Abstract
Rationale
Cardiomyocyte apoptosis is one of the key events in the development and progression of heart failure, and a crucial role for ICER (inducible cAMP early repressor) in this process has been previously reported. ERK5 is known to inhibit cardiac apoptosis after myocardial infarction (MI), especially in hyperglycemic states, via association with CHIP ubiquitin (Ub) ligase and subsequent up-regulation of CHIP ligase activity, which induces ICER ubiquitination and subsequent protein degradation. The regulatory mechanism governing ERK5/CHIP interaction is unknown.
Objective
We previously demonstrated increased p90RSK activation in the diabetic heart. As a logical extension of this work, we now investigate whether p90RSK activation inhibits ERK5-mediated CHIP activation, and subsequently increases ICER levels and apoptosis.
Methods and Results
p90RSK activation inhibits ERK5/CHIP association and CHIP Ub ligase activity. p90RSK and CHIP share a common binding site in the ERK5 C-terminal domain (aa571-807). Overexpression of either p90RSK or an ERK5 fragment (aa571-807) inhibits ERK5/CHIP association, suggesting that p90RSK and CHIP competes for ERK5 binding and that p90RSK activation is critical for inhibiting ERK5/CHIP interaction. We also identified ERK5-S496 as being directly phosphorylated by p90RSK, and demonstrated that an ERK5-S496A mutant significantly impairs Angiotensin II-mediated inhibition of CHIP activity and subsequent increase in ICER levels. In vivo, either cardiac specific depletion of ERK5 or overexpression of p90RSK inhibits CHIP activity and accelerates cardiac apoptosis after MI--a phenomenon fully reversible by activating ERK5.
Conclusion
These data suggest a role for p90RSK in inhibiting CHIP activity and promoting cardiac apoptosis through binding to and phosphorylation of ERK5-S496.
Keywords: MAP kinase pathway, ubiquitin, diabetes mellitus (DM), myocardial infarction (MI), apoptosis
INTRODUCTION
Diabetes mellitus (DM) is an independent risk factor for both mortality and morbidity after myocardial infarction (MI)1, 2. Previously, we have reported that activation of ERK5, an atypical mitogen activated protein kinase with transcriptional activity3-6, inhibits apoptosis and left ventricular (LV) dysfunction in DM mice following MI. Sustained elevation of inducible cAMP early repressor (ICER), a pro-apoptotic transcriptional repressor7, 8, favors apoptosis through inhibition of the cAMP response element binding protein (CREB)-mediated transcription and down-regulation of Bcl-29, 10. The protein level of ICER is regulated by CREB-dependent ICER gene transcription as well as proteasome-dependent ICER protein ubiquitination and degradation11. We reported that ICER levels were significantly increased in diabetic mice after MI (DM+MI), and this increase in ICER levels was blunted in transgenic mice expressing a cardiac-specific constitutively active form of MEK5α (CA-MEK5α-Tg)12. This finding provides a mechanistic framework for understanding the cardio-protective action of ERK5 in DM + MI mice via downregulation of ICER levels and inhibition of apoptosis.
In a subsequent study we demonstrated that ubiquitination was a critical regulatory mechanism linking ERK5 activation and ICER reduction in DM mice. ERK5 positively regulated chaperone-dependent E3 ubiquitin (Ub) ligase CHIP (carboxyl terminus of Hsp70-interacting protein)-mediated ICER ubiquitination and subsequent protein degradation. This down-regulation of ICER levels leads to maintaining Bcl-2 at high levels, and protects cells from apoptosis. Our studies showed that cardiac CHIP Ub ligase activity was significantly decreased after DM + MI and this decrease was rectified in CA-MEK5α-Tg mice13. However, the regulatory mechanism underlying this reduction of CHIP Ub ligase activity in DM + MI mice and how ERK5 activation reversed this phenomenon remains unclear.
In this study, we show that the ERK5-CHIP module is one of the major targets of p90RSK in diabetic hearts. Our data strongly suggest that the activation of p90RSK abrogates ERK5-mediated CHIP Ub ligase activation, and accelerates apoptosis and cardiac dysfunction in the DM+MI condition.
METHODS
Additional information regarding animals, antibodies and reagents, plasmids, adenovirus construction, cell culture, mammalian two-hybrid analysis, immunoprecipitation, western blotting, p90RSK in vitro kinase assay, in vitro ubiquitination assay with GST-ICER, permanent coronary ligation surgery, streptozotocin (STZ) injection, apoptosis assay, echocardiographic analysis, LC-MS/MS analysis of ERK5 phosphorylation by p90RSK, and statistics can be found in the Online Data Supplement at http://circres.ahajournals.org.
RESULTS
Angiotensin II upregulates the ICER protein levels and promotes apoptosis of cardiomyocytes via p90RSK activation
Angiotensin II (Ang II) plays a pivotal role in the exacerbation of heart failure in patients with diabetes after MI14-16. In these patients the ICER protein levels and apoptosis in cardiomyocytes are enhanced17, 18, and p90RSK is also activated19. p90RSKs are a family of serine/threonine kinases, activated by various stimuli including ischemia20, 21, reactive oxygen species (ROS)22-25, Ang II26-28, and DM24. Since ICER is important in the regulation of cardiomyocyte apoptosis12, we have examined whether p90RSK modulates apoptosis via regulation of the ICER protein level. First, we confirmed that Ang II activated p90RSK in cardiomyocytes (Online Figs. IA and IB). Similarly, high glucose (25 mM) also activated p90RSK, whereas mannitol (25 mM), which served as a hyperosmolar control, did not (OnlineFigs. IC and ID).
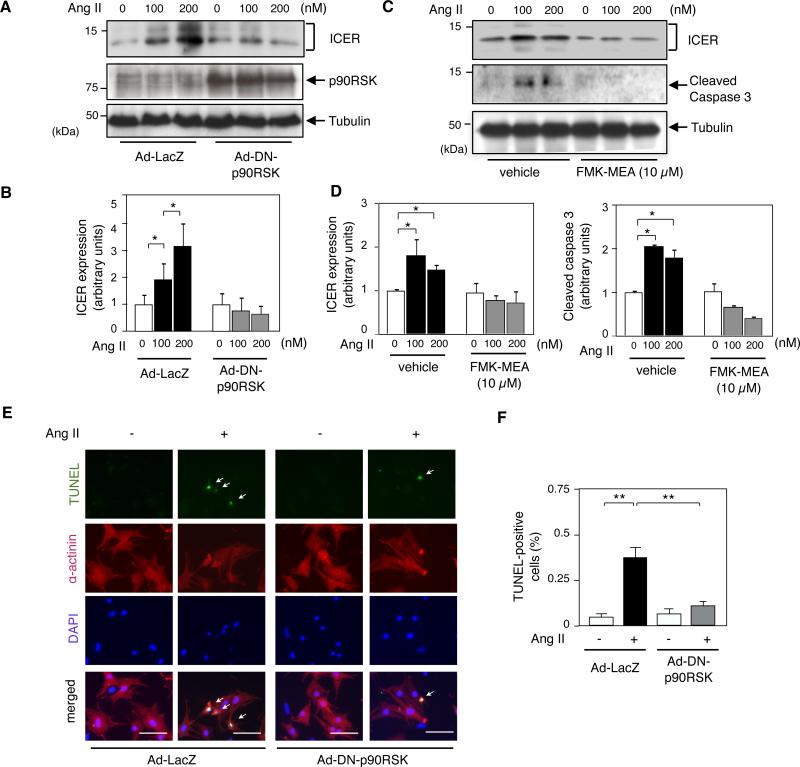
Next, we investigated the involvement of p90RSK activation in cardiomyocyte apoptosis as assayed by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining. When cardiomyocytes were treated with Ang II, both ICER levels (Figs.1A and 1B) and the number of TUNEL-positive cells (Figs. 1E and 1F) increased. However, these increases were inhibited in cells transduced by an adenovirus expressing dominant negative p90RSK (Ad-DN-p90RSK) (Figs. 1A and 1B and Figs. 1E and 1F).
Figure 1. Ang II induced ICER expression and apoptosis via p90RSK activation.
(A) Cardiomyocytes were transduced with adenovirus containing either dominant negative form of p90RSK (Ad-DN-p90RSK) or LacZ (Ad-LacZ) as a control (MOI = 20) for 3 h. They were then stimulated with Ang II (100 nM and 200 nM) for 24 h. ICER, p90RSK and tubulin were detected by Western blotting with each specific antibody. (B) ICER band intensities were quantified (Fujifilm Image Gauge 4.0) and normalized relatively to tubulin band intensity. Results are expressed as fold increase in Ang II-treated compared to the untreated group. * p<0.05, compared to untreated control, mean±S.D., n=3. (C) Cardiomyocytes were stimulated with Ang II for 24 h after 3 h of FMK-MEA pre-treatment, and Western blotting was performed with each specific antibody. (D) ICER and cleaved caspase-3 band intensities are shown relatively to the tubulin band intensity at each point. Shown is mean±S.D. (n=3). *p<0.05 , compared to the vehicle control. (E) Cardiomyocytes were transduced with Ad-DN-p90RSK or Ad-LacZ as in (A) and stimulated with AngII (200 nM) for 24 h, and processed for TUNEL staining. Representative pictures of TUNEL (top),α-actinin (second row), DAPI (third row), and merged (bottom) staining. 40X objective lens. Scale bars: 40 μm. (F) Bar graphs showing percentage of TUNEL positive cells (total of 400-600 cells counted). **p<0.01, *p<0.05, mean±S.D., n=3.
In addition to overexpressing dominant negative p90RSK, we also used 1-(4-amino-7-(3-(2-methoxyethylamino) proxyl)-5-p-tolyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-2-fluoroethanone (FMK-MEA) which is a water-soluble derivative of the previously reported fluoromethyllketone, a selective p90RSK inhibitor.29, 30. Ang II-induced increase in the ICER levels and apoptosis assayed by cleaved caspase 3 expression were significantly inhibited in cardiomyocytes treated by FMK-MEA (Figs.1C and 1D), also supporting the obligatory role of p90RSK activation in these Ang II-mediated effects. We confirmed that Ang II-mediated p90RSK activation was significantly inhibited by Ad-DN-p90RSK transduction and FMK-MEA treatment (Online Fig. II). Furthermore, we performed the converse experiment using Ad-WT-p90RSK and found higher ICER levels in cardiomyocytes (OnlineFigIIIB), also confirming an obligatory role for p90RSK in regulating ICER levels.
Activation of p90RSK reduces CHIP Ub ligase activity induced by Ang II in vitro and after myocardial infarction in diabetic mice in vivo
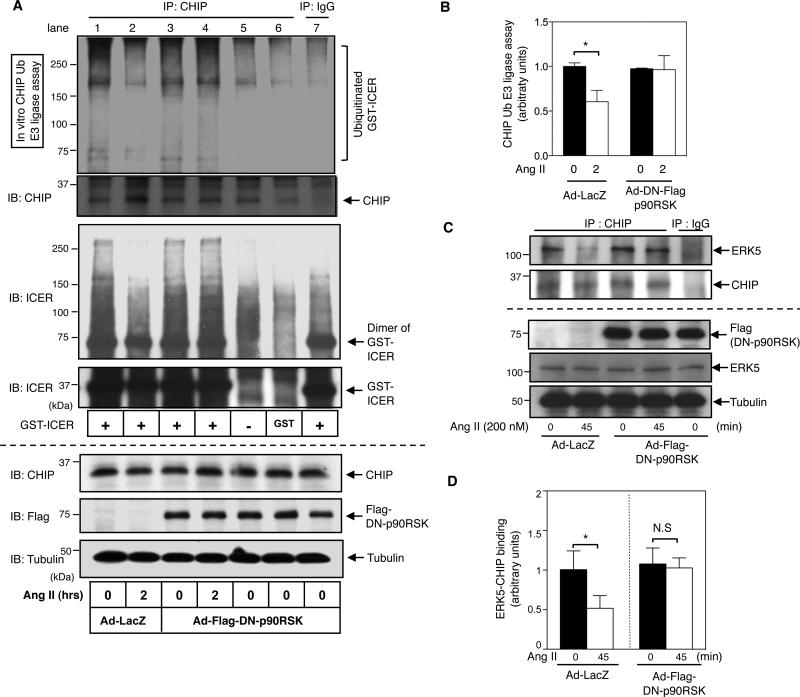
ERK5-CHIP association is critical for the up-regulation of CHIP Ub ligase activity, leading to the ubiquitination and subsequent proteosomal degradation of ICER13. Since p90RSK activation was crucial for an Ang II-mediated increase in ICER levels, we investigated whether p90RSK could negatively regulate CHIP Ub ligase activity and thereby stabilize the ICER protein. To detect CHIP Ub ligase activity, we performed an in vitro ubiquitination assay using a GST-ICER fusion protein as a substrate13. CHIP Ub ligase activity was significantly decreased after Ang II stimulation, and transduction with Ad-DN-p90RSK significantly inhibited this phenomenon (Figs. 2A and 2B).
Figure 2. p90RSK inhibited CHIP Ub ligase activity and ERK5-CHIP association.
(A) Ang II inhibited CHIP Ub ligase activity. Cardiomyocytes were transduced with either Ad-DN-p90RSK or Ad-LacZ for 24 h, followed by stimulation with Ang II (200 nM) for indicated times. Immunoprecipitated CHIP was subjected to an in vitro ubiquitination assay to determine CHIP Ub ligase activity using GST-fused ICER protein as a substrate (lane 1-4). The expression of Flag-tagged DN-p90RSK, CHIP, and tubulin was detected by Western blotting with each specific antibody. In vitro Ub ligase assay of GST-ICER with the Ub conjugation system and immunoprecipitates with IgG control (lane 7), and in vitro Ub ligase assay with GST only (lane 6) or without any GST substrate (lane 5) with the Ub conjugation system and immunoprecipitates with CHIP Ab (lane 1-6) were also shown. Immunobloting with anti-CHIP (2nd from top) and anti-ICER antibody (3rd and 4th from top) after in vitro CHIP Ub ligase assay suggested that the Ub bands we observed in this assay, especially above 120kDa, are mainly due to the ubiquitination of GST-ICER but not from GST, IgG or endogenous CHIP as described in the method. (B) Quantifications of relative CHIP Ub ligase activity from 3 independent experiments (mean±S.D., n=3, *p< 0.05 compared to the Ad-LacZ and untreated control). Results were expressed as the relative ratio of untreated cells in the LacZ control. (C) p90RSK activation inhibited ERK5-CHIP association. Cardiomyocytes were transduced with Ad-DN-p90RSK or Ad-LacZ for 24 h, followed by 45 min Ang II stimulation. Cell lysates were immunoprecipitated with anti-CHIP, and immunoblotted with anti-ERK5. Expression of ERK5, CHIP and Flag-tagged DN-p90RSK was detected by Western blotting using each specific antibody. (D) Relative co-immunoprecipitated ERK5 band intensitites from 3 independent experiments were quantified (mean±S.D., n=3, *p<0.05, compared to untreated control). Results are expressed as fold decrease in ERK5 band intensity in Ang II-treated cells that are expressing either Ad-LacZ or Ad-Flag-DN-p90RSK compared to untreated cells.
Next we investigated whether p90RSK activation could regulate ICER mRNA expression. As shown in Online Fig. IIIA, we found that Ang II increased ICER mRNA expression. However, we could not detect any inhibition of ICER mRNA expression by Ad-DN-p90RSK transduction. These data suggested that p90RSK activation regulates ICER levels at the post-transcriptional rather than the transcriptional level. Since CHIP Ub ligase activity requires ERK5-CHIP interaction13, and since Ang II regulates this process by activating p90RSK, we examined whether Ang II-mediated p90RSK activation could regulate ERK5-CHIP interaction. In quiescent cardiomyocytes, ERK5 activity regulates the association of ERK5 with CHIP 13. Indeed, we found that the ERK5-CHIP association was significantly decreased by stimulation of cardiomyocytes with Ang II ( s. 2C and 2D) and Ad-DN-p90RSK transduction blocked this decrease in cells treated with Ang II (Figs. 2C and 2D). These results indicated a key role for p90RSK in the reduction of ERK5-CHIP association, and subsequent inhibition of CHIP Ub ligase activity in cells stimulated with Ang II.
To explore the role of p90RSK activation in the reduction of CHIP Ub ligase activity in vivo, we utilized the cardiac-specific DN-p90RSK-Tg mice. These mice showed relative resilience to cardiac ischemia/reperfusion injury31, therefore we examined the effects of inhibiting p90RSK in diabetic mice after MI. The cardiac function of DN-p90RSK-Tg mice was unaltered 31, and the ICER levels in these transgenic were similar to that of non-transgenic littermate control (NLC) sham operated mice (Online Fig. IVA). Mice were rendered diabetic by intraperitoneal injection of streptozotocin (STZ). On day 7 after STZ injection, both NLC and DN-p90RSK-Tg mice showed elevated random blood sugar (BS) levels with similar body weight (Online Fig. VB). We have previously shown that preoperative insulin-treatment of STZ-injected mice stabilizes cardiac function after MI, suggesting myocardial derangement in diabetic mice after MI is a function of hyperglycemia rather than a toxicity artifact of STZ32. DN-p90RSK-Tg diabetic mice had improved survival after MI compared to that of NLC mice (Online Fig. VA). One week after MI, the LV weight/TL (tibial length) and lung weight/TL ratios were increased in NLC mice (Online Figs. VC and VD), whereas these parameters were reduced in DN-p90RSK-Tg mice (OnlineFig. VD). Echocardiography performed one week after MI showed increased left ventricular end diastolic diameter (LVEDd) and left ventricular end systolic diameter (LVESd) in diabetic NLC mice with a concomitant decrease in fractional shortening (FS) and ejection fraction (EF) (Online Fig. VE) compared to sham-operated non-MI controls. By contrast, we found that these MI-induced cardiac effects were attenuated in DN-p90RSK-Tg mice (Online Fig. VE).
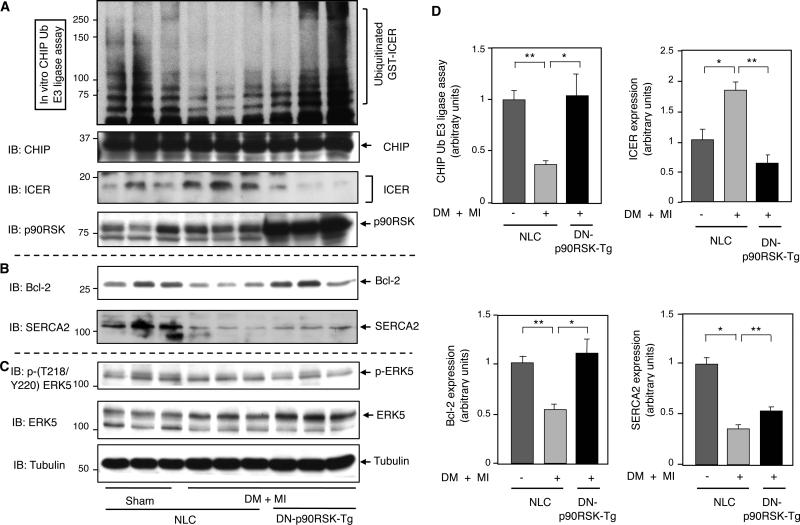
The role of p90RSK activation in regulating CHIP Ub ligase activity, subsequent ICER levels and cardiomyocyte apoptosis in vivo were examined. As shown in Figs. 3A, 3D, and S6, CHIP Ub ligase activity was significantly decreased one week after MI in diabetic NLC mice but not in diabetic DN-p90RSK-Tg mice (Figs. 3A and 3D and Online Fig. VI). Since CHIP Ub ligase activity regulates ICER protein stability in heart failure 12, 13, we examined the ICER protein level as well as the abundance of downstream ICER mediators, such as Bcl-210 and SERCA233. In NLC mice, we found increased ICER levels and a concomitant reduction in Bcl-2 expression after MI in diabetic mice and this was rescued in DN-p90RSK-Tg diabetic mice after MI (Figs. 3A, 3B and 3D). SERCA2 expression was also reduced in NLC diabetic mice after MI. However, the reversal of this effect in DN-p90RSK diabetic mice after MI is less obvious (Figs. 3B and 3D). Interestingly, the level of ERK5 phosphorylation at T218 and Y220 was decreased slightly in DN-p90RSK diabetic mice after MI (Fig. 3C), suggesting a role for activated p90RSK in phosphorylating ERK5 T218/Y220-the same residues phosphorylated by MEK5 responsible for ERK5 activation.
Figure 3. CHIP Ub ligase activity and ICER expression after MI in diabetic DN-p90RSK-Tg mice.
(A) Heart samples from sham control and DM+MI groups in NLC mice and DM+MI group in DN-p90RSK-Tg mice were collected and the CHIP Ub ligase activity assay was performed as described in the Methods (top). Western blots showing in (A) CHIP, ICER, and p90RSK, in (B) Bcl-2 and SERCA2, and in (C) p-(T218/Y220)ERK5, ERK5 and tubulin expression. (D) Quantifications of relative CHIP Ub ligase activity and ICER expression (upper) and the expression of Bcl-2 and SERCA2 (lower) (**p<0.01 *p< 0.05 compared to NLC sham operation control, mean±S.D., n=3,). Results are expressed as the relative ratio of mean value of NLC sham operation.
TUNEL-positive cells significantly increased in diabetic NLC mice after MI when compared with sham controls (Online Figs. VF and VG), supporting the known role of cellular apoptosis in the development of heart failure34. Notably, reduced incidence of apoptosis was found in diabetic DN-p90RSK-Tg mice after MI (Online Figs. VF and VG), suggesting a functional consequence for p90RSK activation in promoting cardiac apoptosis after MI in diabetic animals.
p90RSK activation inhibits ERK5-CHIP association via competitive binding with ERK5 and direct phosphorylation of ERK5-S496
Upon activation, ERK5 exerts its cardio-protective effect by promoting ERK5-CHIP binding, which increases CHIP Ub ligase activity13. Since there is a role for p90RSK activation in Ang II-mediated disruption of ERK5-CHIP binding and subsequently reduction of CHIP Ub ligase activity (Figs. 1 and 2), we investigated how p90RSK regulates ERK5-CHIP association. To determine the protein domain of ERK5 responsible for the p90RSK-ERK5 and ERK5-CHIP interaction, we generated three truncated ERK5 fragments and evaluated their association with p90RSK (Fig. 4A) and CHIP (Fig. 4B) using a mammalian two-hybrid assay. A plasmid containing GAL4-DBD (DNA binding domain) and the full-length p90RSK (Fig. 4A) or CHIP (Fig. 4B) were constructed in the pBIND vector; plasmids to express VP16-ERK5 (full-length or truncated forms) were constructed in the pACT vector. Constitutively active MEK5α (CA-MEK5α) induces forced activation of ERK5 and significantly increases ERK5-CHIP association by decreasing the inhibitory effect of the NH2-terminal kinase domain of ERK5. This ERK5-CHIP association at the helical linker domain of CHIP is fundamental for increasing CHIP Ub ligase activity13. Therefore, we examined the effect of CA-MEK5α expression on the association of ERK5 and CHIP. The mammalian two-hybrid assay demonstrated that the NH2-terminal region of ERK5 (aa1-148) and the COOH-terminal region of ERK5 (aa571-807) associate with CHIP (Figs. 4A and 4B). The latter site overlaps with the p90RSK binding site.
Figure 4. p90RSK kinase activation inhibits ERK5-CHIP association.
(A, B) p90RSK-ERK5 and CHIP-ERK5 association were examined by a mammalian two-hybrid assay in Hela cells. The activation domain VP16 fused to full length or three truncated ERK5 proteins (A-B) and full length p90RSK (A) or CHIP (B) fused to the Gal4 binding domain were cotransfected with the Gal4-responsive luciferase reporter pG5-luc. The total transfected DNA amount was normalized with empty VP16 vector. Binding affinity was assayed by luciferase activity normalized by the renilla luciferase activity. Data are representative of three independent experiments. **p<0.01, mean±S.D., n=3. (C) Both wild type p90RSK (WT-p90RSK) and ERK5-Fr (aa571-807) but not DN-p90RSK disrupted ERK5-CHIP association. After plasmid transfection as indicated, immunoprecipitated ERK5 was immunoblotted with anti-CHIP. Protein expression was determined by immunoblotting with each specific antibody. The blots are representative of data obtained from at least three different blots. (D) Ang II increased p90RSK-ERK5 interaction via p90RSK activation. Cardiomyocytes were stimulated with Ang II after 3h of FMK-MEA pre-treatment. Immunoprecipitated ERK5 was immunoblotted with anti-p90RSK. Each protein expression was detected as described in C. (E) Relative p90RSK-ERK5 binding was quantified as described in Fig.2D. *p<0.05, mean±S.D., n=3.
To examine whether the wild type p90RSK and the ERK5 fragment (ERK5-Fr, aa571-807) alter ERK5-CHIP association in vitro, we utilized an over-expression system. Since CHIP binding site is within the ERK5 aa571-807 region, we focused on ERK5-Fr (aa571-807). ERK5-CHIP binding was increased by CA-MEK5α (Fig. 4C, lane 3). Over-expression of both wild type p90RSK and ERK5-Fr inhibited ERK5-CHIP association (Fig. 4C, lane 5-6 and Online Fig. VIIA, B), suggesting that the ability of WT p90RSK to inhibit ERK5-CHIP association was due to competition between p90RSK and ERK5 for the same binding site on CHIP (Fig. 8E).
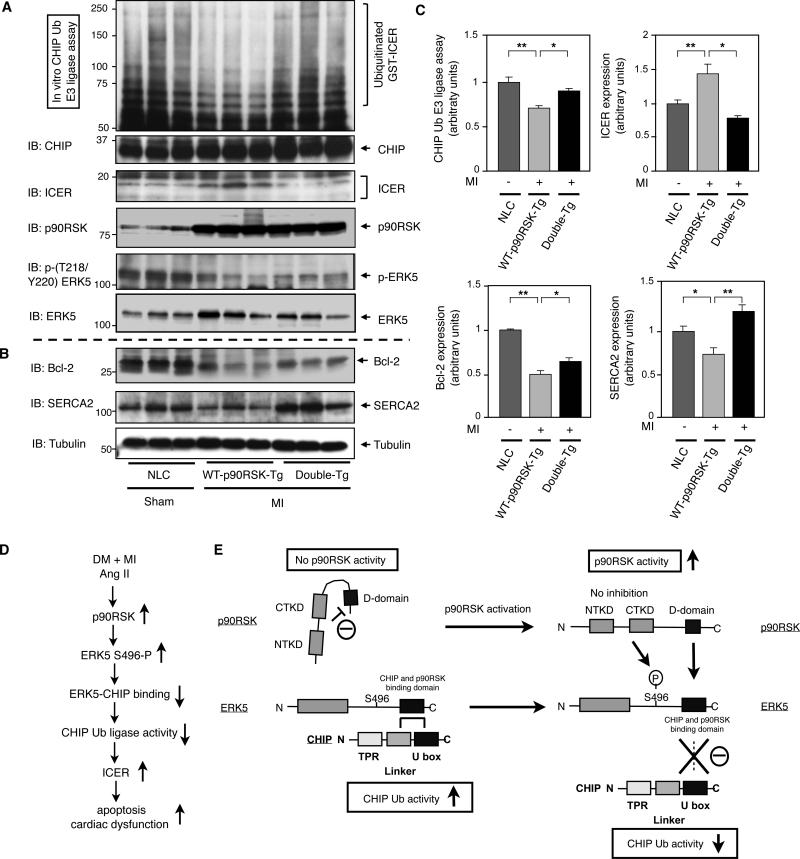
Figure 8. CHIP Ub ligase activity and ICER expression after MI in NLC, WT-p90RSK-Tg, and Double-Tg mice.
(A-C) Heart samples from control and MI groups in NLC, WT-p90RSK-Tg, and Double-Tg mice were collected and CHIP Ub ligase activity assay was performed as described in methods (top). Western blot shows (A) CHIP, ICER, p90RSK, p-ERK5, and ERK5 and (B) Bcl-2, SERCA2, and tubulin expression. (C) Quantifications of relative CHIP Ub ligase activity and ICER expression (upper), Bcl-2 and SERCA2 expression (lower) as described in Fig.2B and Fig.1B. (**p<0.01, * p<0.05, mean±S.D., n=3). (D) A model of DM + MI or Ang II-mediated p90RSK-ERK5-CHIP signal transduction pathway that regulates cardiac apoptosis and subsequent cardiac dysfunction. (E) A scheme depicting p90RSK-mediated regulation of the ERK5-CHIP module. At the basal level, inactive p90RSK inhibits the D-domain to bind with ERK539. p90RSK-free ERK5 associates with CHIP at its linker and U-box domain and maintains its CHIP Ub ligase activity to prevent ICER induction and subsequent apoptosis13. However, once p90RSK gets activated, the inhibition of the kinase domain is released39, and the D-domain of p90RSK associates with the ERK5 COOH-terminal domain, leading to compete with ERK5-CHIP association and ERK5-S496 phosphorylation, which disrupts ERK5-CHIP interaction. The disruption of ERK5-CHIP interaction inhibits CHIP Ub ligase activity13, increases ICER induction, and induces apoptosis13, 10.
Since p90RSK inhibits ERK5-CHIP association via competitive binding with ERK5, we have investigated whether Ang II-mediated p90RSK activation is required for p90RSK-ERK5 association in cardiomyocytes. Ang II increased p90RSK-ERK5 binding, and this was significantly inhibited by FMK-MEA (Figs. 4D and 4E). As p90RSK activation induced by Ang II increased p90RSK-ERK5 binding, we determined the role of p90RSK activation in ERK5-CHIP binding. Wild type p90RSK but not DN-p90RSK completely inhibited ERK5-CHIP association (Fig.4C, lane 4-5, and Online Fig.VIIC and D), supporting the role of p90RSK activation in increased p90RSK-ERK5 association as well as decreased ERK5-CHIP association. Based on these results, we reasoned that since Ang II induced p90RSK, but not ERK5, activation (Online Fig. VIII), the regulation of ERK5-CHIP binding mediated by p90RSK activation most likely occurs by a process that requires direct interaction of the two without ERK5 enzymatic activity being activated (Fig. 8E).
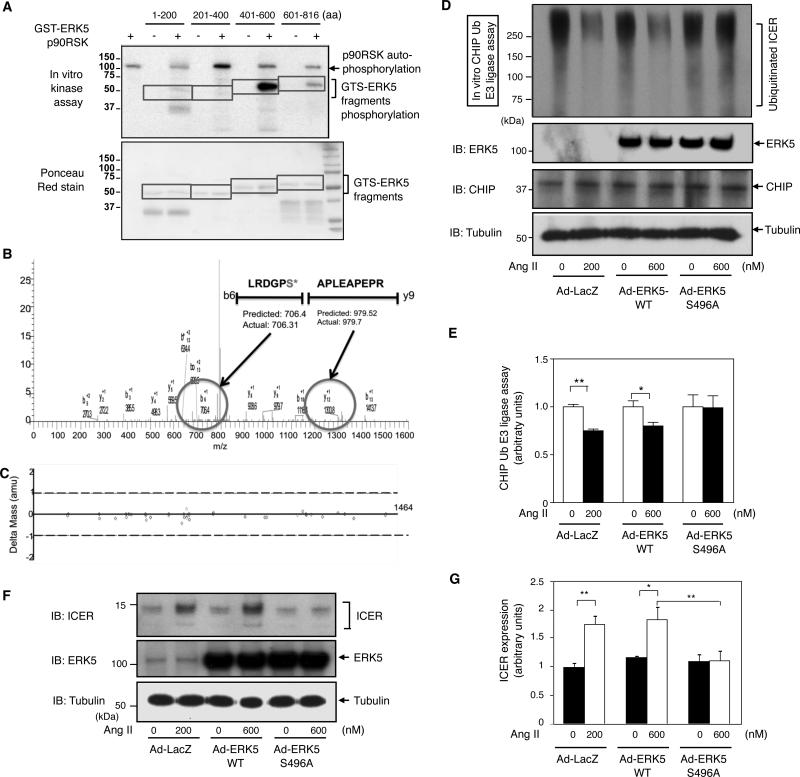
Since p90RSK can associate with ERK5, we asked whether p90RSK directly phosphorylated ERK5 and as a consequence inhibited CHIP Ub ligase activity. To test this possibility, we performed an in vitro kinase assay by incubating recombinant p90RSK with GST-tagged-ERK5 fragments and found that p90RSK phosphorylated two ERK5 fragments (aa401-600 and aa601-816) (Fig. 5A). In addition, liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analyses identified ERK5-S496 as being directly phosphorylated by p90RSK (Figs. 5B and 5C). To determine whether ERK5-S496 phosphorylation is involved in the Ang II-mediated reduction of CHIP Ub ligase activity in cardiomyocytes, we generated an adenovirus expressing the ERK5-S496A mutant (Ad-ERK5-S496A). Cardiomyocytes were transduced with Ad-ERK5-S496A, Ad-ERK5-WT, or Ad-LacZ, followed by Ang II stimulation. Cell lysates were then subjected to an in vitro CHIP ubiquitination assay using a GST-ICER fusion protein as a substrate. Ang II inhibited CHIP Ub ligase activity in cardiomyocytes transduced by Ad-LacZ or Ad-ERK5-WT. In contrast, Ad-ERK5-S496A transduction abolished this inhibitory effect on CHIP Ub ligase activity (Figs. 5D and 5E). In addition, the Ang II-mediated increase in ICER levels was significantly inhibited in cardiomyocytes overexpressing Ad-ERK5-S496A mutant, but not in those expressing the Ad-ERK5-WT or Ad-LacZ (Figs.5F and 5G). These findings indicate that p90RSK-mediated ERK5-S496 phosphorylation is required for inhibiting CHIP Ub ligase activity (Figs. 8D and 8E).
Figure 5. p90RSK phosphorylated ERK5-Ser496 and inhibited CHIP Ub ligase activity.
(A) In vitro p90RSK kinase assay using 4 different GST-ERK5 fragments as substrates. An autoradiogram (A, upper panel) shows strong 32P incorporation into a GST-ERK fragment (aa401-600), and weak incorporation into another fragment (aa601-816). Ponceau staining (A, lower panel) of the membrane demonstrates the position of proteins after separation by SDS-PAGE. (B) LC-MS/MS analysis identified p90RSK-mediated ERK5-S496 phosphorylation with less than 0.05 error. An LC-MS/MS plot of different peptide fragments shows S496 phosphorylation based on mass shift of phosphorylated serine with H2O (+98 Da). The predicted mass of each b/y fragment and the actual value are shown above with the b6 fragment containing the phosphorylated S496 having a predicted mass of 706.4 Da versus an actual value of 706.31 Da. (C) An error plot of the LC-MS/MS data shows the error for calculating each b/y peptide fragment is less than 0.5 Da. (D) Ang II-mediated reduction of CHIP Ub ligase activity was inhibited by ERK5-S496A mutant. Cardiomyocytes were transduced with either Ad-ERK5-WT, Ad-ERK5-S496A mutant, or Ad-LacZ for 24 h followed by 2 h of Ang II stimulation as indicated. (E) Relative CHIP Ub ligase activity was quantified as described in Fig.2B. (**p<0.01, *p<0.05, mean±S.D., n=3). (F) Ang II-mediated ICER induction was inhibited by ERK5-S496A mutant. Cardiomyocytes were transduced as described in D followed by 24 h of Ang II stimulation. (G) Quantification of ICER expression as described in Fig.1B. (**p<0.01, *p<0.05, mean±S.D., n=3).
Cardiac-specific ERK5 knockout mice (ERK5-CKO) showed reduced CHIP Ub ligase activity after MI
To corroborate the necessity of ERK5 activation in increasing CHIP Ub ligase activity and subsequent reduction of ICER, we utilized ERK5-CKO. Since ERK5 activation is cardio-protective13 and ERK5-CKO mice displayed significantly increased levels of cardiac dysfunction after MI under the non-DM condition (Figs. 6A, 6B and 6C), we subjected the mice to MI only. No basal cardiac abnormality was found in ERK5-CKO mice as we reported previously35, and no increase in ICER levels was observed (Online Fig. IVB). After MI, ERK5-CKO mice showed a significant reduction in CHIP Ub ligase activity, and an increase in ICER levels (Figs. 6F and 6G) compared to the NLC mice. As expected, the ERK5-CKO mouse hearts showed a significant increase in the number of TUNEL-positive cardiomyocytes compared with the NLC group (Figs. 6D and 6E). These data further confirmed the importance of ERK5 in regulating CHIP Ub ligase activity and subsequent ICER levels and apoptosis in vivo (Fig. 8D). Since a difference in the infarct size may correlate with post-MI cardiac dysfunction, we compared the permanent coronary ligation-mediated infarct size between NLC (C57BL/6) and ERK5-CKO mice. As shown in Online Fig. IX, we did not find any difference in the infarct size among these groups, although we found significant cardiac dysfunction and an increase in lung weight/TL in ERK5-CKO mice.
Figure 6. CHIP Ub ligase activity and ICER expression after MI in ERK5-CKO mice.
(A) LV weight/tibial length (TL) and (B) lung weight/TL after MI in NLC and ERK5-CKO mice as indicated (* p<0.05, mean±S.D., n=8-9). (C) Echocardiographic data obtained after MI in NLC and ERK5-CKO mice as indicated. FS (fractional shortening), EF (ejection fraction). (** p<0.01, mean±S.D., n=8-9) (D) Cardiomyocyte apoptosis in remote areas was enhanced in ERK5-CKO mice. Animals were subjected to either MI or sham. TUNEL-positive cardiomyocytes were counted among more than 8,000 to 10,000 nuclei in the remote area of each mouse heart after one week of surgery. 40X objective lens. Scale bars, 40 μm. Representative pictures of TUNEL (top), 4’, 6’-diamidino-2-phenylindole (DAPI) (middle), and α-actinin merged with TUNEL and DAPI staining (bottom) of the remote area from NLC and ERK5-CKO mice subjected to MI or sham operation as indicated. (E) A bar graph showing TUNEL-positive cells (%) in various animals. (* p<0.05, mean±S.D., n=3-4). (F) Heart samples from sham and MI groups in NLC mice and in ERK5-CKO mice were collected and CHIP Ub ligase activity assay was performed as described in methods (top). CHIP, ERK5, ICER, and tubulin expressions were detected by Western blot. (G) Relative CHIP Ub ligase activity (left, n=9) and ICER expression (right, n=3) were quantified as described in Fig.2B and Fig.1B, respectively. (** p<0.01, * p<0.05, mean±S.D.).
The reduction of CHIP Ub ligase activity and increased ICER levels were observed in WT-p90RSK-Tg mice after MI but were significantly inhibited in WT-p90RSK-Tg/constitutive active (CA) MEK5α double transgenic mice
Thus far, we have shown the critical role of the p90RSK-ERK5 module in CHIP Ub ligase activity and ICER levels in vitro, but the role of this module in vivo remains unclear. To clarify the function of the p90RSK-ERK5 signaling cascade in vivo, we crossed WT-p90RSK-Tg mice with those expressing constitutive active MEK5α (CA-MEK5α-Tg) to generate double transgenic mice (Double-Tg). Cardiac function is normal in CA-MEK5α-Tg and WT-p90RSK-Tg mice at 8-12 weeks of age as we previously described36, 37. Double-Tg also shows normal cardiac function (FS% = 67.7 ± 3.7, n = 3). No increase in ICER levels was observed in WT-p90RSK-Tg or Double-Tg mice (Online Fig. IVA).
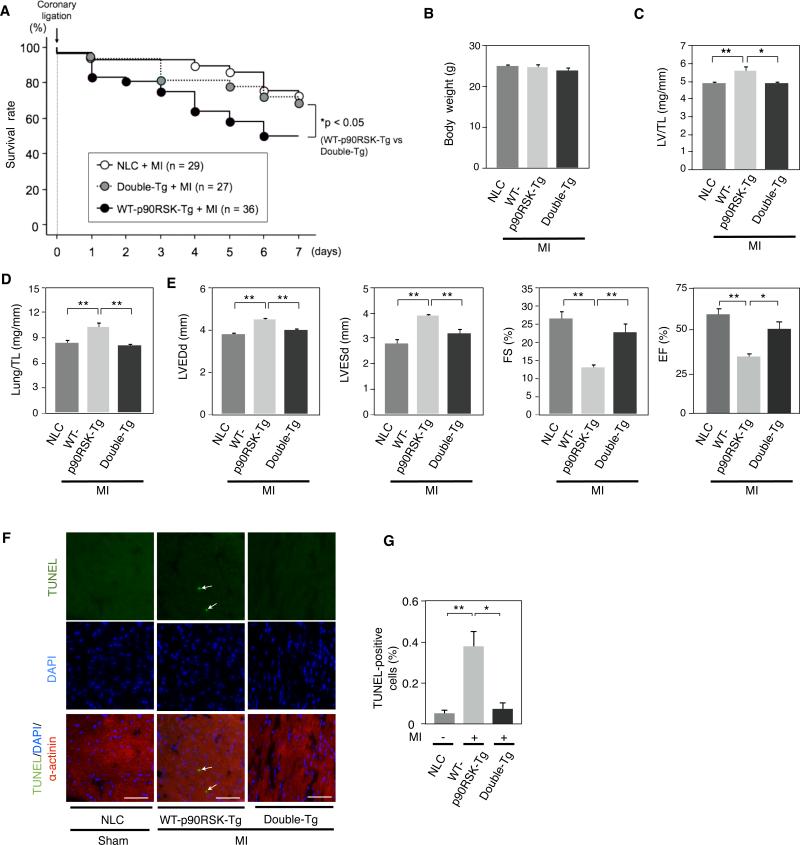
Non-diabetic mice were subjected to a simple MI. Following MI, the mortality of WT-p90RSK-Tg mice was significantly greater than NLC mice. The mortality of Double-Tg was similar to NLC mice (Fig. 7A). There was no difference in body weight among the three groups (Fig. 7B). One week after MI, the LV weight/TL and lung weight/TL ratios were higher in WT-p90RSK-Tg mice compared to NLC mice (Figs. 7C and 7D) but lower in Double-Tg mice compared to WT-p90RSK-Tg mice (Figs. 7C and 7D). Myocardial contractile function as assessed by echocardiography was diminished in WT-p90RSK-Tg mice compared to NLC following MI (Fig. 7E). Myocardial contractile function as assessed by echocardiography was preserved in Double-Tg mice (Fig. 7E). We previously reported that CA-MEK5α-Tg mice develop contractile dysfunction one week after simple MI32. In contrast, we found that activation of ERK5 by CA-MEK5α could reverse the detrimental effect of p90RSK activation on cardiac function after a simple MI, consistent with the idea that p90RSK activation accelerates cardiac dysfunction and heart failure after MI, possibly via inhibiting ERK5 activity. We also assessed the infarct size after permanent coronary ligation in NLC (FVB) mice, in diabetic mice, and in Double-Tg mice. As shown in Online Fig.IX, we found no difference in infarct size among these three groups. However, we noted significant cardiac dysfunction and increase of lung weight/TL in diabetic mice after MI. These data suggest that the major difference in cardiac dysfunction observed after one week of MI stems from cardiac remodeling in the surrounding, non-infarcted area rather than simply from infarct expansion.
Figure 7. ERK5 activation prevented the exacerbation of LV dysfunction after MI in WT-p90RSK-Tg mice.
(A) Kaplan-Meier survival analysis in NLC, WT-p90RSK-Tg, and Double-Tg-WT-p90RSK-Tg/CA-MEK5α-Tg (Double-Tg) after MI. Survival rate in NLC (n=29), WT-p90RSK-Tg (n=36), and Double-Tg mice (n=27) after MI are plotted. Overall survival was significantly higher in Double-Tg compared to WT-p90RSK-Tg mice. * p<0.05 compared to WT-p90RSK-Tg group. (B) Body weight one week after MI in NLC, WT-p90RSK-Tg, and Double-Tg mice. (mean±S.D., n=17-19) (C, D) LV weight/TL (C) and lung weight/TL (D) after MI in NLC, WT-p90RSK-Tg, and Double-Tg mice. (* p<0.05, ** p<0.01, mean±S.D., n=17-19) (E) Echocardiographic data obtained after MI in NLC, WT-p90RSK-Tg, and Double-Tg mice. LVEDd, left ventricular end-diastolic dimension; LVESd, left ventricular endsystolic dimension; TL, Tibial length; FS (fractional shortening); EF (ejection fraction). (* p<0.05, ** p<0.01, mean±S.D., n=17-19) (F) Cardiomyocyte apoptosis in the remote area was increased in WT-p90RSK-Tg mouse hearts, which was inhibited in Double-Tg mice. TUNEL-positive cardiomyocytes were counted in the remote area of each mouse heart as described in Fig.6D. Representative pictures of TUNEL (top), DAPI (middle), and α-actinin merged with TUNEL and DAPI staining (bottom) of the remote area from NLC, WT-p90RSK-Tg, and Double-Tg mice subjected to MI or sham operation. 40X objective lens. Scale bars, 40 μm (G) A bar graph showing TUNEL-positive cells (%) in various animals. (** p<0.01, *p<0.05, mean± S.D., n=3).
Next, we investigated the involvement of CHIP Ub ligase activity in regulating ICER levels. Compared to the sham NLC mice, WT-p90RSK-Tg mice have reduced CHIP Ub ligase activity one week after MI. In contrast, no decrease in CHIP Ub ligase activity was observed in Double-Tg mice (Figs. 8A and 8C). The increase in ICER level and concomitant reduction in Bcl-2 expression in WT-p90RSK-Tg mice were also detected, and these changes were mostly reversed in Double-Tg mice (Figs. 8A, 8B and 8C). Similar to NLC diabetic mice after MI (Figs. 3B and 3D), SERCA2 expression was also reduced in WT-p90RSK-Tg mice after MI (Figs. 8B and 8C). However, restoration of SERCA2 expression in Double-Tg mice after MI was observed (Figs. 8B and 8C), contrasting what was observed DN-p90RSK diabetic mice after MI (Figs. 3B and 3D).
Finally, WT-p90RSK-Tg mice have increased apoptosis one week after MI compared with sham NLC mice, and this phenomenon was reversed in Double-Tg mice (Figs. 7F and 7G). Taken together, ERK5 activation suppresses p90RSK-mediated cardiac apoptosis after MI in vivo.
DISCUSSION
In this study, we demonstrated a critical role for p90RSK activation in the reduction of CHIP Ub ligase activity and subsequent increase in the ICER protein levels (Figs. 8D and 8E). p90RSK activation promotes its association with ERK5 and phosphorylates it at S496. This phosphorylation appears to be a necessary and sufficient step in the ability of p90RSK to promote the dissociation of the ERK5-CHIP complex, which leads to reduced CHIP Ub ligase activity. The physiologic significance of p90RSK activation in diabetic mice after MI lies in its ability to decrease CHIP Ub ligase activity. This mechanism is reversed in diabetic DN-p90RSK-Tg mice, which show increased CHIP Ub ligase activity and recovery of cardiac function.
Depletion of ERK5 inhibited CHIP Ub ligase activity and enhanced ICER levels after MI, suggesting a causal role of ERK5 in controlling CHIP Ub ligase activity and subsequent ICER stability. Supporting the role of ERK5 as a negative regulator of p90RSK-mediated CHIP Ub ligase inhibition, we demonstrate that diabetic WT-p90RSK-Tg mice exhibit diminished CHIP Ub ligase activity after MI, but that co-expressed CA-MEK5α in Double-Tg mice leads to restoration of CHIP Ub ligase activity, and prevents cardiac dysfunction, at least in part by preventing myocardial apoptosis.
Our studies focus on defining the molecular mechanism of cardiac dysfunction after MI, which is a prevalent but poorly understood consequence of diabetes. Therefore, we established a diabetic model of MI in which post-MI biochemical and physiological sequelae could be carefully examined in the immediate post-MI period 32. We observed that p90RSK activation was significantly increased in DM. Consistent with our previous work in WT-p90RSK-Tg mice, we see cardiac dysfunction and significant fibrosis after 8 month of age--a phenotype similar to that seen in diabetic cardiomyopathy37. Furthermore, we found that DN-p90RSK-Tg mice do not suffer the same degree of cardiac dysfunction after MI in a diabetic model, supporting an important role for p90RSK in the development of cardiac dysfunction after MI in diabetes. The ability of p90RSK activation to decrease functionality of the ERK5-CHIP module suggests that cardiac-specific ERK5 knockout mice (ERK5-CKO) mimic the deleterious effect of diabetes on the heart. Therefore, we modeled MI only in WT-p90RSK-Tg (Fig.8) and ERK5-CKO mice (Fig.6). As mentioned above, we found that CHIP Ub ligase activity after MI was inhibited in ERK5-CKO mice compared with NLC mice. We also observed that CHIP activity in the sham operation group is much lower in C57BL/6 than FVB mice. The background of ERK5-CKO mice is C57BL/6, while the background of DN-p90RSK-Tg, WT-p90RSK-Tg, and Double-Tg is FVB. Therefore, this may explain why C57BL/6 mice are much more sensitive to MI than FVB strain and show much higher mortality after MI surgery. Further investigation will be necessary to clarify this issue.
Ranganathan et al. reported that the COOH-terminal domain of the D motif of p90RSK is an ERK5 binding site and that p90RSK activation was required for full association between p90RSK and ERK538. Consistent with this report, in our present study, we found that p90RSK activation is a key event in disrupting ERK5-CHIP interaction by: 1) competitive binding of p90RSK to ERK5 causing displacement of CHIP from ERK5, and 2) phosphorylation of ERK5 at S496. Forced activation of ERK5 induced by IGF-1 or CA-MEK5α inhibits the association of p90RSK with ERK5. In addition, activated ERK5 significantly increases ERK5-CHIP association by relieving the inhibitory effect of the NH2-terminal kinase domain of ERK5 (Figs. 8D and 8E). ERK5-CHIP association at the helical linker domain of CHIP is critical for increasing CHIP Ub ligase activity13. In support of this, the elevation of ERK5 activity in Double-Tg mice reversed the inhibitory effect of p90RSK activation on CHIP Ub ligase activity. Taken together, our data demonstrate that p90RSK activation influences the ability of its own COOH-terminus docking domain to bind ERK5 and phosphorylate residue S496. The p90RSK-ERK5 interaction and ERK5-S496 phosphorylation disrupt ERK5-CHIP association and decrease CHIP Ub ligase activity (Figs. 8D and 8E). It is possible that the association between p90RSK and ERK5 is necessary for p90RSK to phosphorylate S496 ERK5, or that p90RSK-mediated S496 phosphorylation of ERK5 leads to dissociation of p90RSK and ERK5. To determine which of these possibilities is true will require further investigation.
Our data show that p90RSK activation regulates ICER protein stability by regulation of CHIP Ub ligase activity. In support of this, 1) an ERK5-S496A mutant significantly inhibits Ang II-mediated reduction of CHIP Ub ligase activity and subsequent increase in ICER levels (Fig.5), 2) CHIP is critical for ERK5-mediated decrease in ICER level13, and 3) depletion of CHIP increases ICER stability13. CHIP activity can regulate a variety of molecules including FOXO139, ASK140, and p5341, 42, and we cannot exclude the possibility that p90RSK can regulate the expression of these molecules via CHIP, and that dysregulation of these molecules contribute to cardiac dysfunction. In addition, p90RSK and ERK5 can also regulate proteins with CHIP and ICER-independent mechanism including Na+/H+ exchanger28, renin-angiotensin system37, 43, and PPARs activities5, 44. Further investigation is necessary to clarify the role of these molecules in p90RSK/ERK5-mediated cardiac dysfunction, especially in DM, both in vitro and in vivo.
Naito et al. reported that CHIP expression was decreased with concomitant upregulation of p53 expression within four days following MI42. In our study there was no significant decrease in CHIP expression or increase in p53 expression at seven days following MI in diabetic mice (data not shown). Instead, we found a significant decrease in CHIP Ub ligase activity in our diabetic mice following MI. The regulatory mechanism responsible for the down-regulation of CHIP expression was not identified in the previous studies42. Our present study suggests a role for p90RSK activation in regulating ERK5-CHIP association and subsequent CHIP Ub ligase activation. Whether through an early decrease in CHIP expression or a later decrease in its Ub ligase activity, the net effect is promotion of the pro-apoptotic milieu contributing to poor functional recovery in diabetic mice following MI. Based on our results on the role of p90RSK-ERK5-CHIP described in our current work, ERK5 activation may reverse the decrease in Ub ligase activity and ameliorate cardiac dysfunction after MI in DM hearts.
Lastly we close by commenting on the protective function of ERK5 after MI with or without superimposed DM. In this study we showed that cardio-protective effect of ERK5 activation is particularly significant after MI in the context of DM. Previously, we reported that the cardiac function did not improve after one week of MI in the absence of DM in CA-MEK5α-Tg mice compared with NLC mice 32. These data suggest that ERK5 activation does not have a significant cardio-protective role in our MI model one week after permanent ligation. Although the role of ERK5 activation in cardiac remodeling after permanent ligation is limited, we found that cardiac protection was enhanced in hyperglycemic CA-MEK5α-Tg mice 32, suggesting that ERK5 activation has a significant role in DM after MI rather than being a consequence of MI per se, at least during the acute phase (within one week) after MI.
Supplementary Material
Novelty and Significance.
What is known?
Diabetes mellitus (DM) is an independent risk factor for both mortality and morbidity following myocardial infarction (MI) Induction of inducible cAMP early repressor (ICER), a proapoptotic transcriptional repressor, accelerates myocyte apoptosis and subsequent cardiac dysfunction in DM mice after MI.
Activation of ERK5 positively regulates chaperone-dependent E3 ubiquitin (Ub) ligase CHIP (carboxyl terminus of Hsp70-interacting protein)-mediated ICER ubiquitination and subsequent protein degradation, which protects cardiomyocytes from apoptosis.
.
What new information does the article contribute?
ERK5-CHIP module is one of the major targets of p90RSK in diabetic hearts.
Depletion of ERK5 inhibits CHIP Ub ligase activity and increases ICER levels after MI.
Diabetic wild type p90RSK transgenic mice exhibit diminished CHIP Ub ligase activity after MI. However, when these mice co-express a constitutively active form of MEK5α in double transgenic mice, CHIP Ub ligase activity is restored, also restoring cardiac dysfunction, at least in part by preventing myocardial apoptosis.
The activation of p90RSK abrogates ERK5-mediated CHIP Ub ligase activation, and accelerates apoptosis and cardiac dysfunction in the DM+MI condition.
Summary of the Novelty and Significance.
DM reduces survival after MI primarily due to acceleration of heart failure. We have found that the activation of p90RSK abrogates ERK5-mediated CHIP Ub ligase activation, and accelerates apoptosis and cardiac dysfunction in the DM+MI condition. Our data demonstrate that p90RSK activation influences the ability of its own COOH-terminus docking domain to bind ERK5 and phosphorylate residue S496. The p90RSK-ERK5 interaction and ERK5-S496 phosphorylation disrupt ERK5-CHIP association and decrease CHIP Ub ligase activity. The regulatory mechanism responsible for the down-regulation of CHIP expression after MI has not yet been identified. Our present study suggests a role for p90RSK activation in regulating ERK5-CHIP association and subsequent CHIP Ub ligase activation. either through an early decrease in CHIP expression or a later decrease in its Ub ligase activity The net effect is promotion of the pro-apoptotic milieu contributing to poor functional recovery in diabetic mice following MI. ERK5 activation may reverse the decrease in Ub ligase activity and ameliorate cardiac dysfunction after MI in DM hearts. Understanding the role and molecular mechanisms how p90RSK targets ERK5-CHIP ubiquitin E3 ligase activity should provide insights into mechanisms contributing to poor cardiac recovery after MI in DM and possibly reveal a novel therapeutic target.
Acknowledgements
The LC-MS/MS analysis was performed by the University of Rochester Proteomics Center (Dr. Fred K. Hagen, Ph.D). We thank Drs. Jane Sottile, Bradford C. Berk, and Scott Cameron for critical reading and suggestions for the manuscript.
Sources of funding
This work is supported by grants from the Mochida Memorial Foundation for Medical and Pharmaceutical Research to Dr. Tetsuro Shishido, the America Heart Association to Dr. Woo (SDG 0930360N), and from the National Institute of Health to Dr. Abe (HL102746-03, HL088637, and HL108551), Dr. Yan (HL077789 and HL088400), and Dr. Yang (HL088637 and HL108551). Drs. Abe and Yan are recipients of Established Investigator Awards of the American Heart Association (0740013N and 0740021N).
Non-standard Abbreviations and Acronyms
- Ad-DN-p90RSK
adenovirus expressing dominant negative form of p90RSK
- Ang II
angiotensin II
- BS
random blood sugar
- CA-MEK5α-Tg
cardiac specific constitutively active form of MEK5α transgenic mice
- CHIP
carboxyl terminus of Hsc70-interacting protein
- CREB
cAMP response element binding protein
- CTD
C-terminal kinase domain
- DM
diabetes mellitus
- DM + MI
DM mice after myocardial infarction
- Double-Tg
double transgenic mice crossing wild-type p90RSK-Tg (WT-p90RSK-Tg) and CA-MEK5α-Tg
- EF
ejection fraction
- ERK5
extra-cellular signal-related kinase 5
- ERK5-CKO
cardiac-specific ERK5 knockout mice
- ERK5-Fr
ERK5 fragment
- FMK-MEA
1-(4-amino-7-(3-(2-methoxyethylamino)proxyl)-5-p-tolyl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-2-fluoroethanone
- FS
fractional shortening
- ICER
inducible cAMP early repressor
- IGF-1
insulin growth factor-1
- LC-MS/MS
liquid chromatography coupled with tandem mass spectrometry
- LV
left ventricle
- LVEDd
left ventricular end diastolic diameter
- LVESd
left ventricular end systolic diameter
- MI
myocardial infarction
- NLC
non-transgenic littermate control
- p90RSK
p90 ribosomal S6 kinase
- ROS
reactive oxygen species
- STZ
streptozotocin
- TL
tibial length
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- Ub
ubiquitin
- WT-p90RSK-Tg
cardiac specific wild-type p90RSK transgenic mice
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. The finmonica myocardial infarction register study group. Diabetes Care. 1998;21:69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr., Sowers JR. Diabetes and cardiovascular disease: A statement for healthcare professionals from the american heart association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Merritt AJ, Seyfried J, Guo C, Papadakis ES, Finegan KG, Kayahara M, Dixon J, Boot-Handford RP, Cartwright EJ, Mayer U, Tournier C. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan L, Carr J, Ashby PR, Murry-Tait V, Thompson C, Arthur JS. Knockout of erk5 causes multiple defects in placental and embryonic development. BMC Dev Biol. 2003;3:11. doi: 10.1186/1471-213X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, Berk BC, Yan C, Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (ppargamma1) mediates interaction with extracellular signal-regulated kinase 5 and ppargamma1 transcriptional activation: Involvement in flow-induced ppargamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasler HG, Victoria J, Duramad O, Winoto A. Erk5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaworski J, Mioduszewska B, Sanchez-Capelo A, Figiel I, Habas A, Gozdz A, Proszynski T, Hetman M, Mallet J, Kaczmarek L. Inducible camp early repressor, an endogenous antagonist of camp responsive element-binding protein, evokes neuronal apoptosis in vitro. J Neurosci. 2003;23:4519–4526. doi: 10.1523/JNEUROSCI.23-11-04519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of crem: An alternative promoter directs the expression of icer, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 9.Sussman MA. Icer-capades: Putting cardiac cyclic amp signaling “on ice”. Circ Res. 2003;93:6–8. doi: 10.1161/01.RES.0000082770.81721.44. [DOI] [PubMed] [Google Scholar]
- 10.Tomita H, Nazmy M, Kajimoto K, Yehia G, Molina CA, Sadoshima J. Inducible camp early repressor (icer) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ Res. 2003;93:12–22. doi: 10.1161/01.RES.0000079794.57578.F1. [DOI] [PubMed] [Google Scholar]
- 11.Yehia G, Schlotter F, Razavi R, Alessandrini A, Molina CA. Mitogen-activated protein kinase phosphorylates and targets inducible camp early repressor to ubiquitin-mediated destruction. J Biol Chem. 2001;276:35272–35279. doi: 10.1074/jbc.M105404200. Epub 32001 Jul 35220. [DOI] [PubMed] [Google Scholar]
- 12.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe J. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3a/inducible camp early repressor feedback loop. Circ Res. 2007;100:510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo CH, Le NT, Shishido T, Chang E, Lee H, Heo KS, Mickelsen DM, Lu Y, McClain C, Spangenberg T, Yan C, Molina CA, Yang J, Patterson C, Abe JI. Novel role of c terminus of hsc70-interacting protein (chip) ubiquitin ligase on inhibiting cardiac apoptosis and dysfunction via regulating erk5-mediated degradation of inducible camp early repressor. Faseb J. 2010;24:4917–4928. doi: 10.1096/fj.10-162636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuanetti G, Latini R, Maggioni AP, Franzosi M, Santoro L, Tognoni G. Effect of the ace inhibitor lisinopril on mortality in diabetic patients with acute myocardial infarction: Data from the gissi-3 study. Circulation. 1997;96:4239–4245. doi: 10.1161/01.cir.96.12.4239. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson I, Torp-Pedersen C, Kober L, Gustafsson F, Hildebrandt P. Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. Trace study group. J Am Coll Cardiol. 1999;34:83–89. doi: 10.1016/s0735-1097(99)00146-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsusaka H, Kinugawa S, Ide T, Matsushima S, Shiomi T, Kubota T, Sunagawa K, Tsutsui H. Angiotensin ii type 1 receptor blocker attenuates exacerbated left ventricular remodeling and failure in diabetes-associated myocardial infarction. Journal of cardiovascular pharmacology. 2006;48:95–102. doi: 10.1097/01.fjc.0000245405.41317.60. [DOI] [PubMed] [Google Scholar]
- 17.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: Implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, Berk BC, Yan C. A positive feedback loop of phosphodiesterase 3 (pde3) and inducible camp early repressor (icer) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci U S A. 2005;102:14771–14776. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi E, Abe J, Berk BC. Angiotensin ii stimulates p90rsk in vascular smooth muscle cells. A potential na(+)-h+ exchanger kinase. Circulation research. 1997;81:268–273. doi: 10.1161/01.res.81.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal s6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 21.Takeishi Y, Abe J, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal s6 kinase and big mitogen- activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999;85:1164–1172. doi: 10.1161/01.res.85.12.1164. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Abe J, Taunton J, Lu Y, Shishido T, McClain C, Yan C, Xu SP, Spangenberg TM, Xu H. Reactive oxygen species-induced activation of p90 ribosomal s6 kinase prolongs cardiac repolarization through inhibiting outward k+ channel activity. Circ Res. 2008;103:269–278. doi: 10.1161/CIRCRESAHA.107.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seko Y, Tobe K, Ueki K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate raf-1, mitogen-activated protein kinase kinase, mitogen-activated protein kinases, and s6 kinase in cultured rat cardiac myocytes. Circ Res. 1996;78:82–90. doi: 10.1161/01.res.78.1.82. [DOI] [PubMed] [Google Scholar]
- 24.Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, King GL, Walsh RA, Yan C, Abe J. Role of p90 ribosomal s6 kinase (p90rsk) in reactive oxygen species and protein kinase c {beta} (pkc-{beta})-mediated cardiac troponin i phosphorylation. J Biol Chem. 2005;280:24135–24142. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- 25.Abe J, Okuda M, Huang Q, Yoshizumi M, Berk BC. Reactive oxygen species activate p90 ribosomal s6 kinase via fyn and ras. J Biol Chem. 2000;275:1739–1748. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, Takano H, Hiroi Y, Ueki K, Tobe K, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest. 1995;96:438–446. doi: 10.1172/JCI118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki T, Komuro I, Yazaki Y. Molecular aspects of mechanical stress-induced cardiac hypertrophy. Mol Cell Biochem. 1996;163-164:197–201. doi: 10.1007/BF00408658. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi E, Abe J, Berk BC. Angiotensin ii stimulates p90rsk in vascular smooth muscle cells. A potential na+/h+ exchanger kinase. Circ Res. 1997;81:268–273. doi: 10.1161/01.res.81.2.268. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for direct regulation of myocardial na+/h+ exchanger isoform 1 phosphorylation and activity by 90-kda ribosomal s6 kinase (rsk): Effects of the novel and specific rsk inhibitor fmk on responses to alpha1-adrenergic stimulation. Mol Pharmacol. 2007;71:799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 31.Maekawa N, Abe J, Shishido T, Itoh S, Ding B, Sharma VK, Sheu SS, Blaxall BC, Berk BC. Inhibiting p90 ribosomal s6 kinase prevents (na+)-h+ exchanger-mediated cardiac ischemia-reperfusion injury. Circulation. 2006;113:2516–2523. doi: 10.1161/CIRCULATIONAHA.105.563486. [DOI] [PubMed] [Google Scholar]
- 32.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe J. Effects of mek5/erk5 association on small ubiquitin-related modification of erk5: Implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res. 2008;102:1416–1425. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C, Miller CL, Abe J. Regulation of phosphodiesterase 3 and inducible camp early repressor in the heart. Circ Res. 2007;100:489–501. doi: 10.1161/01.RES.0000258451.44949.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wollert KC, Drexler H. Regulation of cardiac remodeling by nitric oxide: Focus on cardiac myocyte hypertrophy and apoptosis. Heart Fail Rev. 2002;7:317–325. doi: 10.1023/a:1020706316429. [DOI] [PubMed] [Google Scholar]
- 35.Kimura TE, Jin J, Zi M, Prehar S, Liu W, Oceandy D, Abe JI, Neyses L, Weston AH, Cartwright EJ, Wang X. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response and promotes pressure overload-induced apoptosis in the heart. Circulation research. 2010 doi: 10.1161/CIRCRESAHA.109.209320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron SJ, Itoh S, Baines CP, Zhang C, Ohta S, Che W, Glassman M, Lee JD, Yan C, Yang J, Abe J. Activation of big map kinase 1 (bmk1/erk5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Lett. 2004;566:255–260. doi: 10.1016/j.febslet.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 37.Itoh S, Ding B, Shishido T, Lerner-Marmarosh N, Wang N, Maekawa N, Berk BC, Takeishi Y, Yan C, Blaxall BC, Abe J. Role of p90 ribosomal s6 kinase-mediated prorenin-converting enzyme in ischemic and diabetic myocardium. Circulation. 2006;113:1787–1798. doi: 10.1161/CIRCULATIONAHA.105.578278. [DOI] [PubMed] [Google Scholar]
- 38.Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The map kinase erk5 binds to and phosphorylates p90 rsk. Arch Biochem Biophys. 2006;449:8–16. doi: 10.1016/j.abb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Xie P, Fan Y, Zhang H, Zheng L, Gu D, Patterson C, Li H. C terminus of hsc70-interacting protein promotes smooth muscle cell proliferation and survival through ubiquitin-mediated degradation of foxo1. J Biol Chem. 2009;284:20090–20098. doi: 10.1074/jbc.M109.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Y, Han C, Huang H, Xin Y, Xu Y, Luo L, Yin Z. Heat shock protein 70 together with its co-chaperone chip inhibits tnf-alpha induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis. 2010;15:822–833. doi: 10.1007/s10495-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 41.Esser C, Scheffner M, Hohfeld J. The chaperone-associated ubiquitin ligase chip is able to target p53 for proteasomal degradation. J Biol Chem. 2005;280:27443–27448. doi: 10.1074/jbc.M501574200. [DOI] [PubMed] [Google Scholar]
- 42.Naito AT, Okada S, Minamino T, Iwanaga K, Liu ML, Sumida T, Nomura S, Sahara N, Mizoroki T, Takashima A, Akazawa H, Nagai T, Shiojima I, Komuro I. Promotion of chip-mediated p53 degradation protects the heart from ischemic injury. Circ Res. 2010;106:1692–1702. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 43.Shi X, Yan C, Nadtochiy SM, Abe J, Brookes PS, Berk BC. P90 ribosomal s6 kinase regulates activity of the renin-angiotensin system: A pathogenic mechanism for ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51:272–275. doi: 10.1016/j.yjmcc.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe J. Erk5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (ppardelta) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.