Eukaryotic cells critically depend on the formation, budding, and scission of membrane-bounded vesicles for many key processes: internalization of cell surface receptors, delivery of cargo proteins to multivesicular bodies (MVBs) and lysosomes for degradation, transport of newly synthesized proteins between intracellular organelles and their delivery to the plasma membrane, and release of microvesicles that function as vehicles for intercellular communication. Although distinct families of proteins and multiprotein complexes have been described that mediate these diverse events in the cell, the field of membrane trafficking remains an active area of investigation. In PNAS, the work by Nabhan et al. (1) describes a role for the arrestin domain-containing protein ARRDC1 in the generation of microvesicles—termed ARRDC1-mediated microvesicles (ARMMs)—that bud directly from the plasma membrane (PM).
Arrestins are well-established regulators of cell signaling pathways, and they act to recruit factors that promote the endocytosis of PM receptors (2). Arrestins can interact directly with ubiquitin ligases, thereby stimulating the ubiquitination of PM receptors and in some cases, the arrestin itself. For example, the work by Lin et al. (3) characterized a family of arrestin-related proteins that recruits the yeast Nedd4-like ubiquitin ligase, Rsp5, to down-regulate the expression of amino acid transporters. Rsp5 was recruited by a PPxY-related motif in the arrestin proteins (3).
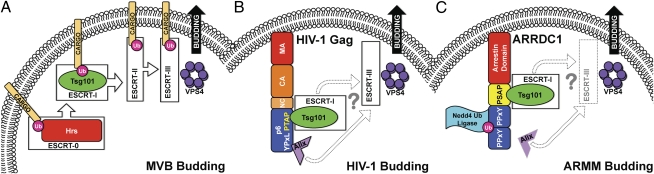
Several recent studies have uncovered an intriguing connection between arrestin domain-containing proteins and components of the endosomal sorting complex required for transport (ESCRT) machinery (3–6). This machinery, which is composed of four multiprotein complexes (ESCRT-0 to ESCRT-III) (Fig. 1A), is required for the delivery of ubiquitinated cargo into vesicles that bud into late endosomes to form MVBs (7). ESCRT machinery also plays a key role in the budding of many enveloped viruses, including HIV-1 and other retroviruses (8). Recruitment of ESCRT machinery by retroviral Gag proteins (the viral structural proteins that drive particle assembly and release) requires the presence of so-called late domains in Gag; PTAP, PPxY, and YPxL late domains recruit the Tsg101 subunit of ESCRT-I, Nedd4 family ubiquitin ligases, and the ESCRT-associated factor Alix, respectively (9). A direct interaction was previously identified between a yeast arrestin-related protein, Rim8, and the yeast homolog of Tsg101, Vps23 (4). This interaction was mediated by binding between monoubiquitinated Rim8 and the ubiquitin E2 variant (UEV) domain of Vps23. Rim8 ubiquitination was dependent on the ubiquitin ligase, Rsp5. Thus, Rim8 functions as an arrestin-related trafficking adaptor to recruit the ESCRT-I complex. Interactions between arrestin-related proteins and ESCRT components—specifically Tsg101 and Alix—have also been described in mammalian cells (6). These studies served as
Fig. 1.
Parallels between MVB, HIV-1, and ARMM budding. (A) Sorting of ubiquitinated (Ub) cargo into vesicles that bud into the MVB uses four evolutionarily conserved, multiprotein ESCRTs. The Hrs subunit of ESCRT-0 initiates the cascade on the early endosomal membrane. Hrs contains a PSAP motif that binds the UEV domain of Tsg101, thereby recruiting ESCRT-I. ESCRT-I subsequently interacts with ESCRT-II, which in turn, recruits ESCRT-III. The ESCRT-III complex acts in concert with the vacuolar protein sorting ATPase Vps4 to catalyze the final membrane scission event that releases the cargo-bound vesicle into the lumen of the MVB. (B) HIV-1 budding requires proper PM targeting mediated by the N-terminal matrix (MA) domain of HIV-1 Gag. Like vesicle budding into MVBs, HIV-1 budding also uses ESCRT machinery. The p6 domain of HIV-1 Gag contains two peptide motifs, PTAP and YPxL, that bind Tsg101 and Alix, respectively. HIV-1 budding requires ESCRT-III and Vps4; however, ESCRT-II is bypassed by an unknown mechanism. Hypothesized mechanisms of recruitment are denoted with light shading and question marks. Capsid (CA) and nucleocapsid (NC) domains of Gag are also labeled. (C) ARMM budding also requires ESCRT machinery, particularly ESCRT-I (Tsg101) and Vps4. The N-terminal arrestin domain of ARRDC1 directs PM targeting. Like Hrs and HIV-1 Gag, ARRDC1 contains a PSAP motif that is required for Tsg101 recruitment. The PPxY motifs of ARRDC1 interact with Nedd4 family ubiquitin ligases (e.g., WWP2). Alix may also be involved in ARMM budding, and given the requirement for Vps4, it is also likely that ESCRT-III plays an essential role.
Nabhan et al. observe that ARRDC1 could be transferred between cells in ARMMs.
an impetus for the investigation of other novel interactions between arrestin-related proteins and the ESCRT machinery.
To this end, the work by Nabhan et al. (1) uses the yeast two-hybrid approach to search for proteins that partner with the Tsg101 UEV domain. This analysis confirms the interaction between ARRDC1 and Tsg101, an interaction that mapped to a highly conserved PSAP motif in ARRDC1. Because, as mentioned above, an equivalent motif (PTAP) in HIV-1 Gag recruits Tsg101 (Fig. 1B), these observations hint at a functional parallel between HIV-1 Gag and ARRDC1. Extending this parallel, the work by Nabhan et al. (1) shows that ARRDC1 is indeed packaged into secreted microvesicles, suggesting that ARRDC1 might be driving microvesicle formation. Because HIV-1 budding takes place primarily at the PM, whereas the ESCRT machinery drives budding events at internal (MVB) membranes, a key question is whether ARRDC1-containing microvesicles are a product of budding events taking place at the PM or in MVBs. The work by Nabhan et al. (1) shows that tagged ARRDC1 is localized almost exclusively to the PM and that overexpression of ARRDC1 induces a relocalization of Tsg101 from the cytosol to the surface. Furthermore, an ARRDC1 mutant deficient in PM localization fails to produce ARMMs, and unlike MVB-derived microvesicles, ARMMs lack endosomal markers. These observations imply that ARRDC1-mediated budding events take place at the PM. Another important question is whether ESCRT machinery is required for ARMM release. The answer seems to be affirmative. First, mutation of the PSAP motif in ARRDC1 blocks Tsg101 recruitment to the PM and abrogates microvesicle production. Second, Tsg101 depletion reduces the release of ARRDC1-containing microvesicles. Finally, a dominant negative mutant of the Vps4 ATPase, which shuts down ESCRT-mediated membrane scission, strongly inhibits ARMM release.
The C-terminal domain of ARRDC1 contains two highly conserved PPxY motifs (Fig. 1C) that were previously implicated in ubiquitination and ESCRT-mediated, PPxY-dependent retroviral budding (6). The work by Nabhan et al. (1) also observes PPxY-dependent ARRDC1 ubiquitination and thus hypothesizes that ubiquitin modification might be required for ARMM release. The authors use a proteomic analysis to identify the Nedd4 family E3 ligase, WWP2, as an ARRDC1-interacting protein. Mutation of the PPxY motifs inhibits the ARRDC1–WWP2 interaction, and the requirement for WWP2 in ARMM budding is confirmed by showing that WWP2 depletion significantly reduces microvesicle release. These observations suggest a model where ARRDC1 interaction with WWP2 through its PPxY motif results in ubiquitination of ARRDC1 and enhanced ARMM release.
The process described in the work by Nabhan et al. (1) represents a type of microvesicle release from the PM that is distinct from exosome release, which originates in a late endosomal compartment. These findings also add another key piece to the puzzle of how evolutionarily conserved cellular processes are hijacked by viruses. The parallels between budding events driven by ARRDC1 and retroviral Gag proteins are striking in several respects: PM location, requirement for ESCRT machinery and Vps4, and involvement of Nedd4 family ubiquitin ligases. The authors propose that retroviral Gag proteins evolved to mimic ARRDC1 release from the PM.
As is the case for most groundbreaking studies, this work raises a number of interesting questions for additional investigation. (i) What is the mechanism by which ARRDC1 is targeted to the PM? Do phosphoinositides play a role like they do for the targeting of Gag proteins of HIV-1 and several other retroviruses (10)? (ii) What other ESCRT components are required for ARRDC1-mediated budding? As depicted in Fig. 1A, ESCRT-mediated MVB budding requires, in many cases, ESCRT-I, -II, and -III, whereas HIV-1 budding is independent of ESCRT-II (11) and requires only a subset of ESCRT-III components (12). Does ARMM budding require ESCRT-II, and which ESCRT-III components are involved? Alix also plays a role, albeit still poorly defined, in HIV-1 budding. The work by Rauch et al. (6) previously showed an interaction between ARRDC1 and Alix, raising the question of whether ARRDC1 interaction with Alix promotes ARMM release (Fig. 1C). (iii) Finally, and perhaps most importantly, what is the physiological function of ARMM release? Membrane vesicles have been shown to function in intercellular communication (13), and Nabhan et al. (1) observe that ARRDC1 could be transferred between cells in ARMMs. The transfer of ARMMs between cells, either as cell-free microvesicles or through a cell–cell route, may represent yet another parallel between ARMMs and retroviral particles.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4146.
References
- 1.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 3.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Herrador A, Herranz S, Lara D, Vincent O. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol. 2010;30:897–907. doi: 10.1128/MCB.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21:2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauch S, Martin-Serrano J. Multiple interactions between the ESCRT machinery and arrestin-related proteins: Implications for PPXY-dependent budding. J Virol. 2011;85:3546–3556. doi: 10.1128/JVI.02045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley JH, Emr SD. The ESCRT complexes: Structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieniasz PD. The cell biology of HIV-1 virion genesis. Cell Host Microbe. 2009;5:550–558. doi: 10.1016/j.chom.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss ER, Göttlinger H. The role of cellular factors in promoting HIV budding. J Mol Biol. 2011;410:525–533. doi: 10.1016/j.jmb.2011.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balasubramaniam M, Freed EO. New insights into HIV assembly and trafficking. Physiology (Bethesda) 2011;26:236–251. doi: 10.1152/physiol.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langelier C, et al. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J Virol. 2006;80:9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita E, et al. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe. 2011;9:235–242. doi: 10.1016/j.chom.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]