Abstract
Mitochondrial iron levels are tightly regulated, as iron is essential for the synthesis of Fe/S clusters and heme in the mitochondria, but high levels can cause oxidative stress. The ATP-binding cassette (ABC) transporter ABCB8 is a mitochondrial inner membrane protein with an unknown function. Here, we show that ABCB8 is involved in mitochondrial iron export and is essential for baseline cardiac function. Induced genetic deletion of ABCB8 in mouse hearts resulted in mitochondrial iron accumulation and cardiomyopathy, as assessed by echocardiography and invasive hemodynamics. Mice with ABCB8 deletion in the heart also displayed mitochondrial damage, and higher levels of reactive oxygen species and cell death. Down-regulation of ABCB8 in vitro resulted in decreased iron export from isolated mitochondria, whereas its overexpression had the opposite effect. Furthermore, ABCB8 is needed for the maturation of the cytosolic Fe/S proteins, as its deletion in vitro and in vivo led to decreased activity of cytosolic, but not mitochondrial, iron–sulfur-containing enzymes. These results indicate that ABCB8 is essential for normal cardiac function, maintenance of mitochondrial iron homeostasis and maturation of cytosolic Fe/S proteins. In summary, this report provides characterization of a protein involved in mitochondrial iron export.
ATP-binding cassette (ABC) proteins compose one of the largest families of proteins (1), and use energy derived from ATP hydrolysis to transport various substrates across a membrane (2). Although many cellular ABC proteins have been characterized, mitochondrial ABC proteins are only beginning to emerge as important regulators of this organelle's function. So far, 11 mitochondrial ABC proteins have been identified: three yeast isoforms, four in mammals, and four in plants. However, only a fraction of them have a known function. Atm1p, a mitochondrial ABC protein, plays a role in the maturation of cytosolic Fe/S clusters in yeast (3–6). The mammalian homologue of ATM1, ABCB7, can complement growth defect caused by ATM1 deletion in yeast, and mutations in ABCB7 are associated with X-linked sideroblastic anemia and cerebellar ataxia in humans (7–9). Both Atm1p and ABCB7 affect mitochondrial iron homeostasis, although the mechanism is not clear. Another yeast mitochondrial ABC protein, MDL1, only partially rescues the ATM1 deletion, regulates mitochondrial iron content and sensitivity to oxidative stress (10), and plays a role in mitochondrial peptide export (11). MDL1 and MDL2 share sequence homology with the mammalian ABCB8 (also known as mABC1) protein (12, 13). ABCB8 is a half-molecule ABC protein that localizes to the inner mitochondrial membrane (14), and contains single transmembrane- and nucleotide binding-domains (15). ABCB8 has been shown to protect against oxidant induced cell death (16); however, its primary function is not known.
Iron is an essential molecule in all organisms from single-cell bacteria to humans. Its ability to easily gain and lose electrons makes it a suitable constituent of hemoglobin, myoglobin, and cytochromes (17). Furthermore, iron is a component of the Fe/S cluster, an ancient biological molecule that is essential for many fundamental processes including cellular respiration, DNA replication and repair, as well as heme synthesis. Iron–sulfur cluster (ISC) assembly occurs in the mitochondria (18, 19), however, cytosolic isoforms of ISC machinery have also been identified (20). Iron is also a component of heme. Heme synthesis starts in the mitochondria, with intermediate steps in the cytoplasm and final reactions, including the incorporation of iron into protoporphyrin IX, in the mitochondria (21). As high levels of mitochondrial iron can be toxic through the formation of reactive oxygen species (ROS), mitochondrial iron levels are tightly regulated (22). Two proteins, mitoferrin 1 and 2, transport iron into this organelle, and mitochondrial ferritin is involved in mitochondrial iron storage (22). However, it is not known how iron is exported out of the mitochondria and whether mitochondrial iron export is needed for the activity of cytosolic Fe/S-containing proteins.
Although other ABC transporters have been studied in the heart, the role of mitochondrial ABC proteins in this organ has not been investigated. To characterize the function of ABCB8 in the heart, we created a mouse model of cardiac-specific ABCB8 KO, and studied the effects of this deletion on cardiac function and mitochondrial iron homeostasis. We demonstrate that cardiac deletion of ABCB8 results in a severe cardiomyopathy and mitochondrial iron accumulation. We also show that ABCB8 is required for maintenance of normal cardiac function, influences mitochondrial iron export, and plays a role in the maturation of cytosolic ISC-containing enzymes.
Results
Genetic Deletion of ABCB8 in Heart.
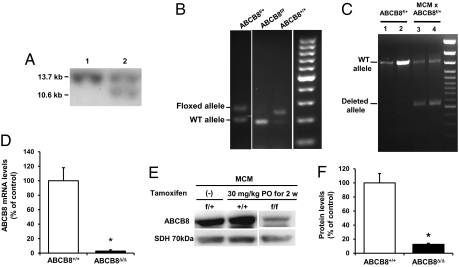
The primary function of ABCB8 is unknown; however, its yeast homologue MDL1 partially restores mitochondrial iron content in Δatm1 cells (10). Thus, we hypothesized that ABCB8 also plays a role in mitochondrial iron homeostasis. To test this, we generated mice with loxP sequences flanking exon 1 of ABCB8 (Fig. 1 A and B and Fig. S1). ABCB8 floxed mice (ABCB8f/f) were then bred with mice that contained a mutated estrogen receptor MER-Cre-MER (MCM) regulated by the cardiac-specific α-myosin heavy chain promoter (23). A 2-wk protocol of oral administration of tamoxifen resulted in successful KO of ABCB8 in the hearts of ABCB8f/f mice. The deletion of ABCB8 was confirmed at the level of genomic DNA (Fig. 1C), mRNA expression (Fig. 1D), and protein levels (Fig. 1 E and F). The residual ABCB8 protein in the hearts of tamoxifen-treated ABCB8f/f mice likely came from noncardiomyocyte sources, such as fibroblasts and endothelial cells, and the degree of gene deletion was comparable to that achieved in a similar tamoxifen-inducible cardiac-specific KO mouse model (24). There was no change in the levels of two additional mitochondrial ABC proteins, ABCB10 (also known as mABC2) and ABCB7 (Fig. S2), and there was no significant reduction in ABCB8 in other tissues (Fig. S3). For all subsequent studies, age- and sex-matched littermates were used as controls.
Fig. 1.
ABCB8 KO in the heart. (A) Southern blot analysis of WT (lane 1) and targeted ES cells (lane 2). Homologous recombination results in a detection of a smaller band after genomic DNA is digested with Sca I restriction enzyme, as shown in A. (B) PCR results of the 5′ arm of ABCB8 heterozygote and homozygote floxed mice. Presence of LoxP sequence results in a larger fragment. (C) Tamoxifen-mediated genetic ablation of ABCB8 in the heart. DNA samples from two untreated (labeled 1 and 2) and two tamoxifen-treated (labeled 3 and 4) mice are shown. (D) ABCB8 mRNA levels are significantly lower in MCM × ABCB8f/f mice 2 wk after treatment with per-oral (PO) tamoxifen compared with WT ABCB8+/+ mice. (E) Levels of ABCB8 protein after treatment of MCM × ABCB8f/f mice with per-oral tamoxifen for 2 wk are significantly lower than in control mice. (F) Summary of densitometry analysis of Western blot (n = 3). Data are presented as mean ± SEM (*P < 0.05).
Loss of ABCB8 Severely Compromises Cardiac Function.
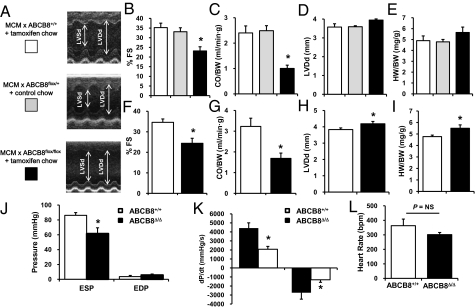
Compared with control mice (MCM transgenic mice treated with tamoxifen chow and MCM transgenic mice in the background of ABCB8f/+ treated with normal chow), ABCB8 KO mice displayed reduced heart pump function, or contractility, after 4 wk. We observed lower fractional shortening [FS; the ratio of left ventricular (LV) diameter in relaxation vs. contraction; Fig. 2 A and B], which correlates with reduced ejection fraction, the measure of LV volume in relaxation vs. contraction. Furthermore, cardiac output (CO; the volume of blood pumped per minute) per body weight (BW) was also reduced as early as 4 wk after tamoxifen treatment (Fig. 2C). However, heart weight (HW)/BW and LV diastolic diameter (LVDd; the measure of cardiac chamber dilation) were not significantly altered (Fig. 2 D and E). When ABCB8Δ/Δ mice were examined by echocardiography 8 wk after tamoxifen treatment, LVDd and HW were significantly elevated in addition to lower FS and CO compared with ABCB8+/+ mice, indicating compromised cardiac pump function (Fig. 2 F–I). Examination of ABCB8Δ/Δ hearts by invasive hemodynamic catheterization 8 wk after tamoxifen treatment showed lower LV end-systolic pressure (measure of systolic function), dP/dtmax (strength of contraction), and dP/dtmin (measure of relaxation) compared with the control animals, thus confirming the depressed contractility and relaxation, whereas no significant change was observed in the heart rate (Fig. 2 J–L). These findings suggest that deletion of ABCB8 results in compromised systolic function as early as 4 wk and systolic and diastolic dysfunction approximately 8 wk after deletion of the gene. However, these mice did not display gross features of heart failure, implying a mild degree of cardiomyopathy or activation of compensatory mechanisms.
Fig. 2.
Cardiac function is compromised in ABCB8 KO mice. (A) M-mode echocardiography images of control and ABCB8 KO mice. Two sets of control animals are used in these studies: MCM × ABCB8+/+ mice treated with tamoxifen chow (white bars) and MCM × ABCB8f/+ mice treated with regular chow (gray bars). (B–E) Control and ABCB8 KO mice were analyzed by echocardiography 4 wk after tamoxifen treatment. Both percentage of FS (measure of ventricular contractility) and CO/BW (the amount of blood pumped by the heart) are significantly reduced, whereas LVDd (measure of chamber dilation) and HW/BW (measure of cardiac size) are not different in ABCB8 KO mice compared with control (n = 6). (F–I) Echocardiographic analysis of control and ABCB8 KO mice at a delayed phase (8 wk after tamoxifen treatment; n = 6). (J) Invasive hemodynamic measurements 8 wk after tamoxifen treatment. (K) dP/dtmax (contractility) and dp/dtmin (relaxation) in WT and ABCB8Δ/Δ mice 8 wk after tamoxifen treatment. (L) Heart rate during hemodynamic measurements in WT and KO mice (n = 6). Data are presented as mean ± SEM (*P < 0.05).
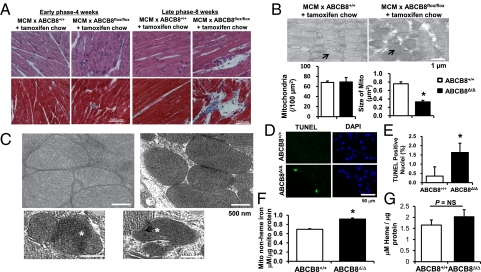
Consistent with deteriorating cardiac function, histological examination of ABCB8Δ/Δ hearts revealed distorted and wavy myofiber architecture when examined 4 wk after tamoxifen treatment, and exacerbated cardiac fibrosis after 8 wk (Fig. 3A). EM demonstrated subcellular disorganization in ABCB8Δ/Δ hearts, with accumulation of vacuoles, mitochondria showing diversity in size, profoundly disrupted alignment, and a rounder and more densely packed appearance compared with the hearts from WT mice (Fig. 3 B and C). Furthermore, ABCB8Δ/Δ hearts displayed loss of mitochondrial cristae, suggestive of the damage to mitochondrial architecture (Fig. 3C). Compared with WT mice, the average size of mitochondria was significantly smaller in ABCB8Δ/Δ mice, although the number was similar (Fig. 3B). Mitochondrial deformities accompanied by electron-dense material were also present in ABCB8Δ/Δ cardiomyocytes (Fig. 3C), and were similar to those observed in iron-loaded mouse hearts (25), suggesting iron aggregation. In addition, ABCB8Δ/Δ hearts displayed greater number of TUNEL-positive nuclei (Fig. 3 D and E), indicating increased apoptosis.
Fig. 3.
ABCB8 KO hearts display structural abnormalities. (A) Histological analysis of hearts from control (MCM × ABCB8+/+ treated with tamoxifen) and ABCB8Δ/Δ mice 4 and 8 wk after tamoxifen treatment. Upper: H&E eosin staining. Lower: Masson trichrome staining. Blue staining represents cardiac fibrosis. (B) Representative EM images of hearts from ABCB8+/+ and ABCB8Δ/Δ mice. Arrows indicate mitochondria. The graph below shows quantification of mitochondrial number and size (n = 5). (C) Representative EM images of hearts from ABCB8+/+ and ABCB8Δ/Δ mice. Hearts from ABCB8+/+ mice show well aligned mitochondria with clearly distinguishable cristae. Mitochondria from ABCB8Δ/Δ mouse hearts show accumulation of electron-dense material (asterisks), disruption of cristae, and deformed morphology. (D) LV myocardial sections from ABCB8+/+ and ABCB8Δ/Δ mice subjected to TUNEL and DAPI (nuclei) staining after 4 wk reveal higher cell death in ABCB8Δ/Δ mice. (E) Quantitative analysis of five fields as shown in D (n = 6). (F) Mitochondrial nonheme iron levels are higher in ABCB8 KO in the heart (n = 5). (G) The levels of mitochondrial heme are not altered in the hearts of ABCB8Δ/Δ mice (n = 3). Data are presented as mean ± SEM (*P < 0.05).
Finally, mitochondrial iron content was elevated in ABCB8Δ/Δ mouse hearts 4 wk after tamoxifen treatment (Fig. 3F and Fig. S4), but there was no difference in mitochondrial heme levels in ABCB8Δ/Δ mouse hearts compared with WT animals (Fig. 3G). Taken together, our results suggest that ABCB8 is important for maintenance of mitochondrial function and integrity, plays a role in mitochondrial iron homeostasis, and its deletion leads to cardiac dysfunction.
Finally, as we showed that deletion of ABCB8 results in cardiac dysfunction, we assessed the levels of this protein in explanted hearts from patients with end-stage cardiomyopathy. The levels of ABCB8 protein were significantly reduced in the explanted hearts, further supporting a role for ABCB8 in normal cardiac function (Fig. S5 A and B).
Modulation of ABCB8 in Vitro Alters Mitochondrial Iron Levels.
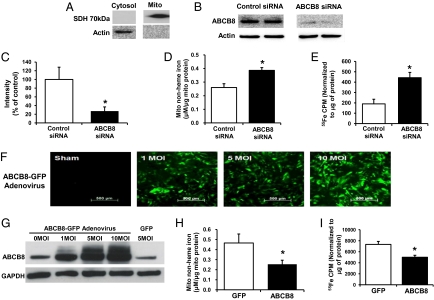
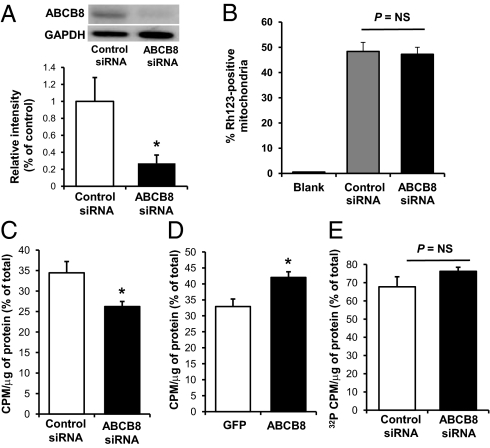
To better characterize the primary function of ABCB8, we measured mitochondrial iron levels after ABCB8 down-regulation with siRNA in neonatal rat cardiomyocytes (NRCMs). We first confirmed that our purification protocol yields a highly enriched mitochondrial fraction by Western blot (Fig. 4A). The ABCB8 siRNA led to a significant decrease in ABCB8 protein levels (Fig. 4 B and C), consistent with a previous work (16), and was associated with a significant accumulation of nonheme iron in the mitochondria, confirming our in vivo findings (Fig. 4D). We also measured mitochondrial iron levels by using radioactive iron. NRCMs were treated with ABCB8 siRNA in the presence of 55Fe, followed by measurement of mitochondrial 55Fe content. Consistently, down-regulation of ABCB8 in these cells resulted in a significant accumulation of 55Fe in the mitochondria (Fig. 4E).
Fig. 4.
ABCB8 down-regulation and overexpression in NRCMs result in an increase and a decrease in mitochondrial iron levels, respectively. (A) Western blot of cytosolic and mitochondrial fractions from NRCMs. (B) Western blot of NRCMs treated with control and ABCB8 siRNA. Actin is used as an internal control (n = 3). (C) Densitometric analysis of the Western blot in B (n = 3). (D) Mitochondrial nonheme iron levels are increased in NRCMs treated with ABCB8 siRNA compared with control (n = 4). (E) Radiotracer (55Fe) in the mitochondrial fraction is in a higher amount in cells treated with ABCB8 siRNA compared with control siRNA (n = 3). (F) Confocal images of NRCMs transduced at a multiplicity of infection (MOI) of 0, 1, 5, and 10 of ABCB8 adenovirus with a GFP coding sequence. (G) Western blot of extracts from NRCMs transduced with increasing amounts of the ABCB8 or GFP adenovirus. (H) Mitochondrial nonheme iron levels are lower in NRCMs transduced at an MOI of 5 of ABCB8 adenovirus compared with GFP-transduced cells (n = 4). (I) Mitochondrial 55Fe levels in NRCMs treated with ABCB8 adenovirus are lower than in GFP (n = 4). Data are presented as mean ± SEM (*P < 0.05).
To provide further evidence for the role of ABCB8 in the regulation of mitochondrial iron homeostasis, we studied the effect of ABCB8 overexpression on mitochondrial iron levels. Transduction of ABCB8-GFP adenovirus in NRCM led to a significant increase in the levels of ABCB8 protein (Fig. 4 F and G) and a significant decrease in the mitochondrial iron content (Fig. 4 H and I).
Accumulation of iron can exacerbate ROS production by catalyzing the Fenton reaction and converting superoxide anion and H2O2 into the most toxic ROS, OH˙ (26). ABCB8Δ/Δ mice displayed higher levels of lipid peroxidation products 8 wk after deletion of ABCB8, suggestive of elevated ROS production and oxidative damage to the heart (Fig. S6A). Unlike the ABCB8Δ/Δ hearts, down-regulation of ABCB8 in NRCM did not change ROS levels at baseline (Fig. S6 B and C), but increased ROS in the presence of exogenously added H2O2 (Fig. S6 D and E). Furthermore, overexpression of ABCB8 reduced ROS levels in NRCM in response to exogenously added H2O2 (Fig. S6 F and G) and is consistent with previously published work showing that ABCB8 overexpression is protective against oxidant-induced cell death (16). These results indicate that a reduction in ABCB8 is associated with increased susceptibility to oxidative stress, whereas overexpression of ABCB8 has the opposite effect, indicative of a link between ABCB8 levels and cellular ROS.
ABCB8 Regulates Mitochondrial Iron Export.
Based on our results that modulation of ABCB8 leads to changes in mitochondrial iron content, we hypothesized that ABCB8 may facilitate iron export out of mitochondria. To address this hypothesis, we developed an in vitro assay to measure the export of radioactive iron from isolated mitochondria. This assay requires large quantities of mitochondria that could not be attained from the primary NRCM cells; thus, the subsequent export studies were performed in established HEK293 cells. Cells were treated with 55Fe, followed by mitochondrial isolation, incubation in an activity-preserving buffer to allow for 55Fe export, and isolation of the soluble fraction to assess mitochondrial iron export. First, we demonstrated effective reduction in ABCB8 levels with the siRNA approach in HEK293 cells (Fig. 5A). There was no difference in the membrane potential of the purified mitochondria with ABCB8 modulation (Fig. 5B), indicating their functional equivalency following the isolation procedure. We then assessed the export of iron from the isolated mitochondria. We observed minimal 55Fe release at time point zero in control and ABCB8 siRNA-treated cells (0.78% vs. 1.05% of total radioactivity for control and ABCB8 siRNA treated cells, respectively; P = 0.28). ABCB8 siRNA treatment of the cells resulted in a significantly lower 55Fe content in the soluble fraction compared with control siRNA after 60 min of incubation (Fig. 5C). Furthermore, overexpression of ABCB8 resulted in a significant increase in 55Fe in the soluble fraction after 60 min, indicating that ABCB8 modulation alters iron export (Fig. 5D). To further confirm that the results are not caused by the nonspecific release of 55Fe, we also measured the effects of ABCB8 siRNA on the export of another radioactive molecule, 32P. ABCB8 siRNA treatment of the cells did not alter 32P release from isolated mitochondria (Fig. 5E), confirming that the observed effects on 55Fe are not caused by the nonspecific leakage. Taken together, our results indicate that ABCB8 regulates mitochondrial iron export, either as the actual transporter, or functioning as a positive regulator of another protein that exports iron.
Fig. 5.
ABCB8 modulation alters mitochondrial iron export. (A) Western blot of HEK293 cells treated with control and ABCB8 siRNA. (B) Flow cytometry analysis of rhodamine 123-stained mitochondria revealed no difference in mitochondrial membrane potential between ABCB8 and control siRNA treated HEK293 cells (n = 3). Unstained mitochondria were used as a blank. (C) Export of 55Fe was reduced in isolated mitochondria from ABCB8 siRNA-treated cells compared with control siRNA-treated cells. Radioactivity in the soluble fraction was measured and normalized to mitochondrial protein and total cellular radioactivity (n = 3). (D) Export of 55Fe was increased in isolated mitochondria from ABCB8 adenovirus-transduced cells compared with GFP (n = 4). (E) ABCB8 siRNA treatment did not alter 32P export, suggesting the effect on 55Fe export is specific to ABCB8 modulation (n = 3). Data are presented as mean ± SEM (*P < 0.05).
Reduction in ABCB8 Results in Decreased Activity of Cytosolic Fe/S Proteins.
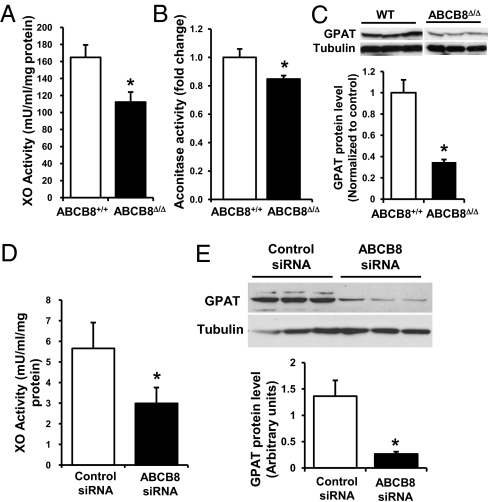
Mitochondria are believed to be essential for the maturation of cytosolic Fe/S cluster proteins (18, 27). Having found a way to modify mitochondrial iron export, we examined the effects of ABCB8 down-regulation on the maturation of cytosolic Fe/S proteins. We hypothesized that a reduction in mitochondrial iron export by ABCB8 would lead to a reduction in cytosolic Fe/S protein activity. In ABCB8 KO mice, the activity of the cytoplasmic Fe/S proteins xanthine oxidase (XO) and cytosolic aconitase, and the levels of glutamate phosphoribosylpyrophosphate amidotransferase (GPAT) were significantly lower than in WT animals (Fig. 6 A–C). The stability of GPAT depends on the presence of Fe/S cluster, and GPAT levels decrease with a reduction in Fe/S cluster assembly (28). In contrast to cytosolic Fe/S proteins, the activity of complex II, a mitochondrial electron transport chain complex dependent on Fe/S-containing proteins for its function, was not altered (Fig. S7A), indicating that the synthesis and availability of Fe/S clusters in the mitochondria are not affected by the reduction of mitochondrial iron export. To confirm these results, we measured the activity and levels of cytosolic Fe/S cluster proteins in NRCM with ABCB8 knockdown. Although ABCB8 down-regulation decreased XO activity and GPAT levels (Fig. 6 D and E), it did not change the activity of mitochondrial respiratory complexes I, II, and IV (Fig. S7 B–D). These results suggest that a decrease in mitochondrial iron export through ABCB8 down-regulation reduces the maturation of cytosolic, but not mitochondrial, Fe/S proteins.
Fig. 6.
ABCB8 knockdown results in the decreased activity of cytosolic Fe/S proteins. (A) XO activity (n = 8), (B) cytosolic aconitase activity (n = 6), and (C) GPAT levels (n = 3) in WT and ABCB8Δ/Δ hearts. (D) XO activity (n = 4) and (E) GPAT levels (n = 3) in NRCMs treated with ABCB8 siRNA. Data are presented as mean ± SEM (*P < 0.05).
Discussion
Mitochondria play an essential role in the synthesis of heme and Fe/S clusters in eukaryotic cells, but iron can also be a source of oxidative stress and cellular damage. Our results show that a reduction in ABCB8 leads to mitochondrial iron accumulation, an increase in ROS production, a decrease in mitochondrial iron export, and a reduction in the activity of cytosolic Fe/S proteins. Furthermore, cardiac-specific deletion of the ABCB8 gene in mice results in cardiomyopathy, mitochondrial iron accumulation, and increased cell death. We conclude that ABCB8 functions to modulate mitochondrial iron export and plays a critical role in mitochondrial iron homeostasis.
Assessment of mitochondrial iron levels and export can be technically difficult, so we used multiple approaches and included adequate control experiments in our studies. We used different methods to assess mitochondrial iron: (i) bathophenanthroline sulfonate and spectrophotometric assay, (ii) radioactive iron, (iii) EM, (iv) Prussian blue staining, and (v) measurement of iron export in isolated mitochondria. In the export studies, to exclude the possibility of nonspecific leakage of radioactivity from damaged mitochondria, we performed overexpression and down-regulation studies and observed consistent results. Furthermore, there was no difference in the membrane potential of the mitochondria isolated from control and ABCB8 siRNA-treated cells. Finally, we assessed the effects of ABCB8 down-regulation on another radioactive molecule (i.e., 32P) and demonstrated no difference in its movement out of the mitochondria with ABCB8 knockdown. These results indicate that the changes in iron export are not caused by differences in mitochondrial integrity or nonspecific leakage of iron, and represent the specific effects of ABCB8 modulation on iron export out of the mitochondria.
It may be argued that the increase in mitochondrial iron with ABCB8 knockdown is a result of ROS production rather than the direct effect of ABCB8 down-regulation. Our data refute this possibility. First, ABCB8 overexpression in NRCMs also led to changes in mitochondrial iron levels in the absence of an increase in cellular ROS levels. Second, our data suggest that an increase in mitochondrial iron levels and the reduction of the activity of cytosolic Fe/S cluster proteins in vitro occur before an increase in ROS. Finally, with ABCB8 down-regulation, the activity of mitochondrial Fe/S cluster proteins and heme levels, which are sensitive to ROS, do not change. Thus, the major and primary effects of ABCB8 knockdown are through the increase in mitochondrial iron levels, rather than the increase in ROS. However, because ABCB8 deletion in mice is associated with mitochondrial iron accumulation, oxidative stress, as well as decreased activity of cytosolic Fe/S cluster proteins, it is not known which of these factors is the primary cause of cardiomyopathy observed in ABCB8 KO mice. Although all three are likely to disrupt cardiac function and may have an additive effect, the relative contribution of each factor to the development of cardiomyopathy needs to be studied.
Our results demonstrate a role for ABCB8 in iron export, but they do not directly implicate ABCB8 as an iron exporter. Although ABCB8 could be directly involved in iron export, it may also function as a positive regulator of another transporter that exports iron. To explore the potential iron export activity of ABCB8, we have tried to purify the protein for the direct assessment of its iron export activity in an exogenous lipid bilayer system. However, our multiple attempts to generate sufficient quantities of the protein were not successful. Furthermore, being a half-transporter, ABCB8 may potentially interact with other mitochondrial ABC proteins, including ABCB7, to form a functional multimeric complex involved in the transport of iron. In fact, ABCB8 was previously shown to exist in a complex with other mitochondrial membrane proteins (15, 29), and these interactions may be indispensable for its iron export function.
The form of iron whose export is regulated by ABCB8 is not known. It is possible that iron is exported: (i) as an iron atom in a chelator-bound form, (ii) as a part of a Fe/S cluster, or (iii) as a complex with other ions. Furthermore, as the activity of cytosolic Fe/S proteins is reduced by ABCB8 down-regulation, this suggests that at least one of the mitochondrial molecules needed for the maturation of cytosolic Fe/S proteins [referred to as molecule X (18)] is an iron-containing molecule.
Similar to yeast, in which both Atm1p and Mdl1p contribute to mitochondrial iron homeostasis, mammalian cells also require both ABCB7 and ABCB8 to maintain their mitochondrial iron levels. It is thought that the function of ABCB7 in the mammalian cells is analogous to that of Atm1p in yeast, whereas the role of ABCB8 may more closely resemble that of Mdl1p. Previous work suggests that, in addition to its role in mitochondrial iron homeostasis, Mdl1 regulates mitochondrial peptide transport (11). Whether ABCB8 also participates in the export of peptides or other substrates besides iron is not known at this point. The role of ABCB7 in mitochondrial iron homeostasis is not clear, as KO of this protein in all organs but liver is embryonic-lethal as a result of a failure in development of multiple tissue types (30). The analysis of livers lacking ABCB7 expression revealed significant iron accumulation in the organ, but no accumulation of iron specifically in the mitochondria. This finding suggests that the role of ABCB7 in iron homeostasis may be different from that of ABCB8. On the contrary, down-regulation of ABCB7 in HeLa cells parallels the mitochondrial iron accumulation phenotype observed with ABCB8 knockdown in NRCM (31), suggesting a functional overlap between the two proteins. Future studies on ABCB7 and ABCB8 and their roles in mammalian mitochondrial iron homeostasis should focus on their potential ability to dimerize and/or interact with other mitochondrial membrane proteins, as well as to examine the effects of these interactions on iron export out of the mitochondria and cytosolic Fe/S protein maturation.
The synthesis of heme requires several enzymatic reactions, some of which occur in the mitochondria, including the last step of incorporating iron into protoporphyrin IX by a Fe/S-containing enzyme ferrochelatase to generate heme (21). We observed no change in mitochondrial heme levels in ABCB8 KO hearts, suggesting that the activity of ferrochelatase is not impaired under these conditions. Consistent with this, we observed no changes in the activites of other mitochondrial Fe/S proteins, including complexes I and II. These results indicate that the increase in ROS as the result of ABCB8 KO is not sufficient to damage Fe/S clusters in the mitochondria.
In summary, our results demonstrate that cardiac deletion of ABCB8 leads to cardiomyopathy and mitochondrial iron accumulation. We also show that ABCB8 is involved in mitochondrial iron regulation and plays a role in the maturation of cytosolic Fe/S cluster-containing enzymes. This represents the characterization of a protein that modulates mitochondrial iron export. Furthermore, our studies demonstrate that mitochondrial iron accumulation as a result of decreased iron export is detrimental to cellular health and can lead to cardiomyopathy.
Materials and Methods
Methods for culturing of NRCMs and ABCB8 modulation in vitro, generation of ABCB8 loxP and ABCB8Δ/Δ mice, cardiac function analysis, histological and transmission EM, assessment of mitochondrial iron content and export, cell viability studies, Fe/S cluster enzyme activity measurements and assessment of ROS levels are presented in SI Materials and Methods and Table S1. Data are expressed as mean ± SEM. Statistical significance was assessed with the unpaired Student t test; a P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Jerry Kaplan for his invaluable advice, support, and important suggestions throughoght the course of this study. We thank Drs. Eric Olson, Patricia Spear, and Jeremy Nathans for their helpful comments and careful review of the manuscript. We also thank Dr. Roland Lill for help with the aconitase assay and helpful discussions. Imaging work was performed at the Northwestern University Cell Imaging Facility, which was supported by National Cancer Institute (NCI) Grant CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. This work was supported by the Northwestern University Flow Cytometry Facility, NCI Cancer Center Support Grant CA060553, and National Institutes of Health Grants K02 HL107448, R01 HL087149, R01 HL104181, and 1P01 HL108795 (to H.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119338109/-/DCSupplemental.
References
- 1.Jones PM, O'Mara ML, George AM. ABC transporters: A riddle wrapped in a mystery inside an enigma. Trends Biochem Sci. 2009;34:520–531. doi: 10.1016/j.tibs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Rees DC, Johnson E, Lewinson O. ABC transporters: The power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett. 1997;418:346–350. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 4.Kispal G, Csere P, Prohl C, Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18:3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leighton J, Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 1995;14:188–195. doi: 10.1002/j.1460-2075.1995.tb06989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schueck ND, Woontner M, Koeller DM. The role of the mitochondrion in cellular iron homeostasis. Mitochondrion. 2001;1:51–60. doi: 10.1016/s1567-7249(01)00004-6. [DOI] [PubMed] [Google Scholar]
- 7.Allikmets R, et al. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A) Hum Mol Genet. 1999;8:743–749. doi: 10.1093/hmg/8.5.743. [DOI] [PubMed] [Google Scholar]
- 8.Bekri S, et al. Human ABC7 transporter: Gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood. 2000;96:3256–3264. [PubMed] [Google Scholar]
- 9.Csere P, Lill R, Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett. 1998;441:266–270. doi: 10.1016/s0014-5793(98)01560-9. [DOI] [PubMed] [Google Scholar]
- 10.Chloupková M, LeBard LS, Koeller DM. MDL1 is a high copy suppressor of ATM1: Evidence for a role in resistance to oxidative stress. J Mol Biol. 2003;331:155–165. doi: 10.1016/s0022-2836(03)00666-1. [DOI] [PubMed] [Google Scholar]
- 11.Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science. 2001;291:2135–2138. doi: 10.1126/science.1056957. [DOI] [PubMed] [Google Scholar]
- 12.Lill R, Kispal G. ABC transporters in mitochondria. In: Holland IB, Cole SP, Kuchler K, Higgins CF, editors. ABC Proteins: From Bacteria to Man. San Diego: Academic; 2003. pp. 515–532. [Google Scholar]
- 13.Lill R, Kispal G. Mitochondrial ABC transporters. Res Microbiol. 2001;152:331–340. doi: 10.1016/s0923-2508(01)01204-9. [DOI] [PubMed] [Google Scholar]
- 14.Ardehali H, Dong P, Machamer C, Marban E. Removal of the mitochondrial targeting signal leads to targeting of mitochondrial membrane proteins to the secretory pathway. Biochem Biophys Res Commun. 2005;338:1143–1151. doi: 10.1016/j.bbrc.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 15.Hogue DL, Liu L, Ling V. Identification and characterization of a mammalian mitochondrial ATP-binding cassette membrane protein. J Mol Biol. 1999;285:379–389. doi: 10.1006/jmbi.1998.2259. [DOI] [PubMed] [Google Scholar]
- 16.Ardehali H, O'Rourke B, Marbán E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ Res. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: Consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 18.Lill R, Mühlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: Mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 19.Napier I, Ponka P, Richardson DR. Iron trafficking in the mitochondrion: Novel pathways revealed by disease. Blood. 2005;105:1867–1874. doi: 10.1182/blood-2004-10-3856. [DOI] [PubMed] [Google Scholar]
- 20.Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- 21.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 22.Richardson DR, et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci USA. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 24.Koitabashi N, et al. Pivotal role of cardiomyocyte TGF-β signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartfay WJ, et al. A biochemical, histochemical, and electron microscopic study on the effects of iron-loading on the hearts of mice. Cardiovasc Pathol. 1999;8:305–314. doi: 10.1016/s1054-8807(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 26.Puntarulo S. Iron, oxidative stress and human health. Mol Aspects Med. 2005;26:299–312. doi: 10.1016/j.mam.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Lill R, et al. The essential role of mitochondria in the biogenesis of cellular iron-sulfur proteins. Biol Chem. 1999;380:1157–1166. doi: 10.1515/BC.1999.147. [DOI] [PubMed] [Google Scholar]
- 28.Sheftel AD, et al. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardehali H, Chen Z, Ko Y, Mejía-Alvarez R, Marbán E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci USA. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pondarre C, et al. Abcb7, the gene responsible for X-linked sideroblastic anemia with ataxia, is essential for hematopoiesis. Blood. 2007;109:3567–3569. doi: 10.1182/blood-2006-04-015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavadini P, et al. RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood. 2007;109:3552–3559. doi: 10.1182/blood-2006-08-041632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.