Abstract
Living coyotes modify their behavior in the presence of larger carnivores, such as wolves. However, little is known about the effects of competitor presence or absence on morphological change in coyotes or wolves over long periods of time. We examined the evolution of coyotes and wolves through time from the late Pleistocene, during which many large carnivorous species coexisted as predators and competitors, to the Recent; this allowed us to investigate evolutionary changes in these species in response to climate change and megafaunal extinctions at the end of the Pleistocene. We measured postcranial skeletal morphologies of wolves (Canis lupus) and coyotes (C. latrans) from Pleistocene-aged tar deposits, as well as early, mid, and recent Holocene populations of both. We found few morphological differences between Pleistocene and Holocene wolf populations. Conversely, we found many differences in coyotes: Pleistocene coyotes were larger and more robust than Holocene populations. However, within 1,000 y of the megafaunal extinctions, coyotes are morphologically indistinguishable from modern populations. We cannot attribute these differences directly to climate change because modern coyotes do not follow Bergmann's rule, which states body size increases with decreasing temperature. Instead, we suggest that Pleistocene coyotes may have been larger and more robust in response to larger competitors and a larger-bodied prey base. Although we cannot separate competition from predator-prey interactions, this study indicates that the effects of biotic interactions can be detected in the fossil record.
Keywords: Pleistocene extinctions, Canidae, Rancho La Brea
Recent studies of coyotes (Canis latrans) and gray wolves (C. lupus) demonstrate competition and antagonistic interactions between these species when they are sympatric (1–7). Coyotes often modify their behavior in response to antagonism from wolves (2, 3, 5, 6). However, coyotes and wolves also respond through body-size convergence when they are in direct competition for small- to medium-sized prey (8).
Antagonism arises from the similar diets in the two species, but wolves and coyotes differ significantly in their hunting styles. Wolves are large-prey specialists that hunt in packs by means of a long, enduring chase (9). In contrast, coyotes are commonly solitary predators of small mammals, such as rodents and rabbits; they kill by a pounce and subsequent shaking that breaks the neck (10). However, coyotes can be opportunistic hunters with highly plastic prey-killing behaviors because of their intermediate size (11) and adaptability (10), and animosity between wolves and coyotes occurs when their diets converge.
Ecologists studying species relationships tend to focus on biotic interactions (12, 13) over, essentially, an instantaneous moment in time, rather than on abiotic factors (for exceptions, see refs. 14–16). Paleoecologists take the principles of ecology and add the concept of deep time with varying levels of resolution (17). This latter approach may focus on biotic interactions but must also examine longer-scale abiotic factors, such as climate change, to explain broader changes in species or assemblages through time (12, 18). Coyotes and wolves have a clear recent ecological record of competition and antagonism. Has this relationship been the same throughout their evolutionary histories? Looking to the past may illuminate how these species interactions have evolved.
Coyotes in the Pleistocene (C. latrans orcutti) were morphologically distinct from extant coyotes. The skulls and jaws of C. l. orcutti are significantly thicker and deeper than in Recent populations. Pleistocene coyotes also had a shorter, broader rostrum and wider carnassial teeth used for processing meat (19–21): all adaptations for killing larger prey and dealing with higher stresses during food acquisition and processing (22–25). These characteristics suggest that C. l. orcutti was more carnivorous than modern coyotes.
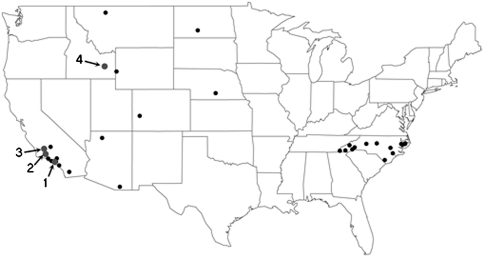
C. l. orcutti is found in large numbers in late Pleistocene (∼40–11 Ka) deposits from three sites in Southern California (SoCal): Rancho La Brea (RLB), Maricopa Brea, and McKittrick tar seeps (21) (Fig. 1). Pleistocene coyotes were part of a carnivorous guild that contained multiple canids, including: foxes, the gray wolf, C. lupus (only at RLB), and the dire wolf, C. dirus, which was larger, more robust, and hunted larger prey than gray wolves (19, 26–28). C. latrans is also present in earliest Holocene-aged pit 10 at RLB (29, 30). Preliminary analyses of cranial material found pit 10 individuals to be smaller than those in older pits (19), suggesting morphological differentiation between Pleistocene and Holocene coyotes. However, to distinguish between biotic or abiotic factors requires, a more quantitative statistical approach.
Fig. 1.
Map of localities for coyote specimens used in this study. Modern sites are in black; fossil sites are in gray. Arrows indicate fossil sites and are numbered: RLB, 1; Maricopa, 2; McKittrick, 3; Idaho caves, 4. See Table S5 for localities.
Changes in gray wolf or coyote morphology between Pleistocene and Holocene populations may be attributable to either species interactions or climate change or both. Here, we examine wolves and coyotes across the Pleistocene–Holocene boundary, taking into account both biotic and abiotic effects that may have influenced their ecologies and morphologies. For our purposes, the key changes that occurred at the end of the Pleistocene are the major warming trend (abiotic) and the megafaunal extinctions (biotic, the cessation of interactions with a now extinct fauna).
We examine the postcranial morphology of wolves and coyotes from the Pleistocene, earliest Holocene, and Recent. We predict that if climate is directly causing morphological change, then a clear correlation will exist between body size and temperature (or latitude), also known as Bergmann's rule, in wolves and coyotes. This effect should not only be present over the Pleistocene–Holocene transition but also in modern species over a climate gradient. It is difficult to assess biotic interactions in the fossil record, but if climate change is not the direct cause of morphological disparity, then competition and predator/prey interactions in the Pleistocene must be considered as major factors.
Results
Wolves.
Few differences were observed between Holocene and Pleistocene gray wolves. Tibial tuberosity length (TiSL) (Tables S1 and S2) was significantly longer in RLB specimens than from Holocene cave sites in Idaho (∼3–5 Ka) and extant populations. Femur breadth (FeB) was significantly larger in RLB wolves than mid-Holocene wolves. No significant differences were found for any of the other 19 measurements.
Coyotes.
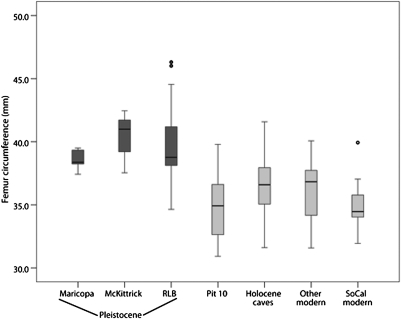
Many significant differences were observed between Pleistocene and Holocene coyotes (Table 1, Table S4, and Fig. 2). SoCal coyotes were significantly smaller than other modern coyotes for 14 out of 21 measurements (Table S3). There were few significant differences within Pleistocene coyote populations; however, Maricopa had smaller means than the other two sites and were not significantly different from Holocene populations. Maricopa coyotes had high variances, potentially reflecting two different morphs in the population (Table 1). Pit 10 coyotes were smallest, followed by modern SoCal coyotes, other modern coyotes, Holocene Cave coyotes, and Maricopa coyotes; McKittrick and RLB coyotes were largest.
Table 1.
ANOVA results for C. latrans comparisons
| SoCal Modern | Other Modern | Holocene Caves | Pit 10 | RLB | McKittrick | Maricopa | |
| Variable | (n = 17) | (n = 23) | (n = 8–10) | (n = 12–15) | (n = 31–58) | (n = 9–12) | (n = 4–7) |
| Humerus | |||||||
| HuL | 154.78 (6.90) | 161.52 (8.41) | 160.72 (6.90) | 153.09 (5.51) | 167.33 (6.70) | 169.95 (7.08) | 162.77 (10.67) |
| HuAPD | 13.01 (0.84) | 13.69 (1.05) | 12.84 (1.22) | 12.62 (0.79) | 15.11 (0.97) | 14.39 (0.86) | 14.38 (1.44) |
| HuMLD | 10.65 (0.70) | 10.75 (0.56) | 10.70 (0.82) | 10.68 (0.73) | 12.25 (0.86) | 11.87 (0.32) | 11.87 (0.66) |
| HuPCL | 59.82 (3.48) | 62.26 (4.45) | 63.64 (6.93) | 60.04 (4.57) | 69.96 (5.00) | 67.89 (3.73) | 62.70 (3.97) |
| HuHTL | 17.79 (1.63) | 19.87 (1.69) | 19.92 (1.37) | 19.80 (0.81) | 22.43 (1.14) | 21.96 (0.97) | 20.66 (1.65) |
| HuEB | 28.26 (1.43) | 29.37 (1.38) | 29.27 (1.23) | 27.89 (2.57) | 32.04 (1.61) | 32.32 (1.49) | 31.14 (2.31) |
| Radius | |||||||
| RaL | 162.87 (5.92) | 168.15 (8.52) | 163.66 (6.63) | 157.27 (6.80) | 173.61 (7.89) | 176.01 (8.34) | 167.12 (4.77) |
| RaAPD | 7.04 (0.58) | 6.87 (0.48) | 6.53 (0.67) | 6.97 (0.66) | 8.22 (0.83) | 8.46 (0.62) | 7.97 (0.11) |
| RaMLD | 11.00 (0.86) | 12.09 (0.75) | 11.42 (0.91) | 10.96 (0.64) | 13.19 (1.00) | 13.17 (0.90) | 13.03 (0.15) |
| Ulna | |||||||
| UlL | 187.01 (8.06) | 193.73 (9.79) | 192.29 (8.17) | 184.38 (12.85) | 192.78 (7.37) | 202.15 (7.51) | None |
| UlOL | 24.42 (3.52) | 25.38 (3.19) | 25.71 (1.28) | 18.85 (1.59) | 19.99 (1.26) | 27.47 (1.05) | None |
| Femur | |||||||
| FeL | 172.03 (6.77) | 176.66 (8.48) | 177.16 (5.98) | 168.99 (8.24) | 185.08 (7.37) | 188.95 (9.58) | 176.20 (9.76) |
| FeAPD | 11.02 (0.79) | 11.45 (0.89) | 11.83 (1.37) | 10.89 (0.99) | 12.52 (0.92) | 12.89 (0.60) | 11.89 (0.33) |
| FeMLD | 11.22 (0.58) | 11.54 (0.78) | 11.49 (0.99) | 11.34 (0.75) | 12.86 (1.12) | 12.87 (0.59) | 12.65 (0.18) |
| FeGTH | 25.48 (4.02) | 44.06 (8.94) | 32.43 (8.09) | 27.17 (2.18) | 29.99 (3.99) | 27.49 (2.66) | 26.22 (3.35) |
| FeHD | 16.03 (0.83) | 16.87 (0.85) | 16.96 (1.14) | 15.92 (0.74) | 17.77 (1.14) | 18.28 (1.07) | 17.39 (0.93) |
| FeB | 27.05 (1.22) | 28.50 (1.39) | 28.92 (1.87) | 26.32 (2.01) | 30.42 (1.80) | 31.11 (1.85) | 29.33 (1.70) |
| Tibia | |||||||
| TiL | 181.84 (8.55) | 190.44 (11.43) | 182.15 (6.56) | 174.76 (7.60) | 193.32 (6.90) | 196.83 (9.77) | 191.46 (14.79) |
| TiAPD | 11.48 (0.94) | 12.14 (1.25) | 11.66 (1.33) | 10.94 (0.90) | 12.99 (0.98) | 13.06 (1.03) | 12.25 (0.93) |
| TiMLD | 11.09 (0.79) | 12.09 (0.64) | 11.14 (0.33) | 10.72 (0.80) | 12.80 (0.94) | 12.32 (0.65) | 11.95 (0.43) |
| TiSL | 29.55 (2.58) | 35.02 (5.04) | 34.91 (4.91) | 31.61 (2.93) | 36.66 (3.02) | 31.19 (2.78) | 31.45 (4.47) |
Mean ± SD for each locality and measurement used in this study. N for fossils is by individual element.
Fig. 2.
Box plot of femur circumference as a representative variable. Horizontal bars indicate the mean value; boxes indicate interquartiles, and whiskers (or dots) represent the extreme values for each group. Pleistocene groups are indicated with a darker shade of gray than Holocene groups.
RLB and McKittrick coyotes were significantly larger than modern coyotes (both groups), and Holocene coyotes for most measurements, but were not significantly different from each other. Surprisingly, all RLB coyotes, including Holocene coyotes from pit 10, had exceptionally short olecranon processes and were significantly different from all other groups. This measurement suggests that coyotes at RLB had exceptional cursorial abilities in both the Pleistocene and the early Holocene, despite the difference in body size between these two time intervals. See Table 1 for mean values for all measurements (all coyotes) and Table S4 for ANOVA P values for all Pleistocene coyotes.
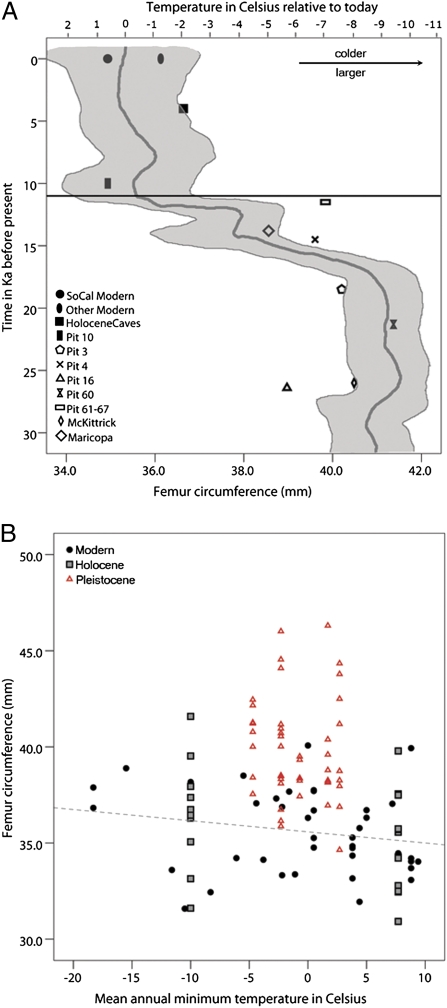
A plot of coyote morphology versus time and climate (Fig. 3A) shows a morphological shift at the end-Pleistocene, concurrent with the megafaunal extinctions and climate change. A linear regression showed no correlation between body size and mean annual minimum temperature for modern coyotes (P value = 0.216; R2 = 0.040) or when Pleistocene specimens were included (P value = 0.077; R2 = 0.029) (Fig. 3B). This demonstrates that coyotes do not follow Bergmann's rule and suggests that climate is not the primary driving factor in coyote body size change.
Fig. 3.
(A) Plot of femur circumference versus time in Ka before present. Data points correspond to morphology and time. All symbols are site averages. Solid symbols indicate Holocene groups, and open symbols indicate Pleistocene groups. The temperature curve was adapted from Jouzel et al. (75), and corresponds to time and temperature relative to the present; the mean is indicated with a gray curved line, and the confidence intervals of the curve are shaded. The horizontal black line indicates the end of the Pleistocene extinction events. (B) Plot of mean annual minimum temperature versus femur circumference to test for Bergmann's rule in coyotes. Points indicate individual specimens. The regression line is nonsignificant, is included only for illustrative purposes (P = 0.216; R2 = 0.04), and includes only modern individuals.
Discussion
Wolves.
Pleistocene wolves from RLB are not morphologically distinct from Holocene specimens. The only difference between the Pleistocene and Holocene populations is a larger tibial tuberosity, the insertion for the quadriceps and hamstring muscles. This suggests that Pleistocene gray wolves had larger and stronger muscles for take-off during a chase, but because there are no concomitant changes in any other measurements, this difference may not have any clear functional implications. Gray wolves are not common at the tar pits, and this may be attributable to behavioral modification or competitive exclusion of gray wolves by dire wolves near the tar seeps. This is also indicated by their absence at McKittrick and Maricopa and is consistent with other studies (31, 32).
A small sample size of gray wolves may give a false-negative result, but Leonard et al. (31) also noted the gracile nature of the gray wolves at RLB (similar to living morphs) compared with end-Pleistocene Alaskan and Beringian wolves. Geist (33) found that living gray wolves in North America show a body size increase with increasing latitude, but only up to 65° N; then, they show a body size decrease again. In wolves, Geist argued that body size trends are not due to climate, but food availability. Other studies also suggest that body size gradients in carnivores are directly dependent upon prey size, prey availability, and interspecific interactions, rather than abiotic factors, such as climate (8, 11, 34–43).
Coyotes.
We find a distinct morphological difference in coyotes across the Pleistocene–Holocene boundary (for a discussion of differences between Pleistocene sites, see SI Results). There was a large shift in morphology concurrent with the megafaunal extinctions and climate warming at the end of the Pleistocene (Fig. 2). However, we observed no relationship between body size and mean annual coldest temperature in modern coyotes (Fig. 3B); this provides additional evidence against Bergmann's rule. Our results are consistent with Thurber and Peterson (44), who found no correlation between latitude and body mass in coyotes.
If climate change was the direct cause of end-Pleistocene body size change in coyotes (that do not follow Bergmann's rule), why would this change not also appear in wolves (that do appear to follow Bergmann's rule) across the same time period? C. l. orcutti was larger and more robust than its extant relatives (Table 1 and Table S4), which can be corroborated by cranial evidence (19, 21). If living coyotes do not follow a simple pattern of larger body size in colder climates (i.e., Bergmann's rule), and we assume that this pattern was not different in the Pleistocene, then end-Pleistocene temperature change would not cause the body size changes seen here. This finding is somewhat unsurprising, considering that biotic interactions are responsible for a multitude of changes and trophic cascade effects that occur in modern ecosystems (for a review see 45). Two possible biotic reasons for the morphological differences seen in C. l. orcutti are: (i) a larger prey base, both in body size and quantity; and (ii) competition with larger carnivores.
Interactions with Prey.
Increased large-sized prey availability in the Pleistocene may have significantly contributed to the size differences seen at the Pleistocene–Holocene boundary.
Schmitz and Lavigne (8) found that both living coyotes and wolves changed in body size in response to prey availability. Coyotes are flexible in their prey-size preferences and increase the proportion of large prey in their diet as they approach 21–25 kg, the size at which carnivores switch from small prey to predominantly large prey (11). Bigger, more robust Pleistocene coyotes were approaching this critical size. Estimated mean mass of coyotes at Maricopa was 18 kg (46), and coyotes from McKittrick and RLB would have been even larger (see Table S4), suggesting C. l. orcutti hunted larger prey than its living conspecifics. In the Pleistocene, there were many prey species from which to choose, including horses, sloths, camels, llamas, and bison. Juveniles of one or more of those large-bodied species may have been suitable prey for coyotes, because modern coyotes are known to kill ungulate neonates when they are available (1).
C. l. orcutti also shows modifications of the crania and dentition for hunting and killing larger prey, including robust jaws and crania to accommodate large forces applied to the cranium and large molars and carnassial teeth for increased bone-cracking and meat-shearing capabilities, respectively (19–21, 24, 25). Van Valkenburgh and Hertel (28) found that coyotes at RLB had significantly more tooth breakage in their canines and premolars than their living counterparts. They concluded that C. l. orcutti had a higher frequency of tooth to bone contact than extant coyotes, from eating larger bones.
Interactions with Competitors.
Extant coyotes change both social structure and diet in response to larger competitors/predators (47). When gray wolves were absent from northwestern Montana between 1980 and 1994, coyotes had minimal pack structure, were usually solitary (66%) or occasionally in pairs (29%), and ate mostly lagomorphs and rodents. Following wolf reintroductions in 1995, coyote pack size increased to pairs (48%) and groups of three or more (33%), and they showed a major shift in diet from small prey to ungulates (47). Arjo and Pletscher (47) hypothesized that this pack structure change was for defense against wolves, and the dietary change was an increase in scavenging of carcasses left by wolves. Increased pack size in coyotes also has important offensive consequences for prey-killing, and many studies have documented that coyotes in large packs kill ungulate prey (10, 48–50).
In the Pleistocene, C. l. orcutti coexisted with both gray wolves and the larger, more robust dire wolf (19). From a competition standpoint, the observed size increase in C. l. orcutti seems suboptimal in the presence of both wolf species. However, if dire wolves competitively excluded gray wolves (31, 32), larger coyotes would have been able to move into the “empty” gray wolf niche. Nowak (21) also suggested that C. l. orcutti was filling a more wolf-like role in the environment. Additional evidence that Pleistocene coyotes were hunting in packs comes from their large sample size at RLB. Coyotes are the third-most common species found at RLB, after dire wolves and Smilodon, the saber-toothed cat. Carbone et al. (51) suggested that Smilodon was a social hunter at RLB because of its numbers at that site. They based this hypothesis on African playback experiments in modern carnivores, where they found that only the largest, pack-hunting species responded to distress calls of herbivores and fighting noises of carnivores. They proposed a similar type of scenario for RLB, where one herbivore would become mired in the tar, drawing attention only from groups big enough to defend themselves from the other carnivores present.
Humans As Competitors/Predators?
Humans migrated to North America at the end of the Pleistocene and may have impacted coyote morphology, either directly or indirectly. Humans were present at RLB, represented by the skeleton of a human female recovered from pit 10, dated at 10.08 Ka (52). Recent humans actively select against the biggest carnivores in an ecosystem, which are seen as a threat to domestic animals and themselves. If Pleistocene coyotes were more wolf-like, they may also have been more aggressive. Therefore, it is possible that many of the larger individuals of C. l. orcutti were killed by humans at the end of the Pleistocene. This would leave smaller individuals behind to repopulate, resulting in the morphology that we see today. However, humans would have also competed with much larger and more ferocious carnivores at the end-Pleistocene, such as saber-toothed cats and dire wolves, rendering coyotes less threatening. Indeed, there is no direct evidence of humans hunting coyotes in the Pleistocene. Instead, humans may have had indirect effects on large coyotes by killing off their prey base.
Conclusions
A large body of literature deals with the evolution of species or communities attributable to species interactions, competition, and predator–prey interactions on decadal time scales (53–57). Still, few studies have demonstrated morphological evolution attributable to species interactions (16, 58, 59). This study shows that relatively rapid evolution in a large mammal can be attributed to biotic interactions.
C. l. orcutti was a larger, more robust, and more wolf-like coyote than Holocene populations. The earliest Holocene coyotes from RLB pit 10 show a distinct change in morphology within 1,000 y of the megafaunal extinctions. We show climate was not directly affecting coyote size; therefore, climate change at the end of the Pleistocene was unlikely the major cause of this morphological shift. We do not dispute the effects of climate change on coyote evolution; our results show that climate change was not the direct cause of morphological change in coyotes. Although climate was not directly responsible for this change, it likely had indirect effects that would be hard to separate from biotic interactions. The end-Pleistocene extinctions are thought to be a product of climate change, which would have caused a trophic cascade in all mammalian assemblages that both survived the event and went extinct (45).
Biotic interactions are the most likely cause for morphological evolution in coyotes, but we cannot discern whether prey disappearance or competitive release was responsible for this shift. Previous studies show that carnivore body size is influenced by both competitors (38–42, 60) and prey species (8, 11, 34–36). In this case, it was probably a combination of both. For example, competition with larger predators causes coyotes to form larger packs for defense (47), which makes them better able to catch larger prey as a team (48–50).
The end-Pleistocene extinctions not only extirpated large mammal species but also had a profound effect on the species that did not go extinct (45). It is important to understand how ecosystems function and change through time, and species interactions are an integral part of any ecosystem (61–64). Some of the carnivore species that were present at RLB are still extant today (e.g., coyotes, gray wolves, mountain lions, etc.). However, the niches that these predators filled in the Pleistocene may have changed because of missing interactions with a now extinct fauna and because abiotic conditions differ.
Modern studies comparing gray wolves to coyotes show that removal of one apex predator has dramatic top-down effects on other predator species, as well as prey species (1–3, 5, 44, 45). C. l. orcutti was a larger-prey specialist than living coyotes, a trait that made C. l. orcutti better able to compete and survive in the carnivore-laden Pleistocene. Both modern ecosystems and Pleistocene ecosystems highlight the importance of species interactions, revealing the delicate balance that exists in both carnivore–carnivore and predator–prey interactions. Eradication of a single (or multiple) carnivore species has profound effects on the remaining species.
Methods
We measured fore- and hind-limbs of coyotes from different localities through time (Table S2 and Fig. S1). See Table S5 for locations and sample sizes. These specimens included C. l. orcutti from the RLB tar pits, from pit 3 (approximate mean age, 18.5 Ka), pit 4 (∼14.5 Ka), pit 16 (∼26.4 Ka), pit 60 (∼21.3 Ka), and pits 61/67 (∼11.5 Ka) (30). An ANOVA was performed comparing individual pits. Significant differences were found in humerus length (pit 4 was significantly larger than pit 61) and radius length (pit 4 was significantly larger than pits 3 and 61/67; pit 16 was also significantly larger than pit 61/67). No other significant differences were found for any measurements between any Pleistocene pits at RLB. Therefore, Pleistocene RLB specimens were treated as one group in subsequent analyses. Additionally, specimens were measured from earliest Holocene pit 10 (∼6–10 Ka) to determine if these specimens are morphologically distinct from older RLB coyotes.
We also measured coyotes from California Pleistocene tar seeps: (i) McKittrick tar pits; and (ii) Maricopa Brea. McKittrick has two radiocarbon dates on plant material at 38 K ± 2.5 Kybp and 12.2 K ± 250 ybp (46, 65). Maricopa Brea also has two radiocarbon dates which range in age from 36 K ± 3.9 Kybp (dire wolf) to 13.8 K ± 420 ybp (wood) (66).
We measured C. latrans from two pre-European Holocene sites in Idaho [Middle Butte Cave (< 8 Ka) and Moonshiner Cave (∼3 Ka) (67)] and from extant populations from Arizona, California, Colorado, Montana, North Carolina, Nebraska, North Dakota, and Wyoming (Fig. 1). Extant subspecies included C. l. latrans, C. l. lestes, C. l. mearnsi, C. l. merriami, C. l. ochropus, and C. l. frustor (n = 40). These subspecies likely represent present diversity in coyotes. We analyzed the SoCal coyote subspecies (C. l. ochropus; n = 17) separately from all other subspecies because this group likely represents the descendants of the Pleistocene coyote populations.
All other coyotes were included together as one group. To determine whether this grouping was acceptable, we compared the total group variation to the variation in the largest non-California group, North Carolina (n = 14/23 total). The sample from North Carolina was large and varied enough that the morphological diversity of the other modern coyotes measured fell within the North Carolina range (>90%) and were as large as coyotes from all of the other states. Given this predominant similarity, we treated all of the remaining extant coyote subspecies as a single group in subsequent analyses.
Although sexual dimorphism is not extreme in canids, it is present (68); thus, for all modern samples, we attempted to measure equal numbers of males and females.
C. lupus was analyzed from RLB, the two Holocene cave sites in Idaho, and recent populations from Alaska, Arizona, Montana, Nebraska, North Carolina, and North Dakota (Table S6). There has been some debate as to whether Eastern wolves are a different species from gray wolves (69). However, the one wolf specimen from North Carolina fell within the range of variation of other gray wolf specimens; thus, all modern wolves were treated as a single group in subsequent analyses. No gray wolf specimens were available from McKittrick, Maricopa, or RLB pit 10.
We chose limb measurements based upon their ability to predict locomotor mode and functional performance in carnivores (70, 71) and rodents (72). We analyzed these measurements with multiple one-way ANOVA tests using Tamhane (for unequal variance) and Scheffé (for equal variance) post hoc procedures. We performed a Levene test of homogeneity of variances to determine which post hoc test was appropriate. We performed all statistical analyses using SPSS version 19 (SPSS). We used raw measurements instead of ratios, and we did not perform any multivariate analyses, because of the fact that fossil specimens are disarticulated and random, and measurements that compare two bones (e.g., radius length to humerus length) would only be possible using averages; this method would effectively reduce the sample size of each group to one. To visualize where each of these groups fell in shape space, we constructed bivariate plots with time on the y axis and morphology on the x axis, with climate overlaid on top (Fig. 3A).
To examine the effects of climate, we plotted femur circumference, a good proxy for body size in canids (73, 74), against mean annual coldest temperature for each locality to look for correlations. We obtained mean annual coldest temperatures for modern specimens by identifying the nearest town (within 5 miles) to the collection locality of each coyote using the most precise data available (either the county in which it was collected or latitude and longitude coordinates) and looking up average temperature data for this town on the National Weather Service website (www.weather.gov). For fossil sites, we identified the weather conditions for the locality in the present, and then using data from Jouzel et al. (75) and the approximate age for the locality, we subtracted the appropriate number of degrees Celsius.
Supplementary Material
Acknowledgments
We thank the following collection managers and curators for access to specimens in their collections: C. Shaw, A. Farrell, and J. Harris (George C. Page Museum); B. Hess and L. Gatens (North Carolina Museum of Natural Sciences); M. Thompson (Idaho Museum of Natural History); L. Gordon (U.S. National Museum of Natural History); J. Dines, X. Wang, and S. MacLeod (Natural History Museum of Los Angeles County); and K. Molina (Dickey Collection, University of California). The manuscript was greatly improved by comments from P. Durst, P. Harnik, M. Kalcounis-Rueppell, J. Logsdon, J. McGuire, D. Polly, T. Roberts, L. Roth, D. Smith, B. Van Valkenburgh, and one anonymous reviewer. This work was supported by National Science Foundation Grant EF-0905606 to the National Evolutionary Synthesis Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113788109/-/DCSupplemental.
References
- 1.Berger KM, Gese EM, Berger J. Indirect effects and traditional trophic cascades: A test involving wolves, coyotes, and pronghorn. Ecology. 2008;89:818–828. doi: 10.1890/07-0193.1. [DOI] [PubMed] [Google Scholar]
- 2.Berger KM, Gese EM. Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol. 2007;76:1075–1085. doi: 10.1111/j.1365-2656.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 3.Merkle JA, Stahler DR, Smith DW. Interference competition between gray wolves and coyotes in Yellowstone National Park. Can J Zool. 2009;87:56–63. [Google Scholar]
- 4.Atwood TC, Gese EM. Importance of resource selection and social behavior to partitioning of hostile space by sympatric canids. J Mammal. 2010;91:490–499. [Google Scholar]
- 5.Atwood TC, Gese EM. Coyotes and recolonizing wolves: Social rank mediates risk-conditional behaviour at ungulate carcasses. Anim Behav. 2008;75:753–762. [Google Scholar]
- 6.Smith DW, Peterson RO, Houston DB. Yellowstone after wolves. Bioscience. 2003;53:330–340. [Google Scholar]
- 7.Krefting LW. The rise and fall of the coyote on Isle Royale. Naturalist. 1969;20:24–31. [Google Scholar]
- 8.Schmitz OJ, Lavigne DM. Factors affecting body size in sympatric Ontario Canis. J Mammal. 1987;68:92–99. [Google Scholar]
- 9.Mech LD. Canis lupus. Mamm Species. 1974;37:1–6. [Google Scholar]
- 10.Bekoff M. Canis latrans. Mamm Species. 1977;79:1–9. [Google Scholar]
- 11.Carbone C, Mace GM, Roberts SC, Macdonald DW. Energetic constraints on the diet of terrestrial carnivores. Nature. 1999;402:286–288. doi: 10.1038/46266. [DOI] [PubMed] [Google Scholar]
- 12.Benton MJ. The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science. 2009;323:728–732. doi: 10.1126/science.1157719. [DOI] [PubMed] [Google Scholar]
- 13.Van Valen L. A new evolutionary law. Evol Theory. 1973;1:1–30. [Google Scholar]
- 14.Keller LF, Grant PR, Grant BR, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin's finches. Evolution. 2002;56:1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 15.Grant PR, Grant BR, Keller LF, Petren K. Effects of El Nino events on Darwin's finch productivity. Ecology. 2000;81:2442–2457. [Google Scholar]
- 16.Grant PR, Grant BR. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- 17.DiMichele WA, et al. Long-term stasis in ecological assemblages: Evidence from the fossil record. Annu Rev Ecol Evol Syst. 2004;35:285–322. [Google Scholar]
- 18.Barnosky AD. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J Vertebr Paleontol. 2001;21:172–185. [Google Scholar]
- 19.Merriam JC. The fauna of Rancho La Brea, part II, Canidae. Memoirs of the University of California. 1912;1:215–272. [Google Scholar]
- 20.Giles E. Multivariate analysis of Pleistocene and Recent coyotes (Canis latrans) from California. Univ Calif Publ Geol Sci. 1960;36:369–390. [Google Scholar]
- 21.Nowak RM. 1979. University of Kansas Museum of Natural History Monograph: North American Quaternary Canis (University of Kansas Museum of Natural History, Lawrence, KS), pp 1–154.
- 22.Meachen-Samuels J, Van Valkenburgh B. Craniodental indicators of prey-size preference in the Felidae. Biol J Linn Soc Lond. 2009;96:784–799. doi: 10.1002/jmor.10712. [DOI] [PubMed] [Google Scholar]
- 23.Van Valkenburgh B, Koepfli K. Cranial and dental adaptations to predation in canids. Symposium of the Zoological Society of London. 1993;65:15–37. [Google Scholar]
- 24.Biknevicius A, Ruff C. The structure of the mandibular corpus and its relationship to feeding behaviors in extant carnivorans. J Zool (Lond) 1992;228:479–507. [Google Scholar]
- 25.Biknevicius AR, Van Valkenburgh B. Design for killing: Craniodental adaptations of predators. In: Gittlleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Vol 2. Ithaca, NY: Cornell University Press; 1996. pp. 393–428. [Google Scholar]
- 26.Fox-Dobbs K, Bump JK, Peterson RO, Fox DL, Koch PL. Carnivore-specific stable isotope variables and variation in the foraging ecology of modern and ancient wolf populations: Case studies from Isle Royale, Minnesota, and La Brea. Can J Zool. 2007;85:458–471. [Google Scholar]
- 27.Bump JK, et al. Stable isotopes, ecological integration and environmental change: Wolves record atmospheric carbon isotope trend better than tree rings. Proc Biol Sci. 2007;274:2471–2480. doi: 10.1098/rspb.2007.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Valkenburgh B, Hertel F. Tough times at La Brea: Tooth breakage in large carnivores of the late pleistocene. Science. 1993;261:456–459. doi: 10.1126/science.261.5120.456. [DOI] [PubMed] [Google Scholar]
- 29.Syverson VJ, Prothero DR. Evolutionary patterns in late Quaternary California condors. PalArch's Journal of Vertebrate Palaeontology. 2010;7:1–18. [Google Scholar]
- 30.O'Keefe FR, Fet EV, Harris JM. Compilation, calibration, and synthesis of faunal and floral radiocarbon dates, Rancho La Brea, California. Contributions in Science (Los Angeles) 2009;518:1–16. [Google Scholar]
- 31.Leonard JA, et al. Megafaunal extinctions and the disappearance of a specialized wolf ecomorph. Curr Biol. 2007;17:1146–1150. doi: 10.1016/j.cub.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 32.Dundas RG. Quaternary records of the dire wolf, Canis dirus, in North and South America. Boreas. 1999;28:375–385. [Google Scholar]
- 33.Geist V. Bergmann's rule is invalid. Can J Zool. 1987;65:1035–1038. [Google Scholar]
- 34.Gittleman JL. Carnivore body size - ecological and taxomonic correlates. Oecologia. 1985;67:540–554. doi: 10.1007/BF00790026. [DOI] [PubMed] [Google Scholar]
- 35.Carbone C, Teacher A, Rowcliffe JM. The costs of carnivory. PLoS Biol. 2007;5:e22. doi: 10.1371/journal.pbio.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbone C, Gittleman JL. A common rule for the scaling of carnivore density. Science. 2002;295:2273–2276. doi: 10.1126/science.1067994. [DOI] [PubMed] [Google Scholar]
- 37.Ml R. Community structure in sympatric Carnivora. J Mammal. 1966;47:602. [Google Scholar]
- 38.Dayan T, Simberloff D, Tchernov E, Yomtov Y. Feline canines - community-wide character displacement among the small cats of Israel. Am Nat. 1990;136:39–60. [Google Scholar]
- 39.Dayan T, Simberloff D, Tchernov E, Yomtov Y. Canine carnassials - character displacement in the wolves, jackals, and foxes of Israel. Biol J Linn Soc Lond. 1992;45:315–331. [Google Scholar]
- 40.Dayan T, Simberloff D. Character Displacement, Sexual Dimorphism, and Morphological Variation among British and Irish Mustelids. Ecology. 1994;75:1063–1073. [Google Scholar]
- 41.Dayan T, Simberloff D. Ecological and community-wide character displacement: The next generation. Ecol Lett. 2005;8:875–894. [Google Scholar]
- 42.Dayan T, Simberloff D. Patterns of size separation in carnivore communities. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Vol 2. Ithaca, NY: Cornell University Press; 1996. pp. 243–266. [Google Scholar]
- 43.Meiri S, Yom-Tov Y, Geffen E. What determines conformity to Bergmann's rule? Glob Ecol Biogeogr. 2007;16:788–794. [Google Scholar]
- 44.Thurber JM, Peterson RO. Changes in body size associated with range expansion in the coyote (Canis latrans) J Mammal. 1991;72:750–755. [Google Scholar]
- 45.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 46.Torres RD. 1989. The mammalian carnivore-herbivore relationships in a late Pleistocene asphalt deposit at Maricopa, Kern County, California. Master's thesis (California State University, Long Beach)
- 47.Arjo WM, Pletscher DH. Behavioral responses of coyotes to wolf recolonization in northwestern Montana. Can J Zool. 1999;77:1919–1927. [Google Scholar]
- 48.Gese EM, Rongstad OJ, Mytton WR. Relationship between coyote group-size and diet in Southeastern Colorado. J Wildl Manage. 1988;52:647–653. [Google Scholar]
- 49.Ogle TF. Predator prey relationships between coyotes and white-tailed deer. Northwest Sci. 1971;45:213–218. [Google Scholar]
- 50.Ozoga JJ, Harger EM. Winter activities and feeding habits of northern Michigan coyotes. J Wildl Manage. 1966;30:809–818. [Google Scholar]
- 51.Carbone C, et al. Parallels between playbacks and Pleistocene tar seeps suggest sociality in an extinct sabretooth cat, Smilodon. Biol Lett. 2009;5:81–85. doi: 10.1098/rsbl.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus LF, Berger R. The significance of radiocarbon dates for Rancho La Brea. In: Martin PS, Klein RG, editors. Quaternary Extinctions: A Prehistoric Revolution. Tucson, AZ: University of Arizona Press; 1984. pp. 159–183. [Google Scholar]
- 53.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett. 2005;8:1114–1127. [Google Scholar]
- 54.Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21:465–477. [Google Scholar]
- 55.Abrams PA, Chen X. The evolution of traits affecting resource acquisition and predator vulnerability: Character displacement under real and apparent competition. Am Nat. 2002;160:692–704. doi: 10.1086/342822. [DOI] [PubMed] [Google Scholar]
- 56.Abrams PA. Character shifts of prey species that share predators. Am Nat. 2000;156:S45–S61. doi: 10.1086/303415. [DOI] [PubMed] [Google Scholar]
- 57.Carroll SP, Hendry AP, Reznick DN, Fox CW. Evolution on ecological time-scales. Funct Ecol. 2007;21:387–393. [Google Scholar]
- 58.Langerhans RB, Layman CA, Shokrollahi AM, DeWitt TJ. Predator-driven phenotypic diversification in Gambusia affinis. Evolution. 2004;58:2305–2318. doi: 10.1111/j.0014-3820.2004.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 59.Rundle HD, Vamosi SM, Schluter D. Experimental test of predation's effect on divergent selection during character displacement in sticklebacks. Proc Natl Acad Sci USA. 2003;100:14943–14948. doi: 10.1073/pnas.2036360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pimm SL, Gittleman JL. Carnivores and ecologists on the road to Damascus. Trends Ecol Evol. 1990;5:70–73. [Google Scholar]
- 61.Fox-Dobbs K, Leonard JA, Koch PL. Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;261:30–46. [Google Scholar]
- 62.Newsome SD, et al. Pleistocene to historic shifts in bald eagle diets on the Channel Islands, California. Proc Natl Acad Sci USA. 2010;107:9246–9251. doi: 10.1073/pnas.0913011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García N, Virgós E. Evolution of community composition in several carnivore palaeoguilds from the European Pleistocene: The role of interspecific competition. Lethaia. 2007;40:33–44. [Google Scholar]
- 64.Werdelin L, Lewis ME. Plio-Pleistocene Carnivora of eastern Africa: Species richness and turnover patterns. Zool J Linn Soc. 2005;144:121–144. [Google Scholar]
- 65.Schultz JR. A late Quaternary mammal fauna from the tar seeps of McKittrick, California. In: Wilson RW, et al., editors. Studies on Cenozoic Vertebrates of Western North America. Washington, D.C.: Carnegie Institution of Washington; 1938. pp. 112–215. [Google Scholar]
- 66.Muleady-Mecham NE. Differential preservation of fossil elements in the Maricopa Brea, California. Bull South Calif Acad Sci. 2003;102:79–88. [Google Scholar]
- 67.Jefferson G, McDonald H, Akersten W, Miller S. 2002. Idaho Museum of Natural History Occasional Paper 37: Catalogue of Late Pleistocene and Holocene fossil vertebrates from Idaho. And Whereas…Papers on the Vertebrate Paleontology of Idaho Honoring John A. White, eds Akersten W, Thompson M, Meldrum D, Rapp R, McDonald H (Idaho Museum of Natural History, Pocatello, ID), Vol 2, pp 157–192.
- 68.Gittleman G, Van Valkenburgh B. Sexual dimorphism in the canines and skulls of carnivores: Effects of size, phylogeny and behavioral ecology. J Zool (Lond) 1997;242:97–117. [Google Scholar]
- 69.Rutledge LY, Bos KI, Pearce RJ, White BN. Genetic and morphometric analysis of sixteenth century Canis skull fragments: Implications for historic eastern and gray wolf distribution in North America. Conserv Genet. 2010;11:1273–1281. [Google Scholar]
- 70.Van Valkenburgh B. Locomotor diversity within past and present guilds of large predatory mammals. Paleobiology. 1985;11:406–428. [Google Scholar]
- 71.Van Valkenburgh B. Skeletal indicators of locomotor behavior in living and extinct carnivores. J Vertebr Paleontol. 1987;7:162–182. [Google Scholar]
- 72.Samuels JX, Van Valkenburgh B. Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol. 2008;269:1387–1411. doi: 10.1002/jmor.10662. [DOI] [PubMed] [Google Scholar]
- 73.Anyonge W. Body mass in large extant and extinct carnivores. J Zool (Lond) 1993;231:339–350. [Google Scholar]
- 74.Anyonge W, Roman C. New body mass estimates for Canis dirus, the extinct Pleistocene dire wolf. J Vertebr Paleontol. 2006;26:209–212. [Google Scholar]
- 75.Jouzel J, et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science. 2007;317:793–796. doi: 10.1126/science.1141038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.