Abstract
We report the successful use of abatacept and sodium thiosulfate in a patient with severe recalcitrant juvenile dermatomyositis complicated by ulcerative skin disease and progressive calcinosis. This combination therapy resulted in significant reduction in muscle and skin inflammation, decreased corticosteroid dependence, and halted the progression of calcinosis.
Juvenile dermatomyositis (JDM) is an inflammatory myopathy with a predilection for proximal muscles and skin. Current treatment of JDM consists of early aggressive use of corticosteroids coupled with steroid sparing agents such as methotrexate, cyclosporine and intravenous immune globulin (IVIG) (1). Delay to diagnosis and inadequate treatment strongly increase the risk of developing calcinosis (2). We describe a case of unremitting JDM with severe skin manifestations that included ulcerations and extensive calcinosis, poorly responsive to conventional therapy. We describe the child's clinical and laboratory response to treatment with abatacept and sodium thiosulfate, and propose this as a novel regimen for recalcitrant JDM complicated by ulceration and calcinosis.
Case Report
A 14 year old Caucasian girl with severe JDM presented at the age of eleven with swelling of the hands and feet, arthralgia, and subsequent heliotrope rash, Gottron's papules overlying extensor joints of the fingers, elbows, and knees, the shawl sign rash, and periungual telangiectasia. Initially, she had mild to moderate weakness of her proximal muscles, elevation of serum lactate dehydrogenase (LDH) to 970 U/L (normal range 470-750 U/L) and aldolase of 10.1 U/L (normal 3.4-8.6 U/L), and negative anti-nuclear antibody. Magnetic resonance imaging (MRI) demonstrated diffuse muscle edema and a skin biopsy of a Gottron's papule demonstrated interface dermatitis. A history of steatohepatitis predating her diagnosis, confirmed on ultrasound and liver biopsy, precluded the use of methotrexate or azathioprine. Despite treatment with daily oral steroids and tacrolimus, as well as monthly IVIG and intravenous pulse methylprednisolone, her illness progressed over twelve months to involve extensive skin ulcerations of the upper extremities and widespread calcinosis (Figure, A).
Figure.
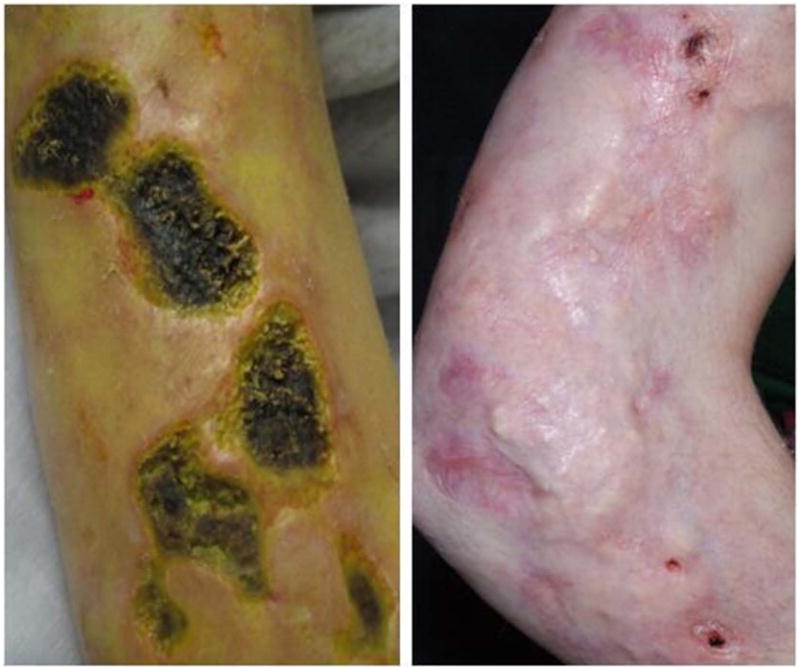
Ischemic cutaneous ulcerations and calcinosis noted at A, 30 months after diagnosis at the start of, and B, 6 months post treatment with topical and IV sodium thiosulfate and abatacept. There was progressive softening of calcinotic areas and healing of associated ulcerations.
Two years into treatment, repeat thigh MRI demonstrated continued muscle edema. The patient had a Childhood Myositis Assessment Scale (CMAS) score of 35 out of 52, Manual Muscle Testing (MMT8) score of 70 out of 80, and Childhood Health Assessment Questionnaire (CHAQ) score of 0.75 out of 3.0. Repeat thigh MRI demonstrated continued muscle edema. Monthly intravenous cyclophosphamide was added to her regimen, with no improvement in skin ulcerations or muscle strength after five months. Several treatments were attempted to halt the progression of her calcinosis, including colchicine, alendronate, and a six month course of infliximab. Despite this, her areas of calcinosis and ulceration continued to spread. At 2.5 years into treatment, the patient also developed worsening weakness and function with a CMAS score of 25, MMT8 score of 62 and CHAQ score of 1.75. Remarkable laboratory studies included LDH of 1163 U/L, and ferritin of 6510 U/L (normal 6-137 U/L).
Due to continued disease progression and increasing liver enzyme concentrations with high doses of corticosteroids, abatacept, at a dose of 10mg/kg was begun, with administration at 0, 2, and 4 weeks, followed by monthly dosing thereafter. To alleviate the patient's significant calcinosis and ischemic ulcerations, treatment with intravenous and topical sodium thiosulfate was also initiated. Topical sodium thiosulfate was used at 3% concentration and increased to 10% concentration over 2 weeks, applied to the open necrotic ulcerations of upper extremities daily under occlusive dressings. In addition, the patient received 10 grams IV sodium thiosulfate 3 times weekly, which was increased to 15 grams, and continued for 3 months.
Following six months of therapy, the CMAS score improved to 45, MMT8 improved to 77, the CHAQ score improved to 0.5. Significant improvement in ulcerations was noted over the course of 6 months, with formation of granulation tissue and epithelialization (Figure, B). Pain medication usage markedly decreased within six weeks of initiating therapy. Within three months, pulses of intravenous methylprednisone were discontinued and the oral prednisone dose was reduced by 30%, with improvement in LDH to 730 U/L and ferritin to 725 U/L. Upper extremity plain x-rays confirmed lack of progression in calcinosis. No significant adverse effects were observed from the infusions.
Discussion
Abatacept is a fusion protein between immunoglobulin and the extracellular domain of cytotoxic T-lymphocyte antigen 4 (CTLA-4), which exerts an anti-inflammatory effect by down-regulating T cell activation (3). The efficacy of this biologic therapy has been established in adult and juvenile rheumatoid arthritis,(4). However, there is no prior experience with abatacept in the treatment of inflammatory myopathies, and this is the first report to suggest a beneficial effect. Abatacept has the added advantage of low renal and hepatic toxicity, a key benefit in our patient's case.
Down regulation of T-cell activation via the use of abatacept may play an important anti-inflammatory role in the pathogenesis of JDM. CD4+ T and B lymphocytes, dendritic cells, and macrophages are among the major cellular components of the inflamed perivascular and perimysial muscle inflammation (5). The co-stimulatory molecules CD28 and CTLA-4, and its counter-receptor CD80, are expressed abundantly on myofibers and inflammatory infiltrates of myositis patients (6). The action of abatacept in JDM may not only be related to the down-regulation of T lymphocytes, but also to the decreased antigen presentation capability of myocytes, as well as inhibition of macrophages, and a resultant decrease in pro-inflammatory cytokines, including IL-6, TNF-α, IL-1β, and TGF-β (7).
Treatment of calcinosis in JDM poses a greater challenge, as no single agent has been found to reproducibly halt or reverse dystrophic calcification. Anecdotal success has been reported with the use of calcium channel blockers, probenecid, colchicine, tumor necrosis factor inhibitors, bisphosphonates, and intra-lesional corticosteroids (2). Several of these agents were tried in our patient, with progression in calcinosis despite their use. Sodium thiosulfate is a potent antioxidant and vasodilator that also chelates and dissolves calcium deposits (8-11). In addition to decreasing the pain associated with ulcerative skin disease and calciphylaxis, it has been shown to decrease dystrophic calcification in calcific uremic arteriolopathy (calciphylaxis), iatrogenic calcinosis cutis, and tumoral calcinosis (8-10). Although it is primarily administered intravenously, topical use has been described for ulcerations associated with lupus calcinosis and uremic calciphylaxis (10). However, the use of this agent for treatment of dermatomyositis-associated calcinosis has not been previously reported.
Sodium thiosulfate promotes vasodilation and vascularization of peripheral neuronal units, resulting in rapid resolution of pain (8). Improved soft tissue vascularization in our patient contributed in the tissue regeneration, skin softening, and resultant healing of ulcerations. In addition, the patient was able to be withdrawn from narcotics relatively soon after initiation of intravenous sodium thiosulfate, which may be attributable in part to vascularization of peripheral neuron units (8). Concomitant use of abatacept may have also played a role in soft tissue vascularization and regeneration. Therefore, it is not possible to determine if our observed effects were secondary to the use of sodium thiosulfate alone, or this combination of therapies.
We conclude that abatacept may be an effective steroid-sparing agent for the treatment of refractory JDM, and sodium thiosulfate may have a valuable role in stabilizing calcinosis, and in diminishing pain and promoting re-vascularization of cutaneous ulcerations. Controlled studies are needed to determine the safety and efficacy of these agents and their role in the therapeutic armamentarium of JDM.
Acknowledgments
Supported by the intramural research program of NIEHS, NIH, Cure JM Foundation, and Mohsen Ziai, MD, Pediatric Endowment at Inova Fairfax Hospital for Children, Inova Health System Foundation.
We thank Drs. James Katz and Nora Taylor for critical review of the manuscript.
List of Abbreviations
- JDM
Juvenile Dermatomyositis
- IVIG
Intravenous Immune Globulin
- LDH
lactate dehydrogenase
- MRI
Magnetic Resonance Imaging
- CMAS
Childhood Myositis Assessment Scale
- MMT8
Manual Muscle Testing
- CHAQ
Childhood Health Assessment Questionnaire
- CTLA-4
Cytotoxic T-Lymphocyte Antigen 4
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense, nor the US Government.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wedderburn LR, Rider LG. Juvenile Dermatomyositis: New Developments in Pathogenesis, Assessment and Treatment. Best Practice and Research Clinical Rheumatology. 2009;23:655–678. doi: 10.1016/j.berh.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider LG. Calcinosis in Juvenile Dermatomyositis: Pathogenesis and Current Therapies. Pediatr Rheum On Line Jour. 2003;1:2. http://www.pedrheumonlinejournal.org/April/calinosis.html.
- 3.Fiocco U, Sfriso P, Oliviero F, Pagnin E, Scagliori E, Campana C, et al. Co-stimulatory modulation in rheumatoid arthritis: the role of (CTLA4-Ig) abatacept. Autoimmun Rev. 2008;8:76–82. doi: 10.1016/j.autrev.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792–802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- 5.Khanna S, Reed AM. Immunopathogenesis of juvenile dermatomyositis. Muscle Nerve. 2010;41:581–92. doi: 10.1002/mus.21669. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraju K, Raben N, Villalba ML, Danning C, Loeffler LA, et al. Costimulatory markers in muscle of patients with idiopathic inflammatory myopathies and in cultured muscle cells. Clin Immunol. 1999;92:161–169. doi: 10.1006/clim.1999.4743. [DOI] [PubMed] [Google Scholar]
- 7.Cutolo M, Soldano S, Montagna P, Sulli A, Seriolo B, Villaggio B, et al. CTLA4-Ig interacts with cultured synovial macrophages from rheumatoid arthritis patients and downregulates cytokine production. Arthritis Res Ther. 2009;11:R176. doi: 10.1186/ar2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlieper G, Brandenburg V, Kettler M, Floege J. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5:539–43. doi: 10.1038/nrneph.2009.99. [DOI] [PubMed] [Google Scholar]
- 9.Raffaella C, Annapaola C, Tullio I, Angelo R, Giuseppe L, Simone C. Successful treatment of severe iatrogenic calcinosis cutis with intravenous sodium thiosulfate in a child affected by T-acute lymphoblastic leukemia. Pediatric Dermatol. 2009;26:311–5. doi: 10.1111/j.1525-1470.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolf EK, Smidt AC, Laumann AE. Topical sodium thiosulfate therapy for leg ulcers with dystrophic calcification. Arch Dermatol. 2008;144:1560–2. doi: 10.1001/archderm.144.12.1560. [DOI] [PubMed] [Google Scholar]
- 11.Amin N, Gonzalez E, Lieber M, Salusky IB, Zaritsky JJ. Successful treatment of calcific uremic arteriolopathy in a pediatric dialysis patient. Pediatric Nephrol. 2010;25:357–62. doi: 10.1007/s00467-009-1313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


