Abstract
Objective
Nutritional status is assessed by measuring BMI or percent body fat (%fat). BMI can misclassify persons who carry more weight as fat-free mass and %fat can be misleading in cases of malnutrition or in disease states characterized by wasting of lean tissue. The fat-free mass index (FFMI) is proposed to assess body composition in individuals who have a similar body composition but differ in height allowing identification of those suffering from malnutrition, wasting or those that possess a relatively high muscle mass. The purpose was to determine whether the FFMI differs in a group of racially/ethnically diverse adults.
Design
Cross-sectional.
Subjects
Subjects were a multi-ethnic sample (Caucasian, CA; African American, AA; Hispanic, HIS and Asian, AS) of 1339 healthy males (n = 480) and females (n = 859) ranging in age from 18–110 years. Total body fat, total fat-free mass and bone mineral density were estimated using dual energy X-ray absorptiometry.
Results
FFMI differed among the four ethnic groups (P ≤ 0.05) for both genders. A curvilinear relationship was found between age and FFMI for both genders although the coefficients in the quadratic model differed between genders (P ≤ 0.001) indicating the rate of change in FFMI differed between genders. The estimated turning point where FFMI started to decline was in the mid 20s for male and mid 40s for female participants. An age × gender interaction was found such that the rate of decline was greater in male than female participants (P ≤ 0.001). For both genders, FFMI was greatest in AA and the least in AS (P ≤ 0.001). There was no significant interaction between race and age or age2 (P = 0.06). However, male participants consistently had a greater FFMI than female participants (P ≤ 0.001).
Conclusions
These findings have clinical implications for identifying individuals who may not be recognized as being malnourished based on their BMI or %fat but whose fat-free mass corrected for height is relatively low.
Keywords: fat-free mass index, fat-free mass, body mass index (BMI), percent body fat, nutritional assessment
Introduction
Assessment of nutritional status is important in several settings. In hospitalized persons, recognition of malnutrition is especially important because nutritional status is linked to longevity and mortality,1,2 directly influences the course of a disease and optimal treatment and influences the length of hospital stay.3 Nutritional status is inferred from the body mass index (BMI; kgm−2). By comparing values of an individual patient to national norms, the health professional is able to assign a level of fatness,3 determine risk level for chronic disease and estimate mortality risk. However, merely expressing body weight relative to height has limitations,3 as a person that carries more weight in lean tissue may be incorrectly classified as possessing an undesirable excess of body fat. Furthermore, data suggest different health effects of fat mass (FM) and fat-free mass (FFM). When only BMI is used as a criterion of nutritional status, these divergent relationships cannot be distinguished.1 In elderly individuals not classified as obese, involuntary weight loss as FM was associated with decreased mortality, whereas weight loss as FFM was associated with increased mortality.1 A second method of nutritional assessment is to estimate the amount of FM stored in the body. But similarly, the use of percent body fat (%fat) to describe the status of the body’s fat stores can be misleading. This is especially apparent in cases of malnutrition or in disease states like AIDS, where individuals may be characterized by normal %fat, but suffer from wasting or reduced FFM.2
The concept of a fat-free mass index (FFMI) is not a new term as it was first described nearly 20 years ago by VanItallie et al.4 They proposed the FFMI to overcome some of the pitfalls associated with merely expressing FM or FFM in absolute terms or as a percentage of total body weight. Although ranges have been established for BMI and suggested for %fat,5 our understanding of what constitutes a normal or healthy amount of FFM is limited. For instance, being classified as obese by BMI or %fat does not necessarily translate into an increased risk of poor health. In fact, a higher %fat has been reported in seemingly healthy and normal functioning persons, termed the ‘healthy obese’.6,7 Conversely, those suffering from wasting such as diseased or hospitalized individuals can present with a normal body weight, but in reality suffer from a loss of FFM while appearing to have preserved FM.2
To further illustrate this point, take as an example two male participants of a similar age, total body weight and body composition, but who differ in height (Table 1). Assume one of these individuals is healthy while the other is malnourished. Even though these two individuals have a similar percentage of weight as FM and FFM and both have a normal BMI, they differ significantly in their nutritional status. Neither BMI nor %fat discloses this difference. However, by calculating the FFMI, a different body composition is revealed for the malnourished individual.4 Calculation of the FFMI will allow a clinician to identify the malnourished individual, whereas interpretation of BMI and %fat may fail to detect the presence of protein–energy malnutrition.4 Although BMI is a useful tool to compare body weights in individuals who differ in height, the FFMI has utility for the comparison of body composition in individuals who differ in height. In effect, numbers describing the body’s content of FM and FFM are of little use in nutritional assessment unless they are normalized for height.
Table 1.
Data for two male individuals of similar age, total body weight and body composition
| Variable | Healthy | Malnourished |
|---|---|---|
| Body fat (%) | 13 | 12 |
| Total body weight (kg) | 70.6 | 69.1 |
| Height (cm) | 170.4 | 185.3 |
| BMI (kgλm−2) | 24.3 | 20.1 |
| FMI (kgλm−2) | 3.1 | 2.4 |
| FFMI (kgλm−2) | 21.2 | 17.7 |
Abbreviations: BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index.
The FFMI has been described previously in a healthy sample of adult Caucasians divided according to age,8 and in a second study that characterized the FFMI by percentiles.9 There has been no report on the FFMI across ethnic groups or how FFMI changes with age within race/ethnic groups. The primary aim of this study was to investigate in a racially/ethnically diverse adult cohort whether, and the extent to which, FFMI is affected by race. A secondary aim, using a cross-sectional design, was to determine the FFMI’s pattern of variation across the adult lifespan.
Materials and methods
Subjects
The sample consisted of healthy multi-ethnic adults recruited to participate in studies performed at the New York Obesity Nutrition Research Center from 1993 to 2002. For this analysis, 1339 participants (480 male and 859 female participants) were analyzed. Participants ranged in age from 18 to 110 years and ranged in BMI from normal to obese. The range in body weight and BMI for the sample was 38.3–147.2 kg and 15.8–50.6 kgm−2, respectively, and the upper weight limit was restricted in accordance with the capacity of the DXA instrument with an upper weight limit (159 kg).
Race was determined by self identification of each subject from the following four categories: Asian, non-Hispanic Black (African American), non-Hispanic White and Hispanic. In addition, subjects were asked to select the category into which their parents and grandparents fell. If all categories were the same, the subject was identified by that category. However, if multiple categories were selected, the individual was classified as ‘other’. For the current analysis, persons classified as ‘other’ were excluded.
Inclusion criteria required that subjects be ambulatory, not exercising vigorously and without orthopedic problems or physical handicaps that would affect the outcomes of any variables being studied in each respective research protocol. Subjects reporting no major health concerns were enrolled as participants. In research protocols requiring further verification of health status, a medical history, physical examination and routine blood studies were completed. Those with any underlying medical conditions were excluded.
Study procedures
Subjects completed all testing at one visit to the Body Composition Unit (New York Obesity Nutrition Research Center) at St Luke’s-Roosevelt Hospital. Body weight and height were measured wearing a hospital gown and foam slippers, with a calibrated scale (Weight Tronix, NY, NY, USA) and stadiometer (Holtain Stadiometer, Crosswell, Wales). Body mass index (BMI) was calculated (kgm−2) from height and weight. FFMI was calculated (kgm−2) using the following formula:4
Using statistical modeling, the following equations were created to calculate the FFMI for male and female participants by race/ethnic group.
Male participants:
FFMI Caucasian (kgm−2) = 20.502 + (0.0271915 × age)−(0.0005795 × age2)
FFMI African American (kgm−2) = 21.3512 + (0.0271915 × age)−(0.0005795 × age2)
FFMI Hispanic (kgm−2) = 20.775 + (0.0271915 × age)−(0.0005795 × age2)
FFMI Asian (kgm−2) = 18.997 + (0.0271915 × age)−(0.0005795 × age2)
Female participants:
FFMI Caucasian (kgm−2) = 14.752 + (0.0929701 × age)−(0.0009674 × age2)
FFMI African American (kgm−2) = 15.602 + (0.0929701 × age)−(0. 0009674 × age2)
FFMI Hispanic (kgm−2) = 15.025 + (0.0929701 × age)−(0. 0009674 × age2)
FFMI Asian (kgm−2) = 13.247 + (0.0929701 × age)−(0. 0009674 × age2)
For male participantss, fat-free mass loss was based on the prediction equations starting at the predicted turning point (23.5 years); for a male participant 182.9 cm tall using the following formula:
(FFMI at 90 years)−(FFMI at 23.5 years)(kgm−2) × (Height)(m2)
For female participants, fat-free mass loss was based on the prediction equations starting at the predicted turning point (48.1 years); for a female participant 170.2 cm tall using the following formula:
(FFMI at 90 years–FFMI at 48.1 years)(kgm−2) × (height)(m2)
Informed consents were obtained before the start of testing. All studies were approved by the Radiation Safety Committee and Institutional Review Board of St. Luke’s-Roosevelt Hospital.
Dual-energy X-ray absorptiometry
Total body fat, lean and bone mineral content (BMC) were measured using the DPXL (GE Lunar, Madison, WI, USA) using software versions 3.6 (88.2% of data), 3.8 (0.8% of data) and 4.7d/e (11% of data). The lean mass component refers to the total body mass less bone and fat. In this paper, the term ‘fat-free mass’ also refers to total body mass less bone and fat. Using specific anatomic landmarks as previously described,10 regions including the arms, legs and trunk were demarcated.
For soft tissue quality control purposes relating to the densitometer, monthly scans were performed using methanol and water bottles with a volume of 8 l to simulate fat and lean soft tissues, respectively.11,12 An anthropomorphic spine phantom made up of calcium hydroxyappetite embedded in a 17.5 × 17.5 cm block was scanned each morning before a subject visit. Phantom scans were also done three times a week and before and after all DXA system manufacturer maintenance visits. Calculated phantom spine bone mineral density was stable throughout the study period. Soft tissue measuring phantoms simulating fat and fat-free mass (methanol and water phantoms) were also scanned as soft tissue quality control markers monthly. The average R-values for methanol and water phantoms for DPXL was 1.287 and 1.369, respectively. The range in measured R-values was 1.253–1.293 (CV = 0.49%) and 1.359–1.375 (CV = 0.22%), for methanol and water, respectively. Testing in our laboratory for our system has shown CV values of 3.4% for %fat, 1.2% for fat-free mass and 1.0% for BMC.
Statistical analysis
Descriptive statistics, including means and s.d. were calculated for all variables for the entire group and by gender. To date, all previously published data to derive an FFMI reference have been developed in a cohort of Caucasian subjects; therefore, we developed models that included variables to adjust for possible ethnic differences. Multiple regression analysis was used to test the main hypotheses. The primary hypotheses were that the four ethnic groups differed for FFMI and that with increasing age, FFMI would decrease in all groups. Dummy variable coding was assigned for gender and race. Linear and quadratic models were explored to determine the best fit model for the relationship between age and FFMI. Gender, age, age2, gender × age interaction and gender × age2 interactions were the independent variables retained in the final model. All statistical calculations were performed using SPSS statistical software (SPSS Institute Inc., Cary, NC, USA version 14.0) for personal computers. The level of significance for statistical tests was 0.05.
Results
A total of 1339 subjects (479 male and 860 female subjects) were included in these analyses. The descriptive characteristics for this study cohort are presented for male and female subjects separately for each race/ethnic group in Tables 2 and 3, respectively.
Table 2.
Descriptive characteristics for male individuals (n = 480)
| Mean ± s.d. | ||||
|---|---|---|---|---|
| Variable | Caucasian (n = 202) |
African American (n = 73) |
Hispanic (n = 147) |
Asian (n = 58) |
| Body weight (kg) | 80.3 ± 13.0 | 81.3 ± 17.5 | 79.6 ± 15.6 | 67.3 ± 8.2 |
| Age (years) | 39.7 ± 19.3 | 52.4 ± 21.7 | 45.4 ± 15.9 | 44.5 ± 20.1 |
| Body fat (%) | 19.2 ± 8.9 | 19.5 ± 9.3 | 23.9 ± 8.6 | 18.4 ± 6.4 |
| Height (cm) | 177.3 ± 7.4 | 174.5 ± 7.6 | 170.0 ± 7.7 | 170.2 ± 6.9 |
| BMI (kgλm−2) | 25.6 ± 3.9 | 26.7 ± 5.1 | 27.5 ± 4.6 | 23.2 ± 2.6 |
| FMI (kgλm−2) | 5.2 ± 3.1 | 5.6 ± 3.7 | 6.9 ± 3.5 | 4.4 ± 1.8 |
| FFMI (kgλm−2) | 20.5 ± 2.1 | 21.1 ± 2.3 | 20.6 ± 2.2 | 18.8 ± 1.8 |
Abbreviations: BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index.
Table 3.
Descriptive characteristics for female individuals (n = 859)
| Mean ± s.d. | ||||
|---|---|---|---|---|
| Variable | Caucasian (n = 404) |
African American (n = 209) |
Hispanic (n = 188) |
Asian (n = 58) |
| Body weight (kg) | 65.2 ± 15.5 | 75.3 ± 17.1 | 69.5 ± 13.8 | 53.3 ± 6.8 |
| Age (years) | 43.2 ± 19.1 | 52.0 ± 20.3 | 53.1 ± 16.3 | 48.5 ± 23.2 |
| Body fat (%) | 30.0 ± 10.8 | 37.1 ± 8.8 | 39.1 ± 7.3 | 29.3 ± 7.1 |
| Height (cm) | 162.7 ± 6.9 | 162.2 ± 7.5 | 155.5 ± 6.5 | 156.8 ± 7.1 |
| BMI (kgλm−2) | 24.6 ± 5.7 | 28.5 ± 5.7 | 28.8 ± 5.7 | 21.7 ± 2.5 |
| FMI (kgλm−2) | 7.9 ± 4.6 | 10.9 ± 4.4 | 11.5 ± 4.1 | 6.4 ± 2.1 |
| FFMI (kgλm−2) | 16.6 ± 1.7 | 17.4 ± 1.9 | 17.0 ± 2.1 | 15.0 ± 1.2 |
Abbreviations: BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index.
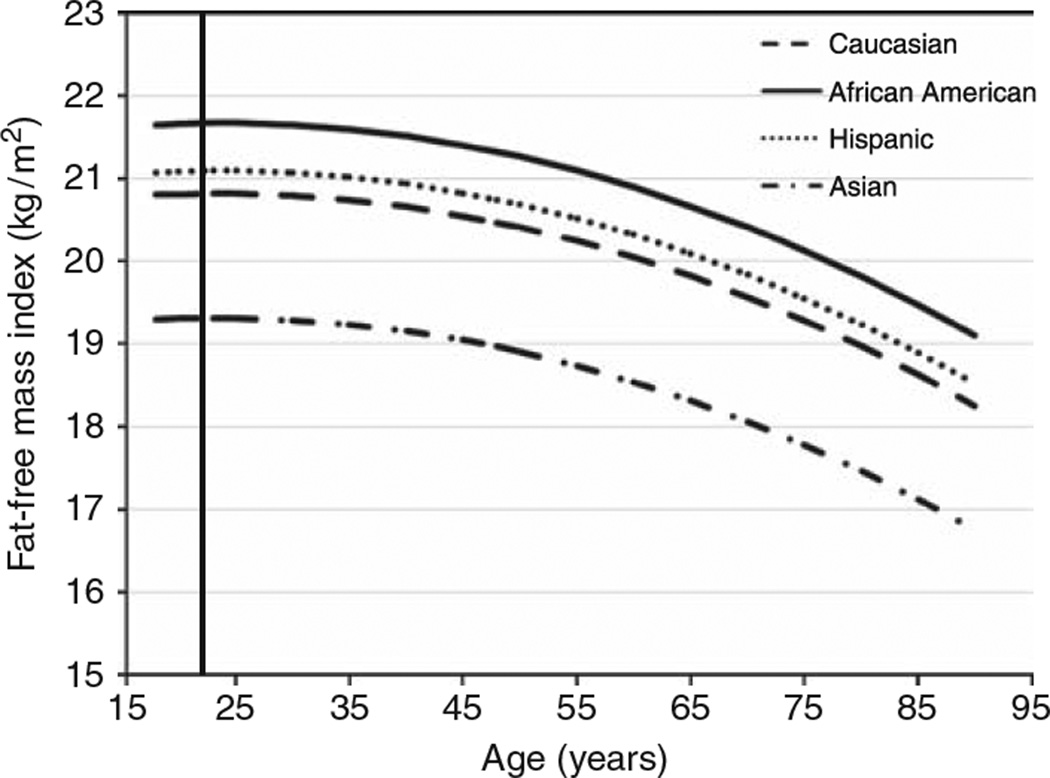
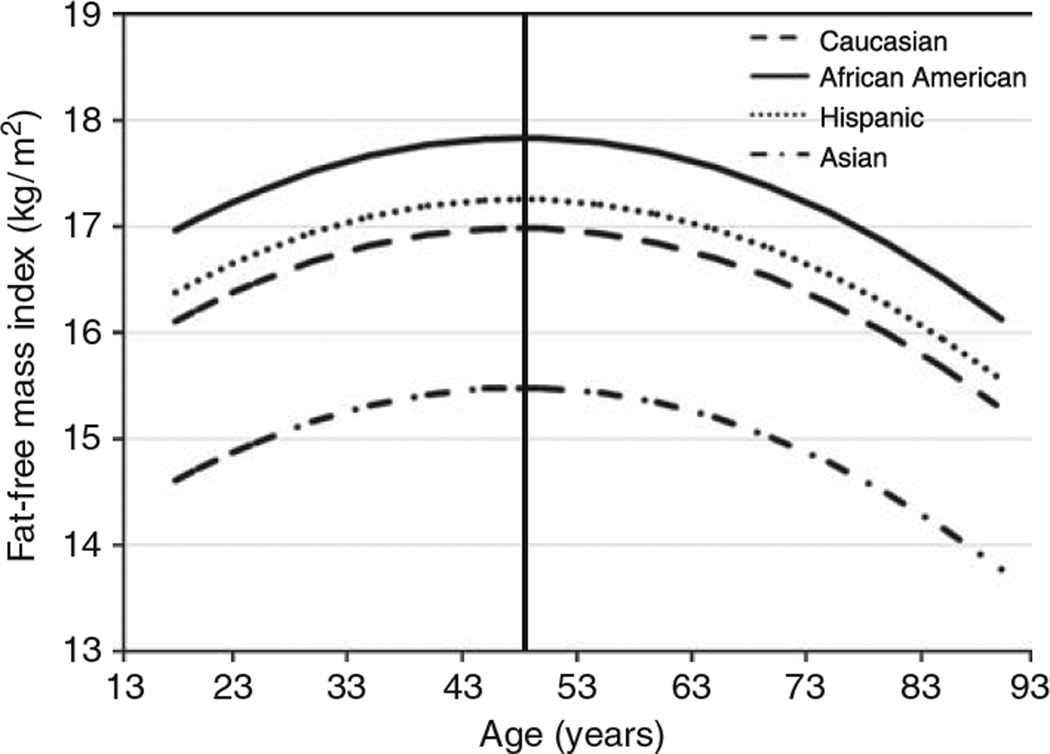
Tables 4 and 5 present the predicted FFMI values for male and female subjects, respectively, by race/ethnicity between ages 20 and 90 years. Figures 1 and 2 depict the relationship between FFMI and age by race/ethnicity in male and female subjects, respectively. All four ethnic groups differed from each other (P ≤ 0.001; except for Hispanic versus Caucasian, where P < 0.04) for both male and female subjects. A curvilinear relationship was found between age and FFMI for both genders though the coefficients in the quadratic model differed between genders (P ≤ 0.001). The estimated turning point where FFMI started to decline was in the mid 20s for male and mid 40s for female subjects. A different age × gender coefficient implies that the rate of decline differed by gender (P ≤ 0.001) indicating the rate varied by age and over the lifespan and the rate of decline was greater in male than female subjects. For both genders, the FFMI was significantly greater in AA and the least in AS (P ≤ 0.001). There was no significant interaction between race and age or age2 (P = 0.06); however, an effect for gender was found (P ≤ 0.001) with male subjects having a greater FFMI than female subjects.
Table 4.
Predicted fat-free mass index (± s.e.) (kgλm−2) for male individuals between the ages of 20–90 years
| Caucasian (n = 202) |
African American (n = 73) |
Hispanic (n = 147) |
Asian (n = 58) |
|
|---|---|---|---|---|
| 20 years | 20.81 ± 0.18 | 21.66 ± 0.21 | 21.09 ± 0.21 | 19.31 ± 0.24 |
| 25 years | 20.82 ± 0.14 | 21.67 ± 0.17 | 21.09 ± 0.16 | 19.31 ± 0.21 |
| 30 years | 20.80 ± 0.12 | 21.65 ± 0.16 | 21.07 ± 0.14 | 19.29 ± 0.19 |
| 35 years | 20.74 ± 0.12 | 21.59 ± 0.16 | 21.02 ± 0.13 | 19.24 ± 0.19 |
| 40 years | 20.66 ± 0.13 | 21.51 ± 0.16 | 20.94 ± 0.14 | 19.16 ± 0.20 |
| 45 years | 20.55 ± 0.15 | 21.40 ± 0.17 | 20.82 ± 0.14 | 19.05 ± 0.21 |
| 50 years | 20.41 ± 0.15 | 21.26 ± 0.17 | 20.69 ± 0.15 | 18.91 ± 0.21 |
| 55 years | 20.24 ± 0.16 | 21.09 ± 0.17 | 20.52 ± 0.15 | 18.74 ± 0.21 |
| 60 years | 20.05 ± 0.16 | 20.90 ± 0.17 | 20.32 ± 0.15 | 18.54 ± 0.21 |
| 65 years | 19.82 ± 0.16 | 20.67 ± 0.17 | 20.09 ± 0.15 | 18.32 ± 0.21 |
| 70 years | 19.57 ± 0.16 | 20.42 ± 0.17 | 19.84 ± 0.17 | 18.06 ± 0.22 |
| 75 years | 19.28 ± 0.19 | 20.13 ± 0.20 | 19.55 ± 0.19 | 17.78 ± 0.23 |
| 80 years | 18.97 ± 0.23 | 19.82 ± 0.23 | 19.24 ± 0.24 | 17.46 ± 0.27 |
| 85 years | 18.63 ± 0.29 | 19.48 ± 0.29 | 18.90 ± 0.30 | 17.12 ± 0.32 |
| 90 years | 18.25 ± 0.37 | 19.10 ± 0.37 | 18.53 ± 0.38 | 16.75 ± 0.40 |
| Percent decline since turning point (%) | −12.3 | −11.8 | −12.1 | −13.3 |
The prediction equations for the four ethnic groups were significantly different from each other (P < 0.001; Hispanic versus Caucasian P < 0.04). FFMI Caucasian (kgλm−2) = 20.502+(0.0271915 × age)−(0.0005795 × age2) FFMI African American (kgλm−2) = 21.3512+(0.0271915 × age)−(0.0005795 × age2) FFMI Hispanic (kgλm−2) = 20.775+(0.0271915 × age)−(0.0005795 × age2) FFMI Asian (kgλm−2) = 18.997+(0.0271915 × age)−(0.0005795 × age2) Percent decline = (FFMI at 90 years−FFMI at 24 years)/(FFMI at 24 years) × 100
Table 5.
Predicted fat-free mass index (± s.e.) (kgλm−2) for female individuals between the ages of 20–90 years
| Caucasian (n = 404) |
African American (n = 209) |
Hispanic (n = 188) |
Asian (n = 58) |
|
|---|---|---|---|---|
| 20 years | 16.22 ± 0.16 | 17.07 ± 0.19 | 16.50 ± 0.19 | 14.72 ± 0.23 |
| 25 years | 16.47 ± 0.12 | 17.32 ± 0.15 | 16.74 ± 0.16 | 14.97 ± 0.20 |
| 30 years | 16.67 ± 0.10 | 17.52 ± 0.14 | 16.94 ± 0.14 | 15.17 ± 0.19 |
| 35 years | 16.82 ± 0.10 | 17.67 ± 0.13 | 17.09 ± 0.13 | 15.32 ± 0.19 |
| 40 years | 16.92 ± 0.10 | 17.77 ± 0.13 | 17.20 ± 0.13 | 15.42 ± 0.19 |
| 45 years | 16.98 ± 0.11 | 17.83 ± 0.14 | 17.25 ± 0.13 | 15.47 ± 0.20 |
| 50 years | 16.98 ± 0.12 | 17.83 ± 0.14 | 17.25 ± 0.13 | 15.48 ± 0.20 |
| 55 years | 16.94 ± 0.12 | 17.79 ± 0.14 | 17.21 ± 17.21 | 15.43 ± 0.20 |
| 60 years | 16.85 ± 0.12 | 17.70 ± 0.13 | 17.12 ± 0.13 | 15.34 ± 0.20 |
| 65 years | 16.71 ± 0.12 | 17.56 ± 0.13 | 16.98 ± 0.13 | 15.20 ± 0.20 |
| 70 years | 16.52 ± 0.12 | 17.37 ± 0.13 | 16.79 ± 0.13 | 15.01 ± 0.20 |
| 75 years | 16.28 ± 0.13 | 17.13 ± 0.14 | 16.56 ± 0.15 | 14.78 ± 0.20 |
| 80 years | 16.00 ± 0.16 | 16.85 ± 0.17 | 16.27 ± 0.18 | 14.49 ± 0.22 |
| 85 years | 15.66 ± 0.21 | 16.51 ± 0.21 | 15.94 ± 0.22 | 14.16 ± 0.25 |
| 90 years | 15.28 ± 0.26 | 16.13 ± 0.27 | 15.56 ± 0.28 | 13.78 ± 0.30 |
| Percent decline since turning point (%) | −10.0 | −9.5 | −9.9 | −11.0 |
The prediction equations for the four ethnic groups were significantly different from each other (P < 0.001; Hispanic versus Caucasian P < 0.04). FFMI Caucasian (kgλm−2) = 14.752+(0.0929701 × age)−(0.0009674 × age2). FFMI African American (kgλm−2) = 15.602+(0.0929701 × age)−(0.0009674 × age2). FFMI Hispanic (kgλm−2) = 15.025+(0.0929701 × age)−(0.0009674 × age2). FFMI Asian (kgλm−2) = 13.247+(0.0929701 × age)−(0.0009674 × age2). Percent decline = (FFMI at 90 years−FFMI at 48 years)/(FFMI at 48 years) × 100.
Figure 1.
The relationship between the fat-free mass index (FFMI) and age in male subjects by race/ethnicity based on prediction equations. The vertical line represents the peak FFMI in the mid 20s. The prediction equations for the four ethnic groups were significantly different from each other (P < 0.001; Hispanic versus Caucasian P < 0.04).
Figure 2.
The relationship between the fat-free mass index (FFMI) and age in female subjects by race/ethnicity based on prediction equations. The vertical line represents the peak FFMI in the late 40s. The prediction equations for the four ethnic groups were significantly different from each other (P < 0.001; Hispanic versus Caucasian P < 0.04).
Presented in Tables 4 and 5 are estimates using the prediction equations for the calculated percent decline of fat-free mass across the adult lifespan for male and female subjects, respectively. In male subjects, the predicted loss of fat-free mass was −11.4 kg, whereas in female subjects, a loss of −7.6 kg was predicted. The percent change for the FFMI in male and female subjects varied by race/ethnic group. In both male and female subjects, the greatest percent change was found in Asians (−13.3 and −11.0%, respectively) followed by Caucasians (−12.3 and −10.0%, respectively), whereas African Americans had the lowest percent change (−11.8 and 9.5%, respectively).
Discussion
The primary aim of this study was to investigate if race/ethnic differences in the FFMI were present in a racially/ethnically diverse adult cohort. A secondary aim was to determine whether the FFMI varied across the adult lifespan within race/ethnic groups. All race/ethnic groups differed from each other and male subjects had a greater FFMI than female subjects. In both male and female subjects, the greatest percent change over time was found in AS followed by CA, whereas AA had the lowest percent change. These findings suggest that race/ethnic specific reference standards for FFMI are mandatory. Furthermore, specific race/ethnic groups may be more susceptible for greater loss of fat-free mass. Apart from our findings with respect to race/ethnic differences in the FFMI and the age-related variations of this index, analysis of these data on the age in life when the FFMI started to decline uncovered a striking gender difference. Declines in the FFMI were found in the early 20s in male subjects whereas decline in female subjects occurred in the late 40s.
FFMI race/ethnic relations
A main effect for race/ethnicity was found where all groups differed from each other. AA had a greater FFMI whereas AS had the least in both genders; however, no interaction between age and race was found. The percent change for the FFMI varied by race/ethnic group due to differences in FFMI between groups; the greatest percent change was found in AS who early in life had the lowest FFMI, whereas AA had the lowest percent change.
Two adult reference data sets are available; one from a group of CA men and women ranging in age from 18 to 98 years9 and a second in subjects ranging in age from 19 to 86 years.8 Though no reference data exists for the FFMI in a diverse cohort, data have been published on how total body potassium (TBK) differs by race.13 TBK is found in skeletal muscle and used as a proxy measure for skeletal muscle mass to provide an estimate of FFM.14 Similar to our findings for both genders, TBK values were greatest in AA and least in AS.13 It is interesting to note that approximately 50% of the subjects used in this analysis were also included in the TBK findings reported above.
These findings highlight racial disparities in body composition and suggest that identification of individuals by race will show greater susceptibility for disease related to loss of fat-free mass. Further metabolic studies are needed to identify or clarify the inter-racial differences in the FFMI in relation to health risk.
FFMI age relations
A curvilinear relationship was found between age and FFMI although the time point at which FFMI started to decline differed by gender. The decline in male subjects began in the mid 20s compared to the mid 40s for female subjects. The rate of decline did not differ between groups. The significant age by gender interaction indicated that the rate varied by age and gender. However, over the course of a normal lifespan, the rate of predicted decline in male subjects was greater than in female subjects. Using the prediction equations in Tables 2 and 3, by the age of 90 years the predicted loss of fat-free mass in male subjects was 11.4 kg and in female subjects was 7.6 kg.
The two previous publications on the FFMI suggest a decline in the FFMI starting later in life.8,9 The overall mean and percentile values were reported for both genders in a group of healthy Caucasian adults across four different age groupings (18–34, 35–54, 55–74 and >75 years).9 Whether examining the data across the age groups or across percentiles within age groups, a similar trend emerged. FFMI increased until the 55–74 year group and then decreased in the >75 year group. Barlett et al.8 measured body density in 813 healthy adults (282 male and 531 female subjects) using hydrodensitometry to estimate the relationship between fat-free mass and height (gcm−1). The ratio remained steady and unchanged until the late 40s in female and mid 50s in male subjects with a decline thereafter through 70 years. In the current analyses, we explored both linear and quadratic equations to determine the best fit model to predict the FFMI. This may be a reason why the results differ in male subjects; Barlett et al. analysis included a two-way ANOVA with post hoc tests to determine gender and age group differences.
FFMI gender relations
Our results found a main effect for gender indicating that gender-specific FFMI values are required and that male subjects had a greater FFMI than female subjects; however by the mid 80s, both genders had similar FFMI values based on prediction equations. Further, an interaction between gender and age indicated that the rate of decline in FFMI was greater in male than in female subjects. The predicted percent decline in male subjects ranged from −11.8% in AA to −13.3% in Asians. The range of percent decline was slightly less in female subjects; ranging from −9.5% in CA to −11.0% in AS. A noteworthy point, the predicted loss of fat-free mass occurred over a much longer time period in male subjects, whereas in female subjects the predicted loss occurred over a shorter time starting in their late 40s. It is unknown how the rate of decline in FFMI levels or the time point in the lifespan when the decline commences predicts or relates to health outcomes or if the later decline in FFMI may help explain the greater longevity in female versus male subjects.
Other studies have found similar results. Barlett and colleagues8 found that the ratio of FFMI/ht peaked in the early 20s for male subjects and mid to late 40s in female subjects. A significant (P = 0.0001) gender difference was detected across the adult lifespan, from the early 20s to late 80s, with male subjects having a greater FFMI/ht ratio. Similarly, Schutz et al.9 found the female FFMI was 20% lower than FFMI in male subjects. However, with increasing age female subjects had a slight but significant (P < 0.0001) increase in the FFMI after 60 years when compared to male subjects.
The gender differences found in peak FFMI were striking and may possibly be explained by known endocrine differences between men and women. In male subjects the hormone testosterone is involved in maintaining muscle mass, bone density and fat mass distribution peaks at age 17 years15 and remains steady until the 30 and 40s when a 1.2% per year decline was found.16 In female subjects, estrogen function is analogous to testosterone function in male subjects.15 Estrogen declines are associated with the end of ovulation, occurring sometimes around the fifth decade of life. In female subjects, the change in FFMI may be explained by mid-life endocrine changes; however, the peak FFMI found in male subjects is still earlier than suggested decline in testosterone. Data sets containing clinical and hormonal endpoints along with body composition data are needed to clarify the gender differences found.
Clinical implications
Assessment of fat-free mass is important since the primary source of the majority of the body’s protein reserve used during catabolic periods, which is used to maintain nutritional needs and bodily function, is stored in lean tissue. With aging and during periods of stress such as starvation or illness, the likely result is a reduction in this critical protein reserve. Accurate assessment of this vital nutritional depot becomes important.
As discussed, the value of BMI to assess nutritional status is limited and measurement of the total body fat can be misleading. In the example presented in Table 1, an assessment based solely on values for percent body fat or BMI would have led to an erroneous conclusion regarding nutritional state. However, use of the FFMI revealed a difference in nutritional status between the two individuals. Furthermore, as suggested by previous research,1 priority should be given to analysis of body composition in relation to mortality in as much as an absolute loss of body weight does not equate with a decrease in mortality. Instead, the loss of the components of total body weight (fat mass and fat-free mass) have opposing effects on health and mortality. The FFMI should prove a beneficial tool to elucidate these relationships.
Previous studies have shown additional ways the FFMI is useful in a clinical setting while exposing the reduced sensitivity of BMI as an indicator for the detection of malnourished individuals in specific subpopulations.17,18 Pichard et al.17 examined the relationship between the FFMI and length of stay in a hospital. A low FFMI was found in 55.6% of hospitalized patients and associated with a length of stay greater than 12 days. Kyle et al.18 demonstrated in overweight individuals that BMI alone was unable to identify malnourished individuals.
The use of the FFMI may also provide insight into sarcopenic obesity, a major public health concern, where BMI may be misleading. Longitudinal data in an elderly population >60 years have shown that total body weight remains stable but masks an increase in total body fat and a decrease in fat-free mass.10 Those individuals with the lowest FFM are more likely to report functional limitation and to have greater susceptibility for falls.19 Falls, functional limitations, immobility and fractures are closely related to morbidity and mortality in elderly populations. The FFMI may prove to be helpful for monitoring the development and progression of sarcopenia, leading to efforts to prevent disability and for the evaluation of rehabilitation programs following a fall or fracture.
Limitations
One limitation is the use of cross sectional data when inferences are being made as to what is occurring longitudinally. Therefore, the FFMI change reported over the lifespan should be interpreted cautiously as factors other than aging may be responsible for the predicted observations. The use of cross sectional data may have limited the ability to detect this occurrence. Future research should investigate whether male subjects who remain closer to their peak FFMI tend to live longer and whether the later decline in the FFMI found in female subjects, may help explain the gender difference in longevity. This data set is unable to determine how the rate of decline in FFMI levels or the time point in the lifespan when declines commence predicts or relates to health outcomes or mortality and whether using this index could provide greater insight into these relationships. These are important public health questions that need to be addressed. Further research is needed to clarify long-term changes and effects.
Conclusions
These data suggest race/ethnic-specific data should be used in situations where the FFMI is used for evaluation. The decline in the FFMI was found to be gender dependent with greater rates of predicted loss in male subjects and beginning much earlier than in female subjects. DXA is a reliable technique for estimation of FM and FFM and is available in most clinical institutions making calculation of the FFMI feasible. The charts and tables included in this paper can be used as a basic scale for evaluation of nutritional studies for research and for clinical purposes.
Acknowledgements
This study was supported by NIH T32-DK007559-19, DK42618, P30-DK-26687, RR24156.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Author Contributions
Hull, Wang and Gallagher were responsible for the conception of this paper. Wang, Pierson, Pi-Sunyer, Heymsfield, Albu, Fernandez and Gallagher participated in data collection. Thornton and Hull were responsible for the analysis and interpretation of data. Hull, Van Itallie, Wang, Thornton, Pierson, Kaleem, Pi-Sunyer, Heymsfield, Albu, Fernandez and Gallagher were responsible for the critical review of the article for intellectual content. Thornton was in charge of the statistical expertise. Gallagher supervised the study.
References
- 1.Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 2.VanItallie TB, Yang MU, Boileau RA, Heymsfield S. Applications of body composition technology in clinical medicine: some issues and problems. In: Kral JG, VanItallie TB, editors. Recent Developments in Body Composition Analysis: Methods and Applications. Great Britain: Smith-Gordon; 1993. pp. 87–97. [Google Scholar]
- 3.Heymsfield SB, Lohman TG, Wang Z, Going SB. Human Body Composition. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- 4.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 6.Heitmann BL. Body fat in the adult Danish population aged 35–65 years: an epidemiological study. Int J obesity. 1991;15:535–545. [PubMed] [Google Scholar]
- 7.Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15–98 years. Nutrition (Burbank, Los Angeles County, Calif) 2001;17:534–541. doi: 10.1016/s0899-9007(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 8.Barlett HL, Puhl SM, Hodgson JL, Buskirk ER. Fat-free mass in relation to stature: ratios of fat-free mass to height in children, adults, and elderly subjects. Am J Clin Nutr. 1991;53:1112–1116. doi: 10.1093/ajcn/53.5.1112. [DOI] [PubMed] [Google Scholar]
- 9.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, et al. Weight stability masks sarcopenia in elderly men and women. Am J physiol. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 11.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obesity Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 12.Vozarova B, Wang J, Weyer C, Tataranni PA. Comparison of two software versions for assessment of body-composition analysis by DXA. Obesity Res. 2001;9:229–232. doi: 10.1038/oby.2001.26. [DOI] [PubMed] [Google Scholar]
- 13.He Q, Heo M, Heshka S, Wang J, Pierson RN, Jr, Albu J, et al. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr. 2003;78:72–77. doi: 10.1093/ajcn/78.1.72. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Zhu S, Wang J, Pierson RN, Jr, Heymsfield SB. Whole-body skeletal muscle mass: development and validation of total-body potassium prediction models. Am J Clin Nutr. 2003;77:76–82. doi: 10.1093/ajcn/77.1.76. [DOI] [PubMed] [Google Scholar]
- 15.Berne RM, Levy MN. Physiology. 4th edn. Mosby: St. Louis; 1998. [Google Scholar]
- 16.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metabol. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 17.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79:613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 18.Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight patients at hospital admission: a controlled population study. Clin Nutr (Edinburgh, Scotland) 2005;24:133–142. doi: 10.1016/j.clnu.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–1645. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]