In Tempo and Mode of Evolution, an early classic of the evolutionary synthesis, George Simpson argued that, although many aspects of evolution can be studied in living populations, rates and patterns of evolutionary change are best documented with the fossil record (1). The fact that fossils can be placed into phylogenetic and stratigraphic frameworks makes it possible to calculate rates of change directly rather than extrapolating them from the inferred history of living organisms. Although Simpson's logic is sound in principle, the task of measuring evolutionary rates from fossils is laborious in practice: Fossils must be taxonomically assigned to species and then assembled into a phylogenetic tree by using methods that distinguish true ancestors from sister groups; the sediments in which fossils are found must be correlated lithologically or biostratigraphically and then packages of rocks must be correlated into a global timescale or otherwise given absolute ages; and finally, the numbers of generations represented by the intervals of time separating the fossils must be estimated (2). Because of the herculean task of gathering these basic data, most paleontological studies of evolutionary rates are narrow in scope, confined to a few lineages or clades, too piecemeal to say much about the broad-scale patterns that are normally the strength of the paleontological record. In PNAS, Evans et al. (3) develop a “shortcut” that allows evolutionary rates to be compared across all mammals over the entire Cenozoic Era (last 65 My), giving a very broad picture indeed. They find some unexpected things, including an evolutionary bias in which body mass appears to decrease faster than it increases. Importantly, their paper puts numbers on the amount of evolutionary time required for body mass changes of different magnitudes.
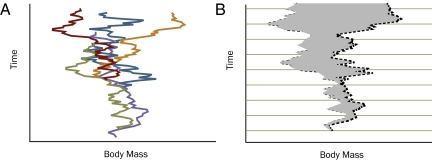
The breadth of Evans et al.'s analysis (3) was achieved first by focusing exclusively on body size, a trait common to all mammals (indeed, to all organisms), and second by focusing not on the rates of evolution of individual lineages, but on changes in the maximum body mass of larger clades. The result is a rather abstract measure called the clade maximum rate (CMR), the rate by which the maximum body size in a clade increases or decreases over time. CMR appears to be one step removed from a true evolutionary rate because it is a measure not of the rate of change within an ancestor-descendant lineage, but of how fast a clade pushes its maximum size boundary (Fig. 1). A clade consists of many evolving lineages, only one of which is the largest at a given time; furthermore, the same lineage is not likely to be the largest at different times. CMR is thus calculated from members of several lineages, each of which happens to be biggest at a particular time; it is therefore a measure of how fast a clade can reach record size, regardless of how many lineages are competing for that record. Even though CMR does not fit the standard definition of an evolutionary rate, it is an intuitively useful metric, because it is often the largest organisms—dinosaurs, whales, sequoias, Titanoboas—whose rate of evolution is of special interest. CMR radically simplifies the task of calculating rates, because phylogeny within each clade does not have to be known. Given that the constituency of most mammalian orders is obvious but their internal phylogenetic details are not, the simplification allows broad patterns to emerge from numerous and complicated data. Alternative strategies for dealing with the complexity of the fossil record, such as sidestepping it by calculating rates based on phylogenies of living mammals (4), are innovative but less satisfying, because they substitute ancestral reconstructions for the empirical temporal data provided by the fossil record. To call CMR a shortcut is doing it a disservice because the amount of data assembled by Evans et al. (3) is massive; their exercise in data reduction avoids a paralyzing morass of taxonomic and temporal uncertainties and allows them to focus on well studied points. After all, the biggest prehistoric animals have received considerable scientific attention and are thus better known than their most medium-sized relatives.
Fig. 1.
The relationship of lineage evolution to CMR. (A) A clade consists of many evolving and bifurcating lineages, only one of which is the largest member of the clade at any one time. (B) CMR is calculated from only the largest members, which are represented by the largest points on the broken black line in each time interval.
Evans et al. (3) found that, in terrestrial mammals, it takes approximately 1.6 million generations for a 100-fold increase in size, approximately 5.1 million generations for a 1,000-fold increase and approximately 10 million generations for a 5,000-fold increase. Rates of maximum size evolution in whales, the largest of mammals, were approximately twice as fast. The latter finding was expected because the remarkably rapid increase in size in early whales has long been noted (5), but less expected was the finding that maximum body size decreases occur, on average, much faster than increases: 10 times faster, in fact. Evans et al. (3) discuss possible biological reasons for this asymmetry, including the possibility that shortening the period of growth before sexual maturity (i.e., pedomorphosis) is an evolutionary easy thing to do, whereas lengthening the period of growth comes with physiological and reproductive costs. The faster rates of maximum size decrease may be related to the commonness of island dwarf species such as foxes, red deer, mammoths, and hippos, compared with island giants such as the Orkney vole and speckled rattlesnakes (6–9). Interestingly, the rates of maximum size evolution found by Evans et al. (3) are slower than those predicted from studies of individual lineages, in which per-generation rates of evolution suggest that the 1,000-fold increase in body mass between a small cat and an elephant could, in principle, happen in fewer than 1 million generations (2, 10). The apparent disjunction between predictions based on rates calculated in microevolutionary studies and the empirical evidence presented by Evans et al. (3) raises questions both mathematical and biological.
One possible explanation for why CMR shows slower rates of evolution than predicted from within-lineage studies is the fact that it is an unusual kind of rate, and so is not comparable to ordinary rates of evolution. Although it is true that CMR is not a typical rate of evolution, it is statistically related to typical rates: If a trait evolves in a Brownian motion pattern, as it would if the intensity and direction of selection was randomly distributed across time and lineages, then the SD across lineages at a given time will increase with the square root of the number of generations elapsed (11). As a trait's maximum is related to its SD—both are metrics of range—CMR is expected to be a function of the average rate of body size evolution in the clade's constituent lineages and should be comparable to them.
Another possible reason for slow macroevolutionary rates is that CMR is biased because it is measured over what could be interpreted as the wrong intervals. A rate is the amount of change divided by the interval over which the change occurred. Stochastic reversals in trait evolution result in a bias because the reversals increasingly cancel out change over longer periods of time: rates measured over longer intervals appear to be lower than those measured over short intervals (12, 13). CMR is measured over deceptively long intervals, longer than the time bin intervals used in its calculation, because the true evolutionary interval between clade maxima is not the interval of time between the bins, but the total phylogenetic time elapsed since the last common ancestor of the two lineages. As Evans et al. (3) point out, this phenomenon means that lineage-specific rates within the clade are
Maximum body size decreases occur, on average, much faster than increases: 10 times faster, in fact.
expected to be higher than CMR. Still, the logic presented in the previous paragraph suggests that a bias related to time interval cannot easily explain the comparatively slow macroevolutionary rates observed by Evans et al. because CMR should still scale directly with within-lineage rates.
Having ruled out these possible sources of bias, the relatively slow rates of CMR are suggested by Evans et al. (3) to result from factors that favor body size decreases over increases. They do not attempt to assess these factors directly, but point to their finding that decreases in maximum body size are 10 times faster on average than increases. They suggest that factors like pedomorphosis, the physiological difficulties associated with large body size, and a bias toward selection favoring small body size and fast reproduction are candidate explanations. Interestingly, Uyeda and colleagues recently analyzed a similar data set of body masses and found that short, rapid bursts of evolution occurred infrequently within lineages, but body size evolution appeared to be constrained over longer intervals (14). Uyeda and colleagues argued that the short bursts were caused by lineages moving from one adaptive zone to another, such as with the origin of whales, but that slower rates were associated with restricted environmental variation within adaptive zones, preventing body size from diverging as rapidly as it did with the initial colonizations of the adaptive zone. Despite the different perspectives of these two studies, their findings are similar in that despite rapid changes over short periods, the overall rate of divergence in mammalian body size appears to be constrained over long time periods, even when the scaling bias in rate measurement has been taken into account. The explanations for this phenomenon offered by the two studies are also broadly compatible in that they both view selection as moving more often in one direction than another, but are focused on different levels of explanation.
These new empirical studies invite renewed investigation of the relative roles of selection and constraint in evolution, and of the sources of difference between broad macroevolutionary patterns and smaller microevolutionary or “mesoevolutionary” scales. Renewed attention should also be given to the way rates of evolution are measured and to the biological theory of how microevolutionary patterns are expected to scale into macroevolutionary ones, if at all. Hansen and colleagues recently pointed out that heritability, a variance-scaled parameter, is unlikely to be a good predictor of long-term evolution because population variance is highly context sensitive (15). Most evolutionary rates, including CMR, are measured as the change in the trait as a proportion of the within-species variance in the trait, making them an example of variance-scaled parameters. The apparent mismatches between rates on large and small scales may deserve a fresh look in light of Hansen and colleagues’ observations. CMR provides yet another way of looking at the evolution of traits, providing even more incentive for renewed attention.
Footnotes
The author declares no conflict of interest.
See companion article on page 4187.
References
- 1.Simpson GG. Tempo and Mode in Evolution. New York: Columbia Univ Press; 1944. [DOI] [PubMed] [Google Scholar]
- 2.Polly PD. Paleontology and the comparative method: Ancestral node reconstructions versus observed node values. Am Nat. 2001;157:596–609. doi: 10.1086/320622. [DOI] [PubMed] [Google Scholar]
- 3.Evans AR, et al. The maximum rate of mammal evolution. Proc Natl Acad Sci USA. 2012;109:4187–4190. doi: 10.1073/pnas.1120774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper N, Purvis A. Body size evolution in mammals: Complexity in tempo and mode. Am Nat. 2010;175:727–738. doi: 10.1086/652466. [DOI] [PubMed] [Google Scholar]
- 5.Uhen MD. The origin(s) of whales. Annu Rev Earth Planet Sci. 2010;38:189–219. [Google Scholar]
- 6.Lister AM. Rapid dwarfing of red deer on Jersey in the last interglacial. Nature. 1989;342:539–542. doi: 10.1038/342539a0. [DOI] [PubMed] [Google Scholar]
- 7.Haynes S, Jaarola M, Searle JB. Phylogeography of the common vole (Microtus arvalis) with particular emphasis on the colonization of the Orkney archipelago. Mol Ecol. 2003;12:951–956. doi: 10.1046/j.1365-294x.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 8.Weston EM, Lister AM. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature. 2009;459:85–88. doi: 10.1038/nature07922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meik JM, Lawing AM, Pires-daSilva A. Body size evolution in insular speckled rattlesnakes (Viperidae: Crotalus mitchellii) PLoS ONE. 2010;5:e9524. doi: 10.1371/journal.pone.0009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingerich PD. Rates of evolution on the time scale of the evolutionary process. Genetica. 2001;112-113:127–144. [PubMed] [Google Scholar]
- 11.Felsenstein J. Evolutionary trees from gene frequencies and quantitative characters: finding maximum likelihood estimates. Evolution. 1981;35:1229–1242. doi: 10.1111/j.1558-5646.1981.tb04991.x. [DOI] [PubMed] [Google Scholar]
- 12.Gingerich PD. Quantification and comparison of evolutionary rates. Am J Sci. 1993;293A:453–478. [Google Scholar]
- 13.Gingerich PD. Rates of evolution. Annu Rev Ecol Evol Syst. 2009;40:657–675. [Google Scholar]
- 14.Uyeda JC, Hansen TF, Arnold SJ, Pienaar J. The million-year wait for macroevolutionary bursts. Proc Natl Acad Sci USA. 2011;108:15908–15913. doi: 10.1073/pnas.1014503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen TF, Pélabon C, Houle D. Heritability is not evolvability. Evol Biol. 2011;38:258–277. [Google Scholar]