Abstract
New neurons are continuously generated in the dentate gyrus (DG) in the adult hippocampus, and new granule cells (GCs) have been shown to be necessary for several aspects of learning and memory. Nonetheless, the limited information available regarding the anatomical and physiological development of synaptic inputs onto maturing neurons has restricted our understanding of how new GCs affect cognition. Here, we use photostimulation to demonstrate the time course by which anatomically isolated inhibitory inputs develop onto maturing GCs. We then show that the gradual development of inhibition is sufficient in a computational model to drive learning of novel information in young neurons. Finally, we validate this model observation by using slice physiology to show how inhibition regulates firing probability and plasticity in young GCs. Combined, these data demonstrate that the unique connectivity of immature GCs affords them a functional role that is different from mature neurons in the DG circuit, a distinction that potentially underlies many of the proposed functions of new neurons in the hippocampal network.
Keywords: neurogenesis, neural network
The incorporation of new granule cells (GCs) into the dentate gyrus (DG) is a gradual process that requires several months for neurons to fully mature (1). This maturation has been proposed to involve several “critical periods” in which they are functionally distinct from mature GCs (2, 3). Nonetheless, the relationship of the network integration of maturing neurons and their unique behavior remains unknown. Glutamatergic synapses begin to form on dendritic spines when young GCs are approximately 16 d old and continue to form for approximately 2 mo (4, 5). Likewise, GABA transitions from depolarizing to hyperpolarizing at a comparable time to the onset of glutamatergic inputs, and the intrinsic responsiveness of young GCs to GABA appears comparable to that of mature GCs by 4 wk (6–10); however, the time course by which actual GABAergic inhibitory inputs develop is less clear.
The development of GABA inputs onto adult-born GCs is likely a significant factor distinguishing young neurons from mature neurons (10). Previous studies performed under artificial conditions in which inhibition was blocked by GABA-A receptor antagonists have shown that immature neurons have altered inward rectifier potassium currents (11), NMDA receptors (2), and T type calcium channels (12). These intrinsic physiological differences contribute to increased excitability and plasticity, which have been suggested to be critical features in theoretical considerations of neurogenesis function (13, 14). However, these mechanisms would not appear to be sufficient for the transition of GCs to their essentially silent mature state in the network. This low baseline activity of mature GCs is widely believed to be a critical aspect of DG function, as sparse coding by GCs has long been thought to underlie the network's proposed pattern separation function.
Although determining the development of GABAergic inputs is important for understanding new neuron function, widespread distribution of diverse populations of interneurons throughout the DG (15) make it hard to precisely measure how inhibitory synapses form onto adult-born neurons by using an extracellular stimulating electrode. Here, we have used caged glutamate to map a time course of GABAergic input development onto young neurons by voltage-clamping neurons at 0 mV and stimulating different regions of the DG. Further, we used an abstract model to demonstrate that this development of inhibition onto maturing neurons is sufficient to focus activity and learning onto immature GCs. The predictions of the model are then validated by using conventional slice physiology to show that GABA development is an important factor underlying the behavior of immature neurons.
Results
Mapping Functional Inhibitory Inputs to Maturing Adult-Born Neurons by Photostimulation.
To examine the development of functional inhibitory inputs from the entire DG, we used focal uncaging of glutamate to excite presynaptic neurons in a spatially restricted region (Fig. S1), thereby avoiding axons en passant and dendrites from distant neurons as well as efficiently stimulating multiple targets in a large area (16, 17). GFP+ retrovirus-labeled GCs were voltage clamped at 0 mV, which allowed us to isolate inhibitory inputs. We recorded inhibitory postsynaptic currents (IPSCs) evoked by photostimulation at more than 800 candidate presynaptic sites across the full extent of the DG in the slice. Based on the amplitude and number of IPSCs, we constructed locations of neurons providing inhibitory inputs to individual GFP+ GCs at different ages after retrovirus injection and compared input strengths across three DG subregions. To characterize the spatial resolution of photostimulation, neurons recorded in whole-cell, current-clamp mode were photostimulated directly by laser flashes (Materials and Methods). Under the experimental condition, spontaneous action potentials (APs) were infrequent. All neurons (n = 11) fired APs when photostimulated within 100 μm from soma, which is consistent with previous findings in the cortex under similar conditions (16). Neurons fired APs only by direct light stimulation, suggesting that monosynaptic inputs were recorded under experimental conditions. Uncaging glutamate at fine spatial resolution could reliably reveal the location of soma of presynaptic neurons (Fig. S2).
Fig. 1 A and B illustrates input maps for a 4-wk postinjection (wpi) neuron and an 8-wpi neuron. Laminar inhibitory synaptic inputs were quantified by the number and the amplitude of IPSCs following photostimulation across sites in the molecular layer, granule cell layer, and hilus (Materials and Methods). Spontaneous IPSCs were measured during no-stimulation control trials. For the cells illustrated in Fig. 1 A and B, the values for stimulation sites in molecular layer, granule cell layer, and hilus were 16 pA, 11.3 pA, and 9.2 pA, respectively, for the 4-wpi neuron, and 49 pA, 25 pA, and 17.7 pA, respectively, for the 8-wpi neuron. The number and amplitude of IPSCs for individual neurons were averaged for each layer, and the number and amplitude of photostimulation-evoked IPSCs were significantly increased between 4 and 6 wpi across all three layers (for 4-, 6-, 8-wpi and GFP− cells, n = 10, n = 8, n = 5, and n = 11, respectively; ANOVA, P < 0.0001 for amplitude, P < 0.0001 for number; Fig. 1 C and D). Consistently, spontaneous IPSC were significant increased after 4 wk (one-way ANOVA, P = 0.0018; Fig. 1E). This developmental change in inhibitory inputs was likely caused by a higher proportion of connections from presynaptic neurons as well as stronger connectivity from each stimulated neuron, caused by changes in the amplitude and number of GABAergic input.
Fig. 1.
Laminar inhibitory input summaries for GCs of different ages. Pseudocolor input maps show representative patterns of inhibitory input to 4-wpi (A) and 8-wpi (B) cells. Color scales demonstrate the amplitude of evoked IPSCs. Summary of amplitudes of IPSCs and numbers of events evoked by photostimulation across ages (for 4-, 6-, 8-wpi and GFP− cells, n = 10, n = 8, n = 5 and n = 11, respectively; ANOVA, P < 0.0001; C and D). (E) Amplitudes of spontaneous IPSCs measured in the no-stimulation trials across ages (for 4-, 6-, 8-wpi and GFP− cells, n = 10, n = 8, n = 5 and, n = 11, respectively; one-way ANOVA, P = 0.0018). GCL, granule cell layer; H, hilus; ML, molecular level.
Synaptic Development Is Computationally Sufficient to Make Young Neurons Excitable.
Although numerous modeling and experimental studies have suggested that immature GCs are more “excitable” than mature GCs, the underlying mechanism of this excitability has been attributed to many different sources. We hypothesize that the gradual addition of synapses may be sufficient to make young neurons the more active GC population, based on the simple statistical observation that neurons that sample many inputs are less influenced by noise than neurons that sample fewer inputs (Fig. 2A and Materials and Methods). Even if mean inhibitory input is greater than mean excitatory input for a given cell, a young neuron with few inputs (1,000 excitatory synapses/250 inhibitory synapses) will still fire occasionally as a result of sampling fluctuations. In contrast, a neuron with a mature cohort of synapses (5,000 excitatory/1,250 inhibitory synapses) will rarely fire under these conditions by chance (Fig. 2B).
Fig. 2.
Model A: fluctuations of synaptic input onto simple neurons with 1,000 excitatory (+)/250 inhibitory (−; green) and 5,000 excitatory and 1,250 inhibitory (black) synapses. Incidents in which a neuron's excitation surpassed inhibition are noted with a filled circle. (B) Relationship of number of synapses to probability of excitation surpassing inhibition at different relative strengths of inhibition. Green and black dots indicate neurons shown in A. (C) Schematic of simple neurogenesis network. Neurons are gradually added to the network, initialized with 100 excitatory/25 inhibitory synapses (green circles) that gradually increase to 5,000 excitatory/1,250 inhibitory synapses (black circles). The network is trained with random patterns of both excitatory (blue circles) and inhibitory (red triangles) inputs. During testing, the network is presented with the trained inputs again as well as a batch of novel inputs. (D) Low-synapse (i.e., young) neurons dominate the trained network's response to novel patterns, whereas high-synapse (i.e., mature) neurons are nonresponsive. The color bar indicates the probability that a neuron responds to an input. Neurons (columns) are ordered by age, although the x axis indicates synapse number, which saturates at 5,000 synapses (therefore, a disproportionate number of neurons have all 5,000 synapses). (E) Effect of synapse number on response to novel inputs. Low-synapse neurons are more responsive to novel inputs at a wide range of relative strengths of inhibition. (F) High-synapse (i.e., mature) neurons are able to respond to a subset of trained events, ordered by when they were initially presented. Low-synapse neurons also respond to most familiar events. (G) Mature neurons convey more information “per spike” than young neurons when calculated across all tested events (both novel and familiar).
To investigate this hypothesis at the network level, we constructed a basic feed-forward neural network model that incorporated new neurons while keeping the ratio of excitatory and inhibitory inputs constant (Fig. 2C). Admittedly, this is a simplified model, as GABA inputs likely precede glutamatergic inputs (6), but the developmental timeline of overall GABA inputs described here is comparable to the rapid increase of dendritic spines from approximately 3 wk to 6 wk (5). Although there are numerically more excitatory than inhibitory inputs onto mature GCs, individual inhibitory synapses are much stronger, typically suppressing GCs. Artificial neuron simulation was also highly simplified in the model: if input excitation was greater than input inhibition, the neuron fired. The assumption of similar development rates for excitation and inhibition and use of binary threshold neurons is clearly a simplification of the neurogenesis process, but the abstract nature of this model is useful for directly investigating the effects of the scaling of synaptic inputs during maturation. Interestingly, the model showed that, when young neurons initially had few synapses, novel inputs were occasionally capable of driving activity despite their “average” inhibited state (Fig. 2D). This increased response to novel inputs suggested that young neurons were better suited for pattern integration as opposed to pattern separation (18). Eventually, neurons matured to a point at which this chance activation did not occur; as a result, mature GCs no longer responded to novel information (Fig. 2E). Importantly, mature neurons were still capable of responding to those familiar events that they learned initially (Fig. 2F). This learning (implemented by using an activity-dependent Hebbian rule) was highly specific, with mature neurons retaining significantly more information about their previous memories than young neurons (Fig. 2G).
Overall, the model demonstrated several directly testable predictions. First, the reduced inhibition in young neurons is sufficient to bias activity in response to novel information to young neurons. Although several models have identified young neurons as being more excitable, the mechanisms underlying this bias have been attributed to many sources. Second, increased activity of young neurons relative to mature neurons will preferentially direct learning to the young neurons, even if learning rates are the same. This result is directly caused by the activity-dependent plasticity used in the model, although this learning rule is consistent with the NMDA-dependent plasticity that has been well characterized in the hippocampus. Finally, because mature neurons will be less likely to be incorporated into new memories, their ability to encode past events will be preserved, allowing mature neurons to retain faithful representations of information in the network without interference from later events, an implication of neurogenesis that has also been predicted by previous models (19). Notably, although the model did not include other details of young neurons (i.e., their intrinsic excitability), this result is consistent with our previous modeling study, in which these intrinsic properties were taken into account with a more sophisticated network model using spiking neurons (18).
Higher Firing Probabilities in Young Neurons.
Although the implications of these model predictions on the population are difficult to demonstrate at the functional time scales of the DG, we sought to validate the model's basic observations about the differences between immature and mature neurons in slice recordings. To investigate input processing, we measured the range of stimulus strengths required to activate immature (4 wpi) and mature GCs (8 wpi). Spikes were detected by using whole-cell recordings under current-clamp mode in response to repetitive stimuli delivered to the media perforant path (mPP) at 2 Hz. Stimuli of increasing intensity elicited spikes with increasing probability (Fig. 3 A and B). A cumulative distribution of the threshold inputs was then used to show activation curves that represent the fractional recruitment of GC populations by stimulating mPP (two-way ANOVA with Bonferroni post-hoc analysis, for 4 wpi, n = 7, P > 0.05; for 8 wpi, n = 10, P < 0.01; Fig. 3B). These results demonstrate a lower threshold for activation of the immature GCs compared with mature GCs. We then examined input–output relationships to determine the strength of synaptic drive required to initiate spiking in 4-wpi vs. 8-wpi neurons. Spikes were recorded in current clamp for a 10-Hz train of stimuli and then, by using voltage clamp, excitatory synaptic currents (EPSCs) were recorded for the same train of stimuli (Fig. 3C) (20). Average EPSCs were paired with their corresponding spike probabilities. The midpoints (EPSC50 is the EPSC amplitude at which the spike probability is 0.5) of input–output relationships corresponded to the strength of synaptic coupling between mPP inputs and spike outputs. We found that less current was required to drive young neurons to fire in the artificial cerebrospinal fluid (ACSF) (11); Bonferroni post-hoc test, P < 0.001, n = 7 for 4 and 8 wpi]. This selective activation at low input strengths might be a result of intrinsic properties of immature GCs or it might be dictated by a more complex interplay between excitation and inhibition. Interestingly, blockade of GABAergic inhibition by picrotoxin (PTX; 100 μM) induced a significant reduction in the input strength required to activate mature but not immature GCs (two-way ANOVA, age–PTX interaction, P < 0.001; Fig. 3 B and D).
Fig. 3.
Higher firing probabilities in young neurons. (A) Example of a current-clamp recording performed on 4- and 8-wpi neurons. The experiment aimed to measure spiking probability in response to mPP stimuli of different intensities without or with PTX in the ACSF. Each trace shows typical membrane potential in response to stimuli. (Scale bars: 100 mV, 100 ms.) (B) Cumulative distribution of firing probability of 4- and 8-wpi cells without and with PTX at all stimulus intensities. (ACSF, for 4-wpi, n = 7; for 8-wpi, n = 10; PTX, for 4-wpi, n = 8; for 8-wpi, n = 10.) (C) Example of current-clamp (Upper) and voltage-clamp (Lower) traces from the same GC to a 10-pulse, 10-Hz train stimuli to mPP. (D) EPSC50 of 4-wpi and 8-wpi cells without and with PTX. Two-way ANOVA revealed significant interaction between ages and application of PTX (P < 0.001). A significant decrease was observed upon applying PTX to 8-wpi cells (Bonferroni post-hoc test, for 4-wpi, n = 4; for 8-wpi, n = 9; P < 0.01) and a significant decrease was observed in 4-wpi cells without PTX (ACSF; Bonferroni post hoc test, P < 0.001; n = 7 for both).
Enhanced Plasticity in Young Neurons.
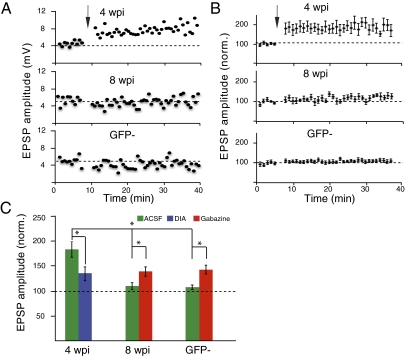
The development of inhibition in the model predicts increased plasticity of immature GCs compared with mature GCs. Indeed, previous studies showed a lower threshold for long-term potentiation (LTP) induction in young neurons, which depends in part on the expression of T type calcium channels and NR2B-containing NMDA receptors (2, 12, 21, 22). However, the role of inhibition in these mechanistic experiments is unclear, as GABA is routinely blocked in plasticity studies. One previous observation suggested that, when GABAergic inhibition is intact, LTP is more robust in young neurons (21). Here we show that, in the absence of GABA blockade, LTP was readily induced in 4-wpi neurons but not in 8-wpi neurons or GFP-negative mature neurons (n = 13, n = 7, and n = 9; Fig. 4 A and B). If inhibition were reduced in mature neurons by approximately half by using 80 nM gabazine (thus mimicking the level of inhibition in young neurons; Fig. S3), LTP could be induced in 8-wpi and GFP− neurons (t test, P < 0.01 for both; for 8-wpi, n = 4; for GFP−, n = 3; Fig. 4C). Notably, complete blockade of GABAergic inhibition using 100 μM PTX did not further increase the extent of potentiation (Fig. S4). To mimic the mature level of GABA on immature neurons, the application of diazepam (1 μM), a benzodiazepine agonist known to enhance activation of GABA-A receptors, decreased the amplitude of LTP in young neurons (n = 8; t test, P < 0.05; Fig. 4C).
Fig. 4.
Enhanced plasticity in young neurons. Example (A) and summary (B) of normalized EPSP amplitude before and after theta-burst stimulation. Arrows indicate application of TBS. Data are expressed as mean SEM. (C) Summary of LTP induction under various conditions: ACSF, 1 μM diazepam (DIA), and 80 nM gabazine. In ACSF after LTP induction, amplitude of EPSPs of 4-wpi cells (n = 13) was significantly higher compared with 8-wpi (n = 7) and GFP− (n = 9) cells (ANOVA, P < 0.001). Diazepam significantly decreased the ESPP amplitude of 4-wpi cells (n = 8; t test, P < 0.05). Gabazine significantly increased EPSP amplitude of 8-wpi and GFP− cells (t test, P < 0.01 for both; for 8-wpi, n = 4; for GFP−, n = 3).
Discussion
Combined, these slice physiology results support the model's observation that immature GCs will be more responsive and show increased plasticity compared with mature GCs, whereas mature GCs show only limited responsiveness. Notably, the relationship of GABA to maturing GCs is complicated; for instance, GABA depolarizes very immature GCs as a result of their higher chloride reversal potential, although it is hyperpolarizing by the ages examined here (8). Furthermore, changes in relative inhibition may have unpredicted consequences on network dynamics that our model did not examine. For instance, sensitivity to feedback and feedforward inhibition may change the temporal relationship of immature neurons to the DG's inputs, which could in turn lead to the observed plasticity results through effects on spike timing-dependent plasticity. Regardless, the strong effects of network GABA on mature GCs, as demonstrated by molecular layer stimulation in the caged glutamate results (Fig. 1 A–D), likely contributes greatly to observation that GCs are notoriously silent in vivo (23, 24), and may help explain why mature GCs appear to “retire” from the DG under certain circumstances (25). The selective regulation of GABA on the plasticity potential of GCs of different ages is of particular importance in considering how the hippocampal network stores and maintains memories throughout life. The ability to balance learning of new associations and preserving existing memories is a nontrivial yet necessary long-term function of neural networks in the brain (26, 27). This is particularly the case for the hippocampus and its proposed role in episodic memories, which requires the network to be able to learn and reliably remember novel information throughout life (28). These data suggest that adult neurogenesis is ideally suited to provide this capability. The development of inhibitory synapses onto maturing GCs is timed such that young neurons can remain responsive to novel cortical inputs that are less capable of activating mature GCs. As suggested by our model and electrophysiology experiments, young GCs are therefore able to integrate across multiple cortical inputs whereas mature GCs remain selective.
This coupling of neurogenesis function with the strong inhibitory network in the hippocampus has several significant implications for understanding the role of neurogenesis in various disorders. The inhibitory circuit within the hippocampus is strongly controlled by subcortical and brainstem sources, such as the serotonergic raphe nucleus and the cholinergic septum and diagonal band (29). As with adult neurogenesis, these subcortical networks have been widely implicated in various neurological and psychiatric conditions, such as Parkinson disease, Alzheimer's disease, and depression (30–32). These data suggest that the hippocampal inhibitory network may be a key player in these relationships.
Furthermore, these data suggest that mismatched excitation and inhibition may have negative implications for network function, even if the imbalance is limited to development. Similar patterns of GABAergic development have been observed in the embryonic and postnatal development of other brain regions (33, 34). For instance, in the developing visual cortex, inhibition gradually increases, preventing the induction of LTP in V1 and setting the critical period for ocular dominance plasticity (35). Temporal disruption of the proper formation of inhibitory networks within the DG might have the potential to result in subtle network differences in a wide range of neurological disorders associated with learning and memory deficits (36).
Materials and Methods
The experimental procedures described in this section are described in further detail in SI Materials and Methods.
Virus Production, Subjects, and Stereotaxic Surgery.
A retrovirus vector based on a Moloney murine leukemia virus expressing GFP under CAG promoter was used in the experiment. The virus was delivered to the DG by stereotaxic equipment.
Slice Preparation, Photostimulation, and Recordings.
Mice were anesthetized between 2 and 8 wk after retrovirus injection. Mice were perfused with section solution and recorded in the ACSF. Photostimulation was achieved by uncaging NMI-glutamate (100 μM; this concentration will not affect a GABAergic synapse) with a 10-ms flash of UV light, which was delivered to the slice through a 40× microscopic objective.
LTP Induction Protocol.
Theta burst stimulation was performed at 10 train/5 Hz. Each train contains 10 pulses for mPP stimuli.
Immunostaining and Confocal Imaging.
After recording, brain slices were fixed in 4% paraformaldehyde (g/vol) overnight and then transferred to 30% sucrose (g/vol) in PBS solution. The recorded cells were identified by staining of biocytin with 1:1,000 Cy3-conjugated streptavidin. Cell morphology was examined by confocal microscope and reconstructed in Neurolucida.
Data Analysis.
A customized program was used to analyze the results of photostimulation. The IPSCs were calculated during a synaptic response window 10 to 150 ms after stimulation. The evoked input strength was calculated by subtracting the mean ± amplitude of the control trial from the mean amplitude of the photostimulation trial. Data are expressed as mean ± SEM, and statistical significance is tested by ANOVA and Student t test.
Model.
The model was programmed and simulated using MATLAB operating on a small 16 CPU Linux cluster, with 500 replicates run for each simulation condition. Full model details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Jamie Simon for illustrations and Ayumu Tashiro, Gong Chen, Wei Deng, Chunmei Zhao, and Mary Lynn Gage for helpful comments. This work was supported by the Human Frontier Science Program, National Institutes of Health Grant R01-MH090258, the Lookout Fund, the Mathers Foundation, and the James S. McDonnell Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120754109/-/DCSupplemental.
References
- 1.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espósito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Mongiat LA, Espósito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS ONE. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 13.Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahay A, Wilson DA, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci. 2009;29:70–85. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nat Neurosci. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- 18.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- 20.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–12607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 23.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 24.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 25.Alme CB, et al. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. doi: 10.1002/hipo.20810. [DOI] [PubMed] [Google Scholar]
- 26.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 27.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Varga V, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 30.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 32.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 33.Hensch TK, et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 36.Chao HT, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.