Abstract
The therapeutic efficacy of most drugs is greatly depends on their ability to cross the cellular barrier and reach their intracellular target sites. To transport the drugs effectively through the cellular membrane and to deliver them into the intracellular environment, several interesting smart carrier systems based on both synthetic or natural polymers have been designed and developed. In recent years, hyaluronic acid (HA) has emerged as a promising candidate for intracellular delivery of various therapeutic and imaging agents because of its innate ability to recognize specific cellular receptors that overexpressed on diseased cells. The aim of this review is to highlight the significance of HA in cancer, and to explore the recent advances of HA-based drug carriers towards cancer imaging and therapeutics.
Keywords: Hyaluronic acid, hyaluronan, tumor targeting, intracellular targeting, nanocarrier, drug delivery, tumor imaging
1. Introduction
Significant research efforts have been devoted over the past few decades to design carrier systems that could specifically deliver active agents to the disease sites, and thereby minimizing the lethal side-effects [1-3]. Recent exciting advances in nanotechnology and our understanding in molecular biology have enabled us to develop a variety of efficient nanocarriers to deliver diagnostic and/or therapeutic agents to the tumor tissue [4-11]. In particular, polymeric nanoparticles have been extensively used for targeted cancer diagnosis and therapy [8, 12]. Macromolecules and nanoparticles have been found to passively accumulate into tumor sites after systemic administration due to their abnormally leaky vasculature and lack of an effective lymphatic drainage system, and this phenomenon is referred to as the enhanced permeation and retention (EPR) effect [13]. However, a number of nanoparticles have not been able to show desirable therapeutic efficacy in vivo because the EPR effect cannot guarantee internalization of the nanoparticles. Even, considerable portion of drugs may be released from the nanoparticles before they are taken up by the tumor cells. Since the therapeutic targets of many anticancer drugs are found inside the cells, effective cancer therapy requires development of nanoparticles that can accumulate in a tumor tissue, penetrate into cancer cells, and release the drugs inside the cells. Intracellular delivery of anticancer drugs is important for enhanced therapeutic effect.
Intracellular delivery has been improved by conjugating tumor-interacting moieties, such as antibodies [14-16], nucleic acids [17-18], proteins [19-22], and various other ligands [23-25], onto the surface of the nanoparticles. Because such nanoparticles can recognize, bind to, and internalize into tumor cells through endocytosis, diagnostic or therapeutic agents loaded within the targetable nanoparticles can be release inside of the tumor cells [10-11, 26-27]. However, many of the tumor-targeting moieties are associated with various complications. For example, the use of antibodies is limited by its immunogenicity, and decrease in the activity due to chemical conjugation processes.
In recent years, hyaluronic acid (HA) has attracted much attention in tumor-targeted delivery because of its ability to specifically bind to various cancer cells that overexpress CD44 receptor [28]. Moreover, HA also possess numerous desirable physicochemical and biological properties such as biocompatibility, biodegradability and non-immunogenicity, for drug delivery applications. Already, a number of drug delivery systems such as drug-conjugates, nanocomplexes, and nanoparticles, using HA as the primary (targeting) constituent have widely investigated. This review consists of three parts: introduce of physicochemical and biological characteristics of HA, including the synthesis, physiological functions, cellular interactions, and degradation in the human body; examination of significant roles of HA in cancer; and comprehensive discussion of the recent advances of HA-based drug delivery systems.
2. Hyaluronic acid – Chemical Structure, biosynthesis, cellular interactions, and biodegradation
2.1. Chemical structure of HA
In 1934, HA was first isolated from the vitreous of bovine eyes by Karl Meyer and John Palmer [29]. The name “hyaluronic acid” was coined by them as a conjugation of two words, hyaloid (vitreous) and uronic acid. After nearly 20 years of research, Meyer's group determined the precise chemical structure of HA [30]. They found that HA is a linear polysaccharide composed of a repeating disaccharide of N-acetyl D-glucosamine (GlcNAc) and D-glucuronic acid (GlcA) with 1→4 interglycosidic linkages, while the disaccharide repeating units are linked by β (1→3) linkages (Figure 1). In normal physiological conditions, the number of repeating disaccharides in a HA molecule ranges from 2,000 to 25,000, which corresponds to a molecular mass of 106-107 daltons (as molecular mass of single disaccharide unit is approximately 400 daltons). As the pK of the carboxyl groups on the GlcA residue is found to be 3-4, the carboxyl groups are predominantly ionized under physiological conditions (pH 7.4) [31]. Thus, HA exists as a polyanion in vivo, and therefore is referred to as hyaluronan. In addition, HA shows an expanded random coil structure in physiological solution. Because of its random-coil structure and high molecular weight (HMW), HA forms very viscose and elastic solution with a large hydrodynamic volume.
Figure 1.

Chemical structure of HA. The arrows represent principal sites for chemical modification.
2.2. Synthesis of HA by HA synthases
HA is synthesized in the inner face of the plasma membrane by enzyme HA synthases (HASs): HAS-1, HAS-2 and HAS-3, which are multipass transmembrane proteins that composed of hydrophobic amino acid clusters and large cytoplasmic loops. These transmembrane enzymes sequentially link GlcA and GlcNAc using their activated nucleotide sugars, UDP-GlcA and UDP-GlcNAc substrates, in alternating β-1,3 and β-1,4 linkages [28, 32-33]. During this synthetic process, HA is secreted outside of the cells onto the cell surface, or into the extracellular matrix (ECM) [28, 34]. ENREF 7 In addition to cell surface and ECM, HA is also found inside the cell. The pericellular HA, which is anchored to the cell surface by the interaction with the HASs or HA receptors, allows the incorporation of extracellular hyaladherins such as aggrecan into the cell surface.
2.3 Hyaluronic acid-binding proteins: hyaladherins
Besides being an important structural component of tissues in all vertebrates, HA is also implicated in several biological functions including intracellular signaling. The unique biological functions of HA is largely attributed to the specific binding and interaction with HA-binding proteins referred to as hyaladherins [35]. Most hyaladherins contain a specific binding domain, also called a link module, which is composed of two α-helices and two antiparallel β-sheets [36]. The HA-binding proteins containing the link modules include diverse proteoglycans, HA receptors and a link protein. The proteoglycan molecules can bind to the HA polymer, to form huge proteoglycan complexes, which can acts as a structural components of diverse tissues such as articular cartilage, blood vessels, skin and brain [37-39]. The best-identified HA receptors, containing the link module, are CD44 and lymphatic vessel endothelial HA receptor (LYVE-1). CD44 is known to have a variety of significant biological roles including maintaining tissue structure via cell-cell and cell-matrix adhesion. It also mediates cell migration during morphogenesis, angiogenesis, and tumor invasion and metastasis. CD44 not only organizes matrix signaling related to cell survival and death, but also mediates the adhesion and rolling of lymphocytes [40-46]. The other HA receptor LYVE-1, expressed on the lymph vessel endothelium, is known to participate mainly in HA degradation [47-49]. In addition to the above hyaladherins, tumor necrosis factor-stimulated gene-6 (TSG-6) has also been well identified as a HA-binding protein. TSG-6 is abundantly found in the synovial fluids of arthritis patients and also detected in the serum of patients with inflammatory or autoimmune diseases. It is also known to be involved in inflammation, leukocyte migration and ECM remodeling [50]. In addition, hyaladherins that do not contain the link module have been also identified. The representative examples include inter-α-inhibitor (IαI), CD38, and receptor for HA-mediated motility (RHAMM) that is also known as CD168. Unlike the other hyaladherins, RHAMM is present in the cytoplasm and nucleus and transiently expressed on the surface of activated leukocytes and fibroblasts. RHAMM is known to mediate cell migration and proliferation [45, 51-53].
2.4. Interactions between HA and CD44
CD44 is a principal cell-surface receptor for HA, which is widely responsible for the interaction between HA and the surface of specific cells. The interaction between the HA and CD44 has been extensively investigated because of its involvement in a wide variety of cellular functions. In particular, CD44 is closely involved in a variety of significant cellular events, including cell proliferation, cell differentiation, cell migration, angiogenesis, and arrangement of cytokines, chemokines and growth factors to the corresponding receptors. The interaction between HA and CD44 is known to mediate signaling for cell survival and endocytosis of HA for its degradation [40, 54-55].
2.4.1. HA-CD44 in cell-cell aggregation
In early 1980, it was recognized that the interaction between HA and a membrane receptor was known to mediate cell-cell aggregation via cross-bridging among pericellular HA matrices and the receptors on the surface of other cells. But the subsequent studies discovered that an integral membrane glycoprotein of 85KDa was responsible for the HA-cell binding and cell-cell aggregation [56-57]. Later, the membrane glycoprotein, referred as gp85 [58], was finally defined to be identical to a leukocyte homing receptor antigen CD44 [59].
2.4.2. HA-CD44 in cell-matrix signaling
The transmembrane CD44 regulates signaling between cell and the matrix HA. The interaction between cell and the matrix activates intracellular signal via the transmembrane receptor CD44. Conversely, the intracellular signals also regulate changes in the ECM. In some cells, binding of the HA molecule to multiple CD44 receptors activate the receptors and induce the extracellular clustering of the receptors. These extracellular clustering of CD44 regulates intracellular organization of cytoskeleton, resulting in the activation of diverse kinases. The secondary signaling finally induces subsequent changes in cellular behavior. However, the multiple binding of HMW HA to CD44 may inactivate CD44. In this case, the disassociation of interactions between HMW HA and CD44 is likely to be result in the clustering of CD44, and thereby initiates CD44 signaling. This disruption of HA-CD44 interaction may occur by presence of Hyals or LMW HA oligomers. Thus, most importantly, the molecular weight (MW) of HA is the crucial factor in CD44-mediated cell-signaling. The monovalent binding of LMW HA fragments can induce different signals from those produced by the multivalent binding of HMW HA to multiple receptors. For example, a study on cell-signaling pathways in macrophage demonstrated LMW HA fragments containing 6-20 saccharide units, but not the native HMW HA, can activate cell-signaling pathways, and induce the expression of various cytokines including IL-1 (interleukin-1), TNF-α (tumor necrosis factor-alpha), IGF-1 (insulin-like growth factor-1) and iNOS (nitric oxide synthase), which are associated with inflammation [31].
2.4.3. HA-CD44 in cell-survival and apoptosis signaling
The HA-CD44 interaction is also significantly involved in cell-survival and apoptosis. In particular, the interaction between CD44 on the cell surface and matrix HA enables to transduce cell-survival signaling. However, when HA oligomer is added to cells that is known to express CD44, such as chondrocyte, the release of nitric oxide (NO) was induced from the cells, resulting in down-regulation of phosphoinositide 3 (PI 3)-kinase and thereafter induce apoptosis [60]. But the addition of HMW HA of 500-760 kDa resulted in the reduction of anti-Fas induced apoptosis in chondrocytes. These findings clearly suggest that the survival of cell is highly associated with the clustering of CD44 resulting from the binding of matrix HA. However, when the LMW HA is added, it competitively binds to the CD44, which simultaneously perturbs the binding of endogenous HMW HA to CD44. The disruption of the matrix HA-CD44 interaction by the LMW HA fragments attenuates vital cellular signals, consequently, inducing apoptosis of the cell. HA oligomers have also been known to stimulate caspase-3 and induce apoptosis in various cancer cell lines, such as breast cancer, colon cancer [61] and glioma cells [62]. It has been demonstrated that treatment of a tiny amount (0-150 μg/ml) of the HA oligomer (approximately 2500 Da) could inhibit PI 3-kinase activity. Besides phosphorylation of serine/threonine-protein kinase (Akt), Bcl-2-associated death promoter (BAD) and forkhead in rhabdomyosarcoma (FKHR) was also inhibited by treatment of the LMW HA. However, the unique biological effect induced by treatment of the HA oligomer was not observed after treatment of HMW HA or chitin, chondroitin sulfate oligomers of similar MW to HA oligomers.
2.4.4. CD44-mediated endocytosis of HA
Several studies have suggested that the interaction between HA and the cell-surface receptor CD44 promotes endocytosis of HA. The distinct internalization of HA is observed in CD44-overexpressing various cell lines, which includes chondrocytes [63], keratinocytes [54] and diverse cancer cells. In an early study, Hua et al. [63] demonstrated experimental evidence for CD44-mediated endocytosis mechanism of HA in chondrocytes. ENREF 41 In that study, the uptake of exogenous fluorescein-labeled HA was monitored after incubating with adult bovine articular chondrocytes. And they found that labeled HA was effectively bound to and internalized into the cells. After removal of the surface bound HA, by treatment with either trypsin or Streptomyces Hyals, the internalized labeled HA was clearly observed inside small intracellular vesicles throughout the cytoplasm. However, fluorescein-labeled dextran of similar MW was not able to internalize into the same cells. Similarly, when CD44 on the chondrocytes was blocked with anti-CD44 antibody or HA hexasaccharide, endocytosis of HA into chondrocytoes was significantly inhibited. Thus, they clearly demonstrated that the internalization of HA into chondrocytes occurred predominantly through the CD44-mediated endocytosis and not by non-specific pinocytosis. The subsequent studies demonstrated that CD44-mediated endocytosis of HA could also mediate the HA turnover [54]. In a study, after selective suppression of CD44 expression in skin keratinocytes and corneal epithelium of mice, abnormal increase in HA accumulation was observed in superficial dermis and corneal stroma of mice. These observations indicated that HA turnover is highly associated with CD44. The results from the above studies and others clearly indicate that internalization/degradation of HA could undergo the following process. First, HA is internalized into the cells that overexpress CD44 via receptor-mediated endocytosis. Second, the internalized HA is delivered to the lysosome. And finally, HA is degraded within the lysosomes by the lysosomal enzyme, Hyal-1.
2.5. Catabolism of hyaluronic acid by hyaluronidases
HA is degraded and removed very fast in the vertebrates. In the human body, approximately 30% of HA is removed and replaced every day [64]. The half-life of HA considerably varies depending on the type of tissues. For instance, the half-life of HA in the human skin is nearly a day [65], whereas it is further extended to 20 days in the cartilage [66] and to 70 days in the vitreous [64]. Circulating HA in the blood stream is mainly catabolized in the liver sinusoidal endothelial cells after internalized by a HA receptor for endocytosis (HARE) receptor [67]. The HA turnover also occurs within the lymph node following internalization of HA into the lymphatic cells by LYVE-1, a lymphatic vessel endothelial HA receptor-1 [47]. This unique degradation process is mainly regulated by specific enzyme known as hyaluonidase (Hyals), which cleave the β-N-acetyl-D-glucosaminidic linkages in the HA backbone [68]. There are six hyaluronidase genes, including HYAL1, HYAL2, HYAL3, HYAL4, HSPAM1 and HYALP1, have been characterized in the human genome. Of the genes, four hyaluronidase genes can express active Hyal proteins. To date, Hyal-1, Hyal-2 and PH-20 have been well characterized. Among the Hyals, Hyal-1 is widely expressed in various somatic tissues. Hyal-1 is also known as lysosomal enzymes due to its sharp optimum pH of around 3.7 although it is found in the human plasma. Hyal-1 exhibits high specific activity, and degrades HA oligomers into small sized fragments such as tetrasaccharides in the lysosome [69]. Although some Hyals, including Hyal-2 and PH-20, account for the extracellular cleavage of HMW HA, the main catabolism of HA is attributed to the intracellular degradation by lysosomal Hyal, i.e., Hyal-1. In particular, the considerable portion of HA degradation is predominantly followed by receptor-mediated internalization into the cells by CD44. Also, there is strong evidence that both the intracellular and extracellular degradation of HA by Hya-1 and Hyal-2 occurs only when CD44 is expressed on the cell surface [44].
3. HA in cancer
In late 1970s, an early study revealed a close relation between HA and tumor invasion [70]. It showed that the higher concentration of HA was present in the adjacent tissues surrounding invasive tumors than in the corresponding tissues of non-invasive tumors. While an another study indicated that HA is highly concentrated in the connective tissues surrounding human breast tumors as compared with HA level in benign regions [71]. In some types of tumors, this abnormal increase in the HA level was found to result from the overproduction of HA in fibroblast cells by the interaction with adjacent cancer cells [72-74]. Similarly, HA also overproduced in the cancer cells themselves possibly due to overexpression of HA in the cancer cells [75]. Consequently, the high level of HA in the tissues can be a significant predictor for estimating malignancy and invasiveness of tumor [76-78]. In some specific types of tumors, such as bladder cancer, the urinary level of HA can also be a marker for detecting cancer as well as for evaluating its grade [79].
To investigate the effect of HA on the tumor progression, studies have manipulated the expression of HA by transfection of HAS-2 and HAS-3 into various cancer cell lines. Several studies have exhibited that the increasing HA level induces the in vitro human fibrosarcoma cell growth [80] and as well as in vivo breast tumor growth of transgenic mice [81]. HAS-3 overexpression also showed promotion of the growth of TSU prostate cancer cells [82]. Some studies have demonstrated that the tumor growth in the nude mice xenografts bearing prostate cancer cells is suppressed by overexpression of HAS-2 and HAS-3 [83]. Interestingly, it was also shown that a slight increase in the production of HA promotes tumor progression; however, excessive HA production suppresses tumor growth [84]. Although many studies suggested that the ectopic overproduction of HA facilitates tumorigenesis and tumor invasion, the implications of HA in tumor progression are very complex and still remains controversial.
The interaction and disruption of endogenous HA and cell surface glycoproteins may induce various intracellular signaling pathways. In particular, there are strong evidences that the perturbation of HA-protein binding inhibits tumor growth and metastasis in vivo. Since LMW HA oligomers of 10-100 kDa can disrupt the HA-protein binding, treatment of LMW HA prevents growth and metastasis of various types of tumor, including lymphoma, melanoma [85], glioma [62, 86], breast cancer and colon cancer cells [61]. ENREF 36 The inhibitory effect is likely due to apoptosis of cancer cells by the disruption of the binding between matrix-HA and receptors on the cancer cells, inducing down regulation of cell survival mechanisms such as PI 3-kinase/Akt signaling pathway. However, LMW HA fragments also show opposite functions in tumor progression. It was also reported that LMW fragments of 20-30 saccharide units generated by tumors stimulate endothelial cell proliferation, promote angiogenesis, and therefore facilitate tumor growth [87]. Moreover, the implications of HA in tumor progression can be significantly dependent on the MW of HA. For instance, it has been reported that the LMW HA oligomer of 8-50 saccharide units stimulate angiogenesis, but HMW HA even inhibits angiogenesis [88-90]. In many cases, only the LMW HA, but not HMW HA, can stimulate intracellular signaling.
3.1. CD44 in cancer
CD44 is closely involved in a variety of cellular events, including cell proliferation, cell differentiation, cell migration, and angiogenesis as well as signaling for cell survival and endocytosis of HA for its degradation, by interacting with HA. Surprisingly, some initial studies have revealed that expression of the CD44 is elevated in many types of malignancies as compared with CD44 levels in the corresponding normal tissues [91-92]. Furthermore, it has been proven that overexpression of CD44 level is closely implicated in tumorigenesis and tumor metastasis in vivo [93-94]. In particular, a specific isoform of CD44 variant, termed CD44v6 was determined as the major CD44 isoform that is overexpressed in various types of malignancy. Overexpression of CD44v6 was detected in metastatic malignancies but was not found in non-metastatic tumors and the corresponding benign tissues [95]. The implication of CD44v6 in tumor metastasis was represented in a preclinical study [96]. In the study, transfection of CD44v6 enhanced the metastatic efficiency of non-metastatic tumor cells; conversely, the metastatic behavior including the growth of lymph node was efficiently inhibited by adding anti-CD44 monoclonal antibody. In a number of clinical studies, direct implication of CD44v6 expression in tumor progression has been proven in various types of tumors, including non-Hodgkin's lymphoma [97], colorectal [98-99], gastric [100], pancreatic [95], renal [101], hepatocellular [102], cervical [103], ovarian [104], non-small lung [105], breast carcinoma [106-107] and melanoma [108]. ENREF 80 This increased CD44v6 expression in various tumors may facilitate internalization of HA into the tumor cells via CD44-mediated endocytosis and subsequent degradation of HA by intracellular Hyals. The enhanced internalization and degradation of matrix-HA have been reported to promote metastasis eventually [109-110]. Owing to the overexpression of CD44v6 in diverse malignancies, it has been considered as a useful marker for detecting cancer and further as a potential target for cancer therapy.
3.2. Hyal in cancer
As discussed above, HMW HA plays pivotal roles as a structural component of ECM and synovial fluid, in which it contains a great amount of proteoglycans. And HMW HA also interacts with the glycoproteins expressed on the cell surface, regulating cell-cell aggregation. The interaction between matrix-HA and cell surface proteins can mediate significant intracellular signaling pathways for cell survival. The abundant expression of HMW HA is also crucial for maintaining homeostasis in the vertebrate. LMW HA formed from degradation of HMW HA by Hyals, mainly by Hyal-1 and Hyal-2, is also found in the vertebrate. Overexpression of LMW HA in the mammalian tissues often indicates high incidence of various diseases. Among mammalian Hyals, Hyal-1 is most commonly expressed in various malignancies, such as bladder [111], prostate [112], and head and neck tumor cells [113]. Furthermore, early studies have shown the positive correlation between the Hyal levels and the tumor progression. For instance, a study has demonstrated elevated Hyal levels in prostate cancer tissues, approximately 3-8 times higher than those in both corresponding normal prostate and benign prostate hyperplasia tissues. In addition, the Hyal levels in high-grade tumors were significantly higher than those in low-grade tumors (36.6±2.9 vs. 9.4±1.4 units/mg protein). Similarly, elevated levels of Hyals were detected in variety of malignant tumors, including prostate [114-115], bladder [116-119], head and neck [113, 120], colorectal [121], lung [122], brain [122-123], non-Hodgkin lymphoma [124], and metastatic breast cancers [42, 125-128]. The expression level of Hyal was found to be elevated in the metastatic tumors compared to the non-metastatic tumors [112, 115]. For example, the Hyal levels of breast metastatic tumors were found to be 4 times higher than the primary breast tumors [125]. Therefore, HA is more rapidly catabolized in malignant tissues [28, 69, 129]. As a result, LMW HA fragments are found in much greater amounts at tumor sites than in normal tissues [112, 130]. ENREF 99
The effect of Hyal on the tumor progression still remains controversial. In some studies, it has been suggested that the overexpression of Hyal promotes the tumor progression. As discussed earlier, LMW HA, generated by Hyals, can promote angiogenesis and thereby facilitate the tumor growth. Since the LMW HA is abundantly present in high grade and metastatic tumor tissues, Hyals can be considered as the tumor promoters. This concept was also supported by some transfection studies [131-132].
Bladder cancer cells were transfected with different cDNA constructs involved in the Hyal production. The study demonstrated that expression of Hyal-1 stimulates tumor invasiveness, vascular development and tumor growth [131]. The other studies demonstrated a direct correlation between the tumor progression and coexpression of both Hyal-1 and HAS-2 [132]. Hyals have also been considered as the tumor suppressor. Several studies have confirmed the effect of Hyal on the tumor suppression. In a study, it was demonstrated that the transfection of HAS-2 induces overproduction of HA, causing an increase in the growth of tumor cells. However, as Hyal-1was overexpressed, the growth rate of the tumor cells was significantly suppressed [133]. Another study also showed the effect of Hyal on the suppression of tumor growth in SCID-bearing xenograft models [134]. Recently, a new concept on the role of Hyals in the tumor progression has been suggested. In a study, the Hyal effect on the tumor progression was examined by varying the concentration of Hyals. More interestingly, at a moderate concentration of Hyal expression, Hyals function as the tumor promoters that stimulate the tumor growth and invasiveness; however, at a high concentration of Hyal, they act as the tumor suppressor. [114]
Although a great number of studies have proved the close relation between HA and cancer, the implications of HA, CD44 and Hyals on tumor progression are complex and remains controversial to date. However, it is clear that CD44 and Hyals are more predominantly expressed in most tumor tissues or cells than in the corresponding normal tissues or cells. Thus, those cancer cells that overexpress CD44 and Hyals can specifically internalize HA via the receptor-mediated endocytosis, and then catabolize it into tetrasaccharide by concerted action of CD44, Hyal-2 and Hyal-1 (Figure 2). Furthermore, this unique interaction between HA and tumors may provide a design clue of potential carriers for tumor diagnosis and therapy.
Figure 2.

Schematic illustration of CD44-mediated signaling transduction and cellular uptake pathways.
4. HA-based carrier system for cancer therapy and imaging
4.1. HA-drug conjugates
The concept of polymer-drug conjugate (or macromolecular-prodrug) was first introduced by Ringsdorf in 1975. In general, polymer-drug conjugates are prepared by covalent conjugation of small molecule drugs to the water soluble polymers via cleavable linkers. Such conjugates could improve solubility, pharmacokinetic profile, and in vivo plasma half-life of the drugs to be conjugated. The linkers are designed to be robust in the bloodstream and cleaved after reaching the target site by simple hydrolysis or by enzymatic degradation. More importantly, these polymer-drug conjugates also take advantage of the EPR effect to accumulate on the tumor.
One of the prerequisites for the polymer drug-conjugate is the availability of adequate functional groups on the polymer for chemical conjugation. Owing to the presence of multiple functional (hydroxyl and carboxylic acid) on the HA backbone, diverse anti-cancer drugs were chemically conjugated to produce HA-drug conjugates. For example, a highly hydrophobic anti-cancer drug paclitaxel (PTX) was chemically conjugated to the adipic dihydrazide modified HA [135]. The resulting HA-PTX conjugate was selectively internalized into the human cancer cell lines including breast, colon and ovarian cancer cells that are known to overexpress HA receptors, but not into mouse fibroblast cell lines that do not overexpress HA receptors. Similarly, the conjugates exhibited selective cytotoxicity to the cancer cells, whereas they did not show significant cytotoxicity to the fibroblast cells. In another study, in vivo anti-tumor activity of the HA-PTX conjugates was evaluated in mouse xenograft models bearing intraperitoneally implants of ovarian cancer cells [136]. The results showed that an intraperitoneal injection of HA-PTX could remarkably improve the survival of the mice implanted with PTX-resistant ovarian cancer cells. Further the conjugate also reduced tumor burdens of the mouse tumor models bearing either NMP-1 or SKOV-3ip cells.
Because of the poor solubility of HA in most organic solvents, the facile conjugation of drug molecules to the HA was often found to be challenging task. Recently, a simple nanocomplexing method, by dissolving HA in dimethylsulfoxide using polyethylene glycol), was set up for the preparation of HA-PTX conjugates [137]. Using this method, about 10.8% (w/w) of PTX were able to conjugated to the HA polymer chain. Similarly, HA-PTX (referred as ONCOFID™-P) with about 20% w/w PTX was prepared by reacting HA-thiobarbituric acid and 2′-PTX-4-bromobutyrate [138]. ONCOFID™-P exhibited higher dose-dependent inhibitory effect against RT-4 and RT-112/84 bladder carcinoma growth than the free PTX. Currently, ONCOFID™-P is undergoing phase II clinical studies in six European countries for the treatment of refractory bladder cancer.
HA-butyric ester (HA-But) conjugate, another type of macromolecular prodrug, was prepared by chemical conjugation of sodium butyrate to HA polymer [139]. Interestingly, bioavailability of sodium butyrate was significantly improved by the chemical conjugation when compared to free sodium butyrate (6 days vs. 5 minutes). In another study, the pharmacokinetics and anti-tumor activity of HA-But was investigated using tumor-bearing mice [140]. The results demonstrated accumulation of HA-But conjugate in the liver and spleen after intravenous (i.v.), intraperitoneal (i.p.) and subcutaneous (s.c.) administration. When HA-But was given by i.p. or s.c. injection to the mouse models bearing intrasplenic implants of Lewis lung carcinoma (LL3) or melanoma (B16-F10) cells, the liver metastases were successfully inhibited in both tumor models. The administration routes did not affect the anti-tumor efficacy. Interestingly, the inhibition efficacy was positively related with expression level of CD44 on the cell surface. The inhibition efficacy of a group bearing B16-F10 cells, which express CD44 in a higher level than those bearing LLC3 cells (68 vs. 87%, respectively), was significantly higher than that bearing LLC3 cells (87 vs. 100%).
4.2. HA-based self-assembled nanoparticles
Polymeric amphiphiles have received enormous attention as drug carrier because they could self-assemble into core-shell nanoparticles, and exhibit distinct physicochemical characteristics in aqueous media. The inner hydrophobic cores were utilized as reservoir for non-covalent encapasulation of therapeutic and/or imaging molecules. The outer hydrophilic shell prevents the unwanted protein adsorption, and thereby evade non-specific uptake by the reticuloendothelial system (RES). Amphiphilic HA derivatives have been prepared by chemical conjugation of hydrophobic moieties including liphophilic molecules such as tetradecylamine or bile acids, oligomers such as oligo(2-(4-(vinylbenzyloxy)-N, N-diethylnicotinamide)) (oligo(VBODENA)), and polymers (poly(caprolactone) (PCL) and poly(lactic-co-glycolic acid) (PLGA)) [141-147]. In most of these studies, these hydrophobic moieties were coupled through the carboxylic acid group of HA through carbodiimide chemistry. The size and the zeta potential of HA nanoparticles, which are important parameters affecting the biodistribution in vivo, have been controlled by varying the degree of substitution (DS) of the hydrophobic moieties. In all the cases, increasing the hydrophobic moieties has resulted in decrease in the size of nanoparticles, due to the enhanced hydrophobicity of the cores. The self-assembled HA-based systems that have been investigated are summarized in Table 1.
Table 1. Amphiphilic HA derivatives for imaging or drug delivery.
| Carrier | Additional hydrophilic substituent | Hydrophobic moiety | Structure of hydrophobic moiety | Drug | Ref. |
|---|---|---|---|---|---|
| HA-g-TDA | - | TDA |
|
- | [142] |
| HA-g-CA | -/PEG | CA |

|
- | [143-144, 148] |
| HA-g-PEG-PCL | PEG | PCL |
|
DOX | [145] |
| HA-g-PEG-PLGA | PEG | PLGA |
|
DOX | [141] |
| HA-g-PLGA | - | PLGA |
|
DOX | [146] |
| HA-g-Oligo(VBODENA) | - | Oligo(VBODENA) |

|
PTX | [147] |
| HA-b-PBLG | - | PBLG |

|
DOX, DTX | [150-151] |
Abbreviations: TDA = tertradecylamine; CA = 5b-cholanic acid; PCL = poly(caprolactone); PLGA = poly(lactic-co-glycolic acid); PEG = polyethylene glycol); oligo(VBODENA) = oligo(2-(4-(vinylbenzyloxy)-N, N-diethylnicotinamide)); and PBLG = poly(γ-benzyl-glutamate).
Effectiveness of any drug carriers largely depends on the ability to perform effectively under in vivo conditions. Hence, it is necessary to understand and characterize the in vivo behavior of nanocarriers. Recently, the in vivo characteristics of near infrared Cy5.5-labeled 5β-cholanic acid conjugated HA nanoparticles (HACA-NPs) were examined in tumor bearing mice using non-invasive imaging techniques (Figure 3a) [143-144]. The particle sizes of HACA-NPs used for the study were in the range of 237-424 nm, which were controlled by varying the DS of CA. The results from the study demonstrated that all HACA-NPs were selectively accumulated on the tumor site, where the smaller size HACA-NPs were able to reach the tumor site more effectively than the larger particles (Figure 3b). The ex vivo imaging of the dissected major organs and tumors after 48 h showed stronger fluorescent signals for all HACA-NPs, while HACA-NP10 (with particle smaller size) exhibited 1.5-fold higher signal intensity than the larger particles (Figure 3c and 3d). At the same time, all the HACA-NPs showed significant fluorescent signals in the liver, and this was attributed by the uptake of HACA-NPs by phagocytic cells of the RES and by liver sinusoidal endothelial cells expressing HARE receptor. This non-specific uptake may seriously limit the practical application of HA nanoparticles for cancer therapy. One of the ways to evade non-specific uptake is imparting stealth characteristics to the nanoparticles. Therefore, in our subsequent study, we prepared a series of PEGylated HA nanoparticles (P-HA-NPs) by varying the grafting density of PEG [148]. To gain clear insights of P-HA-NPs, whether it could overcome preferential liver accumulation, we carried out in vivo biodistribution study for P-HA-NPs in tumor-bearing mice. More interestingly, all P-HA-NPs exhibited lower liver uptake and higher tumor accumulation compared to the bare HA nanoparticles.
Figure 3.
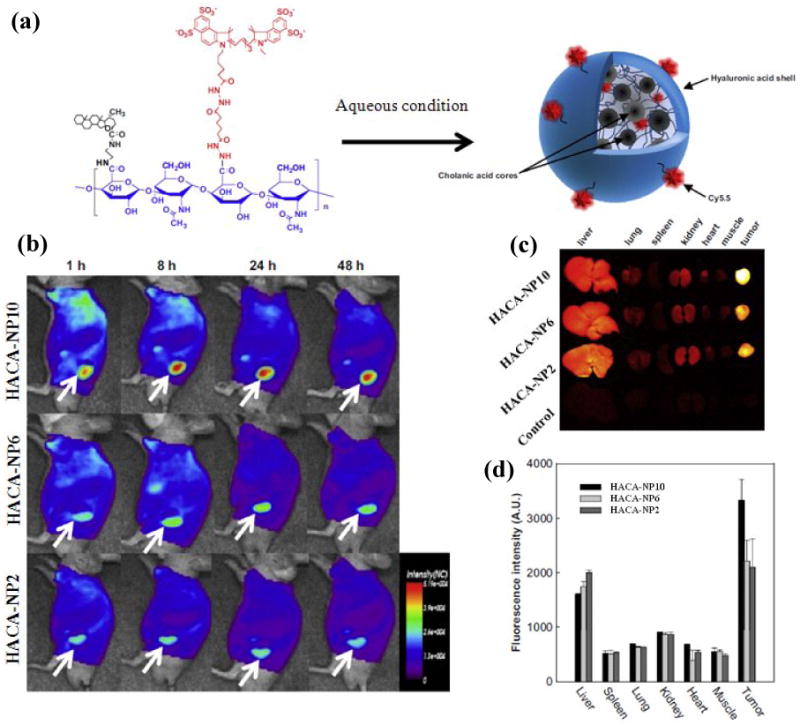
(a) Structure of Cy5.5-labeled HACA-NPs in aqueous solution; (b) Time dependent whole body images of athymic nude mice, after intravenous administration of HACA-NPs (where 2, 6 and 10 represents the number of cholanic acid moeites per 100 sugar residue of HA); (c) Representative Ex vivo fluorescence images of dissected organs and tumor; and (d) Quantified ex vivo characteristics of HACA-NPs. (Adapted with permission from Choi et al. [143]).
The other critical drawbacks of nano-sized drug carriers are low drug loading capacity and low stability in physiological conditions. To address these issues, amphiphilic hydrotropic hyualuronic acid (HydroHA) conjugate were synthesized by conjugation of diethylnicotinamide based hydrotropic oligomer to the HA backbone [147]. Hydrotropic polymers and oligomers were known for increasing the water-solubility of poorly soluble drugs by several orders of magnitudes. In particular DENA based hydrotropes were identified as specific agent for PTX [149]. The resulting HydroHA nanoparticles effectively encapsulated 20.7 wt.% PTX by simple dialysis method.
Very recently, Upadhyay et al. [150] prepared HA-block-poly(γ-benzyl-glutamate) (HA-b-PBLG) copolymer by conjugating PGBLG homopolymer to the reducing end group of HA via Huisgen 1,3-dipolar cycloaddition. This approach is highly advantageous for HA-based drug carriers because it could preserve the integrity and physicochemical characteristics of HA polymer, which is highly influenced in the uptake of the resulting nanocarriers. Interestingly, HA-b-PBLG copolymers formed polymersome structures in aqueous conditions, and loaded up to 12 wt.% of doxorubicin (DOX) and 10 wt.% of docetaxel [150-151]. The DOX loaded polymersomes exhibited significant tumor regression compared to the free DOX in breast cancer model.
4.3. HA-based nanocomplexes and nanogels
In an attempt to improve bioavailability of biological therapeutic agents such as proteins [152-153], oligodeoxynucleotide (ODN) [154], and small interfering RNA (siRNA) [155] for a prolong period of time and to deliver them to the target site, several nanocomplex systems based on HA have been developed. Owing to strong negative charge, HA could readily form ionic nanocomplexes with positively charged polymers. For instance, HA formed ionic nanocomplexes with a positively-charged protein referred to as the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) by simply mixing HA and TRAIL [153]. The HA-TRAIL complexes stabilized TRAIL for a longer period of time when compared with native TRAIL. Furthermore, the bioavailability was circulated in the blood stream for a prolonged period of time (5 days) as compared with the native TRAIL (less than 12h) after they were subcutaneously injected into the rats. To improve the stability and gene inhibition efficiency of ODN, HA was conjugated with ODN via reducible disulfide bonds, and the resulting HA-ODNA conjugates were complexed with protamine to form HA-ODN/protamine nanocomplexes of size about 200 nm. The HA-ODN/protamine nanocomplexes exhibited the improved stability and cellular uptake as compared to naked ODN. In addition, the gene inhibition effect of HA-ODN/protamine nanocomplexes was comparable with PEI/ODN complexes.
Besides polymeric nanocomplexes, HA-based nanogels with a size in the range of 200-500 nm were prepared by an inverse water-in-oil emulsion method [156]. In this method, within aqueous emulsion droplets, HA modified with thiol groups was crosslinked via formation of disulfide bond to form nano-sized gel capable of physically entrapping siRNA. The siRNA entrapped nanogels showed glutathione sensitive release profile, due to the cleavage of the disulfide bonds. The above nanogels were readily taken by CD44 overexpressing cancer cells (HCT-116), and also efficiently transfected to the cancer cells.
4.4. HA-conjugated colloidal nanoparticles
Several colloidal nanoparticles have been surface modified with HA to impart targeting characteristics. For example, many drug-loaded liposomes have been conjugated with HA as a targeting moiety (Figure 4a and 4b). These HA-bound liposomes (HALs) containing anti-cancer agents such as DOX [25, 157] and mitomycin C (MMC) [158] exhibited enhanced targeting ability to the cancer cells and higher therapeutic efficacy compared to free drugs. Like other systems based on HA, HALs also readily bound to and thereafter were taken up by the CD44-overexpressing cancer cells (B16F10), but not by the fibroblast cells (CV-1) known as CD44 negative cells [25]. In addition, DOX loaded HALs showed potent anticancer effect at relatively low DOX concentration as compared with free DOX or DOX loaded non-targeted liposomes. And the IC50 value of DOX-loaded HALs were significantly lower by more than 100 and 5 times as compared to those of free DOX and DOX-loaded non-targeted liposomes, respectively. Similarly, HA-conjugated liposomes (HALs) loaded with MMC showed 100-fold higher anticancer activity in CD44-overexpressing cancer cells [158]. In tumor bearing mouse models, MMC-loaded HALs were able to circulate for a prolonged period of time. With regard to therapeutic responses, tumor progression, metastatic burden and survival, the group treated with MMC-loaded HALs were significantly superior to the other groups without treatment or treated with free MMC (p<0.001). Similarly, drug loaded colloidal nanoparticles prepared from synthetic polymers have also modified with HA either by covalent conjugation of HA or through physical immobilization of HA derivatives (Figure 4c and 4d) [159]. Obviously, chemically functionalized nanoparticles have shown better colloidal stability and higher amount of HA on the surface of the nanoparticles. DOX loaded PLGA nanoparticles chemically conjugated with HA have demonstrated specific uptake in the CD44 expressed human breast cancer cells.[160].
Figure 4.

(a) Chemically HA-conjugated liposome (HAL); (b) a representative component structure of HAL, e.g. HA-conjugated palmitoyl oleoyl phosphatidylglycerol (HA-POPE); (c) non-covalently HA modified polymeric nanoparticle; and (d) covalently HA modified polymeric nanoparticle.
4.5. Hyaluronic acid-based imaging probe
Besides the drug delivery systems based on HA, a few imaging probes based on HA have been developed for detection of tumor cells or various molecular events in the tumor tissues or cells [146, 161-163]. Magnetic resonance imaging (MRI) is a powerful non-invasive diagnostic technique, which is currently limited by the drawbacks of the non-specific imaging agents. To improve the specificity, a HA based tumor-targetable magnetic nanoprobe (HA-MNP) with an average size of 15 nm was prepared by immobilizing HA on the surface of the magnetite nanocrystals using mussel-inspired method (Figure 5a) [162]. The in vitro study showed selective uptake of HA-MNP to the CD44-positive colon cancer cells (HCT-116) but not to the fibroblast cells (NIH-3T3) (Figure 5b).
Figure 5.

(a) Preparation of HA immobilized magnetite nanoparticles (HA-MNP); (b) In vitro T2-weighted MR images of HCT 1116 and NIH3T32 cells after incubated with HA-NMP, where + and − represent HA-MNP treated and non-treated cells, respectively. Adapted with permission from Lee et al. [162] Copyright 2008, John Wiley & Sons, Inc; (c) schematic representation of fluorescence quenching and recovery of HA-based gold nanoprobes (HA-GNP); and (d) In vivo fluorescence images of tumor-bearing mice after tail vein injection of HA-GNP. (Adapted with permission from Lee et al. [163]).
Since highly metastatic tumors and rheumatoid arthritis are known to overproduce Hyals and reactive oxygen species (ROS), respectively. To detect Hyals and ROS, an activatable HA-based gold nanoprobe (HA-GNP) was prepared by immobilizing the NIRF dye labeled HA oligomer (8–20 nm length) on to the surface of gold nanoparticles (16 nm diameter) (Figure 5c) [163]. In vivo studies have demonstrated a time-dependent fluorescence enhancement in both inflammation and tumor sites, followed by systemic administration. This time dependent increase in the fluorescence signals was attributed to the cleavage of HA oligomer by Hyal and ROS. For example, after tail vein-injection of HA-GNP in normal and human ovarian tumor (OVCAR-3) tumor bearing mice, detectable fluorescence signals were observed nearly after 3 h post-injection and the signals gradually increased in the tumor up to 24 h post-injection (Figure 5d). Recently, HA-based MR/optical dual imaging nanoprobe, sensitive to the Hyal, have been prepared [164]. The in vitro Hyal activity has been demonstrated using NIRF technique.
Early and effective monitoring of clinical response is critical in cancer chemotherapy because we could obtain critical information for future medical intervention. As conventional imaging methods like MRI are mainly focused on assessing change in the tumor volume, it is difficult to obtain therapeutic response at the initial stage of the treatment [165]. On the other hand, measuring changes at the molecular or cellular level could be beneficial and more valuable. Since most anticancer drugs kill cancer cells by initiating apoptosis, monitoring the activity of enzymes that involved in apopototic process could be an effective tool to follow the early therapeutic responses. Caspases, intracellular cysteine proteases, play a critical role in apoptosis. To target caspases, the imaging probe needs to be delivered into the cytoplasm of the cell. As HA nanoparticles can effectively internalize into cells and deliver their cargos into the intracellular compartments, a novel activatable polymeric nanoprobe (HA-PNP) based on self-assembled HA nanoparticles were developed by chemically labeling the nanoparticles using dual-quenched caspase-3-sensitive fluorogenic peptides [161]. The results from the study demonstrated that HA-PNP could efficiently transport caspase-3-sensitive fluorogenic peptides into cells, and thereby allowing strong fluorescence amplification for imaging in apoptotic tumor cells in real-time. More interestingly, the tumor apoptosis in tumor-bearing mice was clearly imaged by a non-invasive real-time NIRF imaging technique.
5. Conclusion and perspectives
Owing to its versatile physicochemical and biological properties, such as biocompatibility, biodegradability, non-immunogenicity, and selective uptake by specific cancer cells, HA has been widely utilized as an important constituent in the development of diverse carrier systems for cancer diagnostics and therapeutics. Each of the HA-based systems that have been investigated, so far, has its own advantages and disadvantages that need to be overcome. For example, HA-drug conjugates are limited by the lack of the facile chemical conjugation methods. Moreover, excess amount of drug conjugation to the HA polymer chain also affects the receptor mediated uptake process of the resulting drug conjugate, due to the significant loss of the HA characteristics by the chemical modification. Hence, it is important to produce the drug-conjugates in such a way that it should not alter the HA binding affinity to the receptors.
Similarly, in vivo studies performed using self-assembled nanoparticles have shown preferentially accumulation of HA-nanoparticles at the liver site due to the presence HA receptor on the liver endothelial cells. Currently, PEGylation strategy has been proposed to address this non-specific uptake. In vivo studies have demonstrated reduced liver uptake and higher tumor targetability of PEGylated HA nanoparticles. However, one should also critically consider the density of the PEGylation because excess PEG on the surface of the HA nanoparticles may reduce the targeting affinity of the resulting nanoparticles. Therefore, development of smart PEGylated HA nanoparticles, which could shield the HA surface during circulation and expose them before uptake, could be more beneficial to improve the therapeutic efficacy. As described in this review, several parameters such as molecular weight, chemical modification, size of the nanoparticles and its surface characteristics significantly affect the cellular uptake behavior of the resulting HA formulation. Despite the significant promise, only few drug delivery products based on HA is in the clinical trials. For example, the HA-PTX drug conjugate, ONCOFID™-P, is currently undergoing phase II clinical evaluation for the treatment of refractory bladder cancer. In conclusion, HA-based drug delivery systems have great potential for imaging and treatment of several cancers.
Acknowledgments
This study was supported in part by the grant from NIH (CA129287) and Showalter Trust Research Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 3.Gregoriadis G. Targeting of drugs. Nature. 1977;265:407–411. doi: 10.1038/265407a0. [DOI] [PubMed] [Google Scholar]
- 4.Cheon J, Lee JH. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc Chem Res. 2008;41:1630–1640. doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 6.Farokhzad OC, Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Kim JH, Park H, Kim YS, Park K, Nam H, Lee S, Park JH, Park RW, Kim IS, Choi K, Kim SY, Kwon IC. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J Control Release. 2010;146:219–227. doi: 10.1016/j.jconrel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Lee S, Kim JH, Park K, Kim K, Kwon IC. Polymeric nanomedicine for cancer therapy. Prog Polym Sci. 2008;33:113–137. [Google Scholar]
- 10.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 11.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim YS, Park K, Lee S, Nam HY, Min KH, Jo HG, Park JH, Choi K, Jeong SY, Park RW, Kim IS, Kim K, Kwon IC. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release. 2008;127:41–49. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 14.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. Journal of Controlled Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. Journal of Controlled Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 16.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62:7190–7194. [PubMed] [Google Scholar]
- 17.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 19.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv Drug Deliv Rev. 2004;56:491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2:373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 23.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269:3198–3204. [PubMed] [Google Scholar]
- 24.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 25.Eliaz RE, Szoka FC., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–2601. [PubMed] [Google Scholar]
- 26.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 28.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 29.Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–634. [Google Scholar]
- 30.Rapport MM, Weissmann B, Linker A, Meyer K. Isolation of a Crystalline Disaccharide, Hyalobiuronic Acid, from Hyaluronic Acid. Nature. 1951;168:996–997. doi: 10.1038/168996b0. [DOI] [PubMed] [Google Scholar]
- 31.Lurent TC. Biology and medical applications of hyaluronan and its derivatives (wenner-gren international series, Vol. 72) Portland Press; London: 1998. [Google Scholar]
- 32.Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem. 2008;144:131–137. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- 33.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 34.Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4584. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 35.Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 36.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, Day AJ. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe H, Cheung SC, Itano N, Kimata K, Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272:28057–28065. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 39.Hardingham TE, Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972;279:401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- 40.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lesley J, Hyman R, English N, Catterall J, Turner G. CD44 in inflammation and metastasis. Glycoconj J. 1997;14:611–622. doi: 10.1023/a:1018540610858. [DOI] [PubMed] [Google Scholar]
- 42.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 43.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 44.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282:5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 45.Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- 47.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 49.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, Geginat G, Arnold B, Goerdt S. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 50.Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–156. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 51.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Chang MC, Zylka D, Turley S, Harrison R, Turley EA. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J Biol Chem. 1998;273:11342–11348. doi: 10.1074/jbc.273.18.11342. [DOI] [PubMed] [Google Scholar]
- 53.Assmann V, Jenkinson D, Marshall JF, Hart IR. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J Cell Sci. 1999;112(Pt 22):3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 54.Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997;11:996–1007. doi: 10.1101/gad.11.8.996. [DOI] [PubMed] [Google Scholar]
- 55.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 56.Underhill CB, Toole BP. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3T3 cells. J Biol Chem. 1980;255:4544–4549. [PubMed] [Google Scholar]
- 57.Underhill CB, Thurn AL, Lacy BE. Characterization and identification of the hyaluronate binding site from membranes of SV-3T3 cells. J Biol Chem. 1985;260:8128–8133. [PubMed] [Google Scholar]
- 58.Tarone G, Ferracini R, Galetto G, Comoglio P. A cell surface integral membrane glycoprotein of 85,000 mol wt (gp85) associated with triton X-100-insoluble cell skeleton. J Cell Biol. 1984;99:512–519. doi: 10.1083/jcb.99.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underhill CB, Green SJ, Comoglio PM, Tarone G. The hyaluronate receptor is identical to a glycoprotein of Mr 85,000 (gp85) as shown by a monoclonal antibody that interferes with binding activity. J Biol Chem. 1987;262:13142–13146. [PubMed] [Google Scholar]
- 60.Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D, Bevilacqua C, Facchini A. Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum. 2001;44:1800–1807. doi: 10.1002/1529-0131(200108)44:8<1800::AID-ART317>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 62.Ward JA, Huang L, Guo H, Ghatak S, Toole BP. Perturbation of hyaluronan interactions inhibits malignant properties of glioma cells. Am J Pathol. 2003;162:1403–1409. doi: 10.1016/S0002-9440(10)64273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci. 1993;106(Pt 1):365–375. doi: 10.1242/jcs.106.1.365. [DOI] [PubMed] [Google Scholar]
- 64.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 65.Tammi R, Saamanen AM, Maibach HI, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 1991;97:126–130. doi: 10.1111/1523-1747.ep12478553. [DOI] [PubMed] [Google Scholar]
- 66.Morales TI, Hascall VC. Correlated metabolism of proteoglycans and hyaluronic acid in bovine cartilage organ cultures. J Biol Chem. 1988;263:3632–3638. [PubMed] [Google Scholar]
- 67.Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE) J Biol Chem. 2000;275:37733–37741. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]
- 68.Chain E, Duthie ES. Identity of hyaluronidase and spreading factor. Brit J Exper Path. 1940;21:324–388. [Google Scholar]
- 69.Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Toole BP, Biswas C, Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci U S A. 1979;76:6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertrand P, Girard N, Delpech B, Duval C, d'Anjou J, Dauce JP. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer. 1992;52:1–6. doi: 10.1002/ijc.2910520102. [DOI] [PubMed] [Google Scholar]
- 72.Asplund T, Versnel MA, Laurent TC, Heldin P. Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res. 1993;53:388–392. [PubMed] [Google Scholar]
- 73.Knudson W, Biswas C, Toole BP. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A. 1984;81:6767–6771. doi: 10.1073/pnas.81.21.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calabro A, Oken MM, Hascall VC, Masellis AM. Characterization of hyaluronan synthase expression and hyaluronan synthesis in bone marrow mesenchymal progenitor cells: predominant expression of HAS1 mRNA and up-regulated hyaluronan synthesis in bone marrow cells derived from multiple myeloma patients. Blood. 2002;100:2578–2585. doi: 10.1182/blood-2002-01-0030. [DOI] [PubMed] [Google Scholar]
- 76.Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998;58:342–347. [PubMed] [Google Scholar]
- 78.Setala LP, Tammi MI, Tammi RH, Eskelinen MJ, Lipponen PK, Agren UM, Parkkinen J, Alhava EM, Kosma VM. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer. 1999;79:1133–1138. doi: 10.1038/sj.bjc.6690180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lokeshwar VB, Ek CAN, Pham HT, Wei D, Young MJ, Duncan RC, Soloway MS, Block NL. Urinary hyaluronic acid and hyaluronidase: markers for bladder cancer detection and evaluation of grade. J Urology. 2000;163:348–356. doi: 10.1016/s0022-5347(05)68050-0. [DOI] [PubMed] [Google Scholar]
- 80.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 81.Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N. Hyperproduction of hyaluronan in neuinduced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol. 2007;170:1086–1099. doi: 10.2353/ajpath.2007.060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res. 2001;61:5207–5214. [PubMed] [Google Scholar]
- 83.Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol. 2002;161:849–857. doi: 10.1016/S0002-9440(10)64245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Itano N, Sawai T, Atsumi F, Miyaishi O, Taniguchi S, Kannagi R, Hamaguchi M, Kimata K. Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem. 2004;279:18679–18687. doi: 10.1074/jbc.M313178200. [DOI] [PubMed] [Google Scholar]
- 85.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer. 1998;77:396–401. doi: 10.1002/(sici)1097-0215(19980729)77:3<396::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 86.Gilg AG, Tye SL, Tolliver LB, Wheeler WG, Visconti RP, Duncan JD, Kostova FV, Bolds LN, Toole BP, Maria BL. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 87.Lokeshwar VB, Selzer MG. Differences in Hyaluronic Acid-mediated Functions and Signaling in Arterial, Microvessel, and Vein-derived Human Endothelial Cells. J Biol Chem. 2000;275:27641–27649. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]
- 88.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 89.Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- 90.Rahmanian M, Pertoft H, Kanda S, Christofferson R, Claesson-Welsh L, Heldin P. Hyaluronan oligosaccharides induce tube formation of a brain endothelial cell line in vitro. Exp Cell Res. 1997;237:223–230. doi: 10.1006/excr.1997.3792. [DOI] [PubMed] [Google Scholar]
- 91.Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 92.Stamenkovic I, Aruffo A, Amiot M, Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991;10:343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Birch M, Mitchell S, Hart IR. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991;51:6660–6667. [PubMed] [Google Scholar]
- 94.Sy MS, Guo YJ, Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991;174:859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 96.Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zoller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stauder R, Eisterer W, Thaler J, Gunthert U. CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood. 1995;85:2885–2899. [PubMed] [Google Scholar]
- 98.Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJ, Pals ST. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344:1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 99.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
- 100.Mayer B, Jauch KW, Gunthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993;342:1019–1022. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- 101.Terpe HJ, Storkel S, Zimmer U, Anquez V, Fischer C, Pantel K, Gunthert U. Expression of CD44 isoforms in renal cell tumors. Positive correlation to tumor differentiation. Am J Pathol. 1996;148:453–463. [PMC free article] [PubMed] [Google Scholar]
- 102.Endo K, Terada T. Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, p53 expression, and patient survival. J Hepatol. 2000;32:78–84. doi: 10.1016/s0168-8278(00)80192-0. [DOI] [PubMed] [Google Scholar]
- 103.Kainz C, Kohlberger P, Sliutz G, Tempfer C, Heinzl H, Reinthaller A, Breitenecker G, Koelbl H. Splice variants of CD44 in human cervical cancer stage IB to IIB. Gynecol Oncol. 1995;57:383–387. doi: 10.1006/gyno.1995.1159. [DOI] [PubMed] [Google Scholar]
- 104.Uhl-Steidl M, Muller-Holzner E, Zeimet AG, Adolf GR, Daxenbichler G, Marth C, Dapunt O. Prognostic value of CD44 splice variant expression in ovarian cancer. Oncology. 1995;52:400–406. doi: 10.1159/000227497. [DOI] [PubMed] [Google Scholar]
- 105.Hirata T, Fukuse T, Naiki H, Hitomi S, Wada H. Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res. 1998;58:1108–1110. [PubMed] [Google Scholar]
- 106.Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995;345:615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- 107.Martin S, Jansen F, Bokelmann J, Kolb H. Soluble CD44 splice variants in metastasizing human breast cancer. Int J Cancer. 1997;74:443–445. doi: 10.1002/(sici)1097-0215(19970822)74:4<443::aid-ijc14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 108.Manten-Horst E, Danen EH, Smit L, Snoek M, Le Poole IC, Van Muijen GN, Pals ST, Ruiter DJ. Expression of CD44 splice variants in human cutaneous melanoma and melanoma cell lines is related to tumor progression and metastatic potential. Int J Cancer. 1995;64:182–188. doi: 10.1002/ijc.2910640307. [DOI] [PubMed] [Google Scholar]
- 109.Zahalka MA, Okon E, Gosslar U, Holzmann B, Naor D. Lymph node (but not spleen) invasion by murine lymphoma is both CD44- and hyaluronate-dependent. J Immunol. 1995;154:5345–5355. [PubMed] [Google Scholar]
- 110.Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228–1232. [PubMed] [Google Scholar]
- 111.Lokeshwar VB, Young MJ, Goudarzi G, Iida N, Yudin AI, Cherr GN, Selzer MG. Identification of bladder tumor-derived hyaluronidase: its similarity to HYAL1. Cancer Res. 1999;59:4464–4470. [PubMed] [Google Scholar]
- 112.Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- 113.Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer. 2003;106:438–445. doi: 10.1002/ijc.11252. [DOI] [PubMed] [Google Scholar]
- 114.Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL. HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res. 2005;65:7782–7789. doi: 10.1158/0008-5472.CAN-05-1022. [DOI] [PubMed] [Google Scholar]
- 115.Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of Elevated Levels of Hyaluronidase, a Matrix-degrading Enzyme, with Prostate Cancer Progression. Cancer Res. 1996;56:651–657. [PubMed] [Google Scholar]
- 116.Chao KL, Muthukumar L, Herzberg O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry. 2007;46:6911–6920. doi: 10.1021/bi700382g. [DOI] [PubMed] [Google Scholar]
- 117.Pham HT, Block NL, Lokeshwar VB. Tumor-derived Hyaluronidase: A Diagnostic Urine Marker for High-Grade Bladder Cancer. Cancer Res. 1997;57:778–783. [PubMed] [Google Scholar]
- 118.Hautmann S, Toma M, Lorenzo Gomez MF, Friedrich MG, Jaekel T, Michl U, Schroeder GL, Huland H, Juenemann KP, Lokeshwar VB. Immunocyt and the HA-HAase urine tests for the detection of bladder cancer: a side-by-side comparison. Eur Urol. 2004;46:466–471. doi: 10.1016/j.eururo.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 119.Lokeshwar VB, Schroeder GL, Selzer MG, Hautmann SH, Posey JT, Duncan RC, Watson R, Rose L, Markowitz S, Soloway MS. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer. 2002;95:61–72. doi: 10.1002/cncr.10652. [DOI] [PubMed] [Google Scholar]
- 120.Christopoulos TA, Papageorgakopoulou N, Theocharis DA, Mastronikolis NS, Papadas TA, Vynios DH. Hyaluronidase and CD44 hyaluronan receptor expression in squamous cell laryngeal carcinoma. Biochim Biophys Acta. 2006;1760:1039–1045. doi: 10.1016/j.bbagen.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 121.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Junker N, Latini S, Petersen LN, Kristjansen PE. Expression and regulation patterns of hyaluronidases in small cell lung cancer and glioma lines. Oncol Rep. 2003;10:609–616. [PubMed] [Google Scholar]
- 123.Delpech B, Laquerriere A, Maingonnat C, Bertrand P, Freger P. Hyaluronidase is more elevated in human brain metastases than in primary brain tumours. Anticancer Res. 2002;22:2423–2427. [PubMed] [Google Scholar]
- 124.Bertrand P, Courel MN, Maingonnat C, Jardin F, Tilly H, Bastard C. Expression of HYAL2 mRNA, hyaluronan and hyaluronidase in B-cell non-Hodgkin lymphoma: relationship with tumor aggressiveness. Int J Cancer. 2005;113:207–212. doi: 10.1002/ijc.20562. [DOI] [PubMed] [Google Scholar]
- 125.Bertrand P, Girard N, Duval C, d'Anjou J, Chauzy C, Méard JF, Delpech B. Increased hyaluronidase levels in breast tumor metastases. Int J Cancer. 1997;73:327–331. doi: 10.1002/(sici)1097-0215(19971104)73:3<327::aid-ijc4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 126.Beech DJ, Madan AK, Deng N. Expression of PH-20 in normal and neoplastic breast tissue. J Surg Res. 2002;103:203–207. doi: 10.1006/jsre.2002.6351. [DOI] [PubMed] [Google Scholar]
- 127.Udabage L, Brownlee GR, Nilsson SK, Brown TJ. The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res. 2005;310:205–217. doi: 10.1016/j.yexcr.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 128.Victor R, Chauzy C, Girard N, Gioanni J, d'Anjou J, Stora De Novion H, Delpech B. Human breast-cancer metastasis formation in a nude-mouse model: studies of hyaluronidase, hyaluronan and hyaluronan-binding sites in metastatic cells. Int J Cancer. 1999;82:77–83. doi: 10.1002/(sici)1097-0215(19990702)82:1<77::aid-ijc14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 129.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar S, West DC, Ponting JM, Gattamaneni HR. Sera of children with renal tumours contain low-molecular-mass hyaluronic acid. Int J Cancer. 1989;44:445–448. doi: 10.1002/ijc.2910440311. [DOI] [PubMed] [Google Scholar]
- 131.Lokeshwar VB, Cerwinka WH, Lokeshwar BL. HYAL1 hyaluronidase: a molecular determinant of bladder tumor growth and invasion. Cancer Res. 2005;65:2243–2250. doi: 10.1158/0008-5472.CAN-04-2805. [DOI] [PubMed] [Google Scholar]
- 132.Simpson MA. Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am J Pathol. 2006;169:247–257. doi: 10.2353/ajpath.2006.060032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jacobson A, Rahmanian M, Rubin K, Heldin P. Expression of hyaluronan synthase 2 or hyaluronidase 1 differentially affect the growth rate of transplantable colon carcinoma cell tumors. Int J Cancer. 2002;102:212–219. doi: 10.1002/ijc.10683. [DOI] [PubMed] [Google Scholar]
- 134.Shuster S, Frost GI, Csoka AB, Formby B, Stern R. Hyaluronidase reduces human breast cancer xenografts in SCID mice. Int J Cancer. 2002;102:192–197. doi: 10.1002/ijc.10668. [DOI] [PubMed] [Google Scholar]
- 135.Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem. 1999;10:755–763. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 136.Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, Ravoori M, Kundra V, Freedman RS, Klostergaard J. Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia. 2007;9:479–486. doi: 10.1593/neo.07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee H, Lee K, Park TG. Hyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjugate Chemistry. 2008;19:1319–1325. doi: 10.1021/bc8000485. [DOI] [PubMed] [Google Scholar]
- 138.Rosato A, Banzato A, De Luca G, Renier D, Bettella F, Pagano C, Esposito G, Zanovello P, Bassi P. HYTAD1-p20: A new paclitaxel-hyaluronic acid hydrosoluble bioconjugate for treatment of superficial bladder cancer. Urologic Oncology-Seminars and Original Investigations. 2006;24:207–215. doi: 10.1016/j.urolonc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 139.Coradini D, Pellizzaro C, Miglierini G, Daidone MG, Perbellini A. Hyaluronic acid as drug delivery for sodium butyrate: improvement of the anti-proliferative activity on a breast-cancer cell line. Int J Cancer. 1999;81:411–416. doi: 10.1002/(sici)1097-0215(19990505)81:3<411::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 140.Coradini D, Zorzet S, Rossin R, Scarlata I, Pellizzaro C, Turrin C, Bello M, Cantoni S, Speranza A, Sava G, Mazzi U, Perbellini A. Inhibition of hepatocellular carcinomas in vitro and hepatic metastases in vivo in mice by the histone deacetylase inhibitor HA-But. Clin Cancer Res. 2004;10:4822–4830. doi: 10.1158/1078-0432.CCR-04-0349. [DOI] [PubMed] [Google Scholar]
- 141.Yadav AK, Mishra P, Mishra AK, Mishra P, Jain S, Agrawal GP. Development and characterization of hyaluronic acid-anchored PLGA nanoparticulate carriers of doxorubicin. Nanomedicine: Nanotechnology, Biology and Medicine. 2007;3:246–257. doi: 10.1016/j.nano.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Choi KY, Lee S, Park K, Kim K, Park JH, Kwon IC, Jeong SY. Preparation and characterization of hyaluronic acid-based hydrogel nanoparticles. J Phys Chem Solids. 2008;69:1591–1595. [Google Scholar]
- 143.Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31:106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 144.Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: synthesis, characterization, and in vivo biodistribution. Journal of Materials Chemistry. 2009;19:4102–4107. [Google Scholar]
- 145.Yadav AK, Mishra P, Jain S, Mishra P, Mishra AK, Agrawal GP. Preparation and characterization of HA–PEG–PCL intelligent core–corona nanoparticles for delivery of doxorubicin. Journal of Drug Targeting. 2008;16:464–478. doi: 10.1080/10611860802095494 . [DOI] [PubMed] [Google Scholar]
- 146.Lee H, Ahn CH, Park TG. Poly[lactic-co-(glycolic acid)]-grafted hyaluronic acid copolymer micelle nanoparticles for target-specific delivery of doxorubicin. Macromol Biosci. 2009;9:336–342. doi: 10.1002/mabi.200800229. [DOI] [PubMed] [Google Scholar]
- 147.Saravanakumar G, Choi KY, Yoon HY, Kim K, Park JH, Kwon IC, Park K. Hydrotropic hyaluronic acid conjugates: Synthesis, characterization, and implications as a carrier of paclitaxel. International Journal of Pharmaceutics. 2010;394:154–161. doi: 10.1016/j.ijpharm.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 148.Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Choi K, Jeong SY. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials. 2011;32:1880–1889. doi: 10.1016/j.biomaterials.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 149.Lee J, Lee SC, Acharya G, Chang CJ, Park K. Hydrotropic solubilization of paclitaxel: analysis of chemical structures for hydrotropic property. Pharm Res. 2003;20:1022–1030. doi: 10.1023/a:1024458206032. [DOI] [PubMed] [Google Scholar]
- 150.Upadhyay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, Le Meins JF, Farooque A, Chandraiah G, Jain AK, Misra A, Lecommandoux S. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly([gamma]-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31:2882–2892. doi: 10.1016/j.biomaterials.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 151.Upadhyay KK, Bhatt AN, Castro E, Mishra AK, Chuttani K, Dwarakanath BS, Schatz C, Le Meins JF, Misra A, Lecommandoux S. In vitro and In vivo Evaluation of Docetaxel Loaded Biodegradable Polymersomes. Macromolecular Bioscience. 2010;10:503–512. doi: 10.1002/mabi.200900415. [DOI] [PubMed] [Google Scholar]
- 152.Kim YJ, Chae SY, Jin CH, Sivasubramanian M, Son S, Choi KY, Jo DG, Kim K, Chan Kwon I, Lee KC, Park JH. Ionic complex systems based on hyaluronic acid and PEGylated TNF-related apoptosis-inducing ligand for treatment of rheumatoid arthritis. Biomaterials. 2010;31:9057–9064. doi: 10.1016/j.biomaterials.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 153.Na SJ, Chae SY, Lee S, Park K, Kim K, Park JH, Kwon IC, Jeong SY, Lee KC. Stability and bioactivity of nanocomplex of TNF-related apoptosis-inducing ligand. Int J Pharm. 2008;363:149–154. doi: 10.1016/j.ijpharm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 154.Mok H, Park JW, Park TG. Antisense oligodeoxynucleotide-conjugated hyaluronic acid/protamine nanocomplexes for intracellular gene inhibition. Bioconjug Chem. 2007;18:1483–1489. doi: 10.1021/bc070111o. [DOI] [PubMed] [Google Scholar]
- 155.Jiang G, Park K, Kim J, Kim KS, Hahn SK. Target Specific Intracellular Delivery of siRNA/PEI–HA Complex by Receptor Mediated Endocytosis. Mol Pharm. 2009;6:727–737. doi: 10.1021/mp800176t. [DOI] [PubMed] [Google Scholar]
- 156.Lee H, Mok H, Lee S, Oh YK, Park TG. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 2007;119:245–252. doi: 10.1016/j.jconrel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 157.Eliaz RE, Nir S, Marty C, Szoka FC., Jr Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res. 2004;64:711–718. doi: 10.1158/0008-5472.can-03-0654. [DOI] [PubMed] [Google Scholar]
- 158.Peer D, Margalit R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer. 2004;108:780–789. doi: 10.1002/ijc.11615. [DOI] [PubMed] [Google Scholar]
- 159.Lemarchand C, Gref R, Couvreur P. Polysaccharide-decorated nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58:327–341. doi: 10.1016/j.ejpb.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 160.Hyung W, Ko H, Park J, Lim E, Park SB, Park YJ, Yoon HG, Suh JS, Haam S, Huh YM. Novel hyaluronic acid (HA) coated drug carriers (HCDCs) for human breast cancer treatment. Biotechnology and Bioengineering. 2008;99:442–454. doi: 10.1002/bit.21578. [DOI] [PubMed] [Google Scholar]
- 161.Lee S, Choi KY, Chung H, Ryu JH, Lee A, Koo H, Youn IC, Park JH, Kim IS, Kim SY, Chen X, Jeong SY, Kwon IC, Kim K, Choi K. Real time, high resolution video imaging of apoptosis in single cells with a polymeric nanoprobe. Bioconjug Chem. 2011;22:125–131. doi: 10.1021/bc1004119. [DOI] [PubMed] [Google Scholar]
- 162.Lee Y, Lee H, Kim YB, Kim J, Hyeon T, Park H, Messersmith PB, Park TG. Bioinspired Surface Immobilization of Hyaluronic Acid on Monodisperse Magnetite Nanocrystals for Targeted Cancer Imaging. Adv Mater Deerfield. 2008;20:4154–4157. doi: 10.1002/adma.200800756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee H, Lee K, Kim IK, Park TG. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials. 2008;29:4709–4718. doi: 10.1016/j.biomaterials.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 164.Lee DE, Kim A, Saravanakumar G, Koo H, Kwon I, Choi K, Park J, Kim K. Hyaluronidase-sensitive SPIONs for MR/optical dual imaging nanoprobes. Macromol Res. 2011;19:861–867. [Google Scholar]
- 165.Oishi M, Tamura A, Nakamura T, Nagasaki Y. A Smart Nanoprobe Based On Fluorescence-Quenching PEGylated Nanogels Containing Gold Nanoparticles for Monitoring the Response to Cancer Therapy. Adv Funct Mater. 2009;19:827–834. [Google Scholar]


