therefore, the temporal dilation effect was indeed caused by the biological motion signals rather than the familiarity of the upright global figures.
Fig. 2.
(A) Psychometric function for a typical observer in biological motion condition with a standard duration of 1,000 ms. The graph shows the proportion of the “longer” responses to the upright stimuli as a function of the differences between the presentation durations of the two test stimuli (upright vs. inverted). The red arrow indicates the PSE and the blue arrows indicate the DL. (B) Duration discrimination results from experiment 1. The temporal dilation effect (i.e., minus PSE) of the biological motion stimuli was significantly larger than that of the static figures (*P < 0.05; n.s., not significant). Error bars show standard errors.
Experiment 2: Temporal Dilation Independent of Global Configuration and Subjective Awareness.
To further investigate whether the temporal dilation effect essentially reflects an intrinsic sensitivity of the human visual system to biological motion signals, we adopted the scrambled biological motion sequences in which all the point lights were spatially scrambled and the global configuration was entirely disrupted. In addition to probing the dependence of the temporal dilation effect on the global configuration of biological motion signals, we were also interested in whether such an effect has to rely on observers’ subjective awareness of the biological nature of the stimuli.
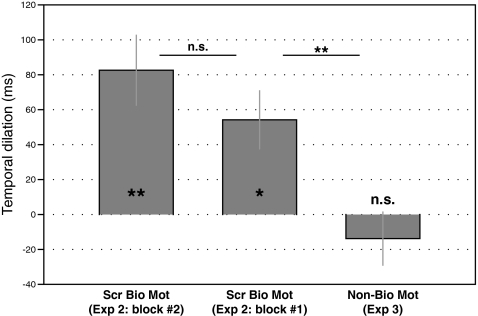
One-sample t tests revealed a significant negative PSE for the scrambled biological motion condition [t(7) = −4.11; P < 0.01], which was not different from the PSE for the intact biological motion stimuli obtained in experiment 1 [t(14) = 0.18; P > 0.1], suggesting that the temporal dilation of biological motion stimuli does not necessarily rely on the global configuration and can be induced by the local motion signals alone. Interestingly, this temporal effect still persisted even when observers were naive to the nature of the stimuli in the first block [t(7) = −3.24; P < 0.02], and there was no significant difference of the temporal effect between the two blocks [t(7) = 1.42; P > 0.1; Fig. 3). These results suggest the existence of a special mechanism of time perception that is tuned to local biological motion signals and operates even without observers’ subjective awareness of the biological nature.
Fig. 3.
Duration discrimination results from experiments 2 and 3. The temporal dilation effect of the scrambled biological motion stimuli in the first block was not different from the second block, but it was significantly larger than that of the nonbiological motion stimuli (*P < 0.05 and **P < 0.01; n.s., not significant). Error bars show standard errors.
Experiment 3: Temporal Dilation Caused by Biological Characteristics.
Finally, we tested nonbiological motion sequences to verify that the observed temporal dilation effect of biological motion signals was indeed driven by the embedded biological characteristics. Results showed that the PSE was not different from zero [t(7) = 0.92; P > 0.1], indicating that the nonbiological motion sequences, although sharing identical moving trajectories with biological motion signals, could not elicit a temporal dilation effect. Moreover, the temporal dilation effect of the scrambled biological motion (i.e., the first block in experiment 2) was significantly larger than that of the nonbiological motion [t(14) = 3.02; P < 0.01; Fig. 3]. Note that the only difference between the scrambled biological motion (experiment 2) and the nonbiological motion (experiment 3) sequences was the presence vs. absence of biological characteristics (i.e., motion acceleration and motion phase). Therefore, the present results provide strong evidence that the observed temporal dilation effect reflects a special mechanism that is essentially triggered by the biological characteristics embedded in the motion signals.
Discussion
People are remarkably adept at recognizing the motions of biological entities in complex visual scenes. The importance of spatial configuration in the perception of biological motion has been widely demonstrated (25, 27–30), whereas only a few have suggested that the temporal properties might also have an impact on the processing of biological motion information (38–43). For example, it was found that varying temporal parameters (e.g., display durations and interframe intervals) of point-light walkers modulated participants’ performance on a direction discrimination task (39, 40). Changing temporal properties also affected the recognition of individuals from point-light biological motion displays (42). Although these studies have suggested that temporal characteristics seem to influence how biological motion is perceived, the temporal encoding of biological motion signals remains unclear. In the present study, we used point-light walkers in a duration discrimination task and found a robust visual perceptual dilation of temporal duration that relies on the automatic encoding of temporal dynamics in biological motion. Specifically, the presentation duration of an upright biological motion sequence was significantly expanded compared with that of an inverted biological motion sequence. Moreover, the effect was not a result of the familiarity of the global configuration, as an upright static human figure showed no such dilation effect compared with an inverted counterpart. This notion was further confirmed with spatially scrambled biological motion stimuli. Unlike intact biological motion displays that had an obvious global configuration, scrambled upright biological motion and its inverted counterpart were equally unfamiliar to naive observers, but the former still exhibited a similar temporal expansion effect. Critically, such an effect completely disappeared when biological characteristics were disrupted from the scrambled stimuli. Therefore, these results suggest that the perceptual dilation of temporal duration observed here is essentially triggered by the specific characteristics of biological motion signals.
A similar temporal dilation phenomenon has been observed with some low-level physical properties of visual stimuli (e.g., size, brightness, and motion) in previous studies (5, 6, 44–47), and it has thus been suggested that the subjective duration of external stimuli mirrors the amount of neural energy used to encode them (i.e., neural energy hypothesis) (3). In other words, more intense stimuli will be perceived longer as they evoke stronger neural responses. Recent brain imaging studies have revealed that upright biological motion signals produce stronger activations in the posterior superior temporal sulcus compared with inverted displays (24), and that activation in this brain region increases in relation to the degree of animacy (48). Therefore, our finding that biological significance shapes visual timing is in accordance with the neural energy hypothesis and clearly demonstrates that the temporal dilation effect could also occur with some certain high-level visual information (see also refs. 47, 49). It is noteworthy that the finding here offers a different view of the distinction between the processing of animate and inanimate motions, which implies an ecological “alerting” function of the neural timing system, allowing living organisms to better prepare themselves for ongoing and upcoming events in social interactions (6, 50).
Notably, such a temporal dilation effect could be extended to local biological motion stimuli without any global configuration, which parallels recent findings on the perception of local biological motion cues (31–33, 37, 51). For example, it has been shown that the ability of direction discrimination and the perception of animacy from spatially scrambled biological motion displays are better with upright than inverted cues (31, 32). However, most previous studies adopted tasks that require the subjects to explicitly process the relevant attributes of biological motion signals (e.g., direction discrimination), and it thus remains possible that the processing of local biological motion cues is dependent on specific tasks. In the present study, observers did not need to rely on any biological motion attributes to complete the task; hence, the observed effect should reflect an intrinsic sensitivity of the human visual system to local biological motion cues. More remarkably, such temporal dilation effects still persisted even when observers were naive to the stimuli, providing compelling evidence that the temporal encoding of biological motion signals is essentially automatic and independent of observers’ awareness of their biological nature. These findings, combined with previous ones (52–55), point to an evolutionarily significant and possibly innate brain mechanism underlying local biological motion processing.
In conclusion, the present study clearly demonstrates that biological motion signals, independent of global configuration and without observers’ subjective awareness of their biological nature, can prolong their perceived temporal duration. These findings suggest the existence of a special and evolutionarily important mechanism of time perception that is specifically tuned to life motion signals. Duration expands when observers are confronted with life motion signals.
Methods
Participants.
A total of 32 observers (17 female) whose ages ranged from 22 to 31 y took part in the study. Sixteen participated in experiment 1, eight participated in experiment 2, and the remaining eight participated in experiment 3. All had normal or corrected-to-normal vision and gave written, informed consent in accordance with procedures and protocols approved by the institutional review board of the Institute of Psychology of the Chinese Academy of Sciences. All observers were naive to the purpose of the experiments.
Stimuli.
Stimuli were generated and displayed by using MATLAB (Mathworks) together with the Psychophysics Toolbox extensions (56, 57). Point-light biological motion sequences, which were created by videotaping a walking actor, were adopted from Vanrie and Verfaillie (28). The segments were digitized, and the head and joint positions in each frame were encoded as motion vectors with initial starting positions. Each motion cycle was 1 s with 30 frames. Static biological motion frames were created by capturing the most extended points of a gait cycle from the biological motion stimuli. Scrambled biological motion sequences were created by randomizing the starting positions of each point within the region approximately covered by the intact biological motion sequences. In the scrambled biological motion sequences, the local motion components remained unchanged, and only the global configural information was entirely disrupted (Fig. 1). Nonbiological motion sequences were derived from the fragments identical to the scrambled sequences but with some critical biological characteristics removed. Specifically, each individual dot moved along a path identical to the scrambled sequences but with a constant speed equal to the average speed of the dot. In addition, the initial motion phase of each individual dot was also randomized. Such manipulations disrupt the natural velocity profile and phase relationship of the scrambled biological motion but keep the motion trajectories of individual point lights unchanged. Inverted biological motion counterparts (i.e., intact, static, scrambled, and nonbiological versions) were created by mirror-flipping all the motion sequences vertically.
Procedure and Data Analysis.
Stimuli were white on a gray background and the viewing distance was 80 cm. In each trial, two stimuli (e.g., an upright biological motion sequence and an inverted biological motion sequence), subtending approximately 4.0° × 6.8° in visual angle, were used and sequentially presented in the center of the screen. One of the stimuli (upright or inverted biological motion) was randomly selected as the standard duration and presented for 500 ms (or 1,000 ms), and the other could be displayed for 200, 300, 400, 500, 600, 700, or 800 ms (or 400, 600, 800, 1,000, 1,200, 1,400, or 1,600 ms), resulting in a total of seven test conditions. In other words, the difference of the presentation durations of the two stimuli (upright vs. inverted) could be −300, −200, −100, 0, 100, 200, or 300 ms (or −600, −400, −200, 0, 200, 400, or 600 ms). A blank interval (with a randomized duration of 400–600 ms) was inserted between the displays of the two stimuli to avoid a potential interference effect. The presentation order of the two stimuli (first vs. second display) was truly randomized across trials. The initial frame of the point-light display was also randomized for each test stimulus and for each trial. Observers were required to make a two-alternative forced choice to indicate, as accurately as possible, which interval (the first or the second) appeared longer regardless of what kind of stimuli was shown. Participants were explicitly told that neither the stimulus content nor its order was predictive of the stimulus presentation duration. The intertrial interval was 1,000 ms.
Experiment 1 adopted a mixed design of two standard durations (500 ms vs. 1,000 ms) and two stimulus conditions (biological motion vs. static figure). Half the observers were assigned to the biological motion condition in which the two types of test stimuli were the upright intact biological motion sequences and the inverted intact biological motion sequences. The remaining observers were assigned to the static figure condition in which the two types of test stimuli were the upright static biological motion frames and the inverted static biological motion frames. They were informed of the nature of the static figures before the experiment. All observers completed two blocks of trials—one in which the standard duration was 500 ms and one in which the standard duration was 1,000 ms. Each block consisted of 140 trials, with 20 trials for each test condition. Test trials were presented in a new random order for each observer, and the order of these two blocks was counterbalanced across observers.
In experiment 2, the two types of test stimuli were the upright and inverted scrambled biological motion sequences, and only the standard duration of 1,000 ms was adopted. There were two blocks in this experiment to test whether the temporal dilation effect relies upon the explicit recognition of the biological information. In the first block, observers were naive to the nature of the scrambled sequences, which was further confirmed in a postsession debriefing. All the observers simply described the scrambled sequences as random motion patterns that they could not recognize, consistent with previous findings (31). At the beginning of the second block, observers were explicitly told that the stimuli were derived from a human walker. In experiment 3, the upright nonbiological motion sequences and their inverted counterparts were used with a standard duration of 1,000 ms.
The results from this two-alternative forced-choice task were fit with a Boltzmann sigmoid function for each individual observer (Eq. 1), and the statistical analyses were conducted on the PSE (i.e., the point at which observers perceived the two stimuli equal in terms of the presentation duration), which is estimated by the midpoint of the Boltzmann function:
A negative PSE means that the upright biological motion sequence is perceived longer (i.e., temporal expansion) compared with the inverted counterpart, whereas a positive PSE indicates the reverse (i.e., temporal compression). In addition, the DL (i.e., half the interquartile range of the fitted function) was used to measure the temporal discrimination sensitivity (a more detailed explanation is provided in refs. 58, 59).
Acknowledgments
This research was supported by grants from the National Basic Research Program of China (No. 2011CB711000), the National Key Technology R&D Program of China (No. 2012BAI36B00), the National Natural Science Foundation of China (No. 31070903), and the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences (No. KSCX2-EW-BR-5 and No. Y1CX302005).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 4043 (volume 109, number 11).
References
- 1.Treisman M. Temporal discrimination and the indifference interval. Implications for a model of the “internal clock”. Psychol Monogr. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- 2.Allan LG. The perception of time. Atten Percept Psychophys. 1979;26:340–354. [Google Scholar]
- 3.Eagleman DM, Pariyadath V. Is subjective duration a signature of coding efficiency? Philos Trans R Soc Lond B Biol Sci. 2009;364:1841–1851. doi: 10.1098/rstb.2009.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SW. Time, change, and motion: The effects of stimulus movement on temporal perception. Percept Psychophys. 1995;57:105–116. doi: 10.3758/bf03211853. [DOI] [PubMed] [Google Scholar]
- 5.Kanai R, Paffen CLE, Hogendoorn H, Verstraten FAJ. Time dilation in dynamic visual display. J Vis. 2006;6:1421–1430. doi: 10.1167/6.12.8. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko S, Murakami I. Perceived duration of visual motion increases with speed. J Vis. 2009;9:14. doi: 10.1167/9.7.14. [DOI] [PubMed] [Google Scholar]
- 7.Beckmann JS, Young ME. Stimulus dynamics and temporal discrimination: implications for pacemakers. J Exp Psychol Anim Behav Process. 2009;35:525–537. doi: 10.1037/a0015891. [DOI] [PubMed] [Google Scholar]
- 8.Mitrani L, Stoianova I. Direct scaling of short time intervals presented with moving and stationary visual stimuli. Acta Physiol Pharmacol Bulg. 1982;8:29–34. [PubMed] [Google Scholar]
- 9.Lhamon WT, Goldstone S. Movement and the judged duration of visual targets. Bull Psychon Soc. 1975;5:53–54. [Google Scholar]
- 10.van Wassenhove V, Buonomano DV, Shimojo S, Shams L. Distortions of subjective time perception within and across senses. PLoS ONE. 2008;3:e1437. doi: 10.1371/journal.pone.0001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse PU, Intriligator J, Rivest J, Cavanagh P. Attention and the subjective expansion of time. Percept Psychophys. 2004;66:1171–1189. doi: 10.3758/bf03196844. [DOI] [PubMed] [Google Scholar]
- 12.Blake R, Shiffrar M. Perception of human motion. Annu Rev Psychol. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Weng X, He S, Jiang Y. Biological motion cues trigger reflexive attentional orienting. Cognition. 2010;117:348–354. doi: 10.1016/j.cognition.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nat Rev Neurosci. 2001;2:561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- 15.Johansson G. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 1973;14:195–204. [Google Scholar]
- 16.Scholl BJ, Tremoulet PD. Perceptual causality and animacy. Trends Cogn Sci. 2000;4:299–309. doi: 10.1016/s1364-6613(00)01506-0. [DOI] [PubMed] [Google Scholar]
- 17.Biermann-Ruben K, et al. Right hemisphere contributions to imitation tasks. Eur J Neurosci. 2008;27:1843–1855. doi: 10.1111/j.1460-9568.2008.06146.x. [DOI] [PubMed] [Google Scholar]
- 18.Jonas M, et al. Observation of a finger or an object movement primes imitative responses differentially. Exp Brain Res. 2007;177:255–265. doi: 10.1007/s00221-006-0660-y. [DOI] [PubMed] [Google Scholar]
- 19.Jonas M, et al. Do simple intransitive finger movements consistently activate frontoparietal mirror neuron areas in humans? Neuroimage. 2007;36(suppl 2):T44–T53. doi: 10.1016/j.neuroimage.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Kessler K, et al. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. Neuroimage. 2006;33:227–238. doi: 10.1016/j.neuroimage.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Shiffrar M, Freyd JJ. Apparent motion of the human body. Psychol Sci. 1990;1:257–264. [Google Scholar]
- 22.Shiffrar M, Freyd JJ. Timing and apparent motion path choice with human body photographs. Psychol Sci. 1993;4:379–384. [Google Scholar]
- 23.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 24.Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Res. 2001;41:1475–1482. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 25.Beintema JA, Lappe M. Perception of biological motion without local image motion. Proc Natl Acad Sci USA. 2002;99:5661–5663. doi: 10.1073/pnas.082483699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beintema JA, Georg K, Lappe M. Perception of biological motion from limited-lifetime stimuli. Percept Psychophys. 2006;68:613–624. doi: 10.3758/bf03208763. [DOI] [PubMed] [Google Scholar]
- 27.Hirai M, Hiraki K. The relative importance of spatial versus temporal structure in the perception of biological motion: An event-related potential study. Cognition. 2006;99:B15–B29. doi: 10.1016/j.cognition.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Vanrie J, Verfaillie K. Perception of biological motion: A stimulus set of human point-light actions. Behav Res Methods Instrum Comput. 2004;36:625–629. doi: 10.3758/bf03206542. [DOI] [PubMed] [Google Scholar]
- 29.Lange J, Georg K, Lappe M. Visual perception of biological motion by form: a template-matching analysis. J Vis. 2006;6:836–849. doi: 10.1167/6.8.6. [DOI] [PubMed] [Google Scholar]
- 30.Lange J, Lappe M. A model of biological motion perception from configural form cues. J Neurosci. 2006;26:2894–2906. doi: 10.1523/JNEUROSCI.4915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troje NF, Westhoff C. The inversion effect in biological motion perception: evidence for a “life detector”? Curr Biol. 2006;16:821–824. doi: 10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Chang DH, Troje NF. Perception of animacy and direction from local biological motion signals. J Vision. 2008;8:1–10. doi: 10.1167/8.5.3. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Zhang K, He S, Jiang Y. Searching for life motion signals. Visual search asymmetry in local but not global biological-motion processing. Psychol Sci. 2010;21:1083–1089. doi: 10.1177/0956797610376072. [DOI] [PubMed] [Google Scholar]
- 34.Pavlova M, Sokolov A. Orientation specificity in biological motion perception. Percept Psychophys. 2000;62:889–899. doi: 10.3758/bf03212075. [DOI] [PubMed] [Google Scholar]
- 35.Proffitt DR, Bertenthal BI. Converging operations revisited: Assessing what infants perceive using discrimination measures. Percept Psychophys. 1990;47:1–11. doi: 10.3758/bf03208159. [DOI] [PubMed] [Google Scholar]
- 36.Sumi S. Upside-down presentation of the Johansson moving light-spot pattern. Perception. 1984;13:283–286. doi: 10.1068/p130283. [DOI] [PubMed] [Google Scholar]
- 37.Chang DH, Troje NF. Acceleration carries the local inversion effect in biological motion perception. J Vision. 2009;9:11–17. doi: 10.1167/9.1.19. [DOI] [PubMed] [Google Scholar]
- 38.Bertenthal B, Pinto J. Global processing of biological motions. Psychol Sci. 1994;5:221–225. [Google Scholar]
- 39.Mather G, Radford K, West S. Low-level visual processing of biological motion. Proc Biol Sci. 1992;249:149–155. doi: 10.1098/rspb.1992.0097. [DOI] [PubMed] [Google Scholar]
- 40.Thornton IM, Pinto J, Shiffrar M. The visual perception of human locomotion. Cogn Neuropsychol. 1998;15:535–552. doi: 10.1080/026432998381014. [DOI] [PubMed] [Google Scholar]
- 41.Hiris E, Humphrey D, Stout A. Temporal properties in masking biological motion. Percept Psychophys. 2005;67:435–443. doi: 10.3758/bf03193322. [DOI] [PubMed] [Google Scholar]
- 42.Hill H, Pollick FE. Exaggerating temporal differences enhances recognition of individuals from point light displays. Psychol Sci. 2000;11:223–228. doi: 10.1111/1467-9280.00245. [DOI] [PubMed] [Google Scholar]
- 43.Jokisch D, Troje NF. Biological motion as a cue for the perception of size. J Vis. 2003;3:252–264. doi: 10.1167/3.4.1. [DOI] [PubMed] [Google Scholar]
- 44.Brigner WL. Effect of perceived brightness on perceived time. Percept Mot Skills. 1986;63:427–430. doi: 10.2466/pms.1986.63.2.427. [DOI] [PubMed] [Google Scholar]
- 45.Fraisse P, Leith J. The Psychology of Time. New York: Harper and Row; 1963. [Google Scholar]
- 46.Terao M, Watanabe J, Yagi A, Nishida S. Reduction of stimulus visibility compresses apparent time intervals. Nat Neurosci. 2008;11:541–542. doi: 10.1038/nn.2111. [DOI] [PubMed] [Google Scholar]
- 47.Xuan B, Zhang D, He S, Chen X. Larger stimuli are judged to last longer. J Vis. 2007;7(2):1–5. doi: 10.1167/7.10.2. [DOI] [PubMed] [Google Scholar]
- 48.Schultz J, Friston KJ, O'Doherty J, Wolpert DM, Frith CD. Activation in posterior superior temporal sulcus parallels parameter inducing the percept of animacy. Neuron. 2005;45:625–635. doi: 10.1016/j.neuron.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 49.Ono F, Kawahara J. The subjective size of visual stimuli affects the perceived duration of their presentation. Percept Psychophys. 2007;69:952–957. doi: 10.3758/bf03193932. [DOI] [PubMed] [Google Scholar]
- 50.Carrozzo M, Moscatelli A, Lacquaniti F. Tempo rubato : Animacy speeds up time in the brain. PLoS ONE. 2010;5:e15638. doi: 10.1371/journal.pone.0015638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirai M, Saunders DR, Troje NF. Allocation of attention to biological motion: local motion dominates global shape. J Vis. 2011;11:1–11. doi: 10.1167/11.3.4. [DOI] [PubMed] [Google Scholar]
- 52.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:e208. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallortigara G, Regolin L. Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol. 2006;16:R279–R280. doi: 10.1016/j.cub.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 54.Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bardi L, Regolin L, Simion F. Biological motion preference in humans at birth: role of dynamic and configural properties. Dev Sci. 2011;14:353–359. doi: 10.1111/j.1467-7687.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 56.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 57.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 58.Luce RD, Galanter E. Discrimination. In: Luce RD, Bush RR, Galanter E, editors. Handbook of Mathematical Psychology. Vol 1. New York: Wiley; 1967. pp. 191–244. [Google Scholar]
- 59.Wearden JH, Ferrara A. Stimulus range effects in temporal bisection by humans. Q J Exp Psychol B. 1996;49:24–44. doi: 10.1080/713932615. [DOI] [PubMed] [Google Scholar]