Abstract
Multimodular polyketide synthases (PKSs) have an assembly line architecture in which a set of protein domains, known as a module, participates in one round of polyketide chain elongation and associated chemical modifications, after which the growing chain is translocated to the next PKS module. The ability to rationally reprogram these assembly lines to enable efficient synthesis of new polyketide antibiotics has been a long-standing goal in natural products biosynthesis. We have identified a ratchet mechanism that can explain the observed unidirectional translocation of the growing polyketide chain along the 6-deoxyerythronolide B synthase. As a test of this model, module 3 of the 6-deoxyerythronolide B synthase has been reengineered to catalyze two successive rounds of chain elongation. Our results suggest that high selectivity has been evolutionarily programmed at three types of protein–protein interfaces that are present repetitively along naturally occurring PKS assembly lines.
A fundamental challenge to our understanding of multimodular polyketide synthases (PKSs) is the ability to explain the unidirectional translocation of growing polyketide chains through these enzymatic assembly lines. The 6-deoxyerythronolide B synthase (DEBS; Fig. 1) is arguably the most well studied example of assembly line PKSs (1–4). Each module of DEBS has an acyl carrier protein (ACP) that collaborates with the β-ketosynthase (KS) domain of the same module to catalyze a single round of polyketide chain elongation (Fig. 2). At this point, the ACP-bound intermediate is precluded from back-transfer to the same KS domain and is instead translocated to the KS domain of the downstream module (Fig. 2).
Fig. 1.
Modular organization of the 6-deoxyerythronolide B synthase. Organized into three homodimeric polypeptides (DEBS1–3), DEBS consists of six modules, each containing a unique set of covalently linked domains. Noncovalent interactions localized to the termini of each polypeptide (matching black tabs) play an important role in chain translocation between modules 2 and 3 and modules 4 and 5. Together, the six modules of this assembly line utilize one propionyl-CoA-derived primer and six methylmalonyl-CoA-derived extender units to synthesize 6-deoxyerythronolide B (6-dEB). DH, dehydratase; ER, enoylreductase; TE, thioesterase; LDD, loading didomain. KR° in module 3 is inactive. The phosphopantetheine prosthetic group of the ACP is shown as a wavy line.
Fig. 2.
Catalytic cycle of a representative minimal module. Every module of an assembly line PKS catalyzes the following sequence of reactions: (1) the polyketide chain is translocated from the ACP domain of the preceding module (grayscale) onto the KS domain of the target module. (2 and 3) Separately, the AT domain catalyzes transfer of a selected extender unit (in this case, a methylmalonyl group) onto the ACP domain. (4) The KS domain then catalyzes chain elongation via decarboxylative condensation, leading to the formation of an ACP-tethered β-ketoacyl product. The oxidation state and stereochemistry of this product is then set by an appropriate combination of KR, dehydratase, and enoylreductase domains (not shown). Eventually, the chain is translocated to the downstream KS as in 1, although it is never translocated back to its own KS.
In the course of our investigations into the mechanism of intermodular chain translocation (Fig. 2, reaction 1) and intramodular chain elongation (Fig. 2, reaction 4) within DEBS, we discovered that the specificity of these two reactions is controlled by protein–protein interfaces involving distinct regions of the ACP domain (5). In the present study, we have used site-directed mutagenesis to identify ACP residues that contribute to the observed specificity. In turn, these residue-level constraints were exploited to validate the proposed structural model for ACP docking during chain elongation (5), as well as to develop an analogous in silico model for chain translocation. Generalization of these models to three naturally occurring PKSs has revealed a programming pattern that establishes a ratchet mechanism that accurately explains the unique chain translocation pathway of each PKS. As a test of this ratcheting mechanism in PKS assembly lines, we engineered a module of DEBS to iteratively catalyze two successive rounds of chain elongation instead of only one.
Results and Discussion
Identification of ACP Residues That Contribute to Chain Elongation Specificity.
All ACPs from fatty acid and polyketide synthases are all-helical bundles comprised of three major α-helices connected by two structured loops (Fig. 3A). In earlier work (5) we showed that, whereas the ACP domain of DEBS module 3 (ACP3) could not effectively partner with the ketosynthase domain of DEBS module 6 (KS6) to catalyze chain elongation, chimeric derivatives of ACP3 harboring loop I of ACP6 (designated SHIV24) had greater preference for KS6 than for KS3 (Fig. 3B). Conversely, ACP6 harboring loop I of ACP3 (SHIV29) had greater preference for KS3 than for KS6. The KS preference of a given ACP was quantified using an established radio-thin-layer chromatography assay (a representative example is shown in Fig. 3C) in which chain elongation is the rate-limiting step (for details, see SI Materials and Methods and Fig. S1A). We therefore sought to identify specific residues within loop I that contribute to the observed preference. Our starting construct was the previously described AYC79 (5), a derivative of ACP3 harboring the shortest loop I swap that had been shown to cause a reversal of KS–ACP domain–domain recognition during chain elongation, resulting in a preference for KS6. As summarized in Fig. 3D, we have systematically mutated the ACP6-derived residues in the predicted LI loop region of AYC79 back to the corresponding ACP3 residues. Most AYC79 derivatives showed a preference for KS6 identical to that of their parent. However, AYC95 and AYC96 harboring altered residues 44 and 45, respectively, each showed notable shifts in their KS preference. A model explaining these results is presented below.
Fig. 3.
Identification of residues that contribute to KS–ACP specificity during chain elongation. (A) Schematic secondary structure of a typical ACP three-helix bundle. The approximate location (S) of the Ser residue onto which the phosphopantetheine arm is attached is shown. The secondary structure elements are mapped onto the tertiary structure in the ACP3 cartoon shown in B. Also shown is a multiple sequence alignment of ACP3, SHIV24, SHIV29, and ACP6 discussed in B. (B) An example of orthogonal KS–ACP recognition between modules 3 and 6 of DEBS. Also shown are two chimeric derivatives in which substitution of loop I of the ACP led to reversal of its preference for a KS partner. (C) Representative radio-thin-layer chromatography assay to quantify the relative preference of a given ACP for alternative KS partners. [KS3][AT3] and [KS6][AT6] refer to the ca. 190-kD homodimeric fragments of modules 3 and 6, respectively, that harbor both the KS and AT domains. (D) Site-directed mutagenesis of AYC79, a minimally altered chimeric derivative of ACP3 with greater preference for [KS6][AT6] than [KS3][AT3]. For the chain elongation activity assay, see SI Materials and Methods. Red, ACP3-derived sequences; green, ACP6-derived sequences. For steric reasons, an H26A mutation was necessary to obtain soluble protein (5).
Identification of ACP Residues That Contribute to Chain Translocation Specificity.
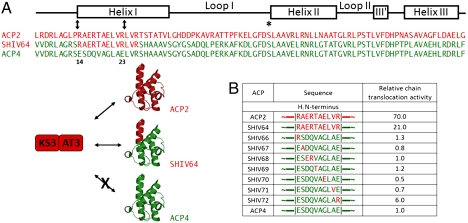
At least two distinct types of protein–protein interactions have been shown to influence intermodular chain translocation in DEBS. On one hand, short peptide sequences flanking the three DEBS proteins (cartooned in Fig. 1 as matching black tabs, also referred to as N- and C-terminal docking domains) permit selective chain translocation between modules 2 and 3 or alternatively modules 4 and 5 (6, 7). At the same time, direct ACP–KS protein–protein interactions at these intermodular interfaces are also selective (5, 8). For example, translocation of a representative polyketide intermediate between ACP2 and KS3 is at least 70-fold faster than the same reaction when ACP4 donates the polyketide chain to KS3 (see comparison of ACP2 versus ACP4 in Fig. 4B). Substitution of a tract of only 10 residues located at the N terminus of helix I of ACP4 with the corresponding 10 amino acid sequence from ACP2 (hereafter referred to as the chain translocation epitope) yielded a chimera with a markedly improved preference for KS3 (Fig. 4 A and B, SHIV64).
Fig. 4.
Identification of residues that contribute to KS–ACP specificity during intermodular chain translocation. (A) KS–ACP specificity during chain translocation between DEBS modules 2 and 3. Whereas ACP2 and KS3 are effective partners, ACP4 cannot substitute for ACP2 in this reaction. However, a chimeric derivative of ACP4 (SHIV64), in which the first 10 residues of HI were replaced with their counterparts from ACP2, led to substantial improvement of its ability to partner with KS3 for chain translocation. (B) Site-directed mutagenesis of SHIV64. Chain translocation activity of each mutant is normalized to that of ACP4. For the activity assay, see SI Materials and Methods. Red, ACP2-derived sequences; green, ACP4-derived sequences. All constructs contain the C-terminal sequence of ACP2 that docks onto an N-terminal coiled-coil on module 3, and is essential for efficient intermodular chain translocation (6).
To identify specific residues within helix I that control chain translocation specificity, we have used a mutagenesis strategy analogous to that described above for analysis of chain elongation specificity. As summarized in Fig. 4B, each residue at the N terminus of helix HI, which differentiates ACP4 from ACP2, was mutated. Most such mutants had low activity for chain translocation to KS3 that was comparable to that of wild-type ACP4. In contrast, SHIV72 harboring an E23R mutation showed sixfold higher activity relative to wild-type ACP4, and was therefore identified as a key determinant of KS–ACP recognition during chain translocation.
Models for KS–ACP Recognition During Chain Elongation and Chain Translocation.
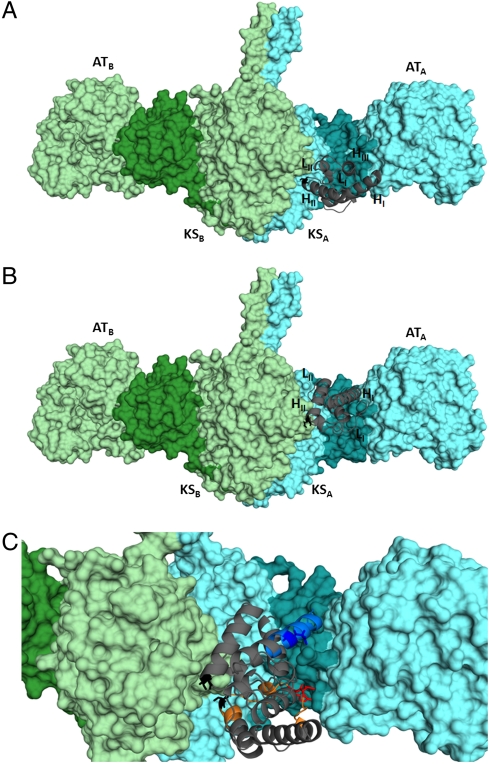
In our earlier study (5), we used computational protein–protein docking (9–12) to derive a model for docking between ACP5 and the core of DEBS module 5 during polyketide chain elongation. Module 5 was targeted for the development of this in silico model because the detailed molecular structure of its core [including the KS and acyltransferase (AT) domains as well as the KS–AT linker, the post-AT linker, and the N-terminal docking domain] has been elucidated by X-ray crystallography (13). Derivation of the computational model was solely constrained by the requirement that the active site Cys sulfhydryl residue of KS5 be within 25 Å of Ser54, the phosphopantetheine attachment site of ACP5. The resulting model (Fig. 5A) is entirely consistent with the site-directed mutagenesis results summarized in Fig. 3, thus highlighting the critical role of residues 44 and 45 in selective intramodular KS–ACP domain–domain interaction. A key feature of our model is that, although the ACP domain from monomer A of the homodimeric module (ACPA) collaborates with KSB during polyketide chain elongation, it docks in a deep cleft defined by the paired KSA domain, the KSA-ATA linker, and the ATA domain.
Fig. 5.
Models for KS–ACP interaction during chain elongation and chain translocation. (A) Docking of ACP5 on the structurally characterized [KS5][AT5] during chain elongation. (B) Docking of ACP4 on the structurally characterized [KS5][AT5] during chain translocation. In both images, the phosphopantetheine attachment site of the ACP is shown in black sticks; it interacts with the KS active site in the light green monomer. (C) Superposition of the two models shown in A and B. Residues 44 and 45 (red) at the chain elongation interface (orange) and residue 23 (deep blue) at the chain translocation interface (blue) are the principal mediators of KS–ACP recognition. The KS–AT linker region of each monomer in A–C is highlighted (KSA-ATA, dark cyan; KSB-ATB dark green).
We also sought to derive a corresponding model for the intermodular KS–ACP interactions that occur during chain translocation (Fig. 2, reaction 1). DEBS module 5 again served as the starting point for the development of this model, due to its extensive structural characterization and in order to facilitate comparison with the experimentally validated docking model for chain elongation, discussed above. To derive the in silico docking model, in addition to the 25-Å constraint on the KS-Cys/ACP-Ser distance, the E23 side chain of ACP4 was constrained to point toward [KS5][AT5] (see SI Materials and Methods for details). The resulting calculated model (Fig. 5B) revealed that ACP4, which collaborates with KS5B during intermodular chain translocation (14), also docks in the same deep cleft of monomer A that is bordered by the KSA domain, the KSA-ATA linker, and the ATA domain, but in a position and orientation distinct from that predicted for KS–ACP interactions during intramodular chain elongation. The N terminus of the ACP4 helix I principally interacts with the KSA-ATA linker of module 5 via an electrostatic interaction between E23 on ACP4 and R551 in KSA-ATA linker. Importantly, even though no constraints were enforced on the position of its C terminus, ACP4 is oriented in a manner consistent with its covalent connection to the structurally characterized C-terminal docking domain of DEBS2 (15) (Fig. S2) via a naturally occurring seven-residue sequence. In contrast, if the same ACP were forced to assume the docking orientation that is utilized for intramodular chain elongation (Fig. 5A), it would then no longer be capable of covalent connection to the C-terminal docking domain of DEBS2 due to the insufficient length of the seven-residue linker between these two domains (Fig. S2). Superposition of the individual docking models for chain elongation and for chain translocation highlights the distinctions between the two different ACP–KS domain–domain interfaces (Fig. 5C).
A Model for Unidirectional chain Translocation in Multimodular PKSs.
An absolutely essential feature of multimodular PKSs is their ability to process growing polyketide chains unidirectionally by a programmed series of alternating chain elongation and chain translocation events. Chain elongation is catalyzed through a partnership between the KS and ACP domains housed within the same module. The resulting ACP-bound intermediate is precluded, however, from intramodular back-transfer to the same KS, in contrast to the mode of action of iterative PKSs and the closely related fatty acid synthases. Instead, vectorial chain growth requires that the product of chain elongation be translocated, either directly or after suitable processing by any accessory domains within the same PKS module, onto the KS domain of the immediately downstream module in the PKS assembly line (Fig. 2). Having identified distinct protein interfaces that control chain translocation versus chain elongation, we sought to determine whether DEBS and other related PKSs have exploited this distinction to ensure unidirectional polyketide processing. Specifically, we wished to test whether the chain translocation docking sites on the ACP domains of consecutive modules have been evolutionarily precluded from both interacting with the same KS domain.
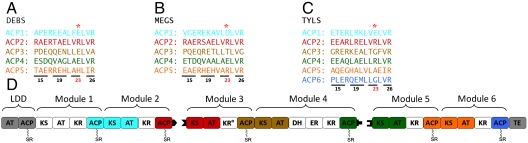
Toward this end, we assumed that any solvent-exposed residue along the N terminus of the predicted helix I of an ACP domain should in principle be able to engage in a noncovalent interaction that might facilitate chain translocation. For example, as shown above, residue 23 (DEBS ACP2 numbering) (16) contributes significantly to the differentiation between ACP2 and ACP4. Because residues 15, 19, and 26 are located one or two helix turns above or below residue 23, they are also well positioned to interact with potential [KS][AT] partners. In fact, examination of the corresponding sets of four residues on helix I of successive ACP domains in several PKSs revealed a striking pattern. As summarized in Fig. 6 A–C for the ACP domains of three PKS systems—DEBS, MEGS (the megalomicin PKS, a distinct PKS that also synthesizes 6-deoxyerythronolide B; ref. 17), and TYLS (the tylosin PKS, which synthesizes tylactone; ref. 18)—in each case, the steric and/or electrostatic features of residue 23 are markedly different for nearly all ACPn-1 - ACPn pairs. In ACP pairs derived from successive modules in which residue 23 is relatively conserved, the other three pairs of residues are markedly different. Thus, consecutive ACP domains may be distinguished based on a sequence fingerprint comprised of residue 23 and, to a lesser extent, residues 15, 19, and 26.
Fig. 6.
A ratchet model for unidirectional translocation of the growing polyketide chain. Sequence alignment of the N terminus of helix (HI) of adjacent ACP domains from (A) DEBS, (B) MEGS (the megalomicin PKS), and (C) TYLS (the tylosin PKS). Residues on this helix that are predicted to be partially or completely solvent exposed are underlined. Residue 23 (DEBS ACP2 numbering) is highlighted with an asterisk, and those that are 1 or 2 helical turns above or below this residue (i.e., residues 15, 19, and 26) are identified. The final ACP from each PKS is omitted because it does not have a downstream KS. (D) A ratchet model for unidirectional chain translocation. The [KS][AT] core of each module is compatible with the N-terminal sequence of helix I of the upstream ACP, but is mismatched to the homologous site on the ACP from its own module. The specificity of protein–protein interactions assures unidirectional translocation of ACP-bound intermediates. LDD, loading didomain.
To elucidate the structural logic of this sequence pattern, we first compared the predicted chain translocation docking site of DEBS [KS5][AT5] and with that of [KS3][AT3] obtained from the above-described computational docking model for chain translocation. The local topology of these regions differs markedly between the two crystal structures (13, 19). In fact, the position occupied by the basic R551 residue from the KS–AT linker in [KS5][AT5] is occupied by the acidic D823 from the AT domain of [KS3][AT3]. (The equivalent Asp residue in module 5 is inaccessible to solvent.) Significantly, in each case, the side chain at this position of the [KS][AT] fragment has a charge opposite to that of residue 23 on the upstream ACP but the same charge as residue 23 of the ACP from its own module (Fig. 6A). Thus, it appears that, notwithstanding their variable sequences, the chain translocation docking sites on the [KS][AT] core of individual modules have evolved to recognize the ACP domain of the upstream module, in preference to the ACP from within their own module. Matching of the chain translocation epitope between ACPn-1 and KSn permits forward translocation of the polyketide chain (Fig. 2, reaction 1). After the subsequent chain elongation reaction (Fig. 2, reaction 4), back-transfer of the ACPn-tethered polyketide to KSn is precluded due to a mismatch in the chain translocation epitopes of ACPn and KSn. This pattern of programmed protein–protein recognition establishes a previously unsuspected ratchet mechanism that ensures orderly transport of the growing polyketide chain along the PKS assembly line (Fig. 6D).
A notable exception to the divergence at residue 23 between consecutive ACP domains is the presence of an E23 residue in both DEBS ACP3 and ACP4 (Fig. 6A). This observation might suggest that the product of module 4 could be transferred back to its own KS. Indeed, the iterative action of DEBS module 4 has been reported, albeit as a rare event (20). This low frequency of back-transfer might be explained by the observation that the three residues located immediately above and below E23 on helix I differ between the ACP3 and ACP4 domains. We also note that the equivalent pair of residues have diverged between ACP3 and ACP4 of MEGS (Fig. 6B), and that back-transfer of the product of module 4 has not been reported for this PKS.
Reprogramming Module 3 of DEBS for Iterative Chain Elongation.
A corollary of the above model for unidirectional chain growth is that helix I of any ACP domain (designated ACPn) within a PKS assembly line is unable to dock onto the [KS][AT] didomain of its parent module (module n), thereby preventing back-transfer of the newly elongated polyketide chain into the active site of its natural KS chain elongation partner (KSn). Iterative use of one or more modules in a PKS would cause the chain length of the resulting polyketide to exceed the total number of modules. Although programmed iterative use of modules has been observed in very rare cases (21–23), the vast majority of individual PKS modules that have been discovered or reconstituted to date catalyze one and only one round of chain elongation.
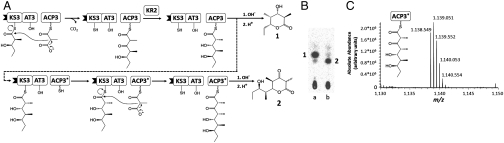
To test our ratchet model for successive ACP–KS recognition, we sought to redesign DEBS module 3 to enable it to catalyze an iterative chain elongation (Fig. 7). As previously reported, upon presentation with a KS3-bound electrophilic substrate that mimics the natural diketide normally synthesized by DEBS module 1, wild-type ACP3 with a bound nucleophilic component derived from methylmalonyl-CoA participates in a single round of chain elongation (Fig. 7A). Furthermore, in a reconstituted system containing recombinant KR2, the ketoreductase domain derived from DEBS module 2, and added NADPH, the initially generated ACP3-bound 3-ketoacyl triketide is diastereospecifically reduced to yield an ACP3-bound intermediate that is identical in structure and stereochemistry to the natural substrate of DEBS module 3 (24). Notwithstanding this identity, the wild-type ACP3 does not appreciably back-transfer the attached triketide to KS3 (Fig. 7B, lane a). By replacing the natural ACP3 domain with an engineered derivative (SHIV78, indicated as ACP3* in Fig. 7A; see SI Materials and Methods for sequence), in which the N terminus of helix I had been swapped with the corresponding helix I sequence from ACP2, we have found that the reconstituted [KS3][AT3], SHIV78, KR2 plus NADPH system can catalyze two rounds of chain elongation to yield a new tetraketide product, as determined by liquid chromatography (LC) high-resolution mass spectroscopy [calculated mass of 3-keto-5,7-dihydroxytetraketide tethered to the tryptic ACP3 peptide 1,138.551 (z = +2); observed 1138.549] (25) (Fig. 7 B, lane b, and C), and supported by the observation of the characteristic phosphopantetheine ejection fragment, [calculated mass of the pantetheinyl fragment corresponding to the 3-keto-5,7-dihydroxytetraketide 489.263 (z = +1); observed 489.264. See Fig. S3] in the derived MS2 spectrum (26). Interestingly, the corresponding pentaketide or longer chain products that would be derived from additional chain elongation cycles could not be detected. Kinetic analysis also verified that the relative rate of chain translocation between SHIV78 and [KS3][AT3] was at least 125-fold higher than the corresponding rate between the natural ACP3 and [KS3][AT3]. Thus, by rational engineering of helix I of the ACP domain, we have demonstrated that an intrinsically noniterative PKS module can be reprogrammed to catalyze an additional round of iterative chain elongation.
Fig. 7.
An engineered chimeric ACP exhibits iterative chain elongation. (A) Schematic representation of the reactions catalyzed by [KS3][AT3] with the wild-type ACP3 (23) and the engineered ACP3 (SHIV78, shown as ACP3*). The reaction involving wild-type ACP3 follows the solid arrows leading to the formation of compound 1 (a triketide ketolactone). The reaction involving ACP3* is predicted to follow the dashed arrow, leading to the formation of compound 2 (a tetraketide ketolactone). (B) Radio thin-layer chromatography for the reaction of [KS3][AT3] with wild-type ACP3 (lane a) and ACP3* (lane b). Compounds 1 and 2 are labeled, and have identical Rf values to those of authentic standards (31) (C) The identity of the tetraketide was confirmed by an established proteolytic LC-high-resolution mass spectroscopy (HRMS) method (24). The HRMS trace (integrated from 60–63 min of the elution gradient) for the ACP3* reaction is shown. The ACP fragment shown in the panel (peptide sequence AFSELGLDS*LNAMALR, where the phosphopantetheine moiety containing the tetraketide thioester is covalently bound to the active serine, S*) has a calculated monoisotopic m/z of 1,138.551 (z = +2). Additional fragment isotopes are labeled and follow the expected isotopic distribution. The precision of the HRMS device is 2 millimass unit.
Conclusion
Understanding the mechanism by which ACP-bound intermediates sequentially access the active sites of successive KS domains is arguably the most fundamental remaining challenge in the study and engineering of assembly line PKS enzymology. We have now shown that at least two distinct protein–protein interactions mediate protein-substrate recognition and processing during intermodular chain translocation—interactions primarily between the C- and N-terminal docking domains (6, 7) of the donor ACP and the acceptor KS domains, and those between the surface of the upstream ACP itself and the downstream KS domain with its appended AT domain and KS–AT linker (8). The former interactions between paired docking domains have been extensively studied and shown to be both architecturally as well as functionally modular (6, 7, 15). In contrast, a general model of ACP–KS protein–protein recognition has proven elusive. Consequently, imperfect KS–ACP interactions between heterologous modules have precluded efficient reprogramming of PKS assembly lines intended to produce unnatural polyketide analogues even when the docking domains were properly matched (2, 27).
Here we have proposed a simple model for ensuring unidirectional growth of a polyketide chain as it is processed on a PKS assembly line. Our model rests on two hypotheses, both of which have been clearly rationalized from a structural standpoint and then experimentally tested. First, three distinct types of protein–protein interactions are proposed to play critical roles in the overall PKS catalytic cycle: (i) interactions between an ACP helix I and the catalytic core of a module; (ii) interactions between short intermodular docking domains; and (iii) interactions between ACP loop I and the catalytic core of the module. The first two types of interactions influence the specificity of intermodular chain translocation, whereas the third interaction affects the specificity of intramodular chain elongation. Second, naturally occurring PKS systems have evolved such that helix I of any ACP domain is preferentially recognized by the catalytic core of the next module in the assembly line rather than by the catalytic core of its own module. Taken together, these two hypotheses suggest that a ratchet mechanism of KS–ACP interactions precludes iterative use of any single module during the overall catalytic sequence of reactions.
This ratchet model should be useful for the rational design of chimeric assembly lines in which PKS modules from heterologous sources can be recombined in a predictable and controlled manner. It may also be useful for reprogramming of selected modules that are found in natural assembly lines in order to purposely catalyze iterative chain elongation, in a manner exemplified by the above-described experiments with the domains of DEBS module 3. Importantly, both envisioned applications rely upon engineering a specific universally conserved secondary structural element in the ACP domain, and thus meet the requirements of architectural as well as functional modularity (2).
Notwithstanding the promising implications for rationally guided biosynthetic engineering, our findings also highlight a number of still unexplained properties of PKS modules that warrant further investigation. As seen in Fig. 7, only one additional iterative round of chain elongation is catalyzed when the KS3 domain of DEBS module 3 is combined with the rationally engineered ACP3 (SHIV78) harboring the helix I sequence from DEBS ACP2 to permit back-transfer of the initially formed chain elongation product to the KS3 domain. Moreover, the added KR2 domain appears to reduce only the 3-ketoacyl-triketide ACP3 intermediate that is generated during the first, normal round of chain elongation, with the second round terminating in formation of the unreduced 3-ketoacyl tetraketide intermediate. The simplest explanation for only a single iteration is that the triketide may be the longest substrate that can be accommodated in the KS3 active site. This hypothesis would be consistent with the well-established mechanism for chain length control by iterative (type II) PKSs (28). Alternatively, the tetraketide may be back-transferred to KS3, but fail to undergo another round of chain elongation, also observed in prior studies on DEBS module 2 in which di- and triketide substrates could be loaded onto the KS domain but could not undergo subsequent chain elongation (29). Notably, such an abortive back-transfer would be expected to result in eventual inactivation of the KS3 domain by the dead-end intermediate. Similarly, the KR2 domain may be unable to process tetraketide or longer substrates, analogous to the previously observed strict specificity of the KR1 domain for only 3-ketoacyl-ACP diketide substrates (30). If these observations are general, then large-scale reprogramming of PKS assembly lines will require consideration of the intrinsic substrate tolerance of both KS and KR active sites in choosing heterologous modules that could be combined to yield unique catalytic cycles.
Materials and Methods
SI Text includes a detailed description of the experimental procedures for the engineering (Tables S1 and S2) and expression of proteins, the assay for chain elongation activity, the assay for chain translocation activity, the molecular docking simulations (Fig. S4), the assay for iterative chain processing, and the LC-MS analysis of ACP-bound polyketide products.
Supplementary Material
Acknowledgments.
We thank Louise Charkoudian for helpful discussions and manuscript preparation. This research was supported by grants from the National Institutes of Health (GM 87934 to C.K. and GM 22172 to D.E.C.), by Stanford Graduate Fellowships to S.K. and A.Y.C., by a National Science Foundation Predoctoral Fellowship to B.L., and by a Naito Foundation Fellowship to S.Y.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118734109/-/DCSupplemental.
References
- 1.Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu Rev Biochem. 2007;76:195–221. doi: 10.1146/annurev.biochem.76.053105.093515. [DOI] [PubMed] [Google Scholar]
- 2.Khosla C, Kapur S, Cane DE. Revisiting the modularity of modular polyketide synthases. Curr Opin Chem Biol. 2009;13:135–143. doi: 10.1016/j.cbpa.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J, Haydock SF, Roberts GA, Bevitt DJ, Leadlay PF. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature. 1990;348:176–178. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- 4.Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Chen AY, Cane DE, Khosla C. Molecular recognition between ketosynthase and acyl carrier protein domains of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci USA. 2010;107:22066–22071. doi: 10.1073/pnas.1014081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gokhale RS, Tsuji SY, Cane DE, Khosla C. Dissecting and exploiting intermodular communication in polyketide synthases. Science. 1999;284:482–485. doi: 10.1126/science.284.5413.482. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji SY, Cane DE, Khosla C. Selective protein–protein interactions direct channeling of intermediates between polyketide synthase modules. Biochemistry. 2001;40:2326–2331. doi: 10.1021/bi002463n. [DOI] [PubMed] [Google Scholar]
- 8.Wu N, Cane DE, Khosla C. Quantitative analysis of the relative contributions of donor acyl carrier proteins, acceptor ketosynthases, and linker regions to intermodular transfer of intermediates in hybrid polyketide synthases. Biochemistry. 2002;41:5056–5066. doi: 10.1021/bi012086u. [DOI] [PubMed] [Google Scholar]
- 9.Mashiach E, Schneidman-Duhovny D, Andrusier N, Nussinov R, Wolfson HJ. FireDock: A web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008;36:W229–232. doi: 10.1093/nar/gkn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhovny D, Nussinov R, Wolfson HJ. In: Guigó R, Gusfield D, editors. Proceedings of the Second International Workshop, WABI 2002 Rome, Italy, September 17–21; Berlin: Springer; 2002. pp. 185–200. Lecture Notes in Computer Science. [Google Scholar]
- 12.Andrusier N, Nussinov R, Wolfson HJ. FireDock: Fast interaction refinement in molecular docking. Proteins. 2007;69:139–159. doi: 10.1002/prot.21495. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Kim CY, Mathews II, Cane DE, Khosla C. The 2.7-Angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci USA. 2006;103:11124–11129. doi: 10.1073/pnas.0601924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao CM, Pieper R, Cane DE, Khosla C. Evidence for two catalytically independent clusters of active sites in a functional modular polyketide synthase. Biochemistry. 1996;35:12363–12368. doi: 10.1021/bi9616312. [DOI] [PubMed] [Google Scholar]
- 15.Broadhurst RW, Nietlispach D, Wheatcroft MP, Leadlay PF, Weissman KJ. The structure of docking domains in modular polyketide synthases. Chem Biol. 2003;10:723–731. doi: 10.1016/s1074-5521(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 16.Alekseyev VY, Liu CW, Cane DE, Puglisi JD, Khosla C. Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase. Protein Sci. 2007;16:2093–2107. doi: 10.1110/ps.073011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volchegursky Y, Hu Z, Katz L, McDaniel R. Biosynthesis of the anti-parasitic agent megalomicin: Transformation of erythromycin to megalomicin in Saccharopolyspora erythraea. Mol Microbiol. 2000;37:752–762. doi: 10.1046/j.1365-2958.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuhstoss S, Huber M, Turner JR, Paschal JW, Rao RN. Production of a novel polyketide through the construction of a hybrid polyketide synthase. Gene. 1996;183:231–236. doi: 10.1016/s0378-1119(96)00565-3. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Chen AY, Kim CY, Cane DE, Khosla C. Structural and mechanistic analysis of protein interactions in module 3 of the 6-deoxyerythronolide B synthase. Chem Biol. 2007;14:931–943. doi: 10.1016/j.chembiol.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson B, et al. Novel octaketide macrolides related to 6-deoxyerythronolide B provide evidence for iterative operation of the erythromycin polyketide synthase. Chem Biol. 2000;7:111–117. doi: 10.1016/s1074-5521(00)00076-4. [DOI] [PubMed] [Google Scholar]
- 21.Olano C, et al. Evidence from engineered gene fusions for the repeated use of a module in a modular polyketide synthase. Chem Commun (Camb) 2003:2780–2782. doi: 10.1039/b310648a. [DOI] [PubMed] [Google Scholar]
- 22.He J, Hertweck C. Functional analysis of the aureothin iterative type I polyketide synthase. Chembiochem. 2005;6:908–912. doi: 10.1002/cbic.200400333. [DOI] [PubMed] [Google Scholar]
- 23.Moss SJ, Martin CJ, Wilkinson B. Loss of co-linearity by modular polyketide synthases: A mechanism for the evolution of chemical diversity. Nat Prod Rep. 2004;21:575–593. doi: 10.1039/b315020h. [DOI] [PubMed] [Google Scholar]
- 24.Castonguay R, He W, Chen AY, Khosla C, Cane DE. Stereospecificity of ketoreductase domains of the 6-deoxyerythronolide B synthase. J Am Chem Soc. 2007;129:13758–13769. doi: 10.1021/ja0753290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnarr NA, Chen AY, Cane DE, Khosla C. Analysis of covalently bound polyketide intermediates on 6-deoxyerythronolide B synthase by tandem proteolysis-mass spectrometry. Biochemistry. 2005;44:11836–11842. doi: 10.1021/bi0510781. [DOI] [PubMed] [Google Scholar]
- 26.Dorrestein PC, et al. Facile detection of acyl and peptidyl intermediates on thiotemplate carrier domains via phosphopantetheinyl elimination reactions during tandem mass spectrometry. Biochemistry. 2006;45:12756–12766. doi: 10.1021/bi061169d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menzella HG, et al. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23:1171–1176. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 28.Keatinge-Clay AT, Maltby DA, Medzihradszky KF, Khosla C, Stroud RM. An antibiotic factory caught in action. Nat Struct Mol Biol. 2004;11:888–893. doi: 10.1038/nsmb808. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Kinoshita K, Khosla C, Cane DE. Biochemical analysis of the substrate specificity of the beta-ketoacyl-acyl carrier protein synthase domain of module 2 of the erythromycin polyketide synthase. Biochemistry. 2004;43:16301–16310. doi: 10.1021/bi048147g. [DOI] [PubMed] [Google Scholar]
- 30.Valenzano CR, Lawson RJ, Chen AY, Khosla C, Cane DE. The biochemical basis for stereochemical control in polyketide biosynthesis. J Am Chem Soc. 2009;131:18501–18511. doi: 10.1021/ja908296m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao CM, Luo G, Katz L, Cane DE, Khosla C. Engineered biosynthesis of structurally diverse tetraketides by a trimodular polyketide synthase. J Am Chem Soc. 1996;118:9184–9185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.