Abstract
Immune cells infiltrating the microenvironment of melanoma metastases may either limit or promote tumor progression, but the characteristics that distinguish these effects are obscure. In this study, we systematically evaluated the composition and organization of immune cells that infiltrated melanoma metastases in human patients. Three histologic patterns of immune cell infiltration were identified, designated immunotypes A, B and C. Immunotype A was characterized by no immune cell infiltrate. Immunotype B was characterized by infiltration of immune cells limited only to regions proximal to intratumoral blood vessels. Immunotype C was characterized by a diffuse immune cell infiltrate throughout a metastatic tumor. These immunotypes represented 29%, 63%, and 8% of metastases with estimated median survival periods of 15, 23, and 130 months, respectively. Notably, all three immunotypes showed increasing proportions of B cells and decreasing proportions of macrophages. Overall, the predominant immune cells were T cells (53%), B cell lineage cells (33%), and macrophages (13%), with NK and mature dendritic cells only rarely present. While higher densities of CD8+ T cells correlated best with survival, a higher density of CD45+ leukocytes, T cells, and B cells also correlated with increased survival. Together, our findings reveal striking differences in the immune infiltrate in melanoma metastases in patients, suggesting microenvironmental differences in immune homing receptors and ligands that affect immune cell recruitment. These findings are important, not only by revealing how the immune microenvironment can affect outcomes but also because they reveal characteristics that may help improve individualized therapy for patients with metastatic melanoma.
Keywords: Melanoma/skin cancers, immune responses to cancer, tumor resistance to immune response, cell motility and migration, metastasis, lymphocyte, survival, tumor microenvironment, tumor infiltrating lymphocytes
INTRODUCTION
Intratumoral immune cells have been associated with improved prognosis in patients with colorectal, mammary, lung and ovarian carcinomas(1–4). In melanoma, some studies show that patients with brisk lymphocytic infiltrate in primary melanomas had lower risk of metastasis and death than patients with sparse or absent infiltrate(5–9). However, despite numerous studies of the prognostic impact of tumor infiltrating lymphocytes (TIL) in primary melanomas(6, 7, 10), comparable studies of melanoma metastases are few and limited in scope. Two studies, primarily in lymph node (LN) metastases, found that increased TIL had prognostic significance(11, 12). A small study also found an association between TIL and response to interferon therapy(13). These studies evaluated T cells and total immune cells, but have not evaluated infiltration of myeloid cells, dendritic cells, B cells, and intratumoral vascular endothelium, which have all been implicated in immunologic dysfunction within the metastatic melanoma microenvironment (MME), and thus may also have prognostic impact(14).
T cells can be cultured from melanoma metastases, and adoptive transfer can induce tumor regressions(15). However, tumor-reactive TIL can be cultured only from a subset of melanoma metastases. In a study of lymph node (LN) metastases, TIL were absent in 46% of metastases, and brisk in only 16%(11). This finding raises questions about factors controlling T cell infiltration of metastases. Circulating immune cells enter peripheral tissues upon engagement of integrins, selectins, and chemokine receptors, and their ligands, at the vascular endothelium. Other integrins and chemokines may be critical for immune cells to move along stromal tissues in the microenvironment and to be retained there. Despite the critical role of vascular endothelium for immune cell infiltration into the MME, prior studies have not addressed the spatial distribution of immune cells relative to the intratumoral vessels, and how that distribution may be associated with the extent of immune cell infiltration.
In the present study, we have evaluated spatial associations between immune cells and intratumoral blood vessels, which help to define patterns of immune cell infiltration (immunotypes). We hypothesized that immunotypes would have prognostic significance. Also, we have evaluated multiple immune cell subsets infiltrating melanoma metastases from patients with clinical followup, and have assessed their prognostic significance. Together, these studies provide a foundation upon which to build a better understanding of the control of immune cell homing and infiltration into the MME, which are critical for immunologic control of melanoma.
MATERIAL AND METHODS
Patients and Database
We reviewed surgical pathology reports of melanoma metastases in the Anatomic Pathology Software System (1982–2007) and selected 183 metastatic melanoma samples from 147 patients with clinical follow-up and ample surgical pathology material to obtain core samples from at least 3–4 tumor regions to construct tissue microarrays (TMAs). The interval from date of surgery to date of last contact (death or last follow-up) ranged from 1 to 358 months (mean, 37; median 13.7 for deceased patients, 63 for alive patients). Histopathologic and clinical findings referenced the 6th edition AJCC Melanoma Staging and Classification(16).
Construction of Tissue Microarrays (TMAs)
Formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved from archives of the Department of Pathology, University of Virginia. Use of human tissues was approved by the UVA IRB (protocol 10598). H&E slides from each block were reviewed by a pathologist (JS) to identify tumor areas. TMAs were constructed with 1.0mm diameter tissue cores from representative tumor areas from the FFPE tissue blocks, transferred into a recipient paraffin block using a semi-automated tissue array instrument (TMArrayer, Pathology Devices, Westminster, MD). Quadruplicate or triplicate tissue cores were taken from each specimen, resulting in nine composite TMA blocks containing tissue cores from 18–27 specimens each. Control tissues from spleen, liver, placenta, and kidney were included in each TMA block. Multiple 4µm sections were cut for H&E and immunohistochemical staining.
Immunohistochemistry
TMA tissue sections were deparaffinized in xylene and rehydrated by sequential incubation in EtOH/water solutions. Heat-induced antigen retrieval was performed in citrate buffer for most antibodies, or in EDTA/high pH buffer for antibodies to CD4, CD8 and CD56. Sections were incubated 60 min at room temperature with antibodies to CD34 (1:100), CD45 (1:400), CD20 (1:400), CD3 (1:400), CD8 (1:200), CD138 (1:100) from Dako (Carpinteria, CA), and to CD4 (1:120, Vector Laboratories, Burlingame, CA), CD56 (1:100, Invitrogen, Grand Island, NY), CD163 (1:50, Santa Cruz Biotechnology, Inc, Santa Cruz, CA), DC-LAMP (1:50, Dendritics, Lyon, France), FoxP3 (1:125, EBioscience, San Diego, CA), and PD-1 (1:100, R&D Systems, Minneapolis, MN). Envision system (enzyme-conjugated polymer backbone coupled to secondary antibodies) and 3,3’-diaminobenzidine chromogen (Dako, Carpinteria, CA) were applied to develop the staining. After rinsing with water, sections were counterstained with Hemotoxylin (Vector Laboratories) and coverslipped with mounting medium (VectaMount, Vector Laboratories). Positive control included LN, and, for CD34 antibody, placenta. Negative control slides used PBS instead of primary antibody, with other conditions constant.
Immunotyping and quantification of TIL
H&E stained sections were reviewed for type, density and location of tumor infiltrating immune cells, tumor cell type, necrosis, hemorrhage, and melanin pigment. Cores with no tumor tissue, >50% necrosis or hemorrhage were excluded. TIL infiltrate was scored by simultaneous review of CD34 and CD45 immunostained sections. TILs were scored as 1, when no or sparse immune cells were present (no more than 50 immune cells in a core); 2, when tumor infiltrating immune cells were present but were limited to perivascular cuffing around intratumoral blood vessels; or 3, when there were immune cells infiltrating diffusely among the tumor cells. A mean score of 2.5 was given when TILs were around blood vessels and also extending into the tumor tissue or there was perivascular cuffing of immune cells as well as infiltration among tumor cells in different areas of the core. Triplicate or quadruplicate cores from each tumor sample were evaluated for homogeneity. Scoring among all cores was consistent for 55% and heterogeneous for 42%. However, 65% of heterogeneic tumors differed in only one core, and 33% had similar scores across multiple cores, such as 2 and 2.5, or 2.5 and 3. Four percent of samples had only one core with adequate tumor. For each sample, a mean score was calculated, then TIL scores for all samples were plotted, and 3 TIL immunotypes were created based on the distribution of mean TIL scores; Immunotype A for <1.75, Immunotype B for 1.75–2.4, and Immunotype C for ≥2.5.
Stained cells were enumerated by a pathologist (GE) on each core in a high power microscope field, excluding cells within blood vessels or in hemorrhagic or necrotic areas. Cell counts were normalized to per mm2. Images were obtained using an Olympus BX51 microscope coupled to an Olympus BP70 digital camera (Olympus America Inc, Center Valley, PA), and software Image ProPlus 4.5 for Windows.
Estimating total cell numbers in the metastases
Total cell numbers (including melanoma cells) per mm2 were estimated on representative histologic sections. Using Aperio software, a box 0.04mm2 in area was drawn, and cell nuclei counted at 20x (SS), and multiplied by 25 to estimate the number per mm2. Results were averaged for 10 samples. We also estimated cell density (per mm3) = number of cells per mm2×the number of tissue sections per mm (1/tissue section thickness in mm) with a correction factor (x slide thickness/cell diameter). This is simplified to: Cells/mm3 = number cells/mm2 × (1/cell diameter (in mm)).
Statistical Analysis
For statistical analyses, averages of immune cell counts (cells per mm2) sampled in quadruplicate were used. An intraclass correlation summarized the relationship between intra- and inter-patient variabilities of total immune cell counts based on multiple samples derived from 29 patients. For the main analyses, only the first sample from each patient was used. Kruskall-Wallis and Wilcoxon tests were used to compare immune cell counts and clinicopathologic variables. Kaplan-Meier curves and log-rank tests were used to assess relationships, in a univariate setting, between categorical clinicopathological parameters and overall survival. Univariate Cox proportional hazards models were fit separately, by immune cell type, to immune cell counts (low vs. high numbers, using median expression as a cutoff) and hazard ratios were obtained. Multivariate Cox proportional hazards models assessed the relationship between survival and the immune cells counts (treated as continuous, using square root transformation) in conjunction with a set of covariates that included NED status, stage, gender, tissue type and age. The production of separate models for each type of immune cell provides information on the relationship between each of them and survival. A similar multivariate Cox proportional hazards model was used to assess survival against immunotype (A vs. B+C) and the set of five covariates noted above. A p-value of less than 0.05 was considered to indicate significance. Analyses were performed with SAS 9.2 or MedCalc software (Version 11.6.1.0, Mariakerke, Belgium).
RESULTS
Patient Demographics and Melanoma Samples
Nine TMA blocks were created using 183 metastatic melanoma specimens from 147 patients (63 female, 84 male). Median age at surgery was 58 years (range 19 – 89). Some patients had 2–5 synchronous or metachronous metastases. At surgery for the earliest specimen, 103 patients had Stage III melanoma (4 IIIA, 40 IIIB, 59 IIIC) and 44 had stage IV melanoma. Of the 183 specimens, 83 were in LNs, 92 in skin and soft tissue, 7 in small intestine, and one peritoneal. Melanoma morphology was epithelioid (80%), mixed (14%), or spindle cell (6%).
The Distribution of intratumoral Immune Cells
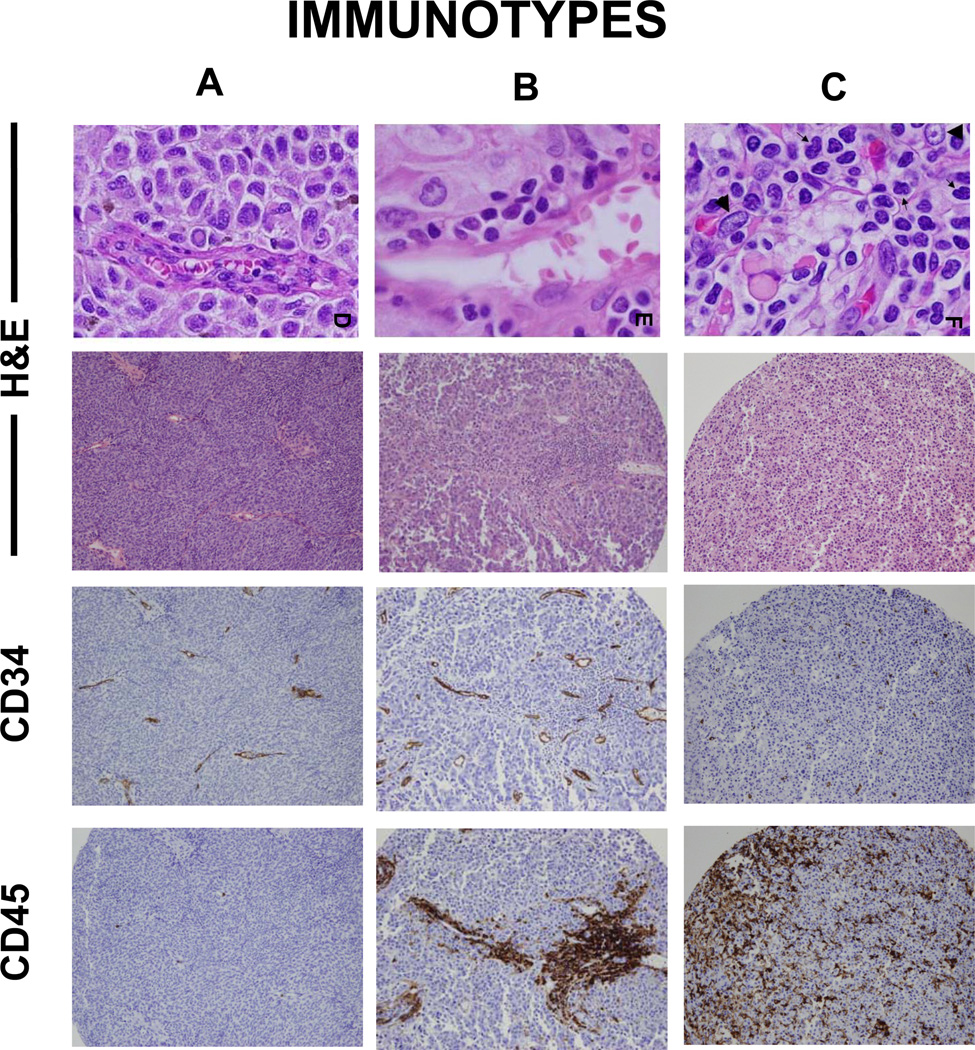
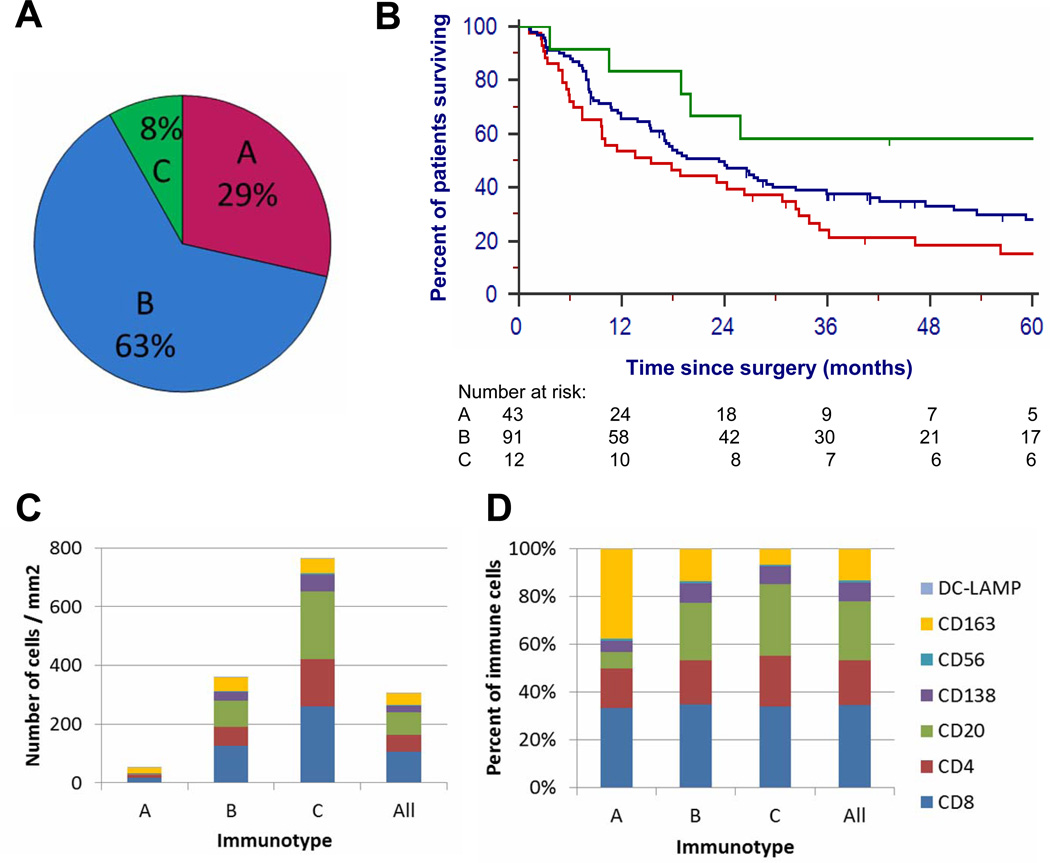
To evaluate the distribution of intratumoral immune cells within melanoma metastases, especially the physical association of immune cells and intratumoral blood vessels, we immunostained TMAs for CD34 (vascular endothelial cells) and CD45 (immune cells). Each specimen was categorized based on the distribution of immune cells in relation to vessels. Examples are shown in Figure 1. Analyses focused on the first metastasis evaluable from each patient. Among these, 29% had no significant immune cell infiltrate; this distribution is referred to as “Immunotype A.” Sixty-three percent had immune cell infiltration limited to perivascular cuffing, referred to as “Immunotype B”. Only 8% had diffuse intratumoral immune cell infiltrate, named “Immunotype C” (Figure 2A). Distributions of Immunotype among clinical subgroups are shown in Table 1. None differed significantly, but 11 of the 12 Immunotype C tumors were in patients rendered clinically free of disease (NED) after surgery.
Figure 1. Immunotypes.
TMAs are stained with routine H&E (400x and 100x), immunostained with CD34 and CD45, and scored for immune cell density and location for immunotyping. Metastatic melanomas are characterized as “Immunotype A” when immune cell infiltrate is absent, “Immunotype B” when immune cell infiltrate is perivascular only, and “Immunotype C” when immune cells are infiltrating among tumor cells beyond the vessels.
Figure 2. Immunotype frequency, prognosis, and cellular composition.
(A) The proportion of patients with each immunotype, in the first surgical specimen in the TMA, (B) Kaplan-Meier survival estimates for patients with each Immunotype, (C) Mean numbers of each immune cell subset, by Immunotype, and for all patients, (D) Proportion of mean total immune cells infiltrating tumor that are represented by each cell subset, by Immunotype.
Table 1.
Distribution of Immunotypes by Clinical factors
| Immunotype | P value* | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| N | (%) | N | (%) | N | (%) | |||
| Total | 42 | (28.6) | 93 | (63.3) | 12 | (8.2) | ||
| Cell Type | E | 28 | (24.3) | 77 | (67.0) | 10 | (8.7) | 0.08 |
| M | 10 | (47.6) | 11 | (52.4) | . | . | ||
| S | 4 | (36.4) | 5 | (45.5) | 2 | (18.2) | ||
| Stage | III | 28 | (27.2) | 66 | (64.1) | 9 | (8.7) | 0.57 |
| IV | 14 | (31.8) | 27 | (61.4) | 3 | (6.8) | ||
| Gender | F | 17 | (27.0) | 43 | (68.3) | 3 | (4.8) | 0.71 |
| M | 25 | (29.8) | 50 | (59.5) | 9 | (10.7) | ||
| Tissue | Lymph Node | 18 | (25.0) | 47 | (65.3) | 7 | (9.7) | 0.35 |
| Peritoneum | . | . | 1 | (100.0) | . | . | ||
| Small Bowel | 3 | (50.0) | 3 | (50.0) | . | . | ||
| Skin/SQ | 21 | (30.9) | 42 | (61.8) | 5 | (7.4) | ||
| Age | <50 | 14 | (27.5) | 34 | (66.7) | 3 | (5.9) | 0.87 |
| >=50 & <70 | 15 | (27.3) | 35 | (63.6) | 5 | (9.1) | ||
| >=70 | 13 | (31.7) | 24 | (58.5) | 4 | (9.8) | ||
| Prior Vax | N | 35 | (28.7) | 78 | (63.9) | 9 | (7.4) | 0.94 |
| Y | 7 | (28.0) | 15 | (60.0) | 3 | (12.0) | ||
| NED after surgery? |
N | 13 | (35.1) | 23 | (62.2) | 1 | (2.7) | 0.31 |
| Y | 29 | (26.4) | 70 | (63.6) | 11 | (10.0) | ||
Chi-square test of association: A vs B+C
Survival association with Immunotype
Kaplan-Meier survival analysis was performed, measuring survival from the metastasis removed earliest. Median survival estimates for Immunotypes A, B, and C are 15.4 months, 23.4 months, and 129.5 months (Figure 2B), respectively. This association with survival was significant across all 3 immunotypes (p = 0.0219, logrank test, Figure 2B). Log rank tests were also performed for differences between pairs of immunotypes: A vs. B+C, (p = 0.02), A+B vs. C (p = 0.0452), A vs. B (p = 0.0590), A vs. C (p = 0.0091), B vs. C (p = 0.0971).
Phenotypes of tumor-infiltrating immune cells
To identify and to quantify the immune cells infiltrating melanoma metastases, immunostained cells in each core were enumerated and reported per mm2 for each metastasis. Not surprisingly, the greatest numbers of immune cells were in Immunotype C tumors, with intermediate numbers in Immunotype B, and rare cells at most in Immunotype A (Figure 2C). Infiltrating cells included CD4+ T cells, CD8+ T cells, NK cells, B cells, plasma cells, macrophages, and dendritic cells. They may contain other cells, but stains for these cells accounted well for the total CD45+ cells. Interestingly, the mean proportions of each cell type varied among 3 immunotypes (Figure 2D). The most notable changes, from Immunotype A to C, are: decreasing proportions of macrophages (CD163+: 40%, 13%, 6%), and increasing proportions of B cells (CD20+: 7%, 24%, 30%, respectively), Table 2.
Table 2.
Numbers of Immune Cell Subsets by Immunotype
| Immunotype A | Immunotype B | Immunotype C | Wilcoxon P value A vs B |
Wilcoxon P value B vs C |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SE | % | N | Mean | SE | % | N | Mean | SE | % | |||
| CD45 | 42 | 28.9 | 6.4 | 59% | 93 | 309.3 | 38.6 | 86% | 12 | 712.9 | 121.5 | 93% | < 0.001 | < 0.001 |
| CD3 | 42 | 22.5 | 5.5 | 46% | 93 | 191 | 19.2 | 53% | 12 | 422 | 74.3 | 55% | < 0.001 | 0.002 |
| CD8 | 41 | 17.5 | 4.4 | 36% | 93 | 125.8 | 10.2 | 35% | 12 | 259.6 | 42.8 | 34% | < 0.001 | 0.003 |
| CD4 | 42 | 8.8 | 4.4 | 18% | 93 | 66.0 | 10.0 | 18% | 12 | 161.7 | 47.4 | 21% | < 0.001 | 0.006 |
| CD20 | 41 | 3.6 | 1.5 | 7% | 93 | 87.2 | 25.4 | 24% | 12 | 230.4 | 88.3 | 30% | < 0.001 | < 0.001 |
| CD138 | 42 | 2.5 | 1.0 | 5% | 93 | 29.5 | 5.4 | 8% | 12 | 57.5 | 23.0 | 7.5% | < 0.001 | 0.15 |
| CD56 | 42 | 0.5 | 0.3 | 1% | 92 | 3.5 | 1.0 | 1% | 12 | 5.1 | 2.6 | 0.7% | < 0.001 | 0.91 |
| CD163 | 42 | 19.6 | 4.0 | 40% | 92 | 47.4 | 6.8 | 13% | 12 | 49.8 | 14.2 | 6.5% | 0.011 | 0.69 |
| DC-LAMP | 42 | 0.2 | 0.1 | 0.4% | 92 | 1.3 | 0.3 | 0.4% | 12 | 1.8 | 0.7 | 0.2% | 0.004 | 0.046 |
| FoxP3 | 42 | 3.2 | 0.9 | 6.5% | 93 | 25.4 | 3.7 | 7% | 12 | 46.5 | 11.3 | 6% | < 0.001 | 0.040 |
| PD-1 | 42 | 2.5 | 1.2 | 5% | 93 | 23.4 | 4.4 | 6.5% | 12 | 34.6 | 14.8 | 4.5% | < 0.001 | 0.16 |
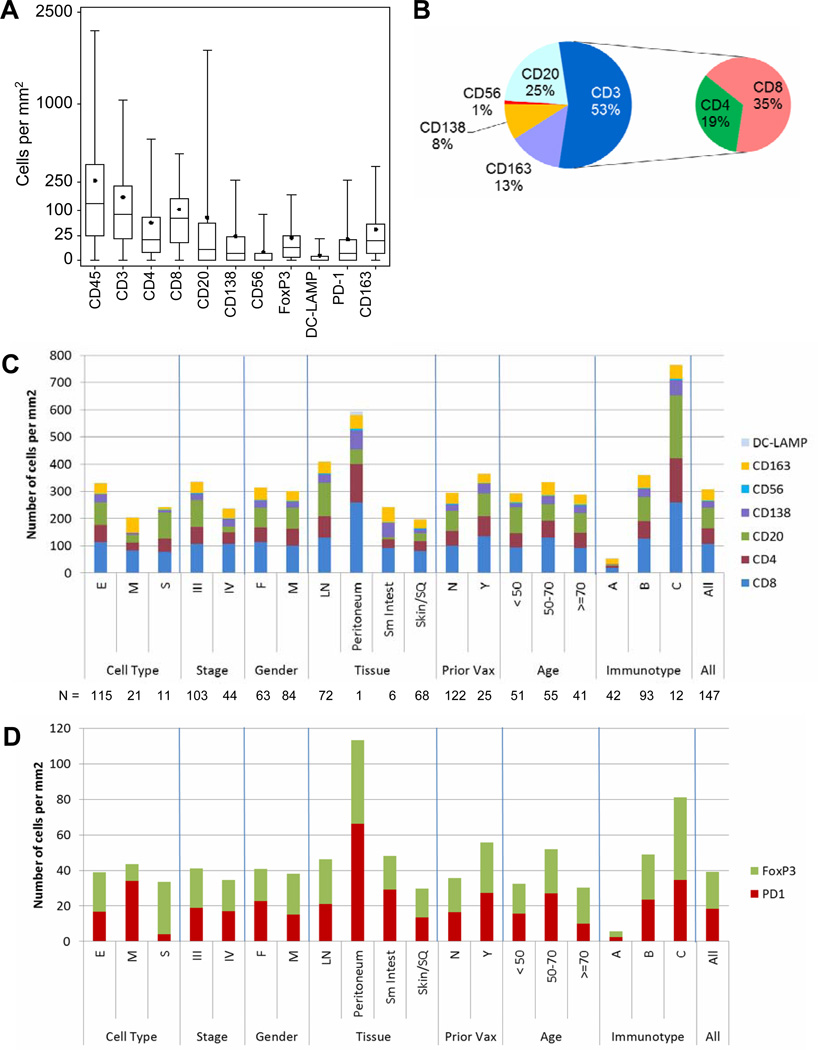
Density of immune cell subsets are summarized in Figure 3A for the first metastases in each patient, and in Supplemental Table 1 for all 183 specimens. Overall, for the 183 melanoma metastases, CD45+ cells ranged from 0 to 2142 per mm2. The most prevalent immune cells in the first metastases (Figure 3B) are T cells (53%), of which CD8 T cells predominate (35%), and CD4 T cells are less common (19%). B cells (CD20) and plasma cells (CD138) are present at high numbers (25% and 8%, respectively). Macrophage lineage cells (CD163) account for 13% of the infiltrates. NK cells (CD56) and mature DC (DC-LAMP) are rare across all samples, with mean proportions of less than 1% each.
Figure 3. Immune cell subsets overall and across patient groups.
(A) Box plots of numbers of immune cell subsets, per mm2, in first melanoma metastases for 147 patients. Boxes represent 25th to 75th percentiles. Middle bar identifies median; solid circle represents mean; whiskers show minimum and maximum, (B) Percentages of tumor-infiltrating immune cells in pie chart, (C) Mean numbers of immune cell subsets for clinical subgroups. Numbers of tumors represented by each bar are shown below the graph (peritoneum = one), (D) Mean numbers of cells expressing FoxP3 or PD1 for clinical subgroups.
There were no statistically significant differences among immune cell counts based on clinical parameters age, gender, tumor stage, or tumor cell type (Figure 3C), except decreased macrophages in spindle cell melanomas (S, p = 0.003), and decreased B cells in Stage IV (p = 0.013). However, there were significant differences based on metastatic tissue site: Melanomas metastatic to LNs contained higher numbers of CD45+, CD3+, CD8+, CD20+, and DC-LAMP+ cells compared to tumors metastatic to skin/soft tissue, small intestine, or peritoneum (p<0.001, p=0.006, p=0.019, p<0.001, and 0.035, respectively, Figure 3C, Supplemental Table 2). CD20+ B lymphocytes were highest in LN metastases and least prevalent in small bowel metastases. Macrophage (CD163) counts were similar among metastatic sites. CD56+ cell counts were very low in all groups, without significant differences (Figure 3C), except across immunotypes.
Variations in immunotype and immune cell numbers within the same patient
There was substantial concordance of Immunotypes among tumors within the same patient (Supplemental Table 3). Each of twenty-nine patients had 2–5 metastases evaluated (19, 8, 1, 1 patients, respectively). Among them, 19 (66%) had identical immunotypes for all or most metastases. The intraclass correlation for total immune cells was high (0.67).
The total number of all cells (including melanoma cells) per mm2 in the metastases was estimated by counting representative sections. In areas 0.04 mm2, counts ranged from 122 to 372 (mean 222, SD 79), representing a mean of 5,558 cells (SD 1,980) per mm2. We estimate the average diameter of melanoma cells to be about 20 microns(17), and of immune cells to be about 10 microns. Using the model presented in the methods, total cell density in metastases was estimated at approximately 280,000/mm3. By comparison, the mean number of CD45+ cells in Immunotype C tumors was 713/mm2 (Table 2); applying the same formula, we estimate about 71,300 CD45+ cells per mm3 (about 25% of the total cell number).
Molecular components of immunologic checkpoints on infiltrating immune cells
Nuclear staining for FoxP3 was detected in 7% of immune cells overall (mean, and median). PD1 was detected in 7% of infiltrating cells (mean; median less than 2%). Both FoxP3 and PD-1 were most numerous in Immunotype C and least in Immunotype A. However, proportions of FoxP3+ cells, and of PD-1+ cells were relatively consistent across immunotypes and across other subgroups. (Table 2, Figure 3D), and numbers of FoxP3+ cells and PD-1+ cells did not differ among metastatic sites (Supplemental Table 2). There was a significant association between numbers of FoxP3+ cells and PD-1+ cells in individual tumors (Spearman correlation, rho = 0.551, p < 0.0001); however, despite this association, there were many cases where FoxP3+ cells were observed in the absence of PD-1+ cells. (Supplemental Figure 1)
Tumor Infiltrating Immune Cell Phenotypes and Survival
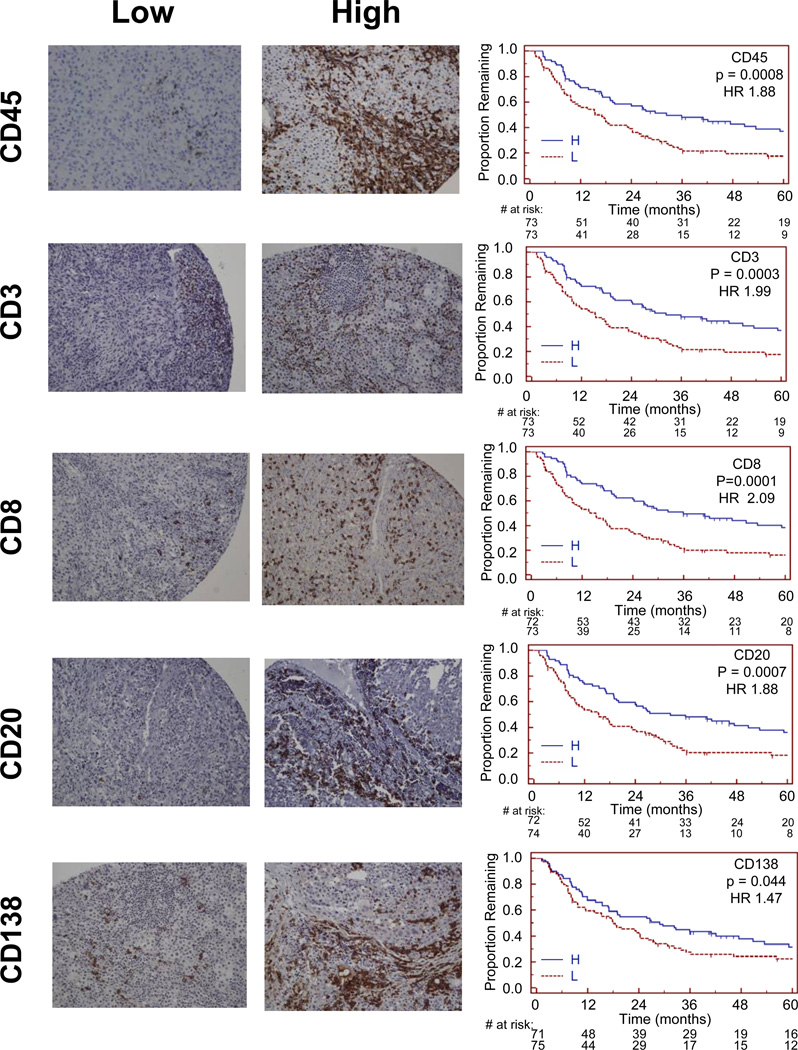
To assess if tumor infiltration by any immune cell population was prognostic for overall patient survival, univariate and multivariate Cox proportional-hazards analyses was performed. Kaplan-Meier curves were produced for high vs. low numbers of each cell type (greater than median vs. median or less), and univariate Cox proportion hazard models suggest longer overall survival for patients with tumors containing high counts of CD45+, CD3+, CD20+, or CD8+ cells than for patients with low counts (all p < 0.001, Figure 4, Table 3). There were trends to better survival for increased plasma cells (CD138, p = 0.044), but numbers of CD4+, CD56+, CD163+, DC-LAMP+, FoxP3+, or PD-1+ cells were not associated with survival (Table 3).
Figure 4. Patient survival and associations with immune cell density.
Representative examples of sections with low- or high- numbers of cells staining for CD45, CD3, CD8, CD20, and CD138 are shown beside Kaplan-Meier survival curves for low and high counts of those markers. Magnification, 200×.
Table 3.
Univariate and Multivariate analyses of association with Survival
| P values | ||
|---|---|---|
| Univariate | Multivariate | |
| Immunotype* | 0.0219 | 0.17 |
| Cell type | 0.41 | ND |
| NED after Surgery | < 0.001 | < 0.001 |
| Stage | <0.001 | 0.80 |
| Gender | 0.58 | 0.18 |
| Tissue type | 0.059 | 0.70 |
| Age | 0.30 | 0.30 |
| CD45 | 0.0008 | 0.004 |
| CD3 | 0.0003 | 0.009 |
| CD8 | 0.0001 | 0.001 |
| CD4 | 0.16 | 0.14 |
| CD20 | 0.0007 | 0.04 |
| CD56 | 0.43 | 0.61 |
| CD138 | 0.044 | 0.01 |
| CD163 | 0.81 | 0.70 |
| DC-LAMP | 0.19 | 0.47 |
| PD1 | 0.77 | 0.03 |
| FoxP3 | 0.215 | 0.13 |
Immunotype A vs B+C
Multivariate Cox proportional models controlled for disease status (no evidence of disease (NED) or not, after surgery), stage, gender, tissue site, and age (Table 3). In a model with these predictors alone, NED status was a significant predictor of survival (p < 0.001), but stage, gender, tissue site, and age were not. Patient numbers were not high enough for one prognostic model to include all the cell types and clinical variables; so counts of each cell type were evaluated in separate models. Adjusting for these covariates, independent association with better survival was observed with higher counts of CD45+ cells (p = 0.004), CD138+ (p=0.01), CD20+ (p=0.04), CD3+ (p=0.009), and CD8+ (p=0.001), and PD-1+ (p = 0.03) cells. In these analyses, there were no significant associations with survival for numbers of CD4+ cells, NK cells, mature DC, macrophages, or FoxP3+ lymphocytes. The small number of immunotype C patients limited multivariate analysis of Immunotype just to comparison of A vs. B+C (p = 0.17).
Associations with long term survivors of vaccine trials
One-third of the patients (49) participated in experimental melanoma vaccine trials at our institution, nine of whom (18%) remained alive more than 5 years and were clinically free of disease at last followup. Of these, tumor was resected before (n=4), or after (n=5), enrolling in a vaccine trial. Immunotypes for these tumors were A (1, 11%), B (5, 56%), and C (3, 33%) and did not change significantly after vaccine trial participation. Mean numbers of infiltrating immune cell subsets (Supplemental Table 4) were high, when reviewed in light of the study population (Supplemental Table 1), the most prominent of which is the high number of B cells.
DISCUSSION
A number of studies have identified immune cell infiltration as an important prognostic factor in primary melanomas(6–10) and in other primary solid tumors(1–4, 18–21), but critical to the future of immunotherapy is understanding the immunobiology of metastases. We have characterized immune cell infiltrates in melanoma metastases, specifically addressing the organization of those cells relative to intratumoral blood vessels, and the prognostic importance of immune cell subsets. Tumor-infiltrating immune cells were absent from 29% of these tumors. This is slightly lower than a prior report (46%), possibly due to higher sensitivity for detecting immune cells with immunohistochemistry. Regardless, infiltrating immune cells are absent in a large subset of melanoma metastases, and patients with these Immunotype A tumors had the worst survival. These tumors likely have a lesion in immune control at the level of endothelium of the tumor vasculature, which appears to act as an effective blood:tumor barrier. Successful immune therapy of Immunotype A tumors likely will require understanding molecular mediators of this blood:tumor barrier at the endothelial interface, for which candidate mechanisms may include overexpression of endothelin B(22), and low endothelial expression of E-selectin, P-selectin, and ICAM-1(23).
Most melanoma metastases (63%) were characterized by perivascular immune cell populations that fail to infiltrate among melanoma cells within the metastatic masses. This phenotype (Immunotype B) has not been described formally, as prior studies have largely reported cell numbers or categories of brisk and non-brisk infiltrates only (11). When these tumors are evaluated on H&E sections, the relationship these cells have to vessels is often unclear, but immunohistochemical stains clarify that most immune cells in the metastases are confined to perivascular regions. Based on these findings, the endothelium in these tumors appears to provide adequate signals to circulating lymphocytes to allow them to roll, arrest, and transmigrate the endothelium and to enter the perivascular areas; however homing of those cells into the tumor microenvironment apparently is arrested at that point. Possible mediators of this phenotype could be a lack of stromal molecules to provide scaffolding for T cell motility within the tumors, lack of lymphocytophilic chemokines in the metastatic melanoma micro-environment (MME), lack of retention integrin induction and engagement, increased emigration of immune cells from the tumor, or failure of the immune cells to survive in the MME. Higher levels of infiltrating T cells has been associated with chemokines CCL2-5, CXCL9, and CXCL10.(24) Production of some of these chemokines by melanoma cells can be increased by interferons(25). In other cancers, intratumoral activation of innate immune pathways by toll-like receptor 7 agonists has increased T cell infiltration; this is linked with activation of interferon signaling and is associated with increased endothelial expression of E-selectin(26–28). However, interferons can have negative effects through activation of immune regulatory processes, especially indoleamine 2,3-dioxygenase (IDO), PD-1 and PD-L1 in peripheral tissues, which may in turn limit T cell infiltration, function, and survival in the MME. Greater understanding of the immunologic signatures of Immunotype B tumors, and net effects of various interventions in the tumor microenvironment will be necessary to dissect these complex interactions and offer opportunities for optimized combination immunotherapies.
Prior work reported brisk TIL in LN metastases of 16% of melanomas(11). We identified only 8% as Immunotype C tumors, characterized by diffuse infiltration among melanoma cells. Diffuse intratumoral infiltrates suggest that the molecular machinery is intact both at the level of the endothelium and within the MME itself, to support homing of T cells and other immune cells into the tumor microenvironment. Patients with these tumors have had the best overall survival, suggesting that this immunobiologic phenotype has clinical relevance. The association with survival is significant in univariate analysis. The multivariate analysis is limited by the small number of patients with Immunotype C tumors, such that a full analysis of the relevance of each immunotype was not possible – the multivariate model only compared immunotype A vs. B+C, and clinical NED status was the most significant prognostic factor in that model. It is of interest that the proportion of patients who could be rendered NED surgically was highest for Immunotype C tumors: 92% (vs. 69% and 75% in Immunotypes A and B, respectively, Table 1). Thus, processes that limit metastatic sites may be explained in part by the immunobiology of Immunotype C tumors. Regardless, the Immunotype constructs can help to focus future research on pathways by which immune cells home to tumor, as well as barriers to infiltration that characterize each phenotype and that may be targeted therapeutically. It is notable that response rates to many systemic melanoma therapies have been about 10–15%, very similar to the frequency of Immunotype C tumors; future work will help to elucidate the extent to which this Immunotype predicts response to immune therapy.
One question is why melanomas with Immunotype C progress, despite T cell infiltration. Many mechanisms of tumor-associated immune dysfunction have been identified(29); so the immunologic advantage of this phenotype is likely limited by effects of tumor cells. Others and we have reported on the evolution of melanoma metastases over time, which likely reflects an ongoing conflict between immunologic control and escape(30, 31). This has been described elegantly in the context of immune surveillance, equilibrium, and editing(32).
Frequencies of immune cells infiltrating tumor differ from those in normal blood and suggest differential recruitment of immune cell populations, likely mediated by differences in homing receptors and their ligands. We found that T cells are the dominant infiltrating immune cells, but the second-most prevalent are B lineage cells (B cells and plasma cells), which increase proportionately from Immunotype A to C. There has been only limited investigation of the role of B cells in melanoma immunobiology. In murine models, B cell lineage depletion can improve immunologic control of experimental solid tumors(33–37), and a subset of regulatory B cells may limit immunologic control of cancer(38). In other murine studies, however, B cells were found to be important for optimal CD8+ and CD4+ T cell tumor immunity, survival of cytotoxic T cells, and tumor control(39, 40). In patients with advanced melanoma, dysfunction of circulating B cells has been observed and has been associated with loss of CD27+ memory B cells(41). Plasma cells in primary melanomas carry negative prognostic significance in a few case series(42, 43); however, we are not aware of detailed reports of B cells and plasma cells in melanoma metastases. In our study, the numbers of B cells correlated positively with overall survival. This opens the door to future studies of the function and dysfunction of B lineage cells in the MME, which appear to have a role in the host:tumor relationship.
Also prevalent were cells of putative immunoregulatory function: PD-1+ cells and FoxP3+ cells, as well as macrophages (CD163+), which may include myeloid populations with immunoregulatory function such as MDSC. FoxP3+ cells account for over 1/3 of the CD4+ cells, and the regulatory molecule PD-1 was expressed on 5–7% of immune cells in metastases of lymph nodes and skin, and 12% of cells infiltrating small bowel metastases. Thus, these regulatory cell populations represent a significant proportion of infiltrating cells and offer a significant potential for tumor-associated immune dysfunction. NK cells were not detected at high frequency using an antibody to CD56; however, an antibody to NKp46 (R&D) may provide more accurate assessments of infiltrating NK cells(44, 45).
CD8+ T cell numbers correlated best with survival. Increased numbers of T cells, specifically activated CD8+ cytotoxic T cells, have correlated with better survival in invasive colon cancer and cervical cancer(1, 19). Interestingly, correlations with survival were not observed for CD4 cells, which may reflect the substantial fraction of FoxP3+ regulatory T cells, and also suggests that the homing receptor/ligand interactions that mediate infiltration of CD4+ T cells differ from those mediating infiltration of other lymphocytes into melanoma metastases. A better understanding of these homing receptors is needed.
It is also unknown to what extent immune infiltrates may be modulated by the genotype of the melanoma cells. Oncogenic BRAFV600E constitutively activates MAP kinase signaling and by complex effects through ERK, microphthalmia-associated transcription factor (MITF) is usually down-regulated in these cells(46). MITF controls pigmentation and expression of melanocytic differentiation proteins (MDP)(47). Conceivably, these changes may decrease the infiltration and antitumor activity of T cells recognizing these common melanoma antigens. Selective inhibition of oncogenic BRAF dramatically increases expression of MDPs, and has been associated with increased T cell infiltration of melanoma metastases(48, 49). We are not aware of other data directly implicating cKit or N-RAS mutations in immune evasion by melanoma, but futures studies are warranted to assess the effect of these and BRAF mutations on Immunotype and immune cell function in the MME.
In conclusion, the density, phenotype and location of immune cells have prognostic value in metastatic melanomas. We describe immunotypes of melanoma that reflect qualitative differences in immunobiology. The prognostic significance of selected immune cell counts is greater than that of the Immunotypes; however, we posit that Immunotypes categorize melanoma metastases into 3 qualitatively distinct and biologically relevant groups. Further investigation is warranted to define the molecular mediators of the blood:tumor barrier in Immunotype A tumors, and of the barriers to emigration from the perivascular regions in Immunotype B tumors. Data are beginning to emerge about homing receptors and ligands that are most critical for immune cell infiltration of melanoma metastases (24, 50). Molecular characterization of these processes in human melanoma will provide new targets to improve infiltration of T cells and other immune cells, which in turn may improve the effectiveness of combination immune therapies for melanoma and other cancers.
Supplementary Material
Acknowledgements
This work was supported in part by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579, Biorepository and Tissue Procurement Facility); and gifts from the Commonwealth Foundation for Cancer Research, the Kincaid Foundation, and from the James and Rebecca Craig Foundation, and the Rebecca Clary Harris Memorial Fellowship (to JS and to SS).
Footnotes
Conflicts of interest related to this study: None.
Reference List
- 1.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eerola AK, Soini Y, Paakko P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Jr, Sober AJ, Kopf AW, Lew RA, Mihm MC, Jr, Hennessey P, et al. A prognostic model for clinical stage I melanoma of the upper extremity. The importance of anatomic subsites in predicting recurrent disease. Ann Surg. 1981;193:436–440. doi: 10.1097/00000658-198104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark WH, Jr, Elder DE, Guerry D4, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. Journal of the National Cancer Institute. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 7.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996;78:427–432. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 10.Spatz A, Gimotty PA, Cook MG, Van Den Oord JJ, Desai N, Eggermont AM, et al. Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study. Journal of Clinical Oncology. 2007;25(18S):8519. [Google Scholar]
- 11.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor-infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Laboratory Investigation. 1996;74:43–47. [PubMed] [Google Scholar]
- 12.Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakansson A, Gustafsson B, Krysander L, Hakansson L. Tumour-infiltrating lymphocytes in metastatic malignant melanoma and response to interferon alpha treatment. Br J Cancer. 1996;74:670–676. doi: 10.1038/bjc.1996.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oble DA, Loewe R, Yu P, Mihm MC., Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9 3.:3. [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De G, V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 18.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 20.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 22.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 23.Weishaupt C, Munoz KN, Buznev E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin Cancer Res. 2007;13:2549–2556. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- 24.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother. 2010;33:965–974. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SJ, Hijnen D, Murphy GF, Kupper TS, Calarese AW, Mollet IG, et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. Journal of Investigative Dermatology. 2009;129:2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panelli MC, Stashower ME, Slade HB, Smith K, Norwood C, Abati A, et al. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol. 2007;8:R8. doi: 10.1186/gb-2007-8-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 30.Coulie PG, Ikeda H, Baurain JF, Chiari R. Antitumor immunity at work in a melanoma patient. Advances in Cancer Research. 1999;76:213–242. doi: 10.1016/s0065-230x(08)60778-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Pressley J, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. JI. 2005;174:6863–6871. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Oizumi S, Deyev V, Yamazaki K, Schreiber T, Strbo N, Rosenblatt J, et al. Surmounting tumor-induced immune suppression by frequent vaccination or immunization in the absence of B cells. J Immunother. 2008;31:394–401. doi: 10.1097/CJI.0b013e31816bc74d. [DOI] [PubMed] [Google Scholar]
- 34.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr, Feng L, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 36.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 37.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 38.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183 doi: 10.1111/j.1749-6632.2009.05137.x. 38–57.:38–57. [DOI] [PubMed] [Google Scholar]
- 39.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deola S, Panelli MC, Maric D, Selleri S, Dmitrieva NI, Voss CY, et al. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol. 2008;180:1362–1372. doi: 10.4049/jimmunol.180.3.1362. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res. 2009;15:4277–4287. doi: 10.1158/1078-0432.CCR-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mascaro JM, Molgo M, Castel T, Castro J. Plasma cells within the infiltrate of primary cutaneous malignant melanoma of the skin. A confirmation of its histoprognostic value. Am J Dermatopathol. 1987;9:497–499. doi: 10.1097/00000372-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Weissmann A, Roses DF, Harris MN, Dubin N. Prediction of lymph node metastases from the histologic features of primary cutaneous malignant melanomas. Am J Dermatopathol. 1984;6 Suppl 35–41.:35–41. [PubMed] [Google Scholar]
- 44.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 45.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 46.Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS ONE. 2008;3:e2734. doi: 10.1371/journal.pone.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vachtenheim J, Borovansky J. "Transcription physiology" of pigment formation in melanocytes: central role of MITF. Exp Dermatol. 2010;19:617–627. doi: 10.1111/j.1600-0625.2009.01053.x. [DOI] [PubMed] [Google Scholar]
- 48.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 49.Wargo JA, Cogdill A, Dang P, Gupta R, Piris A, Boni A, et al. Treatment with a selective inhibitor of BRAFV600E increases melanocyte antigen expression and CD8 T cell infiltrate in tumors of patients with metastatic melanoma. Cancer Research. 2011 Apr 15;71(8) Suppl 1 abstract 958. [Google Scholar]
- 50.Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J Immunol. 2010;184:4079–4086. doi: 10.4049/jimmunol.0901903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.