Abstract
Fructose intake from added sugars correlates with the epidemic rise in obesity, metabolic syndrome, and nonalcoholic fatty liver disease. Fructose intake also causes features of metabolic syndrome in laboratory animals and humans. The first enzyme in fructose metabolism is fructokinase, which exists as two isoforms, A and C. Here we show that fructose-induced metabolic syndrome is prevented in mice lacking both isoforms but is exacerbated in mice lacking fructokinase A. Fructokinase C is expressed primarily in liver, intestine, and kidney and has high affinity for fructose, resulting in rapid metabolism and marked ATP depletion. In contrast, fructokinase A is widely distributed, has low affinity for fructose, and has less dramatic effects on ATP levels. By reducing the amount of fructose for metabolism in the liver, fructokinase A protects against fructokinase C-mediated metabolic syndrome. These studies provide insights into the mechanisms by which fructose causes obesity and metabolic syndrome.
Keywords: ketohexokinase, hepatic steatosis, insulin, leptin
Fructose, present in added sugars such as sucrose and high fructose corn syrup, has been epidemiologically linked with obesity and metabolic syndrome (1–3). Experimental studies have shown that fructose can induce leptin resistance and virtually all features of metabolic syndrome in rats, whereas glucose (or starch) intake does not (4). Clinical studies also support fructose as a cause of metabolic syndrome, especially in overweight individuals. Thus, overweight subjects that consumed a 25% fructose-based diet for 10 wk developed insulin resistance, postprandial hypertriglyceridemia, and visceral obesity, unlike subjects given a glucose-based diet (5). Similarly, the additional administration of 200 g of fructose per day to a normal diet could induce de novo metabolic syndrome in 25% of overweight men in just 2 wk (6). Furthermore, a recent report showed that consumption of fructose-sweetened, but not glucose-sweetened, beverages, which provided 25% of energy requirement with usual ad libitum diet could induce postprandial hypertriglyceridemia (not fasting triglycerides) and increased circulating LDL and apolipoprotein B (Apo-B) levels in young and overweight subjects (7). These data are consistent with a recent metaanalysis that found sugary soft drinks to be an independent risk factor for obesity and diabetes (8).
The mechanism by which fructose induces metabolic syndrome has been shown to be independent of excessive energy intake. In the study by Stanhope et al., the ability of fructose to induce features of metabolic syndrome compared with glucose was independent of changes in weight (5). Our group has also shown that fructose (or sucrose) can exacerbate features of metabolic syndrome compared with pair-fed glucose- or starch-fed controls, even under settings of caloric restriction (9–11). Thus, whereas fructose intake may induce leptin resistance with impaired satiety and weight gain (12, 13), fructose-induced metabolic syndrome may also occur, independent of increases in energy intake or weight gain.
These latter findings suggest there may be unique features involved in fructose metabolism that may predispose to the development of metabolic syndrome. Although fructose can be metabolized by hexokinase similarly to glucose (14), the relative affinity for fructose is substantially less and under most conditions fructose is metabolized by fructokinase (ketohexokinase, KHK), an enzyme that is specific for fructose. KHK is uniquely different from other hexokinases in its ability to induce transient ATP depletion in the cell (15, 16). The mechanism is due to the fact that KHK phosphorylates fructose to fructose-1-phosphate rapidly, resulting in marked ATP depletion, coupled with the absence of a feedback inhibition (such as that which controls glucose catabolism). The ATP depletion is also associated with intracellular phosphate depletion and AMP generation, with stimulation of AMP deaminase and the stepwise degradation of AMP to purine products including uric acid (15, 16). Intracellular ATP depletion in response to fructose occurs with low concentrations (1 mM) of fructose and has been shown in laboratory animals and humans (16–18).
Fructokinase exists in two alternatively spliced isoforms consisting of fructokinase C (KHK-C) and fructokinase A (KHK-A) differing in exon 3 (19, 20). KHK-C is expressed primarily in the liver, kidney, and intestines, whereas KHK-A is more ubiquitous (21). Whereas both KHK-C and KHK-A can metabolize fructose, KHK-C is considered the primary enzyme involved in fructose metabolism due to its lower Km (22). Because of its higher Km, KHK-A is not considered presently to be actively involved in fructose metabolism but rather has been postulated to act on as yet unknown substrates (22).
Recently, we generated mice genetically lacking both KHK isoforms (the KhkΔ/Δ mouse; KHK-A/C KO), and mice lacking only KHK-A (the Khk3a/3a mouse; KHK-A KO) (21, 23). These mice show a normal phenotype under normal diet (23). In the present study, we investigated how they would respond to a diet supplemented with fructose.
Results
Energy Intake and Balance.
At baseline (age 2 mo), there was no significant difference in body weight, liver, and epididymal fat weight, blood pressure, and biochemical analyses between wild-type (WT) mice, KHK-A/C KO mice, and KHK-A KO mice, consistent with previous reports (Table S1). WT mice, KHK-A/C KO mice, and KHK-A KO mice (8-wk-old male) were then fed normal chow diet ad libitum with 15 or 30% fructose in the drinking water, or tap water, for 25 wk (n = 8–9 per group). Energy balance (intake and expenditure) was assessed at 19 wk.
WT mice given 15 or 30% fructose received 32 and 45% of their total energy intake as fructose, respectively (Fig. 1A). Whereas WT mice given fructose in the water reduced their intake of chow, overall cumulative energy intake was increased (Fig. 1A and Fig. S1). Indirect calorimetry performed at week 19 showed an increase in total energy expenditure and a positive energy imbalance in WT mice receiving 30% fructose compared with control WT mice (Fig. 1 D and F), which were associated with an increase in body weight (Figs. 2A and 3A).
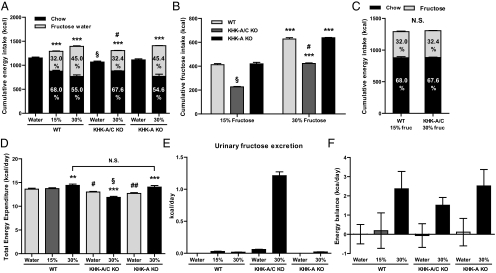
Fig. 1.
Preference for fructose and energy balance. WT mice, KHK-A/C KO mice, and KHK-A KO mice given ad libitum normal chow diet with 15 or 30% fructose water or tap water. (A) Cumulative ad libitum energy intake of normal chow diet with 30% fructose water or tap water (n = 8–9). ***P < 0.001 vs. respective water control. §P < 0.05 vs. WT water or KHK-A KO water. #P < 0.05 vs. WT or KHK-A KO mice given 30% fructose water. (B) Cumulative ad libitum fructose intake (n = 8–9). ***P < 0.001 vs. respective 15% fructose. §P < 0.001 vs. WT or KHK-A KO drinking 15% fructose water. #P < 0.05 vs. WT or KHK-A KO given 30% fructose water. (C) Cumulative ad libitum energy intake of normal chow diet with 15% fructose water in WT mice and with 30% fructose water in KHK-A/C KO mice (n = 9). (D–F) Total energy expenditure (D) over the day at 19 wk. Indirect calorimetry measurements of vO2 and vCO2 were used to calculate metabolic rate (cal/min) every 16 min over 48 h. Average metabolic rate over each day was extrapolated over 24 h to acquire estimates of total energy expenditure (TEE) over the day (kcal/day) (n = 6–8 mice per group, 2 d per mouse). While in the chamber, energy intake over each 24 h was measured and 48-h urine was collected. Urinary fructose excretion (E) was measured, and then daily energy balance was calculated (F). **P < 0.01, ***P < 0.001 vs. respective water control. §P < 0.05 vs. WT or KHK-A KO mice given 30% fructose water. #P < 0.05, ##P < 0.01 vs. WT water. Data represent means ± SEM. Values in the bars mean percentages of energy intake from normal chow diet or fructose water. NS, not significant.
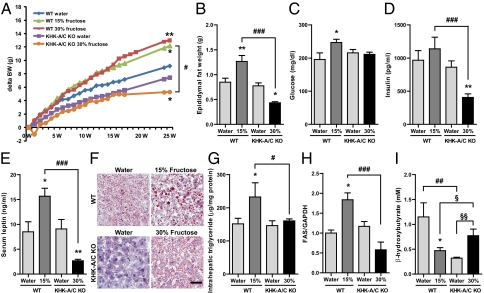
Fig. 2.
Effect of high fructose consumption in WT mice and KHK-A/C KO mice. WT mice and KHK-A/C KO mice given ad libitum normal chow diet with 15 or 30% fructose water or tap water for 25 wk (n = 9). Serum and tissue samples were collected after 6 h fasting. (A) Growth curves of WT mice and KHK-A/C KO mice. (B–E) Epididymal fat weight (B), serum glucose (C), serum insulin (D), and serum leptin (E) (n = 9). (F) Representative images of Oil Red O staining in WT mice and KHK-A/C KO mice. (Scale bar, 50 μm.) (G) Intrahepatic triglyceride levels (n = 7). (H) Western blot analysis of fatty acid synthase (FAS). Relative intensity of FAS to GAPDH in liver (n = 4). (I) Serum β-hydroxy butyrate concentration (n = 5). Data represent means ± SEM *P < 0.05, **P < 0.01 vs. respective water control by ANOVA. #P < 0.05, ##P < 0.01, ###P < 0.001 by ANOVA. §P < 0.05, §§P < 0.01 by t test.
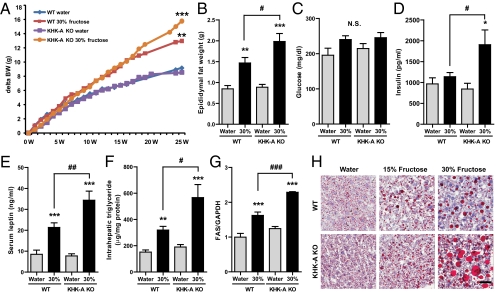
Fig. 3.
Effect of high fructose consumption in WT mice and KHK-A KO mice. WT mice and KHK-A KO mice given ad libitum normal chow diet with 15 or 30% fructose water or tap water for 25 wk (n = 8–9). Serum and tissue samples were collected after 6 h fasting. (A) Growth curves of WT mice and KHK-A KO mice. (B–E) Epididymal fat weight (B), serum glucose (C), serum insulin (D), and serum leptin (E) (n = 9). (F) Intrahepatic triglyceride levels (n = 7). (G) Western blot analysis of FAS. Relative intensity of FAS to GAPDH in liver (n = 4). (H) Representative images of Oil Red O staining in WT mice and KHK-A KO. (Scale bar, 50 μm.) Data represent means ± SEM *P < 0.05, **P < 0.01, ***P < 0.001 vs. respective water control. #P < 0.05, ##P < 0.01, ###P < 0.001. NS, not significant.
Unlike WT mice, KHK-A/C KO mice did not show a preference for fructose water over normal water (Fig. 1B and Fig. S1). As such, cumulative energy intake was lower in fructose-fed KHK-A/C KO mice compared with WT mice (Fig. 1A). Nevertheless, cumulative energy intake was still increased compared with either WT or KHK-A/C KO mice administered water (Fig. 1A). Because humans lacking KHK have been reported to lose significant amounts of fructose in their urine, we performed energy balance studies at week 19. Although urinary fructose was significantly elevated in fructose-fed KHK-A/C KO mice, overall energy balance remained positive after correcting for the urinary fructose excretion (Fig. 1 E and F), possibly related in part to a decrease in total energy expenditure (Fig. 1D). Additionally, the fructose balance in fructose-fed KHK-A/C KO mice was positive (fructose intake, 3.9 kcal/day; urinary fructose excretion, 1.2 kcal/day).
KHK-A KO mice acted similarly to WT KO mice in their total ingestion of fructose, chow, and total cumulative energy intake. Although urinary fructose was slightly increased in KHK-A KO and WT mice on 30% fructose compared with mice not receiving fructose, the levels obtained were much less than that observed with KHK-A/C KO mice (Fig. 1E). Similar to WT mice, fructose feeding in KHK-A KO mice increased total energy expenditure and sustained a positive energy imbalance (Fig. 1 D and F).
Fructose Effects on Wild-Type Mice.
Mice are relatively resistant to the effects of fructose to induce metabolic syndrome (24). Nevertheless, wild-type mice fed fructose showed many features of metabolic syndrome, including increased body weight, total body fat, epididymal fat, liver weight, serum glucose, serum insulin, serum leptin, and serum LDL cholesterol (Fig. 2 A to E and Table S2). However, systolic blood pressure by tail cuff (at 5, 10, and 15 wk), serum triglycerides, HDL cholesterol, liver enzymes, serum uric acid, and serum creatinine (at 25 wk, Table S2) were not different among groups.
Fructose-fed mice also developed progressive hepatic steatosis, associated with increased hepatic triglyceride content (Fig. 2 F and G). This hepatic steatosis was associated with increased fatty acid synthase (FAS) mRNA expression and reduced serum ketones (β-hydroxybutyrate), consistent with both increased fat synthesis and decreased fatty acid oxidation (Fig. 2 H and I).
KHK-A/C KO Mice Are Protected from Metabolic Effects of Fructose.
As mentioned, both total fructose intake and cumulative energy intake were lower in fructose-fed KHK A/C KO mice compared with WT mice fed the same concentration of fructose in the water (Fig. 1A and Fig. S1). However, WT mice receiving 15% fructose ingested the same amount of fructose and overall energy intake as did KHK-A/C KO mice receiving 30% fructose, thus allowing us to determine the role of KHK A/C on features of metabolic syndrome compared with WT mice when intake was identical (Fig. 1C). As shown in Fig. 2 and Table S2, fructose-fed KHK-A/C KO mice were completely protected from the increases in weight gain, total body fat, epididymal fat, serum insulin, serum leptin, serum LDL cholesterol, hepatic FAS expression, and hepatic steatosis. In contrast, glycogen staining of liver showed that fructose-fed KHK-A/C KO mice had much more glycogen accumulation than fructose-fed WT mice (Fig. S2).
Effects of Fructose on KHK-A KO Mice.
Both WT and KHK-A KO mice receiving 30% fructose showed nearly identical energy intake and energy balance (Fig. 1 A and F). However, KHK-A KO mice showed significantly worse features of metabolic syndrome. At 19 wk, weight gain and total fat mass of KHK-A KO mice were higher but not significantly different from WT mice receiving 30% fructose (Fig. 3A and Table S2). However, at 25 wk, epididymal fat mass, serum insulin, serum leptin, intrahepatic triglycerides, and FAS expression were significantly higher in KHK-A KO mice compared with WT mice receiving fructose, whereas fasting serum glucose showed no difference (Fig. 3 A–G and Fig. S3). In particular, hepatic steatosis was much more severe in KHK-A KO mice compared with WT mice when tissues were stained with Oil Red O (Fig. 3H).
Opposing Effects of KHK Isoforms.
KHK-C has been reported to have much greater affinity for fructose than KHK-A, raising the question of whether KHK-A actively metabolizes fructose in vivo (22). We confirmed the lower Km of KHK-C compared with KHK-A using human recombinant proteins (Fig. 4 A and B), but importantly recombinant KHK-A does metabolize fructose at concentrations of 0.5–5 mM (Fig. 4B, Inset). In vitro ATP consumption was significantly lower for KHK-A compared with KHK-C (Fig. 4 C and D), consistent with the slower metabolism. These studies demonstrate that KHK-A is capable of metabolizing fructose.
Fig. 4.
Difference between KHK-A and KHK-C. (A and B) Kinetic analysis of recombinant human KHK-C (A) and KHK-A (B) for d-fructose. Nonlinear regression fit to Michaelis-Menten equation (n = 4). (C and D) ATP consumed by 100 ng or 1,000 ng of KHK-C (C) or KHK-A (D) with 5 mM fructose. No fructose was used as control. Data represent means. **P < 0.01, ***P < 0.001 vs. control. (E–G) Quantitative RT-PCR. Relative comparison of expressions of KHK-C (E) and KHK-A (F) in various organs (n = 2–3) and in liver (G, n = 6) from WT mice.
We next evaluated the expression levels of KHK-C and KHK-A in WT mice. As shown in Fig. 4 E and F, KHK-C was primarily expressed in the liver, intestines, pancreas, and kidney, whereas KHK-A is expressed at these sites as well as many other organs, especially skeletal muscle. In the liver, KHK-C expression was higher than KHK-A (Fig. 4G). Fructose ingestion was found to increase both KHK-C and KHK-A expression in wild-type mice (Fig. 5 A and B). As expected, no KHK expression was observed in KHK-A/C KO mice, whereas only KHK-C was detected in KHK-A KO mice, the latter showing similar increases to WT mice in KHK-C expression with fructose (Fig. 5 A–C and Fig. S3). These data confirm that KHK-A is more ubiquitously expressed compared with KHK-C (21) and that KHK-A is up-regulated with ingested fructose.
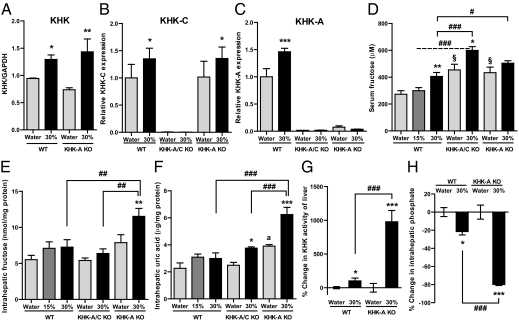
Fig. 5.
Potential mechanisms for opposing effects of KHK-A and KHK-C on metabolic syndrome. WT mice and KHK-A KO mice given ad libitum normal chow diet with 15 or 30% fructose water or tap water for 25 wk (n = 8–9). Serum and tissue samples were collected after 6 h fasting. (A) Western blot analysis of KHK. Relative intensity of KHK to GAPDH in liver (n = 4). (B and C) Quantitative RT-PCR for KHK-C (B) and KHK-A (C) expressions in liver (n = 6). (D) Serum fructose concentration (n = 8–9). (E and F) Fructose content (E) and uric acid content (F) in liver (n = 6). (G and H) Percent change in KHK activity of liver (G, n = 5) and in intrahepatic phosphate content (H, n = 6) with fructose consumption. Data represent means ± SEM *P < 0.05, **P < 0.01, ***P < 0.001 vs. respective water control. #P < 0.05, ##P < 0.01, ###P < 0.001. §P < 0.05 vs. WT water. aP < 0.01 vs. WT water or KHK-A/C water.
To determine whether there is a functional role for KHK-A in in vivo fructose metabolism, we measured serum fructose levels in all groups. As shown in Fig. 5D, serum fructose levels increased in WT mice with increasing fructose in the diet. KHK-A/C KO mice, which can only metabolize fructose via hexokinase, showed higher baseline serum fructose levels, which increased further with high fructose intake, as expected. However, KHK-A KO mice showed baseline serum fructose levels equivalent to that observed in WT mice fed 30% fructose, and levels increased (although not significantly) with higher fructose intake. In this study, we used an enzymatic assay for fructose measurement, which has high sensitivity and reproducibility, but which tends to give higher absolute levels than other methods (Materials and Methods). Using this method, we found increased serum fructose levels in KHK-A/C mice and KHK-A KO mice given tap water compared with WT mice. These studies suggest that in addition to the well-known role of KHK-C in fructose metabolism, KHK-A also has an important role in the metabolism of endogenously produced fructose, such as fructose produced via the polyol pathway.
Whereas KHK-A is a slow metabolizer of fructose, it may still be active in tissues in which KHK-C is not expressed, and hence knocking down KHK-A may result in higher serum fructose levels and greater delivery of fructose to the liver and other sites where KHK-C is expressed. Consistent with this hypothesis, we found significantly higher intrahepatic fructose levels in KHK-A KO mice compared with WT mice receiving 30% fructose (Fig. 5E). Because KHK-C is not negatively regulated by excess substrate (25), the higher fructose delivery should be associated with greater metabolism of fructose. Consistent with this hypothesis, we found both higher KHK activity, intrahepatic uric acid levels (Fig. 5 F and G), and lower intrahepatic phosphate (Fig. 5H) in the KHK-A KO mice receiving 30% fructose compared with WT mice receiving similar concentrations of fructose. No KHK activity was observed in liver from KHK-A/C KO mice on tap water or fructose water.
Discussion
These studies document that excessive fructose intake by WT mice induces several features of metabolic syndrome, including obesity, visceral fat accumulation, fatty liver, and elevated insulin and leptin levels, all of which are blocked in mice genetically deficient in both fructokinase isoforms. It is likely that the increased leptin levels represent leptin resistance (13), which could account for the greater cumulative energy intake in these mice. In addition, recent studies suggest that fructose may have direct effects on the hypothalamus to stimulate energy intake (26–28). However, the effects of fructose to induce features of metabolic syndrome is not simply due to increased energy intake, because KHK-A/C KO mice that had similar fructose intake and energy balance failed to develop these features. These studies are consistent with a key role for KHK in driving metabolic syndrome independent of increased energy intake. Interestingly, the energy balance studies revealed a positive energy imbalance in fructose-fed KHK-A/C KO mice. Although fructose-fed KHK-A/C KO mice did not accumulate lipid in their liver, they did accumulate more glycogen than observed with wild-type mice fed fructose. On the basis of these results, KHK-A/C KO mice are likely metabolizing some fructose via the hexokinase pathway (14).
Whereas KHK-A/C KO mice were protected from the metabolic effects of fructose, KHK-A KO mice showed worse metabolic syndrome compared with WT mice, despite equivalent fructose and total energy intake. The mechanism relates to the fact that although KHK-A is a slow metabolizer of fructose, it likely has an important function in fructose metabolism in tissues that do not express KHK-C. Indeed, serum fructose levels were high in KHK-A KO mice even in the absence of dietary fructose. In turn, the absence of KHK-A allows more delivery of fructose to the liver, resulting in higher KHK activity and greater amounts of fructose being metabolized, as determined by measuring intrahepatic uric acid and intrahepatic phosphate as a surrogate of ATP depletion. These studies are also consistent with data suggesting that KHK-dependent ATP depletion, likely in the liver and hypothalamus, could be important in mediating features of metabolic syndrome in response to fructose (17, 26–28).
The primary importance of these findings is the discovery of an alternative pathway for fructose metabolism that appears to attenuate the effects of KHK-C on fat storage. A caveat is that these mice were ingesting 30–45% of their diet as fructose, which is markedly higher than the 10–15% fructose intake that is commonly observed in Western diets. However, the absorption of fructose is markedly enhanced by the presence of glucose (29, 30) such as is present in sucrose or high fructose corn syrup. Hence, administering 40% sucrose diets (which contains only 20% fructose) can induce fatty liver and diabetes in male breeder rats (10). Fructose absorption is also enhanced by prior exposure to fructose (31, 32). This may explain why clinical studies investigating the effects of fructose on lipids and insulin resistance show greater metabolic effects of fructose on subjects who are overweight or insulin resistant or with a family history of diabetes (5, 6, 33–35), as opposed to those that are healthy and lean (36, 37). In addition, humans may be more predisposed to the metabolic effects of fructose because they do not synthesize vitamin C and also lack uricase, resulting in higher uric acid levels (38, 39). Vitamin C has been found to block some fructose effects (40), whereas uric acid appears to amplify fructose effects (9, 41).
In conclusion, fructose-induced metabolic syndrome is prevented in mice lacking both isoforms, fructokinase C and A, but is exacerbated in mice lacking fructokinase A. These results show that excessive fructose metabolism via fructokinase induces metabolic syndrome and suggest that fructokinase A has an important role in the metabolism of dietary fructose and in attenuation of fructose-induced metabolic syndrome. Whereas studies with mice lacking only fructokinase C would be helpful to understand the role of fructose in metabolic syndrome, the current study provides important insights into the different roles of KHK isoforms in fructose metabolism and in the development of fructose-induced obesity and insulin resistance.
Materials and Methods
KHK-A/C KO mice, which were of C57BL/6 background and were lacking both ketohexokinase A and ketohexokinase C, and KHK-A KO mice were generated as described previously (23). KHK-A/C knockout homozygous mice, KHK-A knockout homozygous mice, and WT litter mates (male, 8 wk old) were used. They were maintained in temperature- and humidity-controlled specific pathogen-free condition on a 12-h dark/12-h light cycle and allowed ad libitum access to normal laboratory chow (Harlan Teklad; no. 2918). WT mice, KHK-A/C KO mice, and KHK-A KO mice were assigned to one of three groups (n = 8–9) respectively, matching mean body weight among the groups. Mice had free access to water containing 15% fructose, 30% fructose, or tap water for 25 wk. Fructose water was prepared by dissolving D-(-)-fructose (Sigma-Aldrich) in tap water. Body weight was measured every week, and energy intake from both normal chow and fructose water was measured three times per week. At 19 wk, energy balance and fuel utilization were assessed with indirect calorimetry as described below, and body composition was measured by quantitative magnetic resonance with an Echo MRI-900 whole body composition analyzer (Echo Medical Systems). At 25 wk, mice were killed after 6 h fasting from food and fructose water. Blood was withdrawn, and tissues were taken and frozen in liquid nitrogen. All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the animal care and use committee of the University of Colorado. Details of methods for serum and tissue analyses are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Takahiko Nakagawa for his valuable comments on this manuscript. We also thank Ginger Johnson for her work on the calorimetric study. This work was supported by Grants HL-68607 and RC4 DK090859-01, Grant DK-038088 and the Nutrition and Obesity Research Center core facilities (DK-48520) from National Institutes of Health, Diabetes United Kingdom Grant RD04/0002833, and startup funds from the University of Colorado.
Footnotes
Conflict of interest statement: Based on the discoveries from this study, T.I., M.A.L., and R.J.J. are listed as inventors on a patent application from the University of Colorado related to developing isoform-specific fructokinase inhibitors in the treatment of disorders associated with obesity and insulin resistance. No other authors have any conflicts of interest.
This article is a PNAS Direct Submission. K.U. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119908109/-/DCSupplemental.
References
- 1.Havel PJ. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 2.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanhope KL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Pozo SE, et al. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 7.Stanhope KL, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96:E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik VS, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa T, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 10.Roncal-Jimenez CA, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60:1259–1270. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gersch MS, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol. 2007;293:F1256–F1261. doi: 10.1152/ajprenal.00181.2007. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro A, et al. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–R1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro A, Tümer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr. 2011;106:390–397. doi: 10.1017/S000711451100033X. [DOI] [PubMed] [Google Scholar]
- 14.Katzen HM, Schimke RT. Multiple forms of hexokinase in the rat: Tissue distribution, age dependency, and properties. Proc Natl Acad Sci USA. 1965;54:1218–1225. doi: 10.1073/pnas.54.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods HF, Eggleston LV, Krebs HA. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970;119:501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977;162:601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode JC, Zelder O, Rumpelt HJ, Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973;3:436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 18.Cortez-Pinto H, et al. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: A pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 19.Bonthron DT, Brady N, Donaldson IA, Steinmann B. Molecular basis of essential fructosuria: molecular cloning and mutational analysis of human ketohexokinase (fructokinase) Hum Mol Genet. 1994;3:1627–1631. doi: 10.1093/hmg/3.9.1627. [DOI] [PubMed] [Google Scholar]
- 20.Hayward BE, Bonthron DT. Structure and alternative splicing of the ketohexokinase gene. Eur J Biochem. 1998;257:85–91. doi: 10.1046/j.1432-1327.1998.2570085.x. [DOI] [PubMed] [Google Scholar]
- 21.Diggle CP, et al. Ketohexokinase: Expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem. 2009;57:763–774. doi: 10.1369/jhc.2009.953190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asipu A, Hayward BE, O'Reilly J, Bonthron DT. Properties of normal and mutant recombinant human ketohexokinases and implications for the pathogenesis of essential fructosuria. Diabetes. 2003;52:2426–2432. doi: 10.2337/diabetes.52.9.2426. [DOI] [PubMed] [Google Scholar]
- 23.Diggle CP, et al. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol Genomics. 2010;42A:235–243. doi: 10.1152/physiolgenomics.00128.2010. [DOI] [PubMed] [Google Scholar]
- 24.Jürgens H, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 25.Van den Berghe G. Fructose: Metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]
- 26.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 27.Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci USA. 2008;105:16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane MD, Cha SH. Effect of glucose and fructose on food intake via malonyl-CoA signaling in the brain. Biochem Biophys Res Commun. 2009;382:1–5. doi: 10.1016/j.bbrc.2009.02.145. [DOI] [PubMed] [Google Scholar]
- 29.Rumessen JJ, Gudmand-Høyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–1168. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ushijima K, Riby JE, Fujisawa T, Kretchmer N. Absorption of fructose by isolated small intestine of rats is via a specific saturable carrier in the absence of glucose and by the disaccharidase-related transport system in the presence of glucose. J Nutr. 1995;125:2156–2164. doi: 10.1093/jn/125.8.2156. [DOI] [PubMed] [Google Scholar]
- 31.Burant CF, Saxena M. Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. Am J Physiol. 1994;267:G71–G79. doi: 10.1152/ajpgi.1994.267.1.G71. [DOI] [PubMed] [Google Scholar]
- 32.Korieh A, Crouzoulon G. Dietary regulation of fructose metabolism in the intestine and in the liver of the rat. Duration of the effects of a high fructose diet after the return to the standard diet. Arch Int Physiol Biochim Biophys. 1991;99:455–460. [PubMed] [Google Scholar]
- 33.Hallfrisch J, Ellwood K, Michaelis OE, 4th, Reiser S, Prather ES. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr. 1986;5:61–68. doi: 10.1080/07315724.1986.10720113. [DOI] [PubMed] [Google Scholar]
- 34.Hallfrisch J, et al. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr. 1983;113:1819–1826. doi: 10.1093/jn/113.9.1819. [DOI] [PubMed] [Google Scholar]
- 35.Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr. 1983;37:740–748. doi: 10.1093/ajcn/37.5.740. [DOI] [PubMed] [Google Scholar]
- 36.Lê KA, et al. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 37.Ngo Sock ET, et al. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr. 2010;103:939–943. doi: 10.1017/S0007114509992819. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RJ, Andrews P, Benner SA, Oliver W. Theodore E. Woodward award. The evolution of obesity: Insights from the mid-Miocene. Trans Am Clin Climatol Assoc. 2010;121:295–305. discussion 305–308. [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson RJ, Andrews P. Fructose, uricase, and the back-to-Africa hypothesis. Evol Anthropol. 2010;19:250–257. [Google Scholar]
- 40.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol Cell Biochem. 2002;241:107–114. doi: 10.1023/a:1020835229591. [DOI] [PubMed] [Google Scholar]
- 41.Lanaspa MA, Tapia E, Soto V, Sautin Y, Sánchez-Lozada LG. Uric acid and fructose: Potential biological mechanisms. Semin Nephrol. 2011;31:426–432. doi: 10.1016/j.semnephrol.2011.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.