Abstract
How fast can a mammal evolve from the size of a mouse to the size of an elephant? Achieving such a large transformation calls for major biological reorganization. Thus, the speed at which this occurs has important implications for extensive faunal changes, including adaptive radiations and recovery from mass extinctions. To quantify the pace of large-scale evolution we developed a metric, clade maximum rate, which represents the maximum evolutionary rate of a trait within a clade. We applied this metric to body mass evolution in mammals over the last 70 million years, during which multiple large evolutionary transitions occurred in oceans and on continents and islands. Our computations suggest that it took a minimum of 1.6, 5.1, and 10 million generations for terrestrial mammal mass to increase 100-, and 1,000-, and 5,000-fold, respectively. Values for whales were down to half the length (i.e., 1.1, 3, and 5 million generations), perhaps due to the reduced mechanical constraints of living in an aquatic environment. When differences in generation time are considered, we find an exponential increase in maximum mammal body mass during the 35 million years following the Cretaceous–Paleogene (K–Pg) extinction event. Our results also indicate a basic asymmetry in macroevolution: very large decreases (such as extreme insular dwarfism) can happen at more than 10 times the rate of increases. Our findings allow more rigorous comparisons of microevolutionary and macroevolutionary patterns and processes.
Keywords: haldanes, biological time, scaling, pedomorphosis
Microevolution and macroevolution characterize two extremes of the evolutionary process, representing evolution below and above the species level, respectively (1, 2). Microevolution often exhibits very fast rates over short timescales (<100 generations). At a typical generation-to-generation rate, evolution by a random walk could hypothetically produce a body mass change from that of a 20-g mouse to that of a 2,000,000-g elephant in fewer than 200,000 generations (3), a relatively brief geological interval. However, such high rates are not sustained over long intervals in the fossil record. Presumably this is because diverse physical, functional, genetic, developmental, and ecological constraints restrict large-scale macroevolution. Because these constraints may operate differently depending on whether an organism is becoming larger or smaller, it is equally important to understand whether the reverse transformation, from elephant to mouse, would be easier. Our question is how quickly such intertwined constraints can be overcome when there is a selective advantage to do so: What is the maximum rate of macroevolution? To paraphrase G. Evelyn Hutchinson “How big was it and how fast did it happen?” (4).
Body mass is the most fundamental animal trait, strongly correlated with most aspects of morphology, life history, physiology, and behavior (5–7). Evolution of body mass influences and is influenced by selection on other traits and is easily characterized. Thus, changes in body size provide some of the best examples of rapid evolution (8, 9).
Evolutionary rates of morphological traits such as size are often quantified in haldanes (h) (10, 11), which measure proportional change in a feature (Mi) between two time points (i) standardized by the available variation (pooled ln SD sp) using a timescale in number of generations (g): h = (lnM2 − lnM1)/(sp × g).
However, most previous measurements of evolutionary rates have been made either for well-defined lineages in a stratigraphic sequence or pairs of time points where an ancestor/descendant relationship is reasonably certain (3, 11, 12). This tends to restrict comparisons to closely related groups with relatively small evolutionary changes and low rates.
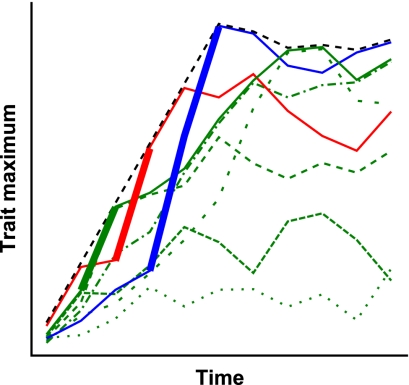
To better characterize major changes in a phenotypic trait within a clade, as opposed to a single lineage, we developed the clade maximum rate (CMR) metric. The clade maximum rate is defined as the rate of change in a specified extreme value of a trait (either the minimum or the maximum) for a clade within a given time interval. Whereas this metric describes the rate at which the maximum of a trait increases, the CMR is normally slower than the maximum rate of evolution of the trait within individual lineages of the clade (Fig. 1). CMR intentionally ignores decreases in the maximum of the trait because these can happen by true evolutionary decreases or extinction of the lineages that achieved the maximum. A major advantage of the clade maximum rate is that a detailed phylogeny is not required, only the recognition of distinct clades.
Fig. 1.
Evolutionary rate of the clade maximum for a trait can underestimate the maximum evolutionary rate of subclades or component lower taxa within the clade. The black dashed line represents the maximum for a clade composed of three subclades represented by green, red, and blue lines. Each of these subclades is composed of lineages of species, shown for the green clade as thin broken lines. When a different subclade becomes the new clade maximum, it must have a higher evolutionary rate than the clade maximum for that interval: the thick lines represent this process.
Here, we investigated the clade maximum rate for maximum body mass. We used a compilation of the maximum body mass (M) for 28 mammal orders on the four largest continents (Africa, Eurasia, and North and South America) and all ocean basins for all subepochs during the last 70 million years, covering the well-documented mammal radiation following the Cretaceous–Paleogene (K–Pg) mass extinction (13). To test for generality of the patterns, we also obtained and analyzed data for North American Artiodactyla at the finer temporal resolution of the North American Land Mammal Age (NALMA) subages. For each clade, we calculated the CMR of body size evolution in haldanes. We supplemented CMR with a reference database from the literature of 1,453 rates of mammalian body mass evolution for many phylogenetic groups at various temporal scales. A third dataset from empirical selection experiments on mouse body size (3, 14) measured evolutionary change over 1–23 generations. Directly comparing rates at different interval lengths is complicated; although a very high rate can be sustained for a short interval, over longer periods, rates tend to vary and the direction of evolution may change (12). Thus, interval length must be incorporated into any analysis.
Generation time is considered the fundamental unit of evolutionary time because evolutionary change cannot happen more quickly than a single generation (10, 11). The use of generation time rather than chronological time is crucial for the calculation of interval length because generation time increases allometrically with mass (i.e., larger species have longer generation times than smaller species). Therefore, evolutionary rates appear to slow in chronological time as the maximum size increases even when they are the same rate in generational time. If generation time were invariant with body mass, then the slope of body mass as a function of chronological time (t) would indicate a true evolutionary rate (Fig. 2A). However, generation time, like many other biological processes such as lifespan, gestation, lactation, and sleep cycle, scales as ∼1/4 power of body mass (M0.259) for placental mammals (Materials and Methods). Thus, plotting M0.259 against time gives a generation time-corrected evolutionary rate in haldanes (Fig. 2B). A straight line relationship here indicates an exponential increase in maximum size over biological time (SI Appendix).
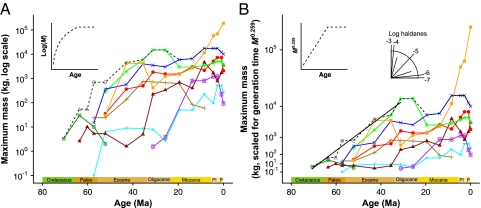
Fig. 2.
Maximum mammalian body mass over time for terrestrial mammals (dashed black line) and separate mammal orders (colored lines). (A) Log(M) vs. Age shows an asymptotic relationship for the mammalian maximum. (B) Mass is scaled to the power of 0.259 on the y axis (given an empirical M0.259 scaling of generation times), so the slope of lines indicates generation time-corrected evolutionary rates as indicated by an angular scale (haldanometer). Inset graphs show how an asymptotic relationship for M vs. Age can result in a linear trajectory for M0.259 vs. Age, as found for terrestrial mammals from 70 to 30 Ma (solid black line in B). Rates were calculated separately for the orders in color; when other orders comprise the maximum size across all mammals, they are shown in gray. Artiodactyls (red circle), carnivorans (red triangle), cetaceans (orange square), creodonts (brown plus sign), multituberculates (green cross in square), perissodactyls (green asterisk), primates (cyan diamond), proboscideans (blue X), rodents (purple star), condylarths (open gray triangle), dinoceratans (open gray diamond), pantodonts (open gray circle). Time units: Paleo, Paleocene; Pl, Pliocene; P, Pleistocene.
Results
We find that the maximum body mass of terrestrial mammals evolved at a near-constant rate from 70 million years ago (Ma), just before the K–Pg, until the appearance of the largest terrestrial mammal, Indricotherium, at about 30 Ma. A linear regression gives an excellent fit to this time interval, with a slope equivalent to 7.1 × 10−6 haldanes (R2 = 0.97; Table 1 and Fig. 2). A similar constancy, but with somewhat different absolute rates, appears in several orders: Cetacea (from Oligocene to Recent), Artiodactyla, Perissodactyla, Proboscidea, and Rodentia, and to a lesser extent the Carnivora and Primates (Table 1). The relative constancy of evolutionary rate for maximum body mass for the 35 million years following the extinction of the nonavian dinosaurs is striking and unexpected. Our results offer a different perspective from a recent analysis of body mass evolution over chronological time, but are consistent with convergence toward an asymptote for maximum body mass globally and within each continent (13) (Fig. 2A).
Table 1.
The maximum body mass for all terrestrial mammals and for several orders increased linearly when generation time is accounted for
| Slope | Haldanes (× 10−6) | R2 | P | |
| Terrestrial maximum | 1.59 | 7.14 | 0.97 | 1.17 × 10−5 |
| Artiodactyla | 0.74 | 3.34 | 0.90 | 3.33 × 10−5 |
| Carnivora | 0.65 | 2.94 | 0.74 | 6.87 × 10−4 |
| Cetacea | 3.25 | 14.60 | 0.83 | 1.70 × 10−3 |
| Perissodactyla | 2.13 | 9.57 | 0.98 | 9.70 × 10−3 |
| Primates | 0.39 | 1.77 | 0.78 | 1.46 × 10−4 |
| Proboscidea | 1.08 | 4.84 | 0.91 | 6.25 × 10−5 |
| Rodentia | 1.21 | 5.45 | 0.93 | 1.74 × 10−3 |
Slope for linear regression of M0.259 vs. Age (Ma) for each group from their origin until their maximum (except for Cetacea, which is for the period of 31 Ma to the Recent). The average rate in haldanes was calculated using the mammalian scaling relationship of generation time with body mass (SI Appendix). These time intervals are plotted as points in Fig. 3B.
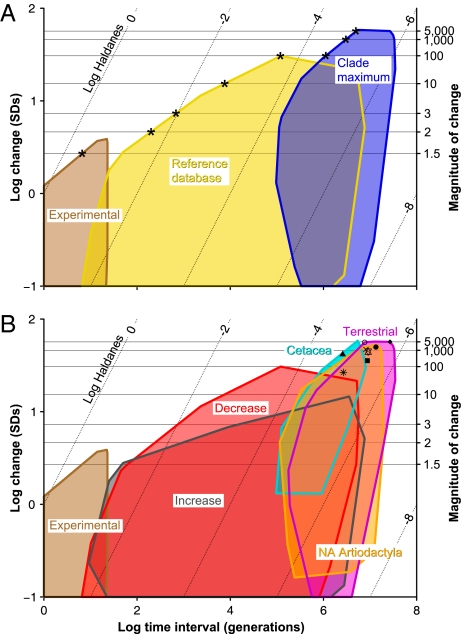
Across all analyzed datasets, we find that the largest changes occur in the clade maximum data (Fig. 3A). The highest magnitudes of change are about 5,000-fold (blue, Fig. 3A), much greater than the 100-fold increases seen in the reference database (yellow, Fig. 3A). This difference occurs despite the considerable overlap between our dataset and the reference data in the time intervals studied. Using the clade maximum rates for all mammals, we estimate the minimum times to evolve 100-, 1,000-, and 5,000-fold increases in body size are 1.1, 3, and 5 million generations, respectively (Table 2) and occur in cetaceans. In contrast, the maximum evolutionary rates for terrestrial mammals are much lower, taking 1.6, 5.1, and 10 million generations, respectively (Table 2).
Fig. 3.
Maximum rates of evolution for large changes in mammalian body mass. Minimum convex polygons of rates plotted as log change in body mass (in units of SD) vs. log time interval (generations). (A) The three datasets compared in this study: experimental rates (3, 14) (brown), 1,453 rates from previous studies (yellow), and clade maximum rates (blue). Asterisks indicate minimum number of generations to evolve a given amount of change. (B) Datasets split into components. Compiled rates are separated into increases (gray) and decreases (red) and clade maximum rates (all of which are increases) into terrestrial orders (pink), cetaceans (cyan), and North American artiodactyls (orange). Points show average rates for linear increase in Table 1 for terrestrial mammals (open circle), artiodactyls (closed circle), carnivorans (square), cetaceans (triangle), perissodactyls (asterisk), primates (diamond), proboscideans (X), and rodents (star). Right-hand y axis and horizontal lines illustrate magnitude of change in body mass. Large decreases (>2-fold) require substantially less time than increases, and maximum rates for very large changes (>100-fold) in cetaceans are about twice those in terrestrial. Diagonal dotted lines are isohaldanes, equal rates measured in log haldanes.
Table 2.
Minimum number of generations (millions) required to evolve various magnitudes of change in mammals
| Magnitude of change | ||||||
| ×3 | ×10 | ×100 | ×1,000 | ×5,000 | ||
| All mammals | Increase | 0.016 | 0.30 | 1.1 | 3.0 | 5.0 |
| Terrestrial mammals | Increase | 0.016 | 0.30 | 1.6 | 5.1 | 10.0 |
| Cetaceans | Increase | 0.10 | 0.40 | 1.1 | 3.0 | 5.0 |
| Insular dwarfism | Decrease | 0.001 | 0.008 | 0.12 | ||
Discussion
Although the global data provide an overall estimate of evolutionary rates across all mammals, there is interesting and likely important variation among the clades and modes of life. The maximum body mass of cetaceans yields the highest long-term rates of any order (Table 1) and higher rates than other mammals (Fig. 3B). This finding may reflect the fewer mechanical constraints on body form and function in the aquatic environment (7). Moreover, a large mass is advantageous for maintaining thermoregulatory balance, so selection pressures for large size may be stronger in an aquatic environment. However, no group yielded macroevolutionary rates approaching those reported from microevolutionary studies.
The discrepancy between microevolutionary predictions for large-scale body size evolution and actual macroevolutionary measurements of rates has long been known (3, 12, 15, 16) but little understood. Although our study cannot definitively address this issue, it does furnish some important insights. We provide strong empirical evidence that the maximum rate of body size evolution decreases with increasing time interval (12, 17). Indeed, we find an approximate linear relationship across the different datasets between the maximum amount of change and the time interval: the maximum log change scales with log time interval with a slope of 0.25 (SI Appendix). Using this scaling relationship, we estimate that the 100,000-fold transformation from mouse to elephant would take 24 million generations. This is substantially longer than 200,000–2 million generations suggested by microevolutionary rates (3, 15).
To investigate the converse transformation of elephant to mouse, we divided our reference data into size increases and decreases. Whereas changes in mass below twofold appear to have similar maximum rates for increases and decreases in size, above this the rates are unequal (Fig. 3B). The largest decreases, such as insular dwarfism, are more than 30 times the rate of increases of the same magnitude (Table 2). This apparent asymmetry is especially surprising given the ample evidence for Cope's rule, a trend for body size to increase consistently and relatively continuously throughout the history of a lineage (18, 19).
The asymmetry between rates can potentially be explained by distinct but not necessarily mutually exclusive mechanisms. One possibility is that there are fewer physical, biological, and environmental constraints to decreasing as opposed to increasing size. Pedomorphic processes are good candidates as mechanisms of size reduction, because all animals must pass through a smaller size during their ontogeny. We hypothesize it is easier to halt the developmental program and reproduce early than to grow larger and delay maturity. Another possibility is that selection favors size decreases because smaller animals have higher rates of reproduction with life histories characterized by rapid maturity, high birth rates, and short lifespans (20). Finally, decreases in size may reflect adaptation to a more generalized ecological niche, whereas increases in size require novel adaptations to obtain more food and space to fuel higher whole-organism metabolic rates.
In the reference dataset, the largest decreases in body size were rates of dwarfing in large mammals after isolation on islands by rising sea levels during the last few million years: elephants on the Mediterranean islands of Sicily, Malta, and Cyprus (9, 21); mammoths on the California Channel Islands (22); and red deer on Jersey (8) (SI Appendix). These island dwarfism cases involve body mass changes of 5- to 100-fold over estimated time intervals of 0.006–0.8 myr or 2,300–120,000 generations. Islands characteristically have fewer predators, competitors, and resources (23), thereby favoring faster life histories and more generalized ecologies and perhaps also leading to higher selection pressures (17).
Our study represents a comprehensive analysis of large-scale macroevolutionary rates for a single trait. Whereas previous work used metrics similar to our clade maximum rates (10, 24, 25) using only two data points, our clade maximum rate metric allows assessment of rates over a range of time intervals and with high temporal resolution. This allows us to make direct quantitative comparisons of microevolutionary and macroevolutionary rates (1, 3, 12, 15, 26). Maximum macroevolutionary rates have important implications for large-scale faunal changes and recovery from mass extinction (13, 19). Our results highlight the comparative difficulty of major changes in body size, especially increasing in size. At least 5 million generations were required for a mammal to increase 1,000-fold in body mass, from the size of a rabbit to the size of an elephant. Compared with an equivalent change at microevolutionary rates, this substantial length of time illustrates just how challenging this great transformation is.
Materials and Methods
We used the compilation (13) of the maximum body mass for each of 28 orders of Mammalia in each subepoch since 70 Ma (Mammoth database v. 1.0). We calculated rates for the mammal maximum and for the nine best sampled orders using the CMR method. The maximum mass of artiodactyls in North America was calculated for 18 families for each North American Land Mammal subage. Natural log body mass SD was estimated to be 0.15 from modern species (27) as used previously (3) (SI Appendix). Generation time was estimated as age at first parturition. Regression equations for body mass vs. generation time calculated from the data for 839 placental mammal species and for 82 marsupial species (28) were used to estimate generation time for extinct taxa on the basis of body size. For each sequence of maxima, all combinations of time points were compared. Only rates of increase in maximum size were calculated for the maximum mammalian body size, as these must be due to evolutionary change. The pattern of increase in maximum body mass of terrestrial mammals (M0.259) from 70 to 30 Ma was assessed with ordinary least squares (OLS), segmented, Gompertz, square root, exponential, and logistic regressions. The OLS regression model was the best fit according to Akaike information criterion (AIC) (SI Appendix). The pattern of increase in maximum size for seven orders was also assessed using OLS regression (Table 1). We calculated evolutionary rates for mammal data in references (3, 17, 29) where sufficient data were present in the original paper to allow estimation of body mass and time intervals. SI Appendix lists the sources of data for body size, generation time, and interval length for the studies used. Data quality for these sources will be variable, depending on factors such as the accuracy of the identification of ancestor-descendant pairs and the date at which the derived morphology was actually attained. Several sensitivity tests were conducted to examine whether the incompleteness of the fossil record and/or binning data by subepoch biased rate calculations. These tests comprised sets of 100 independent random walks in 10 clades for 1,000 steps in 10 intervals. The maximum within each subclade and for the whole clade was calculated for each interval. The rates of change in the subclade and clade maxima were calculated per interval as for the CMR method. Fossilization was simulated by downsampling the data to between 1 and 0.005%. Maxima in each interval and rates of change were then calculated for each subclade and clade. These calculations indicated that the estimated evolutionary rates are not significantly biased due to these effects, although at very low preservation levels variation in measured rates increased.
Supplementary Material
Acknowledgments
We thank G. Evans, M. Burd, D. Dowling, Evolutionary Biology at Monash, P. Smits, G. Sanson, J. Jernvall, F. Whiteman, M. Balk, B. Van Valkenburgh, J. Damuth, A. Lister, and P. D. Polly for discussions and comments on earlier manuscripts. This study was supported by an Australian Research Council Australian Research Fellowship (to A.R.E.), Monash University Monash Research Fellowship (to A.R.E.), National Science Foundation Grant Integrating Macroecological Pattern and Processes across Scales Research Coordination Network (IMPPS RCN) DEB 0541625 (to F.A.S., S.K.L., and S.K.M.E., principal investigators), European Union Marie Curie Grant PIOF-GA-2009-235868 (to D.J.), and a Harold Mitchell Foundation Harold Mitchell Fellowship (to E.M.G.F.). This paper is IMPPS RCN publication no. 18.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 4027.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120774109/-/DCSupplemental.
References
- 1.Simpson GG. The Major Features of Evolution. New York: Simon and Schuster; 1953. [Google Scholar]
- 2.Stanley SM. Macroevolution: Pattern and Process. San Francisco: W. H. Freeman; 1979. [Google Scholar]
- 3.Gingerich PD. Rates of evolution on the time scale of the evolutionary process. Genetica. 2001;112-113:127–144. [PubMed] [Google Scholar]
- 4.Hutchinson GE. Variations on a theme by Robert MacArthur. In: Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. Cambridge: Belknap Press; 1975. pp. 492–512. [Google Scholar]
- 5.Peters RH. The Ecological Implications of Body Size. Cambridge: Cambridge Univ Press; 1983. [Google Scholar]
- 6.Calder WA. Size, Function, and Life History. Cambridge, MA: Harvard Univ Press; 1984. [Google Scholar]
- 7.Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important? Cambridge: Cambridge Univ Press; 1984. [Google Scholar]
- 8.Lister AM. Rapid dwarfing of red deer on Jersey in the last interglacial. Nature. 1989;342:539–542. doi: 10.1038/342539a0. [DOI] [PubMed] [Google Scholar]
- 9.Roth VL. Inferences from allometry and fossils: Dwarfing of elephants on islands. Oxf Surv Evol Biol. 1992;8:259–288. [Google Scholar]
- 10.Haldane JBS. Suggestions as to quantitative measurement of rates of evolution. Evolution. 1949;3:51–56. doi: 10.1111/j.1558-5646.1949.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 11.Gingerich PD. Quantification and comparison of evolutionary rates. Am J Sci. 1993;293A:453–478. [Google Scholar]
- 12.Gingerich PD. Rates of evolution: Effects of time and temporal scaling. Science. 1983;22:159–161. doi: 10.1126/science.222.4620.159. [DOI] [PubMed] [Google Scholar]
- 13.Smith FA, et al. The evolution of maximum body size of terrestrial mammals. Science. 2010;330:1216–1219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- 14.Falconer DS. Replicated selection for body weight in mice. Genet Res. 1973;22:291–321. doi: 10.1017/s0016672300013094. [DOI] [PubMed] [Google Scholar]
- 15.Polly PD. Paleontology and the comparative method: Ancestral node reconstructions versus observed node values. Am Nat. 2001;157:596–609. doi: 10.1086/320622. [DOI] [PubMed] [Google Scholar]
- 16.Kinnison MT, Hendry AP. The pace of modern life II: From rates of contemporary microevolution to pattern and process. Genetica. 2001;112-113:145–164. [PubMed] [Google Scholar]
- 17.Millien V. Morphological evolution is accelerated among island mammals. PLoS Biol. 2006;4:e321. doi: 10.1371/journal.pbio.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanley SM. An explanation for Cope's rule. Evolution. 1973;27:1–26. doi: 10.1111/j.1558-5646.1973.tb05912.x. [DOI] [PubMed] [Google Scholar]
- 19.Alroy J. Cope's rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280:731–734. doi: 10.1126/science.280.5364.731. [DOI] [PubMed] [Google Scholar]
- 20.Sibly RM, Brown JH. Effects of body size and lifestyle on evolution of mammal life histories. Proc Natl Acad Sci USA. 2007;104:17707–17712. doi: 10.1073/pnas.0707725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies P, Lister AM. Palaeoloxodon cypriotes, the dwarf elephant of Cyprus: Size and scaling comparisons with P. falconeri (Sicily-Malta) and mainland P. antiquus. In: Cavarretta G, editor. The World of Elephants: Proceedings of the 1st International Congress, Rome 2001. Rome: Ufficio Pubblicazioni; 2001. pp. 479–480. [Google Scholar]
- 22.Lister A, Bahn PG. Mammoths: Giants of the Ice Age. Rev. Ed. Berkeley: Univ of California Press; 2007. [Google Scholar]
- 23.Lomolino MV. Body size evolution in insular vertebrates: Generality of the island rule. J Biogeogr. 2005;32:1683–1699. [Google Scholar]
- 24.Colbert EH. Evolution of the horned dinosaurs. Evolution. 1948;2:145–163. [Google Scholar]
- 25.Stanley SM. Rates of evolution. Paleobiology. 1985;11:13–26. [Google Scholar]
- 26.Estes S, Arnold SJ. Resolving the paradox of stasis: Models with stabilizing selection explain evolutionary divergence on all timescales. Am Nat. 2007;169:227–244. doi: 10.1086/510633. [DOI] [PubMed] [Google Scholar]
- 27.Silva M, Downing JA. CRC Handbook of Mammalian Body Masses. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 28.Hamilton MJ, Davidson AD, Sibly RM, Brown JH. Universal scaling of production rates across mammalian lineages. Proc R Soc Lond Ser B. 2011;278(1705):560–566. doi: 10.1098/rspb.2010.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt G. The relative importance of directional change, random walks, and stasis in the evolution of fossil lineages. Proc Natl Acad Sci USA. 2007;104:18404–18408. doi: 10.1073/pnas.0704088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.