Abstract
Although protein S-nitrosylation is increasingly recognized as mediating nitric oxide (NO) signaling, roles for protein denitrosylation in physiology remain unknown. Here, we show that S-nitrosoglutathione reductase (GSNOR), an enzyme that governs levels of S-nitrosylation by promoting protein denitrosylation, regulates both peripheral vascular tone and β-adrenergic agonist-stimulated cardiac contractility, previously ascribed exclusively to NO/cGMP. GSNOR-deficient mice exhibited reduced peripheral vascular tone and depressed β-adrenergic inotropic responses that were associated with impaired β-agonist–induced denitrosylation of cardiac ryanodine receptor 2 (RyR2), resulting in calcium leak. These results indicate that systemic hemodynamic responses (vascular tone and cardiac contractility), both under basal conditions and after adrenergic activation, are regulated through concerted actions of NO synthase/GSNOR and that aberrant denitrosylation impairs cardiovascular function. Our findings support the notion that dynamic S-nitrosylation/denitrosylation reactions are essential in cardiovascular regulation.
Keywords: excitation-contraction coupling, nitroso-redox imbalance
Guanosine 3′,5′-cyclic monophosphate (cGMP)-dependent and -independent signaling by nitric oxide (NO) has been described in many organ systems, including the cardiovascular (CV) system (1, 2). Accumulating evidence indicates that the principal non-cGMP signal is effected by the covalent attachment of NO to the thiol group of cysteine (Cys) residues (S-nitrosylation) (3) and that this posttranslational modification may influence cardiac contractility (4) and peripheral vascular resistance (5) through effects on ion channels (6) and adrenergic receptors (7). Because deletion or inhibition of NO synthase (NOS) diminishes all forms of NO bioactivity and thus impairs both cGMP and S-nitrosylation signaling, it has been difficult to elucidate the exact roles of S-nitrosylation vs. cGMP in CV regulation.
Investigation of the role of S-nitrosylation in cellular signaling has been aided by discovery of enzymes that metabolize S-nitrosothiols (SNOs) without affecting NOS activity or levels of NO itself (mammalian enzymes that directly metabolize NO have not been identified) (8–10). In particular, S-nitrosoglutathione reductase (GSNOR), an enzyme involved in the removal of NO groups from Cys thiols in proteins (SNO-proteins) through metabolism of S-nitrosoglutathione (GSNO, which is in equilibrium with SNO-proteins), has been ascribed an indispensable role in regulating S-nitrosylation in the CV system (9). Although GSNOR does not affect baseline blood pressure, it mitigates hypotension induced by anesthetics and infectious agents (5) and plays an essential role in regulating both β-adrenergic receptor expression and responsiveness in the heart (7). These studies suggest that SNOs may exert physiological roles in the control of systemic hemodynamics and cardiac contractility.
In the CV system, endothelial NOS (NOS3, eNOS) and neuronal NOS (NOS1, nNOS) subserve endothelium-dependent vasodilation (11–13) and regulate β-adrenergic receptor signaling and intracellular calcium handling (7, 14–16), thereby influencing peripheral vascular resistance and cardiac inotropic responses. Although a role for cGMP in these effects is well established (17), the regulatory functions of S-nitrosylation have not been well-elucidated.
Here, we demonstrate that GSNOR−/− mice exhibit a CV phenotype characterized by decreased vascular tone and impaired cardiac inotropic response to β-adrenergic stimulation. Our data identify GSNO, the substrate of GSNOR, as a physiologic second messenger in the CV system and indicate that protein denitrosylation by GSNOR is essential for CV regulation. Our studies thus support the notion that CV disease, which is generally attributed to impaired NOS activity, may additionally or alternatively reflect dysregulated S-nitrosylation.
Results
Integrated Hemodynamic Measurements.
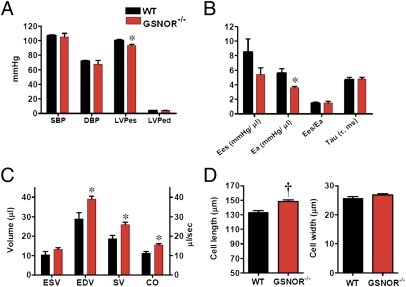
To establish whether GSNOR participates in physiological regulation of the CV system, we measured integrated CV performance in female mice homozygous for a deletion of GSNOR (GSNOR−/−) and in corresponding WT C57BL/6 mice by using a combined left ventricular (LV) pressure–volume catheter (16) (Fig. 1). GSNOR−/− mice, as previously described (5), have similar blood pressure to WT mice (Fig. 1A). In addition, basal cardiac contractility [maximum rate of change in left ventricular pressure (dP/dtmax) and ratio of dP/dtmax to instantaneously developed pressure (dP/dt-IP); Table S1], ventricular elastance (Ees), a load-independent measurement of contractility, and coupling of the cardiac to the vascular system [ratio of Ees to arterial elastance (Ea), Ees/Ea; Fig. 1B] are normal. The Ees/Ea assesses the interaction between ventricular peak stiffening and vascular tone (18). The optimal Ees/Ea value, indicative of flow output at a minimal energy cost, is between 1 and 2 (19). Thus, GSNOR−/− mice manifest normal cardiac mechanical efficiency. Importantly, however, GSNOR−/− mice exhibit a greatly decreased Ea (Fig. 1B; 5.6 ± 0.6 mmHg/μL in WT vs. 3.6 ± 0.2 mmHg/μL in GSNOR−/−, P < 0.05), indicating a state of systemic vasodilatation. Blood pressure is thus maintained in the normal range by increases in cardiac output (Fig. 1C; 11.1 ± 1 μL/s in WT vs. 15.4 ± 0.7 μL/s in GSNOR−/−, P < 0.05). Cardiac chamber size [end diastolic volume (EDV)] is also increased in GSNOR−/− mice (Fig. 1C; 28.9 ± 3.1 μL in WT vs. 39.1 ± 1.4 μL in GSNOR−/−, P < 0.05) because of an increase in myocyte length (Fig. 1D). Thus, the GSNOR−/− mouse evidently adapts to chronic reduction in vascular tone (systemic vasodilatation) with cardiac chamber enlargement and increased cardiac output.
Fig. 1.
Integrated hemodynamics reveal reduced vascular tone and increased cardiac output in female GSNOR−/− mice. (A) Systolic (SBP) and diastolic (DBP) blood pressure of GSNOR−/− mice is indistinguishable from WT, although the LV-end systolic pressure (LVPes) is decreased in GSNOR−/− mice, which is a reflection of lower systemic vascular resistance. LVPed, LV-end diastolic pressure. (B) GSNOR−/− mice have reduced afterload. Vascular elastance is markedly reduced (Ea; P < 0.05 vs. WT), a finding consistent with increased vascular NO bioactivity. The coupling of Ees/Ea and myocardial lusitropy (Tau) are similar between the strains. (C) Integrated CV function. GSNOR−/− mice compensate by increasing chamber size (EDV), thereby increasing stroke volume (SV) and cardiac output (CO) (all P < 0.05 vs. WT). End systolic volume (ESV) is similar between strains. (D) Myocyte length is increased in GSNOR−/− mice, consistent with the increased chamber size. *P < 0.05 vs. WT, †P < 0.001 vs. WT, t test.
GSNOR Localization and Activity.
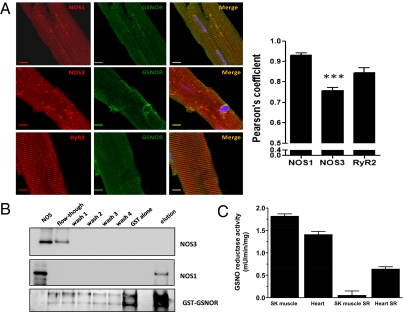
Previous work has established that regulation of β-adrenergic inotropic responses (7, 16, 20) and sarcoplasmic reticulum (SR) Ca2+ cycling (21) by NOS3 and NOS1, respectively, is achieved via their subcellular compartmentalization at the plasma membrane and SR (14). At the plasma membrane NOS3 interacts with target proteins, including caveolin and β-arrestin, whereas at the SR NOS1 interacts with cardiac ryanodine receptor 2 (RyR2) (14, 21). Immunoblotting (Fig. S1A) and confocal microscopy (Fig. S1C and Fig. 2A) showed that, although GSNOR is present in the hearts of mice (and humans) and widely distributed throughout the myocardium, a significant proportion of immunoreactivity is distributed in a T-tubular pattern and colocalizes with RyR2. RyR2 expression is similar in WT and GSNOR−/− myocytes (Fig. S1B), whereas GSNOR is absent from GSNOR−/− myocytes (Fig. S1 A and C). GSNOR activity, assayed in cardiac and skeletal muscle SR-enriched fractions and crude homogenates, is enriched in SR of cardiac but not skeletal muscle (Fig. 2C). This difference is consistent with the observation that GSNO has a primary role in regulating cardiac versus skeletal Ca2+ release (22). These results also reveal that the GSNOR denitrosylase is, in significant part (∼35% of whole-cell activity), localized to the cardiac myocyte SR, the organelle that subserves inotropic Ca2+ signaling and the principal site of NOS1 in the heart.
Fig. 2.
GSNOR colocalizes with NOS1 and RyR2 in the heart. (A) Confocal fluorescent microscopy images depict colocalization of GSNOR with NOS1 but not NOS3 and colocalization of GSNOR with RyR2 along the T-tubular invaginations of the cardiac myocyte. (Scale bars: 10 μM.) (B) GSNOR binds NOS1 but not NOS3 in vitro. Aliquots of recombinant NOS1 and NOS3 were incubated with GST or GST-GSNOR fusion proteins. The first well corresponds to the positive control, being either NOS1 or NOS3 (2.5 ng). The second well corresponds to the first flow through the column after binding. Consecutive washes of the column were then performed before elution. The bottom gel shows the profile (silver staining) of elution of GST-GSNOR (∼67 kDa) in the NOS1 binding experiment. (C) GSNOR enzymatic activity in total skeletal and heart muscle homogenates and in purified SR preparations. GSNOR activity is enriched in SR of cardiac but not skeletal muscle.
Interaction of GSNOR and NOS.
We next examined whether there is an interaction between GSNOR and NOS. Confocal fluorescent microscopy images show colocalization of GSNOR with NOS1 but not NOS3 (Fig. 2A). The Pearson correlation coefficient, used for quantitative analysis of colocalization, is significantly decreased in the GSNOR and NOS3 dual-stained myocytes.
To test for a direct interaction between GSNOR and NOS1, immunoprecipitation experiments with GSNOR and NOS1 or NOS3 were performed, but the results were confounded by nonspecific binding of proteins. Therefore, as an alternative approach, we immobilized GST-tagged GSNOR to a glutathione resin and incubated the complex with recombinant human NOS1 or NOS3. After elution, the complexes were examined by Western blot analysis. NOS3 was seen only in the flow-through, whereas NOS1 was found only in the elution fraction (Fig. 2B). These results indicate that NOS1, but not NOS3, bound to GST-GSNOR, and they support the view that enzymatic machinery for both S-nitrosylation and denitrosylation is resident in cardiac SR.
Effect of S-Nitrosylation on β-Adrenergic Response and Diastolic Ca2+ Leak.
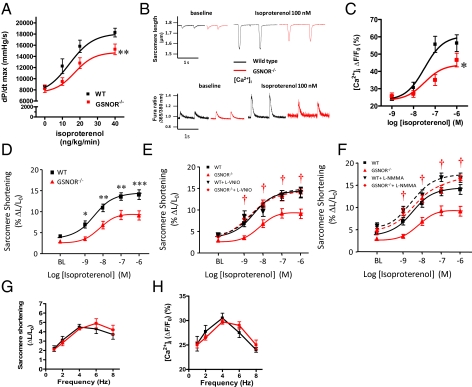
Motivated by these findings, we evaluated the role of GSNOR in regulation of excitation–contraction coupling. To this end, mice (prepared as for integrated hemodynamics) received i.v. infusions of increasing concentrations of the β-adrenergic agonist isoproterenol (ISO). This treatment produced increases in myocardial contractility (peak dP/dtmax) that were significantly attenuated in GSNOR−/− mice (Fig. 3A).
Fig. 3.
β-Adrenergic inotropic response is blunted in GSNOR−/− mice. (A) Dose–response curve for the in vivo effect of ISO on cardiac contractility in WT (n = 12) and GSNOR−/− (n = 11) mice. *P < 0.05, two-way ANOVA. (B) Representative traces of sarcomere shortening and [Ca2+]i in myocytes isolated from WT and GSNOR−/− hearts at baseline and after ISO (100 nM). (C and D) [Ca2+]i and sarcomere shortening dose–response to ISO in isolated myocytes. GSNOR−/− myocytes (n = 11–13, 2–10 cells per heart) displayed a blunted response to ISO vs. WT (*P < 0.05, **P < 0.01, ***P < 0.001; n = 11–14, 2–4 cells per heart). (E and F) Specific inhibition of NOS1 with l-VNIO (100 μM) as well as pan-NOS inhibition with l-NMMA (100 μM) normalized the β-adrenergic responses in GSNOR−/− myocytes (†P < 0.05 vs. control). (G and H) Evaluation of the force–frequency response (sarcomere shortening and the amplitude of the [Ca2+]i) in isolated myocytes stimulated at 1–8 Hz. The force–frequency relationship is preserved in GSNOR−/− cardiomyocytes.
Next, we assessed the β-adrenergic inotropic response in isolated myocytes paced at 1 Hz. Although electrically evoked baseline Ca2+ transients ([Ca2+]i) and sarcomere shortening were similar in myocytes isolated from WT and GSNOR−/− mice (Fig. 3B), GSNOR−/− myocytes demonstrated substantial blunting of both [Ca2+]i and sarcomere shortening in response to ISO (Fig. 3 C and D). Specific inhibition of NOS1 with N5-(1-imino-3-butenyl)-l-ornithine (l-VNIO) as well as NOS inhibition with NG-monomethyl-l-arginine monoacetate (l-NMMA) normalized the β-adrenergic responses in GSNOR−/− myocytes (Fig. 3 E and F), verifying the role of GSNO in the impaired inotropic responses. By contrast, treatment with KT5823 (1 μM), a PKG inhibitor, did not restore responsiveness to ISO (Fig. S2). Increases in contractility and [Ca2+]i stimulated by force–frequency were not different between strains (Fig. 3 G and H).
We performed several experiments to show that blunting of β-adrenergic contractility by NO/SNO was not caused by impairment of signaling through the β-adrenergic receptor nor mediated by changes in the phosphorylation state of phospholamban or RyR2 (7). In particular, ISO-mediated phosphorylation of phospholamban at Ser-16 was similar in GSNOR−/− and WT strains, and the degree of phosphorylation of Thr-17 was neither different nor changed by ISO (Fig. S3A). In addition, phosphorylation of RyR2 at Ser-2809 was increased similarly by ISO in GSNOR−/− and WT mice (Fig. S3B). The levels of guanylyl cyclase and cGMP were also examined and showed no differences between strains (Fig. S3C). Altogether, these results suggest that the blunted inotropic responses in GSNOR−/− mice are unrelated to NOS3/cGMP or to a defect in β-adrenergic receptor signaling (note that β-adrenergic receptor density is actually higher in hearts from GSNOR−/− mice) (7) and point to aberrant Ca2+ cycling as the cause. Furthermore, any molecular explanation for NO/SNO effects must account for the observation that Ca2+ cycling is only impaired after stimulation by agonists.
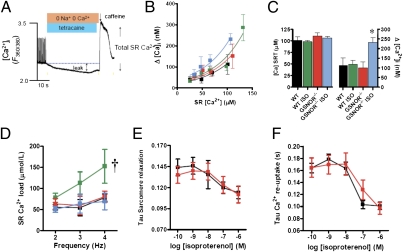
Calcium leak is well known to be associated with conditions of impaired cardiac and skeletal muscle function (23, 24), and increased S-nitrosylation of RyR1 has been implicated in a Ca2+ leak that underlies the pathogenesis of skeletal muscle disorders, including malignant hyperthermia, exercise-induced fatigue, and central core disease (25, 26). On the other hand, decreased S-nitrosylation of RyR2, caused by nitroso–redox imbalance, has also been linked with diastolic Ca2+ leak and depressed contractility in a rodent model of heart failure (21, 27). We therefore considered the possibility that aberrant S-nitrosylation of RyR2 would produce a Ca2+ leak that would compromise excitation–contraction coupling. We used a tetracaine–caffeine protocol (Fig. 4A) to measure Ca2+ leak from RyR2 under basal conditions and after stimulation by ISO. Notably, whereas the GSNOR−/− mouse showed intact load–leak relationships at rest, a substantial increase in SR Ca2+ leak was detected after ISO stimulation in GSNOR−/− vs. WT mice (Fig. 4 B and C), an effect that has direct bearing on SR Ca2+ content (Fig. 4D). Although WT myocytes increased SR Ca2+ content with ISO, GSNOR−/− myocytes failed to augment SR Ca2+ load (Fig. 4D), consistent with SR leak because measurement of reuptake parameters, including Tau sarcomere relaxation and Tau Ca2+ reuptake, was not different between groups (Fig. 4 E and F). Thus, GSNOR, by regulating RyR2 activity, controls SR Ca2+ levels during β-adrenergic stimulation in a manner that is independent of the reuptake systems.
Fig. 4.
Increased diastolic Ca2+ leak in GSNOR−/− cardiomyocytes. (A) Protocol used to estimate diastolic SR Ca2+ leak. (B) Load–leak relationship in WT (n = 12) and GSNOR−/− myocytes (n = 10 animals, 4–9 cells per data point) in the presence or absence of ISO (100 nM) shows a leftward shift in GSNOR−/− myocytes stimulated by ISO. (C) Comparison of the amount of diastolic Ca2+ leak against a matched load for both strains in the presence or absence of ISO. GSNOR−/− myocytes manifest a substantial increase in SR Ca2+ leak after ISO stimulation (*P < 0.05, ANOVA). (D) SR Ca2+ content at different frequencies in the presence or absence of ISO (†P < 0.05, ANOVA). GSNOR−/− myocytes fail to augment SR Ca2+ load in response to ISO. (E) Sarcomere relaxation in response to ISO was similar in WT versus GSNOR−/− cardiac myocytes. (F) The Tau constant of [Ca2+]i decline, a measurement of Ca2+ reuptake, was similar in WT vs. GSNOR−/− myocytes. Black, WT; green, WT treated with ISO; red, GSNOR−/−; blue, GSNOR−/− treated with ISO.
Effect of S-Nitrosylation on Regulatory Heart Proteins.
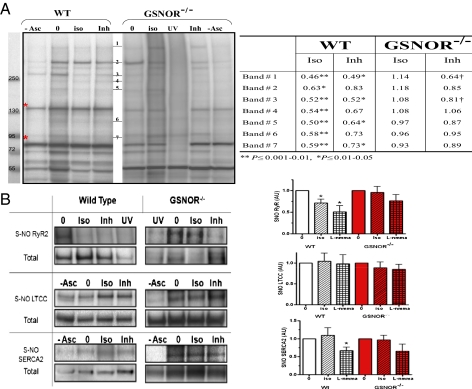
It has been reported that both the L-type Ca2+ channel (LTCC) (28) and the RyR (29) are targets of S-nitrosylation, and a regulatory effect of GSNOR on either or both Ca2+ channels would, in principle, be consistent with our findings. We performed biotin-switch assays for S-nitrosylation on total cardiac extracts from WT and GSNOR−/− hearts, followed by immunoprecipitation or streptavidin-affinity and Western blotting strategies to establish the identity of cardiac SNO proteins underlying cardiac regulation (Fig. 5A and Table S2). This approach identified RyR2, SR Ca2+ ATPase (SERCA2a), and LTCC as physiological substrates of S-nitrosylation (Fig. 5B), whereas other calcium-handling proteins such as the plasma membrane sodium–calcium exchanger (NCX) were not detected. Several steps were taken to verify these results, including the use of prephotolysis (UV) to eliminate cellular SNOs (30) (Fig. 5 A and B) and validation of RyR2 and SERCA2a nitrosylation by MS/MS (Tables S2 and S3). Consistent with findings in other organ systems (5, 31), there were no differences in basal SNO modification of RyR2, SERCA2a, and LTCC between WT and GSNOR−/− hearts. In addition, the NOS inhibitor l-NMMA reduced the levels of S-nitrosylated proteins in both WT and GSNOR−/− animals (Fig. 5 A and B).
Fig. 5.
Effect of ISO and NOS on S-nitrosylation of proteins in the heart and cardiomyocytes. (A) S-nitrosylated protein band clusters in WT and GSNOR−/− mice heart homogenates by Western blot analysis. Each lane represents biotin-switched homogenate of a whole heart perfused with Krebs–Henseleit solution (0), in the presence of 2.5 nM ISO (iso) or the NOS inhibitor (Inh) l-NMMA (100 μM). Elimination of sodium ascorbate (−Asc) or exposure to UV-photolysis (UV) are controls for the biotin-switch technique (30). Red asterisks indicate endogenously biotinylated proteins. The table shows densitometric quantification of SNO protein band clusters normalized to baseline (0). ISO and l-NMMA reduced S-nitrosylation levels in WT whereas this effect is blocked or attenuated in GSNOR−/− mice (*P ≤ 0.01–0.05 and †P ≤ 0.001–0.01; ANOVA with Student Newman–Keuls post hoc test; WT 0 and ISO, n = 11; l-NMMA, n = 5; GSNOR−/− 0 and ISO, n = 9; l-NMMA, n = 3). (B) Representative Western blot images of immunoprecipitated SNO-RyR2, LTCC, and SERCA2 after biotin-switch assay, with cumulative measurements displayed graphically. Each graph shows the effect of ISO and l-NMMA on SNO levels of RyR2, LTCC, and SERCA2 in WT and GSNOR−/− mice hearts. The SNO level was detected by anti-biotin antibody normalized to the total protein load as detected with its specific antibody (*P ≤ 0.01–0.05; ANOVA with Student Newman–Keuls or Kruskal–Wallis posttest). Western blot images are representative of n = 4–6 RyR2, n = 7–9 LTCC, and n = 4–6 SERCA2. In the GSNOR−/− heart, ISO-induced denitrosylation of RyR2 was substantially abrogated.
We also examined the consequences of β-adrenergic receptor stimulation (2.5 nM ISO for 10 min) on protein nitrosylation in situ. Remarkably, ISO tended to decrease the steady-state levels of multiple SNO-proteins, generally matching the effect of NOS inhibition with l-NMMA (Fig. 5A). These data suggest that ISO induces the denitrosylation of multiple proteins that are actively S-nitrosylated by constitutive NOS. Separate measurements were conducted on Ca2+-handling proteins to explore the basis of the Ca2+ leak in GSNOR−/− mice. These studies showed that, among the endogenously S-nitrosylated Ca2+-handling proteins, only RyR2 S-nitrosylation was linked to stimulation of the β-adrenergic receptor (Fig. 5B). More specifically, in WT animals, RyR2 (but not SERCA2 or LTCC) underwent denitrosylation in response to ISO, and decreases in SNO-RyR2 mediated by ISO were comparable to those elicited by l-NMMA (Fig. 5B). Thus, RyR2 undergoes cycles of NOS-mediated S-nitrosylation that are followed by ISO-induced denitrosylation. By contrast, in the GSNOR−/− mouse, ISO-induced denitrosylation was substantially abrogated (and the NOS-inhibitor l-NMMA more modestly lowered the levels of SNO-RyR2). S-nitrosylation/denitrosylation of RyR2 is thus regulated by the concerted actions of nNOS and GSNOR, and deletion of GSNOR leads to impaired denitrosylation of RyR2.
Denitrosylation induced by ISO was confirmed by confocal fluorescence microscopy images of cardiomyocytes in WT mice compared with GSNOR−/− mice (Fig. S4 A–C). In these studies, nitrosylation was measured as fluorescence intensity (Fig. S4B) of cardiac myocytes by using an anti–Cys-NO antibody with verified specificity (Fig. S5). Merged images were deconvolved and analyzed by computing the Pearson correlation coefficient for quantitative colocalization (Fig. S4C). In addition, l-NMMA (2 mM) decreased the SNO signal, whereas NO donor S-nitroso-N-acetylpenicillamine (SNAP; 1 mM) increased it. Collectively, our results implicate dynamic S-nitrosylation and denitrosylation of RyR2 as critical to β-adrenergic inotropic responses, the central mechanism underlying cardiac contractile reserve.
Discussion
S-nitrosylation of Cys residues has emerged as a major signaling pathway through which NO modifies protein activity and thereby elicits diverse effects (32–34). A major mechanism of S-nitrosylation involves transnitrosylation by GSNO (32, 34). GSNOR selectively metabolizes GSNO and thereby depletes the levels of S-nitrosylated proteins in equilibrium with GSNO (9). In the present paper, we have demonstrated that mice deficient in GSNOR exist in a persistent state of systemic vasodilatation. Blood pressure is thus maintained by increased cardiac output. In addition, basal cardiac contractility and mechanical efficiency are normal, but the cardiac inotropic response to β-adrenergic stimulation is impaired. Thus, GSNOR governs two essential CV responses, systemic vasodilatation and β-agonist–induced inotropic responses, indicating that GSNO plays a key a role in classic physiology customarily ascribed to NO/cGMP (2, 11–13).
S-nitrosylation modulates the function of ion channels that regulate excitation–contraction coupling and thereby subserve normal systolic and diastolic function (32, 34). These channels may also undergo alternative redox-based modifications (35–37), including S-glutathionylation and higher S-oxidations, particularly under pathological conditions (29, 38–40). In animal models of heart failure, hyponitrosylation with concomitant oxidation of the RyR2 leads to SR Ca2+ leak, which is corrected by antioxidants, with improvement in cardiac function (27, 36, 37). We have also shown that a low concentration of NO donor, which restores the nitrosylation state of the channel, increases cardiac contractility (4, 21, 27). Collectively, these observations highlight the importance of nitroso–redox balance within the SR in determining cardiac function. The current study adds perspective by showing that the steady-state levels of RyR2 nitrosylation may be determined by the activity of denitrosylases. Impaired denitrosylation of RyR2 in response to β-adrenergic stimulation resulted in diastolic Ca2+ leak, indicating that RyR2 function is governed by dynamic cycles of nitrosylation and denitrosylation. Therefore, hypo- and hypernitrosylation of RyR2 may have similar consequences on RyR function. Consistent with this idea, increased S-nitrosylation of RyR2 in a mouse model of Duchenne muscular dystrophy (mdx)-associated dilated cardiomyopathy resulted in diastolic SR Ca2+ leak and triggered cardiac arrhythmias (41). In addition, mice with constitutive PKA hyperphosphorylation of RyR2 develop age-dependent cardiomyopathy associated with progressive oxidation and S-nitrosylation of RyR2 and with diastolic SR Ca2+ leak (42).
It has been shown that β-adrenergic agonists directly stimulate NO production via RyR2-associated NOS1 (43, 44), which leads to an increase in RyR2 S-nitrosylation and activity. We now demonstrate a likely interaction between GSNOR and NOS1, which suggests that enzymatic machinery for both S-nitrosylation and denitrosylation is resident in the SR within a ternary complex with RyR2. We also show that levels of RyR2 S-nitrosylation after agonist stimulation are set primarily by GSNOR (rather than NOS1), by direct analogy to the role of phosphatases in setting levels of phosphorylation, and that impaired denitrosylation of RyR2 may compromise the contractile response to β-adrenergic agonists. Denitrosylation by GSNOR thus prevents excessive RyR2 activity by preventing NOS1-induced hypernitrosylation, as evidenced by the increased SR Ca2+ leak and failure to augment SR Ca2+ load after ISO stimulation in GSNOR−/− myocytes. The mechanism by which GSNOR activity is evidently coupled to β-adrenergic stimulation and the identity of the full complement of proteins that undergo ISO-mediated denitrosylation remain to be determined.
Our results have important physiological ramifications. In particular, systemic hemodynamic responses at rest and after β-adrenergic stimulation are evidently regulated through concerted actions of NOS1/GSNOR (that enhance Ca2+ cycling in SR) (14) and NOS3/GSNOR (that influence peripheral vascular tone), respectively (5). A substantial quantity of literature describes dysregulated NOS activity in CV and other diseases. Although our results do not in any way diminish the essential role of cGMP in NOS action (45), they establish an additional level of regulation of CV function through protein S-nitrosylation/denitrosylation, which may have implications for dysregulated S-nitrosylation in disease (26, 46, 47). In fact, impairments in CV denitrosylation manifest in GSNOR−/− mice have been linked to increased mortality after endotoxic challenge, hypotension under anesthesia, and survival after myocardial infarction (5, 48). Aberrant protein denitrosylation, which can result from altered GSNOR activity or expression, may thus play a previously underappreciated role in diseases such as hypertension and heart failure.
Materials and Methods
A detailed description of the materials and methods is in the SI Materials and Methods. Briefly, GSNOR−/− mice were generated as described (5) and compared with age-matched WT (C57BL/6; 4–8 months old). Myocardial systolic and diastolic performance was assessed from pressure-volume data, as described previously (16). Isolated myocyte sarcomere shortening (SL) and Ca2+ transients ([Ca2+]i were measured as described previously (21), and SR Ca2+ leak was determined using tetracaine and caffeine pulses as described previously (49). For the GST-GSNOR binding column, we used full-length recombinant GSNOR with a GST tag at the N terminus (10 μg; Abnova Corporation). Biotin-switch assay was performed as described in Forrester et al. (30), as modified from Jaffrey et al. (50). In addition, biotinylated proteins from whole extracts were separated with streptavidin-agarose beads and electrophoretically resolved, then bands at 550 and 110 KDa were submitted for MS/MS identification (Tables S2 and S3), and determined to be RyR2 and SERCA2a, respectively. GSNOR activity was assayed as described previously (31) in cardiac and skeletal muscle SR-enriched fractions and crude homogenates. For the immunofluorescence experiments, myocytes were fixed in 2% paraformaldehyde and dual-stained for RyR2 and Cys-NO and, in separate experiments, dual-stained for GSNOR and nNOS, eNOS, or RyR2. cGMP was measured using an enzyme immunoassay kit (Cayman, Ann Arbor, MI). For statistical analyses, data are reported as mean ± standard error of the mean (SEM). Concentration-effect relationships were analyzed by two-way analysis of variance (ANOVA) with interaction terms between isoproterenol concentration and genotype. Comparisons at baseline between C57Bl/6 and GSNOR−/− mice were performed using Student's t test.
Supplementary Material
Acknowledgments
This work was funded by National Heart, Lung, and Blood Institute Grants R01 HL-094849 and R01 HL-65455 (to J.M.H.) and HL91876, HL095463, HL059130, and P01 HL-075443 (to J.S.S.). J.M.H. is also funded by RO1 HL110737, HL107110, and HL084275.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113319109/-/DCSupplemental.
References
- 1.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 3.Gow AJ, et al. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 4.González DR, et al. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide. 2008;18:157–167. doi: 10.1016/j.niox.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 6.Carnes CA, et al. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. doi: 10.1074/jbc.M704893200. [DOI] [PubMed] [Google Scholar]
- 7.Whalen EJ, et al. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 10.Forrester MT, Eyler CE, Rich JN. Bacterial flavohemoglobin: A molecular tool to probe mammalian nitric oxide biology. Biotechniques. 2011;50:41–45. doi: 10.2144/000113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 12.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 13.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: An overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Barouch LA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa K, et al. S-nitrosylation of β-arrestin regulates β-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varghese P, et al. β3-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest. 2000;106:697–703. doi: 10.1172/JCI9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senzaki H, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates β-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 18.Hare JM, Kass DA, Stamler JS. The physiological response to cardiovascular ‘orphan’ G protein-coupled receptor agonists. Nat Med. 1999;5:1241–1242. doi: 10.1038/15193. [DOI] [PubMed] [Google Scholar]
- 19.Naeije R, Huez S. Reflections on wave reflections in chronic thromboembolic pulmonary hypertension. Eur Heart J. 2007;28:785–787. doi: 10.1093/eurheartj/ehm040. [DOI] [PubMed] [Google Scholar]
- 20.Massion PB, et al. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates β-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, et al. Regulation of the cardiac muscle ryanodine receptor by O2 tension and S-nitrosoglutathione. Biochemistry. 2008;47:13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 24.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 25.Bellinger AM, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durham WJ, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 30.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 31.Que LG, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulman IH, Hare JM. Regulation of cardiovascular cellular processes by S-nitrosylation. Biochim Biophys Acta. April 16, 2011 doi: 10.1016/j.bbagen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen RI, Wilson D, Liu SF. Nitric oxide modifies the sarcoplasmic reticular calcium release channel in endotoxemia by both guanosine-3′,5′ (cyclic) phosphate-dependent and independent pathways. Crit Care Med. 2006;34:173–181. doi: 10.1097/01.ccm.0000194722.12260.f9. [DOI] [PubMed] [Google Scholar]
- 36.Mochizuki M, et al. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez G, Pedrozo Z, Domenech RJ, Hidalgo C, Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol. 2005;39:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Aracena-Parks P, et al. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 39.Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine receptor by different mechanisms. An allosteric function for O2 in S-nitrosylation of the channel. J Biol Chem. 2003;278:8184–8189. doi: 10.1074/jbc.M211940200. [DOI] [PubMed] [Google Scholar]
- 41.Fauconnier J, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan J, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saraiva RM, et al. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: Role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 44.Khan SA, et al. Nitric oxide regulation of myocardial contractility and calcium cycling: Independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 45.Cawley SM, et al. sGCα1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling. Am J Physiol Heart Circ Physiol. 2011;301:H157–H163. doi: 10.1152/ajpheart.01273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima B, et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci USA. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 50.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.