Abstract
This NIH-funded multicenter randomized study of focal segmental glomerulosclerosis (FSGS) treatment compared the efficacy of a 12-month course of cyclosporine to a combination of oral pulse dexamethasone and mycophenolate mofetil in children and adults with steroid-resistant primary FSGS. Of the 192 patients enrolled, 138 were randomized to cyclosporine (72) or to mycophenolate/dexamethasone (66). The primary analysis compared the levels of an ordinal variable measuring remission during the first year. The odds ratio (0.59) for achieving at least a partial remission with mycophenolate/dexamethasone compared to cyclosporine was not significant. Partial or complete remission was achieved in 22 mycophenolate/dexamethasone- and 33 cyclosporine-treated patients at 12 months. The main secondary outcome, preservation of remission for 26 weeks following cessation of treatment, was not significantly different between these two therapies. During the entire 78 weeks of study, 8 patients treated with cyclosporine and 7 with mycophenolate/dexamethasone died or developed kidney failure. Thus, our study did not find a difference in rates of proteinuria remission following 12 months of cyclosporine compared to mycophenolate/dexamethasone in patients with steroid-resistant FSGS. However, the small sample size might have prevented detection of a moderate treatment effect.
Keywords: focal segmental glomerulosclerosis, proteinuria, randomized controlled trial
Focal segmental glomerulosclerosis (FSGS) is one of the leading causes of end-stage kidney disease (ESKD). The 5-year kidney survival rates approach 100% occur following complete proteinuria remission, 90% after partial remission and 60% with treatment resistance.1–5
The initial treatment of primary FSGS usually involves corticosteroids3,6 and results in proteinuria remission in ~ 25% of patients.4,7,8 In observational and uncontrolled trials, prolonged prednisone therapy (mean 9 months) in adults resulted in complete and partial remission rates of 33% and 29%, respectively.9 Smith et al.10 evaluated pulse oral dexamethasone (DEX) over 32 weeks in 15 adults with primary FSGS and nephrotic range proteinuria. One complete remission and six partial remissions (urine protein/creatinine (Up/c) <2 grams per grams (g/g)) were observed, yielding a combined response of 47%, which fell to 20% with longer follow-up. Uncontrolled data from a single center suggest improved control of FSGS with long-term high-dose pulse corticosteroid therapy in conjunction with cytotoxic agents compared with historic controls.11 Together, these studies suggest that long-term corticosteroid therapy may improve the partial and complete remission rate in patients with FSGS and resistance to a standard short course of corticosteroids.
The only medications that have been evaluated in randomized clinical trials (RCTs) and have shown to increase the rate of partial and complete remission are cyclosporine (CSA) coupled with low-dose prednisone.12,13 The high relapse rate following discontinuation of CSA12 and its side-effect profile, including nephrotoxicity, have stimulated a search for alternative therapy. Mycophenolate mofetil (MMF), which reduces proteinuria in steroid-resistant FSGS with less toxicity than CSA,14,15 has not been tested in a large RCT. Thus, there is an urgent need to evaluate available and new therapies for FSGS in a systematic manner. The National Institutes of Health (NIH)-funded FSGS Clinical Trial (FSGS CT) is a multicenter RCT that compared the efficacy of a 12-month course of CSA to a combination of oral pulse DEX and MMF in children and adults with steroid-resistant primary FSGS. This article presents the primary and main secondary outcomes of this trial. A previous publication has provided details about the study design and cohort.16
RESULTS
Patient disposition
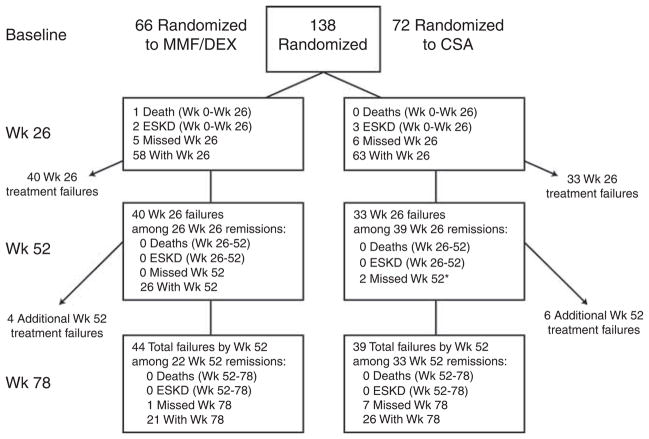
A total of 192 patients were enrolled and 138 were randomized. The most common reasons for exclusion of the 54 enrolled but ineligible patients were lack of FSGS lesion on kidney biopsy (N = 18), Up/c <1 (N = 20), and estimated glomerular filtration rate (eGFR) <40 ml/min per 1.73 m2 (N = 5). Patient randomization, allocation and follow-up are summarized in Figure 1. Over the study period, 6 (4%) died or reached ESKD by week 26, 12 (9%) died or reached ESKD by week 52, and cumulative 15 (11%) died or reached ESKD before week 78. Missing visits from patients who had not reached a primary outcome of treatment failure, ESKD, or death occurred in 11 patients (8%) at week 26, 2 (1%) at week 52, and 8 (6%) at week 78 visits. The two patients who missed all three outcome assessment visits did not receive study drug following randomization (one MMF/DEX and one CSA). One of these patients developed diabetes between the screening and randomization visit, which was detected immediately after randomization. The other was lost to follow-up immediately after randomization, but was observed to be in remission 13 months after the initial evaluation. Both patients were assigned a level 6 (failure) primary outcome and were included in the intent-to-treat primary outcome analysis.
Figure 1. Summary of randomized subject disposition.
Treatment failure was declared if a subject either met a final outcome status of no remission (failure to achieve at least a partial remission by week (Wk) 26 (category 5 and 6) or failure to achieve a partial or complete remission by week 52) or reached a protocol-defined study stop point. *2 missing week-52 visits were assigned treatment failure outcome and are included in the 6 week-52 treatment failures. CSA, cyclosporine; DEX, dexamethasone; ESKD, end-stage kidney disease; MMF, mycophenolate mofetil.
Baseline characteristics
Baseline characteristics were balanced between the 72 patients randomized to CSA and 66 randomized to MMF/DEX (Table 1). The randomized cohort included 93 (67%) children aged <18 years, 53 (38%) blacks, 73 (53%) males, 48 (35%) with baseline eGFR ≤90 ml/min per 1.73 m2, 33 (24%) and 36 (26%) with baseline Up/c <2 g/g and 2–4 g/g, respectively, and 77 (56%) with a serum albumin ≤3.0 mg/dl.
Table 1.
FSGS CT baseline characteristics comparing the randomized treatment arms MMF/DEX and CSA
| Variable | MMF/DEX (n=66) N (%) or median (IQR: Q1, Q3) | CSA (n=72) N (%) or median (IQR: Q1, Q3) |
|---|---|---|
| Age (years) | ||
| 2–12 | 20 (30.3%) | 23 (31.9%) |
| 13–17 | 25 (37.9%) | 25 (34.7%) |
| 18+ | 21 (31.8%) | 24 (33.3%) |
| Race | ||
| Black | 26 (39.4%) | 27 (37.5%) |
| White | 38 (57.6%) | 40 (55.6%) |
| Other | 2 (3.0%) | 5 (6.9%) |
| Hispanic | 12 (18.2%) | 14 (19.4%) |
| Male | 33 (50.0%) | 40 (55.6%) |
| Study baseline eGFR (ml/min per 1.73 m2)a | 110.1 (80.6, 169.6) | 112.8 (75.6, 194.2) |
| Up/c (g/g) | ||
| 1–1.99 | 13 (19.7%) | 20 (27.8%) |
| 2–3.99 | 22 (33.3%) | 14 (19.4%) |
| 4–7.99 | 12 (18.2%) | 19 (26.4%) |
| 8+ | 19 (28.8%) | 19 (26.4%) |
| Albumin (g/dl) | 2.7 (2.1, 3.7) | 3.0 (2.3, 3.7) |
| Cholesterol, total (mg/dl)b | 312 (260, 455) | 283 (241, 390) |
| Hemoglobin | 13.8 (12.9, 15.0) | 14.0 (13.0, 15.2) |
| FSGS pathology subtype | ||
| NOS | 43 (65.2%) | 51 (70.8%) |
| Perihilar | 4 (6.1%) | 6 (8.3%) |
| Cellular | 2 (3.0%) | 2 (2.8%) |
| Tip | 10 (15.2%) | 4 (5.6%) |
| Collapsing | 7 (10.6%) | 9 (12.5%) |
| Duration of FSGS (months) | 6.5 (3.2, 16.2) | 7.1 (4.1, 16.3) |
| Previous steroid exposure (months)c | 3.0 (2.0, 6.0) | 3.0 (2.0, 4.0) |
| Hypertension | 39 (59.1%) | 41 (56.9%) |
| Family history of kidney diseased | 6 (9.8%) | 8 (11.6%) |
| BMI at screeninge | 24.2 (20.4, 27.6) | 23.0 (19.3, 29.9) |
Abbreviations: BMI, body mass index; CSA, cyclosporine; DEX, dexamethasone; eGFR, estimated glomerular filtration rate; FSGS CT, focal segmental glomerulo-sclerosis Clinical Trial; IQR, interquartile range; MMF, mycophenolate mofetil; NOS, not otherwise specified; Up/c (g/g), urine protein/creatinine grams per grams.
The study baseline is calculated using the average of serum creatinine measured at screening and at the time of randomization.
In all, 65 CSA patients and 62 MMF/DEX patients had total cholesterol measurement available at baseline.
A total of 68 CSA patients and 59 MMF/DEX patients had data available on months of previous steroid exposure at baseline.
A total of 69 CSA patients and 61 MMF/DEX patients had data available on family history of kidney disease at baseline.
BMI is the measured BMI at screening. The obesity exclusion criterion for study participation had to be met by BMI before first steroid exposure for nephrotic syndrome.
Drug dosing
The mean time-averaged prescribed dose plus or minus s.d. for the CSA arm was 4.6±1.7 (range 1.6–9.0) mg/kg/day and for MMF was 26.2±6.1 (range 13.4–41.2) mg/kg/day.
Patients were treated with either angiotensin-converting enzyme inhibitor lisinopril (85.5% (118/138)) or angiotensin receptor blocker losartan (7.9% (11/138)), 4.3% (6/138) were switched from lisinopril to losartan during the study, 1.4% (2/138) did not receive any study drug, and 0.7% (1/138) did not receive angiotensin-converting enzyme or angiotensin receptor blocker because of allergies but was treated with the main study interventions. Additional antihypertensive therapies were not restricted by study protocol. The time-averaged dose of lisinopril was 0.36±0.12 (range 0.04–0.56) mg/kg/day and of losartan was 1.10±0.50 mg/kg/day (range: 0.55–2.69).
Blood pressure
Systolic blood pressure (median (inter-quartile range)) for adults in the MMF arm at baseline was 120 mm Hg (16), at week 26 was 120 mm Hg (16), at week 52 was 121mm Hg (17), and at week 78 was 121mm Hg (22). Median systolic blood pressure in the CSA arm for adults at baseline was 129 mm Hg (18), at week 26 was 124 mm Hg (18), at week 52 was 125 mm Hg (22), and at week 78 was 118 mm Hg (21). In children, the median systolic blood pressure in the MMF arm at baseline was 117 mm Hg (22), at week 26 was 113 mm Hg (22), at week 52 was 110 mm Hg (52), and at week 78 was 110 mm Hg (23). For children in the CSA arm, the median (interquartile range) systolic blood pressure at baseline was 115 mm Hg (19), at week 26 was 109 mm Hg (22), at week 52 was 111mm Hg (14), and at week 78 was 114 mm Hg (19). The differences in blood pressure between the CSA and MMF/DEX groups were not statistically significant at any time point.
Primary and main secondary analyses
The distributions of the primary and main secondary outcomes are displayed in Tables 2 and 3. As shown, 33 (46%) CSA patients and 22 (33%) MMF/DEX patients had a primary outcome score of 1, 2, or 3, indicating achievement of at least a partial remission at 1 year. Only 14 (19%) CSA and 6 (9%) MMF/DEX patients had scores of 1 or 2, indicating a complete remission. The primary analysis comparing the mean level of the primary outcome between the CSA and MMF/DEX groups was not statistically significant (P = 0.11). The odds of at least a partial remission at week 52 were lower for MMF/DEX than for CSA (odds ratio (OR) = 0.59, 95% confidence interval (CI) 0.30–1.18) but did not reach statistical significance. No randomized patients were treated with the protocol-defined relapse therapy during the week 0–52 study treatment period. During the 78 week study period, 8 (14%) CSA and 7 (11%) MMF/DEX subjects reached kidney failure or death (P = 0.56).
Table 2.
FSGS CT primary analysis comparing proteinuria remission by week 52 between the randomized treatment arms
| Primary outcome level | Frequencies by primary outcome level
|
Cumulative frequencies at indicated level or better
|
Odds ratio* | 95% CI | ||
|---|---|---|---|---|---|---|
| MMF/DEX | CSA | MMF/DEX | CSA | |||
| 1 | 2 (3.0%) | 4 (5.6%) | 2 (3.0%) | 4 (5.6%) | 0.53 | (0.09–3.03) |
| 2 | 4 (6.1%) | 10 (13.9%) | 6 (9.1%) | 14 (19.4%) | 0.41 | (0.15–1.15) |
| 3 | 16 (24.2%) | 19 (26.4%) | 22 (33.3%) | 33 (45.8%) | 0.59 | (0.30–1.18) |
| 4 | 4 (6.1%) | 6 (8.3%) | 26 (39.4%) | 39 (54.2%) | 0.55 | (0.28–1.08) |
| 5 | 15 (22.7%) | 6 (8.3%) | 41 (62.1%) | 45 (62.5%) | 0.98 | (0.49–1.96) |
| 6 | 25 (37.9%) | 27 (37.5%) | 66 (100%) | 72 (100%) | — | — |
Abbreviations: CI, confidence interval; CSA, cyclosporine; DEX, dexamethasone; FSGS CT, focal segmental glomerulosclerosis Clinical Trial; MMF, mycophenolate mofetil.
P-value for primary analysis: 0.109. Odds ratios compare odds of achieving a primary outcome score equal to or better than the indicated level for MMF/DEX compared with CSA. Odds ratios <1 indicate greater odds of better scores for CSA. The odds ratio of 0.59 for a primary outcome level of ≥3 corresponds to a ratio in the probabilities of a level of ≥ 3 of 0.73, 95% CI (0.48–1.11).
Table 3.
FSGS CT main secondary analysis comparing sustainable proteinuria remission from study weeks 52–78 between the randomized treatment arms
| Main secondary outcome level | Frequencies by main secondary outcome level
|
Cumulative frequencies at indicated level or better
|
Odds ratio* | 95% CI | ||
|---|---|---|---|---|---|---|
| MMF/DEX | CSA | MMF/DEX | CSA | |||
| 1 | 3 (4.6%) | 5 (6.9%) | 3 (4.5%) | 5 (6.9%) | 0.64 | (0.15–2.78) |
| 2 | 1 (1.5%) | 1 (1.4%) | 4 (6.1%) | 6 (8.3%) | 0.71 | (0.19–2.63) |
| 3 | 13 (19.7%) | 10 (13.9%) | 17 (25.8%) | 16 (22.2%) | 1.21 | (0.56–2.66) |
| 3.5 | 1 (1.5%) | 6 (8.3%) | 18 (27.3%) | 22 (30.6%) | 0.85 | (0.41–1.78) |
| 4 | 4 (6.1%) | 11 (15.3%) | 22 (33.3%) | 33 (45.8%) | 0.59 | (0.30–1.18) |
| 5 | 44 (66.7%) | 39 (54.2%) | 66 (100%) | 72 (100%) | — | |
Abbreviations: CI, confidence interval; CSA, cyclosporine; DEX, dexamethasone; FSGS CT, focal segmental glomerulosclerosis Clinical Trial; MMF, mycophenolate mofetil.
P-value for the main secondary analysis was 0.358. Odds ratios compare odds of achieving a main secondary outcome score equal to or better than the indicated level for MMF/DEX compared with CSA. Odds ratios <1 indicate greater odds of better scores for CSA.
Main secondary outcome: sustainable remission
Of the 33 CSA patients who achieved at least a partial remission at week 52, 11 (33%) relapsed after withdrawal of immunosuppressive therapy, 16 (48%) retained at least a partial remission, and 6 (18%) had an unknown week 78 remission status. Of the 22 MMF/DEX patients with a partial or complete remission at week 52, 4 (18%) relapsed, 17 (77%) retained at least a partial remission, and 1 (5%) had an unknown status at week 78 (P = 0.38). The OR comparing MMF/DEX to CSA for maintaining at least a partial remission at week 78 was 1.21 (95% CI 0.56–2.66). This analysis excludes the seven patients assigned a main secondary outcome level of 3.5 without Up/c measurements after week 52; if these patients are included and assumed to have sustained remissions, the OR becomes 0.85 (95% CI 0.41–1.78).
Subgroups
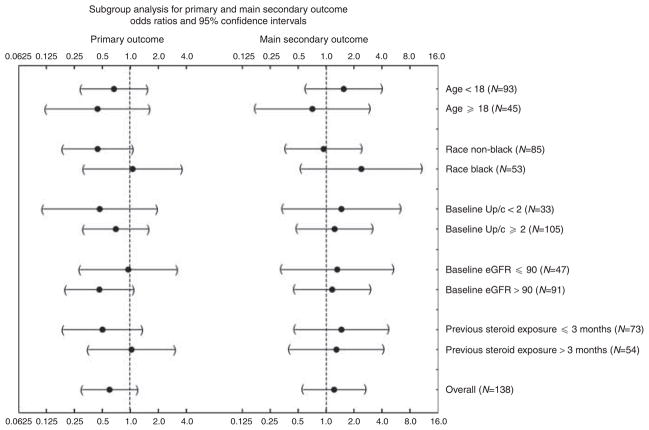
There were no significant differences in the treatment effects between subgroups defined by patient age, race (black vs non-black), baseline Up/c, baseline eGFR, or months of previous steroid exposure (Figure 2). A trend for a beneficial effect of CSA compared with MMF/DEX among patients with baseline Up/c ≤4 g/g was present for the primary outcome, but a similar trend was not observed for the main secondary outcome. There was also no significant difference in the treatment effect on the primary or main secondary outcomes between those with a baseline Up/c <2 vs ≥2 g/g (data not shown). There were 14 patients with a family history of kidney disease: 8 in the CSA arm and 6 in the MMF arm. Four of the CSA patients achieved at least partial remission at week 52. Of them, two maintained the partial remission at week 78, and one had a 3.5 as a secondary outcome. All the six MMF patients with a positive family history were treatment failures.
Figure 2. Subgroup analyses for the primary outcome, proteinuria remission by 52 weeks, and main secondary outcome, sustained remission between 52 and 78 weeks.
Shown are odds ratios and 95% confidence limits comparing the odds of a primary outcome score of ≥3 (left) and of a main secondary outcome score of ≥3 (right) between the MMF/DEX and CSA interventions for prespecified subgroups. CSA, cyclosporine; DEX, dexamethasone; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; Up/c, urine protein/creatinine.
Other analyses of Up/c and eGFR
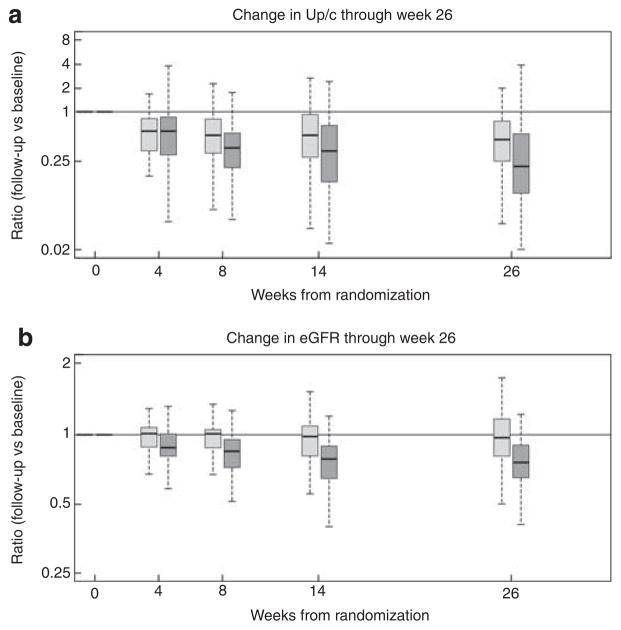
As shown in Figure 3, the median ratio (25th–75th percentiles) of week 26 to baseline Up/c was smaller in the CSA than the MMF/DEX group: CSA 0.24 (0.10–0.54) vs MMF/DEX 0.46 (0.25–0.81) (P = 0.039). The smaller median ratio indicates a larger decline of Up/c in the CSA group than the MMF/DEX group. However, the median ratio of week 26 eGFR to baseline eGFR was also smaller in the CSA than the MMF/DEX group, CSA 0.73 (0.63–0.89) vs MMF/DEX 0.95 (0.79–1.14) (P = 0.001), indicating a better preservation of eGFR in the MMF/DEX-treated patients.
Figure 3. Change in proteinuria and eGFR over time.
(a) Ratio of week 26:week 0 Up/c. Dark gray represents the CSA arm and light gray represents the MMF/DEX arm. Shown are box plots (indicating 5th, 25th, 50th, 75th, and 95th percentiles) of the ratio of follow-up to baseline Up/c values. (b) Ratio of week 26:week 0 eGFR. Dark gray represents the CSA arm and light gray represents the MMF/DEX arm. Shown are box plots (indicating 5th, 25th, 50th, 75th, and 95th percentiles) of the ratio of follow-up to baseline eGFR values. CSA, cyclosporine; DEX, dexamethasone; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; Up/c, urine protein/creatinine.
Adverse events
The summary of adverse events reported during the trial is presented in Table 4. We report week 26 cumulative events, as this is the time point where all participants have equal opportunity to contribute an event. The number of adverse events was similar in the two arms, and the observed differences mainly reflect the known side-effect profile of the test treatments.
Table 4.
Summary of adverse events comparing the randomized treatment arms
| Event | Weeks 0–26
|
Weeks 0–52
|
||||||
|---|---|---|---|---|---|---|---|---|
| MMF/DEX (N=66)
|
CSA (N=72)
|
MMF/DEX (N=66)
|
CSA (N=72)
|
|||||
| N pts with events (N of events reported) | % of pts | N pts with events (N of events reported) | % of pts | N pts with events (N of events reported) | % of pts | N pts with events (N of events reported) | % of pts | |
| Serious infection requiring | 7 (12) | 10.6 | 5 (5) | 6.9 | 9 (14) | 13.6 | 7 (12) | 9.7 |
| hospitalizationa | ||||||||
| Serious CVb | 0 | 0.0 | 0 | 0.0 | 1 | 1.5 | 0 | 0.0 |
| Hospitalizationa | 14 (34) | 21.2 | 12 (25) | 16.7 | 18 (43) | 27.3 | 17 (36) | 23.6 |
| Deathb | 1 | 1.5 | 0 | 0.0 | 2 | 3.0 | 0 | 0.0 |
| Gastrointestinalb | 47 | 71.2 | 47 | 65.3 | 49 | 74.2 | 50 | 69.4 |
| Coughb | 49 | 74.2 | 43 | 59.7 | 53** | 80.3 | 45** | 62.5 |
| Dermatologic conditionb | 29 | 43.9 | 43 | 59.7 | 33 | 50.0 | 44 | 61.1 |
| Hypotension/orthostasis/ | 31 | 47.0 | 27 | 37.5 | 35 | 53.0 | 29 | 40.3 |
| dizzinessb | ||||||||
| Infectiona | 27 (65) | 40.9 | 23 (43) | 31.9 | 30 (81) | 45.5 | 29 (70) | 40.3 |
| Painb | 24 | 36.4 | 22 | 30.6 | 27 | 40.9 | 29 | 40.3 |
| Neuropsych conditionb | 16 | 24.2 | 22 | 30.6 | 16 | 24.2 | 23 | 31.9 |
| Gingival hyperplasiab | 0** | 0.0 | 11** | 15.3 | 0** | 0.0 | 17** | 23.6 |
| Anemiab | 10 | 15.2 | 11 | 15.3 | 11 | 16.7 | 16 | 22.2 |
| Hypertensionb | 6 | 9.1 | 11 | 15.3 | 7 | 10.6 | 12 | 16.7 |
| Hyperlipidemiab | 6 | 9.1 | 10 | 13.9 | 6 | 9.1 | 13 | 18.1 |
| Hyperkalemiab | 2 | 3.0 | 6 | 8.3 | 2 | 3.0 | 9 | 12.5 |
| Non-serious CVb | 6 | 9.1 | 4 | 5.6 | 7 | 10.6 | 4 | 5.6 |
| Hyperglycemiab | 6** | 9.1 | 0** | 0.0 | 6 | 9.1 | 2 | 2.8 |
| Decreased muscle strengthb | 5 | 7.6 | 3 | 4.2 | 5 | 7.6 | 3 | 4.2 |
| Cataractb | 3 | 4.6 | 0 | 0.0 | 4 | 6.1 | 1 | 1.4 |
| Angioedemab | 2 | 3.0 | 0 | 0.0 | 2 | 3.0 | 0 | 0.0 |
| Thromboembolismc | 1 | 1.5 | 1 | 1.4 | 1 | 1.5 | 1 | 1.4 |
| Adrenal insufficiencyb | 1 | 1.5 | 0 | 0.0 | 1 | 1.5 | 0 | 0.0 |
| Alopeciab | 1 | 1.5 | 0 | 0.0 | 1 | 1.5 | 0 | 0.0 |
| Pregnancya | 1 | 1.5 | 0 | 0.0 | 1 | 1.5 | 0 | 0.0 |
| Abdominal crampsb | 1 | 1.5 | 0 | 0.0 | 1 | 1.5 | 0 | 0.0 |
| Decreased ANCa | 0 (0) | 0.0 | 0 (0) | 0.0 | 1 (1) | 1.5 | 0 | 0.0 |
| Generalized tonic–clonic | 0 (0) | 0.0 | 0 (0) | 0.0 | 0 (0) | 0.0 | 1 (4) | 1.4 |
| seizurea | ||||||||
Abbreviations: ANC, absolute neutrophil count; CSA, cyclosporine; CV, cardiovascular; DEX, dexamethasone; MMF, mycophenolate mofetil; pts, patients.
Multiple reports were recorded for these adverse events during the indicated time period of the study.
Only the first occurrence of these adverse events was recorded during the indicated time period of the study.
In one case the thrombus was intracardiac.
P<0.02, Fisher’s exact test.
Seven patients reached the 50% GFR decline and/or dialysis stop point during the 78 week intervention phase. Six patients, who reached the 50% decline in GFR point, including 5 from the CSA arm at study weeks 0, 20, 38, 38, and 52, subsequently progressed to end-stage renal disease (ESRD). One meeting this criterion was in the MMF arm and did not proceed to ESRD. One patient in the MMF arm progressed to dialysis at week 32 after discontinuation of prednisone (week 8), lisinopril (week 14), and MMF and DEX (week 20; see below for details). One patient experienced a pregnancy that was terminated.
Individual medications were discontinued within the study intervention phase in 20 patients. MMF was discontinued in one patient, mentioned above, who had gastrointestinal toxicity (week 20) and who subsequently progressed to ESRD (week 32). CSA was discontinued in four patients, including one with gout (week 14), one with hirsutism (week 14), one with severe dyslipidemia (week 14), and one patient choice (week 8). DEX was discontinued in five patients. Three of these patients discontinued at predefined stop points, according to protocol, including one with MMF for gastrointestinal toxicity (week 20, see above) and two because of hypergly-cemia (weeks 2 and 2). Two others discontinued because of fever (weeks 6 and 20), which subsequently resolved but the drug was not restarted by physician/patient choice. Of the two who discontinued for hyperglycemia, one required insulin therapy and one progressed to ESRD at month 24.
Prednisone was discontinued in six patients, two at the predefined stop point of hyperglycemia (weeks 2 and 8), both of whom were on the MMF/DEX arm. Of these two, one required insulin therapy and one progressed to ESRD (week 32; see above). Additionally, two patients discontinued prednisone because of cataracts (weeks 14 and 14), one with severe dyslipidemia (week 14; see above), and one for anxiety (week 20). Lisinopril/losartan therapy was discontinued in 10 patients, 7 of whom stopped at predefined stop points, including 1 with angioedema (week 4), 1 with hypotension (week 26), and 5 with declining GFR (weeks 14, 14, 20, 26, and 65). In the latter group, one progressed to ESRD at week 32 and is counted above and two progressed to ESRD in month 24. Three patients discontinued lisinopril/losartan therapy for other indications, including two patients because of patient choice (weeks 8 and 65) and one patient with transient oliguria (week 0).
Patients who reached a final outcome variable of treatment failure at week 26 (level 6) were monitored every 6 months according to the protocol. At week 52, 8 of 37 (22%) week 26 MMF treatment failures and 8 of 30 (27%) week 26 CSA treatment failures did not have week 52 urine protein results documented. Of the week 26 treatment failures completing the week 52 visit, 1 (3%) MMF failure had complete remission at week 52, 3 (8%) of MMF and 2 (7%) of CSA failures had partial remission at week 52 (Up/c between 0.2 and 2 g/g and reduction to <50% of baseline value), and 2 (5%) of MMF and 4 (13%) of CSA arm reached ESRD by week 52. One patient in the MMF arm died between week 26 treatment failure and week 52. Between weeks 26 and 52, locally prescribed therapy for week 26 treatment failures included: 2 calcineurin inhibitors, 5 MMF, 0 rituximab, 0 adalimumab, and 23 renin-angiotensin-aldosterone antagonists. At week 52, the majority were off immunomodulating therapies with 2 calcineurin inhibitors, 1 MMF, 0 rituximab, 0 adalimumab, and 6 remained on renin-angiotensin-aldo-sterone antagonists.
DISCUSSION
This report describes the outcome of the largest RCT ever performed in a cohort of pediatric and adult patients with steroid-resistant primary FSGS. This sample represents a well-characterized group of mainly incident patients following initial corticosteroid therapy. Therefore, our findings should be valid and generalizable to the population of patients with newly recognized steroid-resistant primary FSGS. The primary comparison between the CSA and MMF/DEX groups failed to demonstrate a treatment difference in remission rates between these interventions at 52 weeks. The 95% CI interval of 0.30–1.18 for the OR for achieving at least a partial remission with MMF/DEX compared with CSA extended over a wide range, and the lower limit of 0.30 leaves open the possibility of a substantial benefit of CSA compared with MMF/DEX that was undetected because of the trial’s limited sample size. However, the proportions of patients with complete or partial remission at week 52 were low in both treatment arms—46% CSA and 33% MMF/DEX—indicating that neither intervention consistently induced remissions. Moreover, only a quarter of the patients in the two groups had a sustained response 6 months following discontinuation of immunosuppressive medications.
Although there were no significant treatment effects on the primary or main secondary outcomes, which were defined based on the occurrence and persistence of large declines in proteinuria to designated thresholds, CSA was significantly more effective than MMF/DEX in reducing proteinuria, expressed as a continuous variable, over the first 26 weeks of therapy. However, the implications of this result must be tempered by the deleterious impact of CSA on eGFR and the proteinuria relapse rate subsequent to the discontinuation of the either the CSA or MMF/DEX therapy. This combined reduction in proteinuria and eGFR in response to CSA tended to resolve after the study medications were stopped. However, the findings raise questions about the prognostic implications of short-term changes in proteinuria and eGFR on clinically relevant outcomes like progression to ESKD or death. A long-term observational study of the FSGS cohort is planned to extend the follow-up period and answer this important question.
Previous studies have evaluated calcineurin inhibitors in FSGS and suggest benefit. In the largest published clinical trial (n = 49), a 6-month course of CSA yielded a complete or partial remission in 69% compared with placebo therapy (4%) in adults with steroid-resistant FSGS. The relapse rate was 8 of 18 (44%) within 6 months after discontinuation of CSA treatment. A 50% decline in kidney function was reported in ~5% of CSA and 12% of the placebo arm at study week 26 and kidney function continued to worsen in the patients with more extended observation. The 4-year renal survival rate was not statistically different (72% CSA vs 49% placebo, P = 0.1) but this interpretation may be subject to type II error because of the small sample size.12
Our rate of remission in the MMF/DEX arm (33%) was less than that reported by Tune and Mendoza (66%)17 and Smith et al. (47%).10 This may reflect a national tendency to treat patients with a longer course of corticosteroids before defining steroid resistance and is reflected by the median 3-month cumulative exposure reported in patients at FSGS CT study entry. MMF has been evaluated only in small patient series that provide limited information for comparison.14,15
Our study has several implications for the treatment of primary FSGS. Because high-dose DEX pulse treatment was incorporated into the experimental arm, it is unlikely that increasing the strength or duration of steroid therapy will be effective in achieving a consistently high rate of complete or partial remission. Although this study did not demonstrate a beneficial effect, appropriate use of CSA may merit continued evaluation, especially in light of recent evidence that this group of drugs may be acting on podocytes in a nonimmune manner.18 The variable responses in proteinuria that ranged from a sustained complete remission to treatment resistance, in relationship to changes in eGFR and progression to CKD, highlight the need to develop biomarkers of early FSGS that can be used to define prognosis, predict response to treatment, and provide a basis for selection of therapeutic regimens on an individual basis.
It is important to acknowledge the strengths of this trial. First, the diagnosis was confirmed by central review of biopsy. Nearly 10% of the patients who were considered to have FSGS based on local interpretation of their biopsies were excluded when the specimens were reviewed by the central pathologist. In addition, the study cohort was diverse with respect to age, race, and ethnicity that may allow application of results to similarly constructed populations.
The trial also had several weaknesses. First, based on information that was available at the time of study development, subjects were not classified according to the presence of genetic mutations. Genetic testing has been conducted as ancillary studies and will be reported separately. The study incorporated no assessment of adherence beyond patient reporting and CSA levels. Enrollment into the trial was significantly lower than projected, so that moderate but clinically important treatment effects may have gone undetected. The sample size shortfall may have resulted from an overestimation of the number of cases anticipated at each site, the stringent definition of primary FSGS, and conflict between the requirements of the protocol and site investigators’ assessment of the risks and benefits of the test agents. Because of the enrollment challenges, the study Up/c eligibility criterion was decreased from >2 g/g to >1 g/g early in the study, the enrollment window was increased, and extensive efforts were made to improve patient access and participation. The study was closed with agreement from the NIH study sponsor and Data Safety Monitoring Board following review of the revised power analysis.
In conclusion, this trial did not demonstrate a difference in proteinuria remission among patients with steroid-resistant primary FSGS who were treated with CSA or MMF/DEX. However, because of the limited sample size, a beneficial treatment would have needed to increase the absolute remission rate by ~20% to provide 80% power; smaller effects may have gone undetected. While either regimen might be selected for future use with a similar success rate, the overall low rate of remission raises the important question of whether immunologically targeted treatment is an optimal approach for this patient population. The results of this trial underscore the need to identify new biomarkers to delineate prognosis, potentially indicate new therapeutic targets, and allow better appraisal of response to treatments. It is hoped that the network of sites and the infrastructure that was created for the FSGS CT can be sustained and applied to future studies of this disorder and other glomerular diseases.
MATERIALS AND METHODS
Trial design
The FSGS CT is a multicenter, prospective, open-label RCT that compared two treatment regimens: CSA and MMF/DEX pulses. The study design is presented in a previous publication.16 CSA was designated as the control arm based on the results of previous clinical trials.12,13 DEX in combination with MMF (MMF/DEX) was considered the experimental therapy.10,14,15
Eligibility criteria
Pediatric and adult patients were eligible if they had primary FSGS confirmed by central pathology review of stored kidney biopsy material, age of proteinuria onset and current age between 2 and 40 years, eGFR ≥40 ml/min per 1.73 m2 assessed at a single study visit, Up/c >1 g/g, and corticosteroid resistance defined as persistent proteinuria following a minimum of 4 weeks of corticosteroid therapy. Details of the inclusion and exclusion criteria are provided in the Appendix and previous publication.16
Physical examination and laboratory assessments
Interval history, occurrence of adverse events, weight, and edema status were assessed at each visit. Edema was classified as the highest score of the following: 1 =extremity, 2 = facial, 3 = ascites, or 4 = anasarca. Blood pressure was measured in the sitting and standing positions. Blood and urine assays were conducted in the Spectra East Core Laboratory. DNA was obtained at baseline, and urine, serum, and plasma were obtained at baseline and weeks 26, 52, and 78 for storage in the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) Biosample and Genetic Repositories.
Urine protein monitoring
Two first morning urine samples were required at screening, baseline, and week 26, 52, and 78 visits. If the two Up/c values differed by > 50%, a third urine sample was collected and the results included in the average baseline Up/c. A single urine specimen was collected at every other study visit.
Estimation of GFR
Participant eGFR was estimated by the Schwartz formula for age < 18 years19 and by the Cockroft–Gault formula adjusted for body surface area for age ≥18 years.20 The measured weight was used in the Cockroft–Gault calculations as it was not possible to accurately estimate dry weight in edematous patients.
Randomization
Randomization schedules using randomly permuted blocks of random sizes were prepared by the Data Coordinating Center. Study investigators were blinded to these schedules. Allocation to the two treatment groups was designed to be equal and stratified by Core Coordinating Center, baseline eGFR (< 90 vs ≥90 ml/min per 1.73 m2), and participant race (black vs non-black).
Monitoring schedule
Eligibility was confirmed based upon the screening visit, and laboratory and pathology data before randomization. Treatment was initiated after the baseline visit (week 0) and subsequent visits occurred at weeks 2, 4, 6, 8, 14, 20, 26, 32, 38, 44, 52, 65, and 78. Long-term monitoring visits were conducted at 6-month intervals until study closeout.
CSA
A dose of 5 to 6 mg/kg per day (maximum initial dose 250 mg/day) was divided into two doses. The CSA dose was adjusted based on drug levels in order to achieve a 12-h trough concentration of 100–250 ng/ml. CSA dose adjustments were made in 30% decrements for prespecified toxicities.16
MMF and DEX
Participants were treated with MMF, 25–36 mg/kg per day (maximum 2 g/day), divided into two daily doses and DEX, 0.9 mg/kg per dose (maximum 40 mg), daily on two consecutive days at the start of weeks 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 30, 34, 38, 42, 46, and 50 for a total of 46 doses. MMF and DEX dose adjustments were made in 30% decrements for prespecified toxicities.10,16
Common therapy in both treatment arms
All participants were treated with prednisone (prednisolone for children taking liquid preparation): 0.3 mg/kg per dose (maximum 15 mg) every other day for the first 6 months of the treatment period. Lisinopril was provided for 18 months to a target dose of 0.4 mg/kg (40 mg maximum) daily. Doses were initiated at 25% of the target dose and increased every 2 weeks until the target dose was reached. Losartan was provided for angiotensin-converting enzyme inhibitor-intolerant subjects (50 mg maximum initial dose and 100 mg maximum full dose). All other treatments, including management of edema and control of blood pressure to achieve standard target readings in pediatric and adult patients, were left to the discretion of the site investigator.
Optional relapse therapy
The protocol allowed for a maximum of 2 weeks of prednisone therapy dose of 2 mg/kg/day (max 60 mg/day) to treat a relapse. This relapse therapy was permitted up to 2 times during study weeks 0–52 and was optional.
Time-averaged dose
Drug exposure for CSA, MMF, and lisinopril was expressed as a time-averaged dose calculated through the 52 week visit. For week 26 treatment failures, the time-averaged dose was calculated through week 26 only.
Stop points
Study stop points included a 50% decline from baseline eGFR to eGFR ≤75 ml/min per 1.73 m2, dialysis, pregnancy, or prespecified severe medication-related toxicity. When a stop point was confirmed, serum chemistries, CSA level, complete blood cell count, lipids, and Up/c were obtained.
Primary and main secondary outcomes
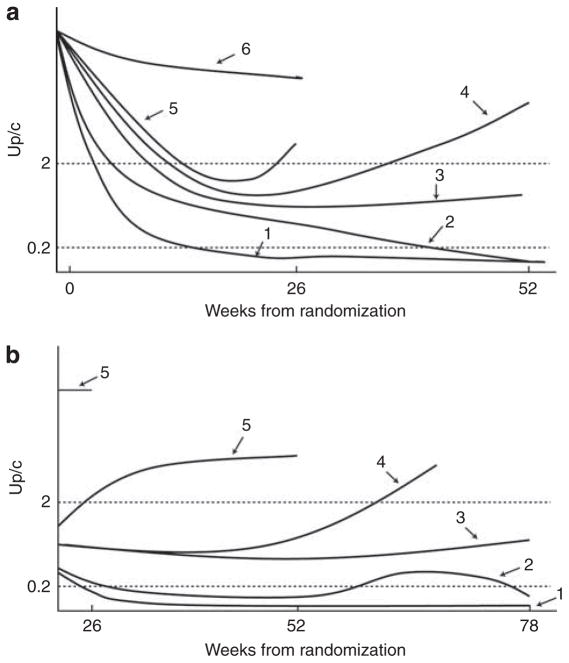
The primary outcome was defined by a six-level categorical assessment of proteinuria remission during the first 52 weeks after randomization. The scores ranged from 1 (most favorable) to 6 (least favorable) (Figure 4a) and were assigned as follows. Level 6 was failure to achieve partial or complete remission between weeks 2 and 26; level 5 was assigned if a partial or complete remission was achieved at least once between weeks 2 and 20, but not at week 26. The primary outcome for patients with scores of 5 or 6 was fully determined by the week 26 visit. If a partial or complete remission was achieved at week 26, patients were assigned to a score of 4 if a partial or complete remission was not achieved at week 52; 3 if a partial remission was achieved at week 52; 2 if a complete remission was achieved at week 52 but Up/c ≥0.2 at least once between weeks 26 and 52; and 1 if Up/c <0.2 for all Up/c measurements between weeks 26 and 52.
Figure 4. FSGS CT outcome based on longitudinal control of proteinuria.
(a) Ordinal classification of FSGS CT proteinuria primary outcome. Category 1: patients who achieved a complete remission by week 26 that was sustained to week 52; category 2: patients who achieved a partial remission at week 26 and then a complete remission at week 52; category 3: patients who achieved a partial remission by week 26 that was sustained to week 52; category 4: patients who achieved a partial remission at week 26 and then had recurrence of proteinuria before week 52; category 5: patients who achieved a partial remission before week 26 and then had a recurrence of proteinuria before week 26; category 6: patients who never had a Up/c reduction of >50% and an absolute value below 2 g/g. (b) Ordinal classification of FSGS CT main secondary outcome: sustainable remission of proteinuria. Participants with a primary outcome level 4 to 6 were assigned level 5. If the primary outcome was level ≤3, the main secondary outcome was assigned to levels 1, 2, 3, or 4 as follows: Level 4 was assigned if the participant failed to maintain at least a partial remission from week 52 through week 78. Participants who maintained at least a partial remission from week 52 through week 78 were assigned to level 3 if they had a partial remission at week 78, to level 2 if they had a complete remission at week 78 but had at least one Up/c between 0.2 and 2.0 between weeks 52 and 78, and to level 1 if they maintained a complete remission from week 52 through week 78. FSGS CT, focal segmental glomerulosclerosis Clinical Trial; Up/c, urine protein/creatinine.
The main secondary outcome was defined by a five-level ordinal categorical variable characterizing maintenance of remission between weeks 52 and 78 following withdrawal of immunosuppressive therapy (Figure 4b). A 5 (the worst outcome) was assigned to patients who failed to achieve a partial or complete remission at week 52. A 4 was assigned if no better than a partial remission was maintained at all visits between weeks 52 and 78, a 3 was assigned if a partial remission was achieved at week 78, a 2 was assigned if a complete remission was achieved at week 78 but Up/c was ≥0.2 at least once between week 52 and Week 78, and a 1 was assigned if the patient maintained a complete remission from weeks 52 to 78.
The multilevel ordinal categorical outcomes were used instead of simpler dichotomous classifications for remission in order to increase statistical power and to allow patients to switch to alternative therapy if a remission was not achieved by week 26. Furthermore, the definitions of the primary and main secondary end points required large clinically significant declines in Up/c in order for positive outcomes to be recorded, thus reducing the susceptibility of these outcomes to minor hemodynamic changes in Up/c.
Additional secondary outcomes were quality of life, adverse events, and preservation of kidney function. Clinical sites were not blinded to the results of the central Up/c measurements for subjects under their care. Study investigators were blinded to results of interim analyses done for the Data and Safety Monitoring Board.
Treatment failure
Treatment failure was declared if a subject either met a final outcome status of no remission (failure to achieve at least a partial remission by week 26 (category 5 and 6) or failed to achieve a partial or complete remission by week 52) or reached a protocol-defined stop point. Following the confirmation of treatment failure status, the study medications were discontinued and therapeutic decisions were deferred to the local nephrologist. These patients continued in the study for observation and monitoring until the close of the study.
Statistical methods
In accordance with the intention-to-treat principle, all randomized patients were assigned nonmissing scores for both the primary and main secondary outcomes. This was accomplished by treating all scheduled Up/c measurements as nonremissions for the definition of the primary and main secondary outcomes if they occurred after death, ESKD, a 50% declining eGFR stop point, or early withdrawal from this study before week 26. Patients who were in remission at week 26 and subsequently lost to follow-up were assigned a primary outcome score between 1 and 4 using a last-value-carried-forward procedure. Patients who achieved a remission at week 52 but failed to provide Up/c measurement after week 52 were assigned a score of 3.5 for the main secondary outcome, which was treated as intermediate between scores of 3 and 4.
For both the primary and main secondary outcomes, the mean outcome score was compared between the CSA and MMF/DEX groups within each of the four randomization strata defined by baseline eGFR and race. The results of these comparisons were pooled across the strata using weights proportional to the strata sample sizes. Standard errors for hypothesis tests were obtained as linear combinations of multinomially distributed proportions of participants in the respective remission categories. An estimate of the effect size for the primary outcome with 95% CI was obtained as the Mantel–Haenszel OR for achievement of at least a partial remission at 1 year (levels 1, 2, or 3 on the primary outcome) between the CSA and MMF/DEX groups with stratification for the four randomization strata. Similarly, the treatment effect on the main secondary outcome was expressed as the Mantel–Haenszel stratified OR for maintenance of at least a partial remission until week 78 (levels 1, 2, or 3 on the main secondary outcome).
In secondary analyses, changes in log-transformed Up/c and eGFR from baseline to each follow-up visit through the week 26 assessment were summarized using box plots and compared between treatment groups using the Wilcoxon rank-sums test, stratified by the four randomization strata. In this analysis, an eGFR value of 10 ml/min was imputed for five patients who reached ESKD.
Adverse events corresponding to defined classes were tabulated first for weeks 0–26 and again for weeks 0–52. Depending on the nature of the adverse event, either the proportions of randomized patients with at least one event or the rate of events (expressed as number of events per patient-year) counting multiple events per patient were compared between the treatment groups. Fisher’s exact test was used to assess the statistical significance of comparisons at the patient level and overdispersed Poisson regression was used for comparisons of event rates where multiple events were counted for each patient. All hypothesis tests were performed using a two-sided α = 0.05, without adjustment for multiple comparisons.
Power
The FSGS CT was originally designed to randomize 500 patients and would have provided 80% power at a two-sided α-level of 5% to detect an absolute increase in remission probability ranging from 10.8% (from 32.5 to 43.3%) to 11.5% (from 60 to 70.5%) under these remission rate scenarios.16 A total of 138 were actually randomized, providing 80% power at the α-level of 5% to detect an absolute increase in the probably of remission ranging from 20.9 to 18.2%. One formal interim analysis was performed using an O’Brien-Fleming stopping boundary at 52% of the final achieved information time.
Acknowledgments
This study was sponsored by the NIH/NIDDK Grants U01- DK063385, DK063490, DK063455, and DK063549. This study was supported by many Clinical and Translational Science Award/NIH-funded institutions for the conduct of study visits, nursing, laboratory, and outpatient research facilities throughout the trial. We express our thanks to all the site coordinators who assisted with patient identification, enrollment, treatment, and follow-up.
APPENDIX
FSGS Clinical Trial group
Steering Committee—Aaron Friedman, Chair; NIDDK—Marva Moxey-Mims, Yining Xie; Data Coordinating Center—Jennifer Gassman, Tom Greene, Milena Radeva, June McMahan, Karen Brittain, Martin Drabik, Minwei Li, Sandra Watkins, Ronald Hogg; Data Safety and Monitoring Board—John Sedor, Chair, James Balow, Marie Diener-West, Agnes Fogo, Norman Fost, Julie Ingelfinger, Andrew Levey, John E Lewy*; Central Biochemistry Laboratory—James Zazra, Dorothy Van Pelt, Kelly Connell; Biospecimen Repository—Fisher BioServices: David Toke, Heather Higgins, Sandra Ke, Phillip Cooley; FSGS Clinical Trial Clinical Sites—Amira Al-Uzri, Doernbecher Children’s Hospital, Oregon; Gerald Appel, Columbia University Presbyterian Medical Center—Internal Medicine; Mazen Arar, University Texas Health Science Center, San Antonio; Bettina Ault, University Tennessee Health Science, Memphis; William Baker, Blue Ridge Nephrology Associates; Sharon Bartosh, University Wisconsin; Lorraine Bell and Martin Bitzan, Montreal Children’s Hospital; Nadine Benador, University California San Diego; Mark Benfield, University Alabama, Birmingham; Andrew Bland, Renalcare Associates of Peoria; Milos Budisavljevic, Medical University of South Carolina; Brad Carter, Kidney Specialists of Central Oklahoma; Daniel Cattran, Toronto General Hospital; Deepa Chand, Cleveland Clinic; Chandran Chandra, St Joseph Regional Medical Center; Demetrius Ellis, Children’s Hospital, Pittsburgh; Robert Fildes, Fairfax Hospital for Children; Danny Fischer, Kidney and Hypertension Center, Cincinnati; Joseph Flynn, Children’s Hospital, Seattle; John Foreman, Duke University Med Center—Peds; Debbie Gipson and Ronald Falk, University North Carolina, Chapel Hill; Larry Greenbaum, Emory University; German Hernandez, Texas Tech University HSC; Jeffrey Hoggard, Eastern Nephrology Associates; Robert Holleman, University South Carolina; Mohammad Ilyas, University Florida, Jacksonville; Eunice John, University Illinois, Chicago; Valerie Johnson, Cornell Medical College NY Presbyterian Hospital; Joshua Kaplan, University of Medicine and Dentistry of New Jersey; Frederick Kaskel, Montefiore Medical Center; Jerome Lane, Children’s Memorial Hospital, Chicago; Jen-Jar Lin, University Michigan; John Mahan, Ohio State University—Peds; Susan Massengill, Carolinas Medical Center—Peds; Douglas Matsell, Children’s Hospital, British Columbia; Tej Mattoo, Children’s Hospital, Michigan; Lawrence Mcgee, Spartanburg Nephrology; Susan Mendley, University Maryland Hospital; Julian Midgley, Alberta Children’s Hospital; Asha Moudgil, Children’s National Medical Center; Dianne Muchant, University Louisville; Jerome Murphy, Children’s Hospital Central California; James Musgrave, University Hawaii School of Medicine; Richard Neiberger, University Florida, Gainesville; Victoria Norwood, University Virginia Children’s Med Center; Luis Ortiz, Medical College of Georgia; Cynthia Pan, Children’s Hospital, Wisconsin; Ana Paredes, Miami Children’s Hospital; Dechu Puliyanda, Cedars-Sinai Medical Center; Virginia Savin, Medical College of Wisconsin; Jon Scheinman, University Kansas; Jeffrey Schelling, Case Western Reserve, Internal Medicine Nephrology; Morris Schoeneman, SUNY Health Science Center, Brooklyn; Mouin Seikaly, Children’s Medical Center, Dallas; Ganesh Shidham, Ohio State University Medical Center; Ajay Singh, Brigham and Women’s Hospital; Michael Somers, Children’s Hospital, Boston; James Springate, Women’s and Children’s Hospital of Buffalo; Howard Trachtman, Cohen Children’s Medical Center; Matti Vehaskari, Children’s Hospital, New Orleans; Brad Warady, Children’s Mercy Hospital, Kansas City; Robert Weiss, Westchester Medical Center; Lynne Weiss, Robert Wood Johnson Med School; Dilys Whyte, SUNY at Stony Brook; Craig Wong, UNM Children’s Hospital; Jonathon Woods, Southeastern Nephrology Associates Wilmington; Ora Yadin, Mattel’s Children’s Hospital UCLA.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
DEDICATION
We would like to dedicate this work to Dr Norman Siegel. His leadership of the FSGS Clinical Trial Steering Committee and commitment to advancing the progress toward successful therapeutic options for children and adults with FSGS was an example to us all.
ETHICS
This study was registered in clinicaltrials.gov, identifier NCT00135811, and monitored by a Data and Safety Monitoring Board. The study protocol was reviewed and approved by the Institutional Review Board at each participating site. Informed consent and, when appropriate, assent was obtained before enrollment.
Deceased.
References
- 1.Troyanov S, Wall CA, Miller JA, et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 2.Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 3.Rydel JJ, Korbet SM, Borok RZ, et al. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis. 1995;25:534–542. doi: 10.1016/0272-6386(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 4.Abrantes MM, Cardoso LSB, Lima EM, et al. Predictive factors of chronic kidney disease in primary focal segmental glomerulosclerosis. Pediatr Nephrol. 2006;21:1003–1012. doi: 10.1007/s00467-006-0138-y. [DOI] [PubMed] [Google Scholar]
- 5.Bakir AA, Share DS, Levy PS, et al. Focal segmental glomerulosclerosis in adult African Americans. Clin Nephrol. 1996;46:306–311. [PubMed] [Google Scholar]
- 6.Braun N, Schmutzler F, Lange C, et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2008;(3):CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moranne O, Watier L, Rossert J, et al. Primary glomerulonephritis: an update on renal survival and determinants of progression. QJM. 2008;101:215–224. doi: 10.1093/qjmed/hcm142. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos E, Stangou M, Papagianni A, et al. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:1348–1356. doi: 10.1093/ndt/15.9.1348. [DOI] [PubMed] [Google Scholar]
- 9.Chun MJ, Korbet SM, Schwartz MM, et al. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 10.Smith D, Branton M, Fervenza F, et al. Pulse dexamethasone for focal segmental glomerulosclerosis. J Am Soc Nephrol. 2003;13:526a. [Google Scholar]
- 11.Tune BM, Mendoza SA. Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults. J Am Soc Nephrol. 1997;8:824–832. doi: 10.1681/ASN.V85824. [DOI] [PubMed] [Google Scholar]
- 12.Cattran DC, Appel GB, Hebert LA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7:56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 14.Nayagam S, Ganguli A, Rathi M, et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol Dial Transplant. 2008;23:1926–1930. doi: 10.1093/ndt/gfm538. [DOI] [PubMed] [Google Scholar]
- 15.Cattran DC, Wang MM, Appel G, et al. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin Nephrol. 2004;62:405–411. doi: 10.5414/cnp62405. [DOI] [PubMed] [Google Scholar]
- 16.Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2010;79:678–685. doi: 10.1038/ki.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tune BM, Mendoza SA. Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults. J Am Soc Nephrol. 1997;8:824–832. doi: 10.1681/ASN.V85824. [DOI] [PubMed] [Google Scholar]
- 18.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Haycock GB, Edelmann CM, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]