Abstract
Noonan syndrome (NS), a genetic disease caused in half of cases by activating mutations of the tyrosine phosphatase SHP2 (PTPN11), is characterized by congenital cardiopathies, facial dysmorphic features, and short stature. How mutated SHP2 induces growth retardation remains poorly understood. We report here that early postnatal growth delay is associated with low levels of insulin-like growth factor 1 (IGF-1) in a mouse model of NS expressing the D61G mutant of SHP2. Conversely, inhibition of SHP2 expression in growth hormone (GH)-responsive cell lines results in increased IGF-1 release upon GH stimulation. SHP2-deficient cells display decreased ERK1/2 phosphorylation and rat sarcoma (RAS) activation in response to GH, whereas expression of NS-associated SHP2 mutants results in ERK1/2 hyperactivation in vitro and in vivo. RAS/ERK1/2 inhibition in SHP2-deficient cells correlates with impaired dephosphorylation of the adaptor Grb2-associated binder-1 (GAB1) on its RAS GTPase-activating protein (RASGAP) binding sites and is rescued by interfering with RASGAP recruitment or function. We demonstrate that inhibition of ERK1/2 activation results in an increase of IGF-1 levels in vitro and in vivo, which is associated with significant growth improvement in NS mice. In conclusion, NS-causing SHP2 mutants inhibit GH-induced IGF-1 release through RAS/ERK1/2 hyperactivation, a mechanism that could contribute to growth retardation. This finding suggests that, in addition to its previously shown beneficial effect on NS-linked cardiac and craniofacial defects, RAS/ERK1/2 modulation could also alleviate the short stature phenotype in NS caused by PTPN11 mutations.
Keywords: growth hormone insensitivity, signaling
Noonan syndrome [NS; Mendelian inheritance in man (MIM) no. 163950] is one of the most common autosomal dominant developmental disorders and characterized by heart defects, facial dysmorphism, and short stature (1). These distinctive traits are shared, with variable severity, with related diseases—notably Costello syndrome, cardiofaciocutaneous syndrome, LEOPARD syndrome, and neurofibromatosis type I. Collectively, this group of disorders is now termed RASopathies, in view of the identification of causative mutations in several genes encoding components of the RAS/MAPK pathway (2, 3).
In the case of NS, half of the patients carry a mutation in the PTPN11 gene, encoding the tyrosine phosphatase SHP2 (4, 5). SHP2 is a ubiquitous protein recruited downstream of many tyrosine kinase-dependent receptors that, once activated, dephosphorylates phosphorylated tyrosines (6). The best-defined function of SHP2 is its positive role in the activation of the RAS/MAPK ERK1/2 pathway [for review, see Dance et al. (7)]. In addition, SHP2 reportedly regulates other key signaling pathways, including the phosphoinositide 3-kinase (PI3K) pathway, the Src family kinase (SFK), the target of rapamycin (TOR) kinase, or the Janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5) module. SHP2 thus plays a fundamental role in organism development and homeostasis, as exemplified by the severe phenotypes of total or tissue specific knockouts of the Ptpn11 gene (8–13).
Given the pleiotropic roles of SHP2, elucidating how its mutations can alter its function(s) is a key question to better understand NS pathogenesis. Biochemical studies have revealed that NS-causing PTPN11 mutations result in hyperactivation of SHP2 catalytic activity by disrupting the inhibitory interaction between its catalytic and SH2 domains (14). Moreover, functional studies have shown that expression of NS-causing mutants can induce ERK1/2 hyperactivation in vitro and in vivo (14–16). More interestingly, recent studies using mouse models for different NS-causing Ptpn11 mutations have demonstrated that pharmacological or genetic inhibition of ERK can alleviate NS-associated cardiac and craniofacial defects (17–20).
In contrast to the above-cited clinical features, the origin of growth retardation in NS is poorly understood, although short stature, reported in more than 70% of affected patients, is one of the main clinical symptoms of NS (1). One proposal is that growth defects could be due to partial growth hormone (GH) insensitivity at the postreceptor level (21, 22). Indeed, most NS patients, notably PTPN11-mutated cases, display normal-to-elevated GH serum levels associated with low serum levels of insulin-like growth factor 1 (IGF-1), the biological mediator of GH acting on different target tissues, including the growth plate. However, whether IGF-1–regulating signaling pathways downstream of the GH receptor (GH-R) are impaired in NS has not been established.
By using a mouse model of NS expressing the D61G mutant of SHP2 (Ptpn11D61G/+) and different cellular approaches, we explored whether and how NS-causing SHP2 mutations alter GH response. We observed that growth retardation in young NS mice is associated with low IGF-1 levels and that NS mutants induce ERK1/2 hyperactivation upon GH stimulation in vitro and in vivo. Moreover, we provide evidence that SHP2 negatively regulates GH-induced IGF-1 release through a RAS/ERK-dependent mechanism. Consistently, pharmacological MAPK/ERK kinase (MEK) inhibition at least partially rescued IGF-1 deficiency in NS mice, resulting in a significant improvement of growth.
Results
Postnatal Growth Retardation in NS Mice Is Associated with Reduced IGF-1 Serum Levels.
Male Ptpn11D61G/+ mice display decreased body length and weight by 3 wk of age, which continues for at least 16 wk after birth (16). To further document this phenotype, we measured the anal–nasal lengths of male and female Ptpn11D61G/+ (NS) mice and compared them with their Ptpn11+/+ (WT) littermates from birth to 6 wk of age. As shown in Fig. 1 A and B, whereas growth parameters in NS and WT mice were similar at birth and at 1 and 2 wk of age, a significant reduction of body length and weight was then observed by 3 wk of age for NS mice, which remained stable until 6 wk of age, in agreement with a previous report (16). These results show that male and female NS mice display early postnatal growth retardation.
Fig. 1.
Growth retardation in NS mice correlates with lower IGF-1 level. (A and B) NS and WT mice were measured (A) and weighted (B) at indicated ages. (C) Blood IGF-1 levels of NS and WT mice were determined by ELISA at the indicated ages. Individual values and mean ± SEM are shown. Statistical significance was assessed by the ANOVA plus Bonferroni posttest. (D) Sera from 3-wk-old NS and WT mice were analyzed for IGF-1, IGF-BP3, insulin, and cortisol levels by ELISA. No. of animals (WT/NS): week 1: 18/10; week 2: 13/10; week 3: 23/14; week 4: 21/15; week 5: 15/13; week 6: 17/10.
Because postnatal growth is primarily controlled by the GH/IGF-1 axis, we monitored IGF-1 serum levels in growing NS and WT mice. As shown in Fig. 1C, IGF-1 levels transiently raised during the first weeks of age of WT animals, with a peak at 4 wk of age. Interestingly, though IGF-1 levels were similar in 1- and 2-wk-old WT and NS animals, they became significantly lower in NS mice during the third, fourth, and fifth week. IGF-1 levels then normalized in 6-wk-old mice, confirming previous results (16) (Fig. 1C). Therefore, this finding suggests that the transient raise in IGF-1 levels is impaired in NS mice, and that their growth retardation is associated with IGF-1 deficiency during the early postweaning growth period.
Moreover, serum levels of IGF binding protein-3 (IGF-BP3), but not those of insulin or cortisol, were reduced significantly in 3-wk-old NS mice compared with their WT littermates (Fig. 1D). Because IGF-1 and IGF-BP3 are major GH target genes, these data suggest that GH response is altered in NS animals.
SHP2 Negatively Regulates GH-Induced IGF-1 Production.
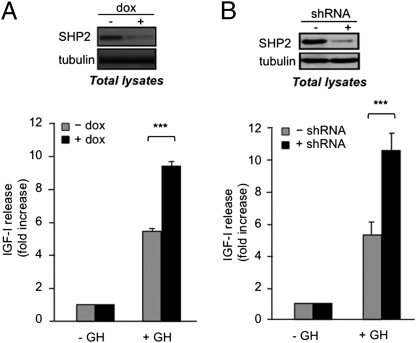
We next investigated the role of SHP2 in GH-induced IGF-1 production. We took advantage of the GH-responsive 3T3F442A cell line, in which we inducibly interfered with SHP2 function using a doxycycline-dependent shRNA (3T3F442A ΔSHP2ind; Fig. S1 and Fig. 2A, Upper). Interestingly, GH-induced IGF-1 secretion was significantly increased in SHP2-deficient cells (+dox) compared with control cells (−dox; Fig. 2A, Lower). To exclude a possible side effect of doxycycline, we compared doxycycline-treated 3T3F442A ΔSHP2ind cells (+shRNA) and 3T3F442A GFPind (−shRNA; Fig. S1C). In response to doxycycline, only the 3T3F442A ΔSHP2ind cells repressed SHP2 expression (Fig S1D and Fig. 2B, Upper). Notably, we again observed increased IGF-1 production in SHP2-deficient cells (Fig. 2B, Lower). Taken together, these results suggest that SHP2 negatively regulates GH-induced IGF-1 production in 3T3F442A cells.
Fig. 2.
SHP2 negatively regulates GH-induced IGF-1 expression. (A) Confluent, insulin-primed, 3T3F442A ΔSHP2ind cells were treated with doxycycline (+dox) or left untreated (−dox), serum deprived, and stimulated with GH for 24 h. (Upper) Aliquots of cells were processed for Western blot analysis as indicated. (Lower) IGF-1 concentrations in the media were measured by ELISA. (B) Same experiment as in A using doxycycline-treated 3T3F442A ΔSHP2ind cells (+shRNA) and 3T3F442A GFPind (−shRNA).
SHP2 Promotes GH-Evoked ERK1/2, but Not JAK2/STAT5 or PI3K/AKT Activation.
To understand how SHP2 participates in GH-induced IGF-1 production, we then monitored the activation of the main signaling pathways mobilized downstream of the GH-R—namely, the JAK2/STAT5, PI3K/AKT, and RAS/ERK1/2 pathways in untreated and doxycycline-treated 3T3F442A ΔSHP2ind cells. SHP2 down-regulation did not affect the expression of the main components of these pathways used as readouts (Fig S1D). Neither GH-induced STAT5 nor AKT phosphorylation was modified significantly in the absence of SHP2 (Fig. 3A). In contrast, phosphorylation of ERK1/2 was inhibited by >50% in SHP2-deficient cells (Fig. 3 A and B), suggesting that SHP2 plays a positive role in GH-induced ERK1/2 activation.
Fig. 3.
SHP2 promotes GH-evoked ERK1/2 activation, but does not influence the JAK2/STAT5 or PI3K/Akt pathways. (A and B) 3T3F442A ΔSHP2ind cells were treated with doxycycline (+dox) or left untreated (−dox), serum-starved, and then stimulated with GH during the indicated times, lysed and processed for Western blot analysis. (C and D) HEKGHR-GFP or HEKGHR-shRNA cells were processed as in A. (E and F) 3T3F442A cells were infected with adenoviruses encoding GFP, His-tagged SHP2-WT or SHP2-C459G, and then processed as in A. (B, D, and F) Quantifications of P-ERK1/2 Western blots from experiments described in A, C, and E.
To ensure that these effects were not limited to 3T3F442A cells, we carried out similar experiments in HEK293 cells stably expressing GH-R (HEKGHR) and expressing the shRNA targeting SHP2 expression (HEKGHR-sh) or GFP (HEKGHR-GFP) as a control. SHP2 expression was strongly reduced in HEKGHR-sh cells (Fig. 3C and Fig. S2). Again, GH-induced STAT5 and AKT phosphorylation were insensitive to SHP2 down-regulation, whereas GH-stimulated ERK1/2 phosphorylation was strongly repressed (Fig. 3 C and D).
To confirm this result by another approach, we used a catalytically inactive mutant of SHP2 (C459G), which displays a dominant negative activity on SHP2 function (23). As shown in Fig. 3E, expression of the C459G mutant of SHP2 did not alter the phosphorylation of STAT5 or AKT, but strongly repressed ERK1/2 phosphorylation (Fig. 3 E and F). These data further confirm that SHP2 positively regulates GH-evoked ERK activation, possibly through its catalytic activity.
SHP2 Participates in GH-Induced RAS/ERK1/2 Activation by Dephosphorylating RAS GTPase-Activating Protein Binding Sites on Grb2-Associated Binder-1.
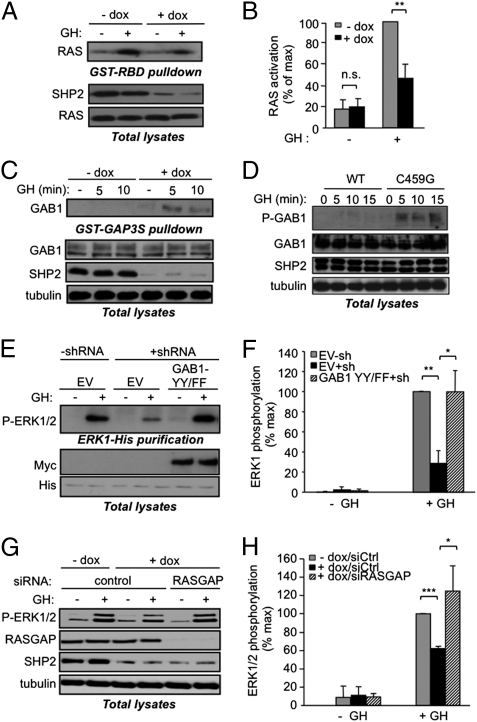
We next evaluated how SHP2 promotes ERK1/2 activation in response to GH. First, we compared GH-evoked RAS activation in untreated and doxycycline-treated 3T3F442A ΔSHP2ind cells using a pull-down assay for activated RAS [GST-Ras binding domain (RBD)]. As shown in Fig. 4 A and B, GH-induced RAS activation was reduced by ∼50% in SHP2-deficient cells, indicating that SHP2 acts upstream of RAS in GH-R signaling.
Fig. 4.
SHP2 controls GH-induced RAS activation by inhibiting RASGAP recruitment. (A and B) 3T3F442A ΔSHP2ind cells were processed as in Fig. 3A and GH stimulated for 10 min. Activated RAS was isolated from cleared lysates using a GST-RBD fusion protein. An anti-RAS Western blot was performed on the GST-RBD pull-downs and on lysates. (C) Cells were treated as in A, except that a GST-RASGAP fusion protein was used and an anti-GAB1 Western blot was performed. (D) 3T3F442A cells were infected with adenoviruses encoding SHP2-WT or SHP2-C459G, stimulated for the indicated times, lysed, and processed for indicated Western blotting (P-GAB1: phospho Y307). (E and F) HEKGHR (−shRNA) and HEKGHR-sh (+shRNA) cells were transfected with ERK1-His and empty vector (EV) as a control or a Myc-tagged GAB1 Y307F/Y317F mutant (GAB1-YYFF), serum starved, and stimulated with GH for 10 min; indicated Western blots were performed on purified ERK1-His (E Upper) and on lysate aliquots (E Lower). (G and H) 3T3F442A ΔSHP2ind were treated with doxycycline (+dox) or left untreated (−dox), transfected with control or anti-RASGAP siRNA, serum starved, then GH stimulated for 10 min and processed for Western blot analysis. (B, F, and H) Quantification of immunoblots described in A, E, and G.
It has been proposed that SHP2 can promote RAS activation by dephosphorylating specific residues involved in the recruitment of the RAS inhibitor RAS GTPase-activating protein (RASGAP), notably on the docking protein Grb2-associated binder-1 (GAB1) (24). We thus assayed the ability of GAB1 to interact with a GST fusion protein containing the SH2 domains of RASGAP in control and SHP2-deficient cells. GAB1 was barely detected in RASGAP pull-downs performed from GH-stimulated control cell lysates (−dox), but was readily precipitated from SHP2-deficient cell lysates (+dox; Fig. 4C). Moreover, GAB1 phosphorylation on one of its RASGAP binding sites (Y307) was more prominent in cells expressing the C459G mutant of SHP2 (Fig. 4D). These data suggest that, upon GH stimulation, SHP2 dephosphorylates the RASGAP binding sites on GAB1, thereby favoring RAS activation. To assess this model, we took advantage of a mutant of GAB1 defective for RASGAP binding (GAB1 Y307F/Y317F). This mutant, when overexpressed, is recruited in lieu of endogenous GAB1, but is constitutively dephosphorylated on its RASGAP binding sites, so that RASGAP cannot be recruited to the vicinity of RAS (24). Interestingly, in HEKGHR-sh coexpressing the GAB1 Y307F/Y317F mutant and a His-tagged ERK1, ERK1 phosphorylation was increased to a level similar to that of control cells (Fig. 4 E and F). We then tested whether RASGAP inhibition could rescue ERK1/2 activation in SHP2-deficient cells. As shown in Fig. 4 G and H, siRNA-mediated RASGAP depletion in SHP2-deficient cells increased ERK1/2 phosphorylation to an extent similar to that observed in control cells. Together, these data suggest that ERK1/2 inhibition in SHP2-deficient cells was due to sustained RASGAP recruitment, and that SHP2 participates in GH-induced RAS/ERK1/2 activation by dephosphorylating GAB1 on its RASGAP binding sites.
NS Mutants Enhance GH-Induced ERK1/2 Activation in Vitro and in Vivo.
To assess the impact of NS mutations in GH signaling, we then studied the effects of two mutants: the D61del variant, a strong mutant which is closely related to the D61G allele borne by the NS mouse, and the N308D mutant, a weak allele frequently found in NS patients. Transient overexpression of either mutant in HEKGHR caused enhanced phosphorylation of a coexpressed His-tagged ERK1 compared with control cells (Fig. 5 A and B). Likewise, overexpression of the N308D or the D61del mutant in 3T3F442A cells via adenoviral infection resulted in increased GH-induced ERK1/2 phosphorylation (Fig. 5 C and D). Then, we mimicked pathophysiological, heterozygous conditions (i.e., 50% SHP2 WT/50% SHP2 NS), by expressing shRNA-resistant forms of the D61del or N308D mutants in 3T3F442A cells while decreasing endogenous SHP2 expression (Fig S3). Interestingly, GH-induced ERK1/2 phosphorylation was significantly enhanced in doxycycline-treated cells compared with controls (Fig. 5 E and F). Hence, expression of two different NS-associated mutants of SHP2, even in a functional heterozygosity condition was sufficient to sustain ERK1/2 activation under GH stimulation. Noticeably, in the various experiments, there was no significant difference between the effects of both mutants, despite variable tendencies depending on the type of experiment or cell lines.
Fig. 5.
NS-associated SHP2 mutants enhance GH-induced ERK1/2 activation. (A and B) HEKGHR cells were cotransfected with ERK1-His and His-tagged SHP2 WT, D61del, or N308D variants, or the empty vector (−), and then processed as in Fig. 4E. (C and D) 3T3F442A cells were infected with adenoviruses encoding GFP, SHP2-N308D, or SHP2-D61del, serum starved, then GH stimulated for 10 min and processed for indicated Western blot analysis. (E and F) Untreated (−dox) or 0.005 μg/mL doxycycline-treated (+dox) 3T3F442A ΔSHP2/N308D and 3T3F442A ΔSHP2/D61del cells were GH stimulated for 10 min, lysed, and processed for indicated Western blot analysis. (G and H) Fasted WT and NS mice were injected i.p. with 8 μg/g GH (+) or PBS (−) for 10 min, and then euthanized. Equal amounts of protein from liver extracts were processed for Western blot analysis. (B, D, F, and H) Quantification of immunoblots described in A, C, E, and G. Only significant differences vs. control are indicated.
We then aimed at evaluating whether GH-evoked ERK1/2 phosphorylation was also increased in NS mice. For this, NS animals and their WT littermates were injected for 10 min with GH or vehicle, and phosphorylation of ERK1/2, STAT5, and AKT were measured in the liver, a major GH target organ for IGF-1 production. Upon GH treatment, ERK1/2 phosphorylation was strongly increased in the liver of NS animals compared with their WT littermates, whereas STAT5 and AKT were phosphorylated to the same extent in animals from both genotypes (Fig. 5 G and H). From these results, we concluded that NS-driving SHP2 mutants up-regulate GH-induced ERK1/2 activation in vitro and in vivo.
MEK Inhibition at Least Partially Restores IGF-1 Levels and Growth in NS Mice.
We next sought a possible connection between the positive function of SHP2 in GH-induced ERK1/2 activation and its negative role in IGF-1 production by asking whether inhibition of ERK1/2 activation affected GH-induced IGF-1 release. Treatment with 50–500 nM U0126, a selective inhibitor of MEK, induced partial ERK1/2 inhibition, similar to that seen in SHP2-deficient cells (Fig. 6A). Interestingly, U0126 at these doses also enhanced GH-induced IGF-1 production to an extent similar to that caused by SHP2 deficiency (Fig. 6B). Thus, partial ERK1/2 inhibition could stimulate IGF-1 release upon GH treatment, suggesting that SHP2 may negatively regulate GH-induced IGF-1 production through activation of the RAS/ERK1/2 pathway.
Fig. 6.
MEK inhibition partially restores IGF-1 level in vitro and in vivo and increases growth of NS mice. (A) 3T3F442A cells were serum starved overnight, then treated with the indicated doses of U0126, stimulated with GH for 10 min, lysed, and processed for indicated Western blot analysis. (B) Confluent, insulin-primed 3T3F442A cells were treated with the indicated doses of U0126, stimulated with GH for 24 h, and then IGF-1 concentrations were measured by ELISA. (C–E) At 5 d after delivery, nursing females were injected i.p. daily for 20 d with U0126 or vehicle, and then blood IGF-1 levels in the pups were determined by ELISA (C), as was their body length (D) and weight (E). Four litters were used, with equivalent animal numbers and body weights at birth (no. of animals: WT/PBS: 5; WT/U0126: 5; NS/PBS: 9; NS/U0126: 8).
Therefore, we hypothesized that decreased IGF-1 levels in NS mice might result from ERK1/2 hyperactivation. To test this possibility, we treated cohorts of young NS and WT mice with U0126, by injecting U0126 or vehicle as a control, to nursing females during the first 3 wk of life of their litters. At the end of the treatment period, IGF-1 levels of the young mice were quantified. Importantly, U0126 treatment significantly increased IGF-1 blood levels in NS mice, suggesting that ERK1/2 inhibition could enhance IGF-1 production in NS mice (Fig. 6C). Moreover, U0126-treated NS mice displayed a significant increase in body length compared with untreated NS mice (Fig. 6D), whereas body weight was not altered (Fig. 6E). However, U0126-treated NS animals did not catch up in IGF-1 levels or in size to WT animals. Together, these results suggest that ERK1/2 inhibition can, at least partially, rescue growth retardation in NS mice, most likely by restoring appropriate IGF-1 production.
Discussion
In this study, we sought a molecular mechanism that could explain how NS-causing SHP2 mutants induce growth retardation, a major feature of NS. First, we demonstrated that short stature in young NS mice was acquired shortly after birth, in accordance with clinical data that reveal normal growth parameters at birth in human (25). Second, we found that growth delay was associated with low IGF-1 levels during the early postweaning growth phase. These findings suggest that IGF-1 production is impaired in growing NS animals and could contribute to growth retardation in NS, in agreement with clinical data showing that NS patients, notably those carrying a PTPN11 mutation, display low IGF-1 levels (21, 22).
Of note, IGF-1 levels are only reduced by 40% in NS mice, whereas studies of several models of Igf-1 gene disruption in liver, the main source of IGF-1 in blood, have shown that even a 75% reduction of IGF-1 levels does not cause growth retardation. This suggests that autocrine/paracrine IGF-1 production (and GH response) may be altered, notably at the bone level, and contribute to impaired growth in NS. Moreover, dysregulation of IGF-1 cofactors [e.g., acid labile subunit (ALS) or IGF-BP3] in NS, resulting in a more decreased IGF-1 bioactivity, could also participate in this apparent discrepancy, because double knockout mice with total Als and liver-specific Igf-1 inactivation are 20% shorter than control animals (26). Interestingly, we observed that IGF-BP3 levels were reduced in NS animals, and it has been shown that NS patients display low levels of ALS (22). Finally, our data do not exclude that other processes, independent of the GH/IGF-1 axis, could be impaired in NS mice and result in growth retardation. Indeed, skeletal and growth abnormalities have been reported in inducible and neuronal-specific SHP2 knockout models (27, 28). Further studies will be necessary to elucidate the part of those different mechanisms in NS-associated short stature.
Our finding of decreased IGF-1 levels in Ptpn11-mutated mice suggests an involvement of SHP2 in GH signaling. Indeed, SHP2 down-regulation in 3T3F442A cells caused sustained GH-induced IGF-1 release. Although we failed to monitor GH-induced IGF-1 release in vivo, our combined results suggest that SHP2 may negatively regulate the production of IGF-1 under GH control. This regulation might be restricted to the growing period, because NS mice show a normalization of IGF-1 levels after 6 wk, and because liver-specific deletion of SHP2 did not result in an increase of hepatic Igf-1 mRNA levels in older mice (8).
Next, we investigated how SHP2 regulates GH-induced IGF-1 production, by monitoring the activation of signaling pathways involved in IGF-1 synthesis. It has been reported that SHP2 can negatively regulate the JAK2/STAT5 pathway or the PI3K/AKT pathway (29, 30). However, we did not observe any effect of SHP2 modulation on GH-evoked STAT5 and AKT phosphorylation, although we cannot exclude that our experimental design does not allow monitoring modulation of STAT5 or AKT phosphorylation upon SHP2 inhibition. Moreover, other phosphatases, notably PTP1B, might be more critically involved in the hepatic regulation of the JAK2/STAT5 module under GH stimulation (31). In contrast, we found that SHP2 participates in GH-induced RAS/ERK1/2 activation, and that NS-driving SHP2 mutants hyperactivate this signaling pathway in response to GH in vitro and in vivo, suggesting that dysregulation of GH-induced ERK1/2 activation could contribute to growth retardation in this syndrome.
We next assessed whether the SHP2-mediated IGF-1 inhibition was dependent on ERK1/2 activation. We demonstrated that partial ERK1/2 inhibition induced an increase in GH-stimulated IGF-1 production in vitro and in vivo. Moreover, we observed that U0126 treatment increased linear growth of NS mice. These data suggest that normalization of NS mutant-induced ERK1/2 hyperactivation can improve their IGF-1 deficiency, which could contribute, at least in part, to the alleviation of the NS-linked growth phenotype. Of note, given the partial effect of U0126 treatment on IGF-1 levels, further studies will be necessary to determine if IGF-1 deficiency in NS is only partially due to ERK1/2 hyperactivation or if the pharmacokinetics of the U0126, which displays a short half-life in vivo (∼2 h), should be improved.
Finally, our results provide direct evidence that growth retardation in PTPN11-mutated NS may be due to partial GH insensitivity (21, 22), which may have important therapeutic fallouts. So far, only recombinant human GH (rhGH) treatment has been proposed to increase stature in NS, but its efficiency is still debated (32, 33). Our data suggesting that NS-associated PTPN11 mutations cause partial GH insensitivity could explain the moderate efficacy of rhGH treatment, and emphasizes the need for alternative therapies. First, in view of the beneficial effects of rhIGF-1 treatment in patients with complete GH insensitivity, there may be a rationale for rhIGF-1 therapy in PTPN11-mutated NS patients (34). Second, our results suggest that ERK1/2 inhibition could improve growth velocity in NS, possibly by restoring IGF-1 levels. Interestingly, several reports have demonstrated that treatment with MEK inhibitors can rescue growth defect in three different NS models (Ptpn11Q79R/+, Raf1L613V/+, and Sos1E846K/E846K) and in several models of RASopathies, and can also alleviate other clinical traits, notably cardiac and craniofacial defects (17–20, 35, 36). This, combined with our own study, provides further insights into the fact that RAS/ERK1/2 inhibition could be a potent strategy to treat RASopathies, including NS. Additional studies will be required to precisely assess the potency and safety of such treatments.
Materials and Methods
siRNA and Plasmid Transient Transfection.
For siRNA transfections, 40% confluent 3T3F442A cells were incubated overnight with a transfection mix containing 1 mL of Opti-MEM, 5 μL of RNAiMAX (Invitrogen), and 20 nM of the indicated siRNA. For plasmid transfections, subconfluent HEKGHR cells were incubated overnight with a mixture containing 6 μL of FuGene6 reagent (Roche) and 2 μg of total DNA, according to the manufacturer's instructions.
In Vivo Experiments.
For GH-evoked signaling in vivo, animals were injected i.p. with GH (8 μg/g body weight) or PBS for 10 min, then euthanized. Livers were excised and samples were homogenized using a Precellys 24 automated biological sample lyser. For U0126 treatment, U0126 (5 mg/kg per day) or vehicle [PBS, 40% (vol/vol) DMSO] was injected i.p. into nursing females daily for 20 d. All procedures were performed following the guidelines of the Midi-Pyrénées Ethics Committee on Animal Experimentation and the French Ministry of Agriculture license.
IGF-1 Measurements.
Confluent 3T3F442A cells were grown for 7 d in DMEM supplemented with 10% (vol/vol) FBS and 50 nM insulin, and treated with 0.05 μg/mL doxycycline for the last 5 d or left untreated. Cells were then serum starved overnight, treated with U0126 or left untreated, and stimulated with 400 ng/mL GH when indicated for 24 h. Conditioned media were collected and processed for IGF-1 ELISAs. To measure IGF-1 levels in blood, blood samples collected from the retro-orbital sinus of isoflurane-anesthetized animals were allowed to clot overnight at 4 °C; they were then centrifuged, and plasma was harvested and processed for IGF-1 ELISAs.
Statistics.
In vitro data are representative from at least three independent experiments. Western blots were quantified using ImageJ software. Results are expressed as mean ± SEM and are compared using paired Student t test, unless otherwise indicated. P < 0.05 was considered significant [nonsignificant (n. s.), P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001].
Supplementary Material
Acknowledgments
We thank C. Fourreau, O. Farrugia, H. Lulka, C. Nevoit, M. Toulouse, and Y. Barreira for help with animal handling; D. Daviaud, N.Malet, C. Roland, and C. Vigouroux for technical help; and J. Ragab, C. Racaud, E. Gouze, F. Conte, J.-P. Combier, S. Caula, and J.-S. Saulnier-Blache for helpful discussions. This work was supported in part by Agence Nationale de la Recherche, ERA-Net E-Rare program (NSEuroNet), Association pour la Recherche sur le Cancer, Institut National du Cancer, Ligue Nationale Contre le Cancer grants, Direction de l'Hospitalisation et de l'Organisation des Soins Institut National de la Santé et de la Recherche Médicale and Pfizer Grant 07.8RN.062, National Institutes of Health Grant R37CA49152, Canadian Institutes of Health Research Grant 106526, Ontario Ministry of Health and Long Term Care, and the Princess Margaret Hospital Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119803109/-/DCSupplemental.
References
- 1.Romano AA, et al. Noonan syndrome: Clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126:746–759. doi: 10.1542/peds.2009-3207. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab. 2011;25:161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tidyman WE, Rauen KA. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tartaglia M, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 5.Edouard T, et al. How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell Mol Life Sci. 2007;64:1585–1590. doi: 10.1007/s00018-007-6509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neel BG, Gu H, Pao L. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 7.Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008;20:453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, et al. Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J Biol Chem. 2010;285:39750–39758. doi: 10.1074/jbc.M110.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxton TM, et al. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat Genet. 2000;24:420–423. doi: 10.1038/74279. [DOI] [PubMed] [Google Scholar]
- 10.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke Y, et al. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J Biol Chem. 2006;281:34374–34380. doi: 10.1074/jbc.M607325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontaridis MI, et al. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008;117:1423–1435. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jopling C, van Geemen D, den Hertog J. Shp2 knockdown and Noonan/LEOPARD mutant Shp2-induced gastrulation defects. PLoS Genet. 2007;3:e225. doi: 10.1371/journal.pgen.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keilhack H, David FS, McGregor M, Cantley LC, Neel BG. Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J Biol Chem. 2005;280:30984–30993. doi: 10.1074/jbc.M504699200. [DOI] [PubMed] [Google Scholar]
- 15.Fragale A, Tartaglia M, Wu J, Gelb BD. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat. 2004;23:267–277. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- 16.Araki T, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, et al. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araki T, et al. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc Natl Acad Sci USA. 2009;106:4736–4741. doi: 10.1073/pnas.0810053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krenz M, et al. Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. Proc Natl Acad Sci USA. 2008;105:18930–18935. doi: 10.1073/pnas.0806556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura T, Gulick J, Pratt R, Robbins J. Noonan syndrome is associated with enhanced pERK activity, the repression of which can prevent craniofacial malformations. Proc Natl Acad Sci USA. 2009;106:15436–15441. doi: 10.1073/pnas.0903302106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder G, Neuer K, Ranke MB, Wittekindt NE. PTPN11 mutations are associated with mild growth hormone resistance in individuals with Noonan syndrome. J Clin Endocrinol Metab. 2005;90:5377–5381. doi: 10.1210/jc.2005-0995. [DOI] [PubMed] [Google Scholar]
- 22.Limal JM, et al. Noonan syndrome: Relationships between genotype, growth, and growth factors. J Clin Endocrinol Metab. 2006;91:300–306. doi: 10.1210/jc.2005-0983. [DOI] [PubMed] [Google Scholar]
- 23.Edouard T, et al. Functional effects of PTPN11 (SHP2) mutations causing LEOPARD syndrome on epidermal growth factor-induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3beta signaling. Mol Cell Biol. 2010;30:2498–2507. doi: 10.1128/MCB.00646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagner A, et al. A novel role for Gab1 and SHP2 in EGF-induced Ras activation. J Biol Chem. 2005;280:5350–5360. doi: 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- 25.Ranke MB, et al. Noonan syndrome: Growth and clinical manifestations in 144 cases. Eur J Pediatr. 1988;148:220–227. doi: 10.1007/BF00441408. [DOI] [PubMed] [Google Scholar]
- 26.Yakar S, Wu Y, Setser J, Rosen CJ. The role of circulating IGF-1: Lessons from human and animal models. Endocrine. 2002;19:239–248. doi: 10.1385/ENDO:19:3:239. [DOI] [PubMed] [Google Scholar]
- 27.Bauler TJ, et al. 2011. Development of severe skeletal defects in induced SHP-2-deficient adult mice: A model of skeletal malformation in humans with SHP-2 mutations Dis Models Mech 4:228–239.
- 28.Banno R, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Qu CK. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SQ, et al. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu F, et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–3762. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raaijmakers R, et al. Response to growth hormone treatment and final height in Noonan syndrome in a large cohort of patients in the KIGS database. J Pediatr Endocrinol Metab. 2008;21:267–273. doi: 10.1515/jpem.2008.21.3.267. [DOI] [PubMed] [Google Scholar]
- 33.Romano AA, et al. Growth response, near-adult height, and patterns of growth and puberty in patients with Noonan syndrome treated with growth hormone. J Clin Endocrinol Metab. 2009;94:2338–2344. doi: 10.1210/jc.2008-2094. [DOI] [PubMed] [Google Scholar]
- 34.Chernausek SD, Backeljauw PF, Frane J, Kuntze J, Underwood LE. GH Insensitivity Syndrome Collaborative Group Long-term treatment with recombinant insulin-like growth factor (IGF)-1 in children with severe IGF-I deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab. 2007;92:902–910. doi: 10.1210/jc.2006-1610. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J Clin Invest. 2011;121:1009–1025. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen PC, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome–associated Sos1 mutation. J Clin Invest. 2010;120:4353–4365. doi: 10.1172/JCI43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.