Abstract
Dendritic spines, small bulbous postsynaptic compartments emanating from neuronal dendrites, have been thought to serve as basic units of memory storage. Despite their small size (~0.1 femtoliter), thousands of species of proteins exist in the spine, including receptors, channels, scaffolding proteins and signaling enzymes. Biochemical signaling mediated by these molecules leads to morphological and functional plasticity of dendritic spines, and ultimately learning and memory in the brain. Here, we review new insights into the mechanisms underlying spine plasticity brought about by recent advances in imaging techniques to monitor molecular events in single dendritic spines. The activity of each protein displays a specific spatiotemporal pattern, coordinating downstream events at different microdomains to change the function and morphology of dendritic spines.
Introduction
In the central nervous system, most excitatory postsynaptic terminals reside in dendritic spines. A mature spine forms a mushroom-shaped structure: a small spherical head (~0.5 μm in diameter) connected to the dendrite through a thin neck (~0.1 μm in diameter) [1]. The neck limits the diffusion of cytoplasmic and membrane molecules in and out of the spine head [2–5]. Elevation of Ca2+ concentration in spines (~ micromolars [4, 5]) initiates biochemical signal transduction that leads to the expression of various forms of synaptic plasticity, including long-term potentiation (LTP) and depression (LTD) [6]. At Schaffer Collateral synapses in the hippocampus, synaptic plasticity is associated with morphological plasticity of dendritic spines: spines display long-term enlargement [7–9] and shrinkage [10] during LTP and LTD, respectively. Signaling involved in LTP and the associated spine enlargement in these synapses has been especially well studied as a prominent memory model. It has been revealed that LTP is caused by a combination of many postsynaptic processes coordinated in time and space, including reorganization of actin cytoskeleton, exocytosis from endosomes and insertion of AMPA receptors (AMPARs) into synapses [11, 12]. In turn, these events lead to an increase in the sensitivity of postsynaptic sites to glutamate [11–13] or increasing the release probability from the presynaptic terminal [14–16]. Depending on the stimulation paradigm, LTP and associated spine enlargement can be maintained for more than several hours [17, 18]. This form of LTP requires synthesis of new proteins [17–20]. Signaling mechanisms regulating these events have also been extensively studied, and tens of signaling proteins have been identified to be important for LTP [21].
Recent progress in imaging techniques has enabled molecular events at the level of the single-synapse to be visualized, and such studies have provided new insights into the molecular mechanisms underlying LTP and associated spine enlargement. In this review, we summarize recent findings that have revealed the spatiotemporal dynamics of molecular processes that occur in dendritic spines during the initial ~30 min of morphological and functional plasticity.
Molecular reorganization in spines during LTP
During LTP induction in Schaffer Collateral synapses in response to repetitive uncaging of caged-glutamate [7], high frequency electrical stimulation[7] or theta-burst electrical stimulation [19], spine morphology has been shown to dramatically change, growing over 2–5 fold in size within ~1 min (Figure 1). This is followed by a decay in volume over the next few minutes, followed by stabilization (for more than 1 hr) at a volume 1.5–2 times as large as the original volume [7, 19]. Recent imaging studies have revealed some of the mechanisms of this amazingly dynamic process, and it has become clear that the induction of LTP and spine enlargement require many cellular events that regulate the actin cytoskeleton, membrane, and postsynaptic density (PSD) within ~1 min, perhaps reorganizing the whole spine structure.
Figure 1. Visualization of signaling molecules and spine volume changes in stimulated spines.
(a) Visualization of Ca2+, Ca2+/calmodulin-dependent kinase II (CaMKII), Ras homolog A (RhoA), and Cell division cycle 42 (Cdc42) activation during morphological plasticity in single spines using 2-photon fluorescence lifetime imaging microscopy combined with 2-photon glutamate uncaging. Warmer color indicates higher levels of activation. The white arrowheads indicate stimulated spines. Scale bars (white) are 1 μm. Images adapted, with permission from [39] (Ca2+ and CaMKII panels), [36] (Cdc42 and RhoA), and [84] (spine volume changes).
(b–d) Time-courses of signaling activity and spine volume changes in stimulated spines. Please note that (c) and (d) represent the same data, illustrated at time-intervals immediately after (d) and much later after (c) stimulation. The time courses of Ca2+ and CaMKII were adapted from [36], and RhoA, Cdc42, and spine volume changes are from [84]. The timecourses of CaMKII in (c) and (d) were originally taken under the condition of 45 pulses at 0.5 Hz [36], but the data points corresponding to the 30th–45th pulses were removed so that the plot approximately represents the one in response to 30 pulses stimulation. The time course of mutant CaMKII (T286A) was normalized to the peak of the wildtype CaMKII [36]. Autophosphorylation at T286 results in CaMKII activation independent of Ca2+/calmodulin, and thus, the activity decays slower than Ca2+ [36]. Unlike wildtype, the T286A mutant fails to integrate Ca2+ signals [36]. These findings indicate that CaMKII activation peaks rapidly after Ca2+ stimulation, followed by RhoA and Cdc42 activation. Subsequent changes in spine volume then occur.
Reorganization of the actin cytoskeleton
The actin cytoskeleton plays an essential role in sustaining and modulating the morphology of spines [12, 22]. In spines, actin filaments undergo rapid treadmilling by adding actin monomer at one end (burbed end) and depolymerizing at the other end (pointed end) [23] . The dynamics of actin treadmilling in dendrites and spines has been studied by measuring fluorescence recovery after photobleaching (FRAP) of green fluorescent protein (GFP)-tagged actin monomer [23] or fluorescence decay after photoactivation of photoactivatable GFP (paGFP)-tagged actin [24]. These studies revealed that the treadmilling process resulted in an exchange between the actin monomers in the spines with those in dendritic shaft within 1 min (dynamic pool). In addition, there is a stable pool that is not exchanged for many minutes [23, 24]. The actin treadmilling produces a net flow of actin monomer from the tip toward the neck of the spine [24]. Single particle tracking of individual actin monomers revealed that the orientation of actin filaments is not well-regulated in spines: each actin monomer moves in all directions, but net ensemble flow is from the tip to the neck [25, 26]. Consistent with these data, direct imaging of actin filaments in spines using platinum replica electron microscopy (EM) revealed that actin filaments are not oriented regularly, but rather appear like that of tangled yarn [27].
During spine enlargement, rapid actin polymerization perhaps provides mechanical force required for pushing out the membrane of the stimulated spine [7, 8]. An imaging study using fluorescence resonance energy transfer (FRET) between yellow fluorescent protein (YFP)-actin and cyan fluorescent protein (CFP)-actin also supported this idea: the filamentous (F-) / monomeric (G-) actin equilibrium rapidly shifts to F-actin within ~5 min of LTP induction, and the change is maintained for more than 30 min [8]. Also, an analysis of paGFP-actin displayed that the stable pool at the spine neck grows within ~2 min and is stabilized over an hour [24].
Reorganization of PSD proteins
Recent technical advances have dramatically improved our understanding of the architecture of the postsynaptic density (PSD) and its regulation during LTP. The Proteomic analyses have identified hundreds of proteins in the PSD (reviewed in [28]). Further, EM tomography reconstruction has enabled the visualization of non-labeled molecules in the PSD directly, and revealed that PSD95, the major PSD scaffold, forms vertically oriented filaments against the membrane, linked by unknown horizontal filaments [29, 30]. By using super-resolution optical imaging, the precise location of proteins in the PSD, as well as presynaptic terminals, has been measured [31]. Live imaging using GFP-tagged PSD95 has revealed that the shape of the PSD is not static, but is constantly changing on a timescale of minutes[32]. This morphological change is actin-dependent [32], suggesting that actin reorganization during LTP may have an impact on the conformation changes that occur at the PSD.
The dynamics of PSD proteins in single spines during LTP has been imaged using paGFP-tagged PSD95 and Shank [33]. Tagged proteins were photoactivated in a single spine, and the movement of these molecules was monitored following 2-photon glutamate uncaging at the same spine. Under basal conditions, these molecules stayed in the spine for more than ~30 min. Upon uncaging, however, both proteins rapidly diffused out of the spine and were exchanged by non-photoactivated proteins. The phosphorylation of PSD95 at Serine 73 by Ca2+/Calmodulin-dependent kinase II (CaMKII) was found to be responsible for its dissociation from the PSD [33].
Surprisingly, the number of labeled proteins (ie. PSD95 and Shank) within the PSD were not changed during spine enlargement, even after ~30 min of stimulation [33]. This is in sharp contrast to CaMKII [34–36] and AMPARs [37–39], of which enrichment occurs at the same time and to the same degree with the volume change. Because the size of the PSD and spine volume are well correlated under basal conditions [40], PSD size may increase at a much later time-point. Consistent with this view, it has been reported that PSD95 enrichment in newly formed spines occurs many hours after spine formation in slices [41, 42] and in vivo [43]. In contrast to the decoupling in the timing of spine volume and PSD size increases during LTP and spinogenesis, PSD shrinkage and loss occurs at the same time (within ~1 min) with spine shrinkage and loss of spines [44].
Endosome trafficking and AMPAR insertion
Spine enlargement during LTP requires the addition of membrane area to the spine. This may be done by diffusion of excess membrane from the dendrite [45] or exocytosis of endosomes [9]. Ultrastructural studies have shown that some spines contain a relatively large fraction of internal membrane in endosomes [9]. Further, inhibition of postsynaptic exocytosis inhibits spine enlargement and LTP [9, 19, 46–48]. These results suggest that the additional membrane required for spine enlargement may come from exocytosis. The exocytosis of endosomes is also important for providing AMPARs to the surface of the spine, which is an important process of LTP [11, 13].
Recently, a technique to image individual exocytosis using a superecliptic pHluorin (SEP)-tagged GluA1 AMPAR subunit, or transferrin receptor, has been developed. SEP fluorescence is quenched in acidic conditions within endosomes and is de-quenched by exocytosis [9, 37, 49]. Thus, by pre-bleaching the existing surface SEP-tagged receptors, one can image exocytosis of single endosomes [38, 39, 48, 50–52]. It has been shown that chemically-induced LTP increases the rate of exocytosis of GluA1-containing endosomes in spines and dendrites [38, 48, 50]. Further, during LTP induced in a single dendritic spine with 2-photon glutamate uncaging, the rate of exocytosis of GluA1-containing endosomes was observed to increase in the stimulated spine as well as in the adjacent dendrite within 5 μm of the stimulated spine [38, 39]. Due to the lateral diffusion of AMPARs into the stimulated spine as well as direct exocytosis, the total number of AMPARs in the stimulated spines increased within ~ 1 min following LTP induction [38, 39]. The recruitment of AMPARs into the PSD requires CaMKII-dependent phosphorylation of stargazin, an auxiliary subunit of AMPARs, and subsequent trapping of phospho-stargazin within the PSD [53–55].
The molecular machinery involved in the endosome trafficking during LTP has also been extensively studied. Two subtypes of soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins have been identified as being important for plasticity: syntaxin 13 [46], which directs traffic from early endosomes to the recycling endosome, and syntaxin 4 [48], which is involved in exocytosis at the plasma membrane. Rab small GTPases [9, 46, 56] and the motor proteins Myosin Va [57] and Vb [58] have also been shown to be important for regulating endosome trafficking during LTP.
Spatiotemporal activation of signaling molecules during plasticity of single dendritic spines
The recent development of 2-photon fluorescence lifetime imaging microscopy (2pFLIM) and new FRET sensors (Box 1) has enabled the visualization of signaling activity triggered by Ca2+ elevation in single dendritic spines. This has revealed the detailed signaling processes linking Ca2+ and molecular reorganization during LTP. We discuss such findings for Ca2+ and a number of downstream signaling molecules in the following sections.
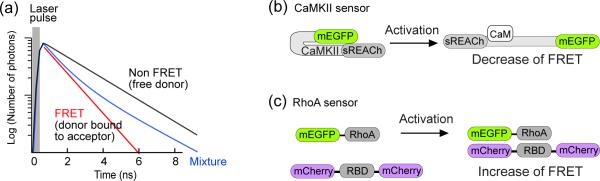
Box 1 Figure I. Fluorescence resonance energy transfer (FRET) sensor for fluorescence lifetime imaging (FLIM).
(a) Theoretical fluorescence lifetime curves of fluorescent protein (i.e. GFP as donor). The free donor at the excited state typically decays mono-exponentially (black line). When FRET occurs by the binding of acceptor to donor, the donor lifetime in the excited state is shortened (red line). For mixed population, the decay curve follows a multi-exponential curve (blue line). Thus, the population of donor bound to acceptor can be calculated from the curve.
(b) Schematic illustration of a CaMKII sensor. CaMKII takes compact form when it is inactive, but the binding of Calmodulin (CaM) induces the opening of CaMKII, increasing the distance between donor (monomeric EGFP or mEGFP) and acceptor (sREACh) and decreasing FRET [36].
(c) Schematic illustration of a RhoA sensor. The activation of mEGFP-RhoA induces the binding of Rho binding domain of Rhotekin (RBD) flanked by two mCherry molecules, and increases FRET [84].
Ca2+
Development of 2-photon glutamate uncaging combined with 2-photon Ca2+ imaging has greatly facilitated the study of Ca2+ signals in dendritic spines [5, 59, 60]. Glutamate uncaging at a dendritic spine with a 2-photon laser can activate glutamate receptors on the spine with kinetics and amplitude similar to those evoked by presynaptic glutamate release [61]. When the Mg2+ block of NMDA receptors (NMDARs) is released by removing extracellular Mg2+ or depolarizing the neuron, glutamate uncaging can produce Ca2+ elevation to the micromolar level in the stimulated spine (Figure 1) [5, 36]. The Ca2+ elevation lasts only ~0.1 s, and is largely restricted to the stimulated spine [5, 36]. By repeating glutamate uncaging (0.5–2 Hz, 30–60 pulses), LTP and associated spine enlargement can be induced in the stimulated spine but not in adjacent spines (Figure 1a) [7]. During this process, Ca2+ elevations show a train of Ca2+ transients [36] (Figure 1b).
CaMKII
CaMKII is one of the most abundant proteins in the PSD [62], and is required for hippocampal LTP and some forms of learning and memory [63]. A holoenzyme of CaMKII consists of 12 subunits (mainly α and β in spines [62]), arranged in two hexameric rings [64]. When [Ca2+] increases, Ca2+ binds to calmodulin, and Ca2+/calmodulin binds to a CaMKII subunit [63, 64]. This causes a conformational change of CaMKII to expose its kinase site, activating the subunit. When two adjacent subunits are activated, they undergo transautophosphorylation at Thr-286 [63, 64], which enable the subunits to maintain its activity even after calmodulin dissociation [63]. It has been hypothesized that this “autonomous” CaMKII activity persists for more than hours [65, 66], and may act as a biochemical memory to maintain LTP [67].
CaMKII activity during LTP was imaged using 2pFLIM in combination with a FRET based CaMKII sensor (Box 1) [36]. When a single spine is stimulated with 2-photon glutamate uncaging to induce LTP, CaMKII is rapidly activated within ~10 s in the stimulated spine, displaying a pattern that is restricted to the spine [36]. Contrary to the theory of persistent CaMKII activity, the activity decayed rapidly after cessation of uncaging with time constants of 6 s and 45 s (Figure 1d). Interestingly, when the autophosphorylation site was mutated (T286A), the activity completely returned to the basal state within a second and thus failed to accumulate its activity during repetitive glutamate uncaging (Figure 1b) [36]. These results suggest that T286 phosphorylation is required for sustaining the activity of CaMKII for the time scale of seconds, not hours, and thus for the integration of the short (~0.1 s) Ca2+ elevation (Figure 1b). However, this experiment does not discard the possibility that a small fraction of CaMKII (for example, a pool of molecules bound to NMDARs [68, 69]) have persistent kinase activity.
During the short CaMKII activation, CaMKII may phosphorylate PSD95 and stargazin, leading to PSD disassembly and AMPAR confinement within the PSD, respectively [33, 53] (but see [70]) (Figure 2). In addition, CaMKII activation may directly regulate actin organization. Because CaMKIIβ can bind to actin filaments, dodecameric holoenzyme can bundle actin filaments to stabilize spine structure [71]. When CaMKIIβ is activated, it dissociates from actin filaments, thereby losing the ability to bundle actin filaments [71]. Thus, CaMKII activation during the induction of LTP may destabilize actin, allowing later actin extension [71]. CaMKIIβ knockout mice displayed deficits in LTP and learning while kinase-dead knock-in (R303A) mice displayed normal LTP and learning behavior [72], further supporting the idea that CaMKIIβ mainly plays a structural role rather than an enzymatic role.
Figure 2. Signal transduction underlying spine morphological plasticity and long-term potentiation (LTP).
Spatiotemporal regulation of signaling cascades triggered by NMDAR activation in single dendritic spines in response to glutamate uncaging. NMDAR activation increases spine Ca2+ concentration, leading to activation of Ca2+/Calmodulin-dependent kinase (CaMKII) [36]. It further activates downstream Rat sarcoma (Ras) [77], Cell division cycle 42 (Cdc42) and Ras homolog A(RhoA) [84]. CaMKII phosphorylates postsynaptic density 95 (PSD95) and causes dissociation of the postsynaptic density (PSD). Rho kinase (ROCK) and p21-activated kinase (PAK) are activated downstream of Rho and Cdc42, respectively. Exocytosis of AMPARs show similar patterns as Ras activation [38, 39] and requires Ras activation [39]. Trapping of diffused AMPARs into the PSD requires stargazin phosphorylation by CaMKII [53]. Fluorescence lifetime images are adapted from [77, 84] with permission. Color-coded intensity map of AMPAR exocytosis is adapted from [38] with permission. Warmer color indicates higher levels of activation/ receptor exocytosis. The white arrowheads indicate stimulated spines.
Ras
One of the CaMKII downstream targets is Rat sarcoma (Ras) [73]. Ras was initially identified as an oncogene protein, and the function of Ras signaling in cell growth, division and survival has been extensively studied [74]. In neurons, Ras is known to be important for regulating various forms of neuronal plasticity and adaptation, including synapse formation, spine morphological plasticity, plasticity of neuronal excitability, dendritic protein synthesis, and gene transcription [73, 75]. Ras is active when bound to GTP, and inactive when bound to GDP [74]. This GTP-GDP cycle is regulated via the interaction with GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) [74]. GAPs promote the hydrolysis of GTP to GDP to inactivate Ras, whereas GEFs promote the exchange of GDP for GTP to activate Ras [74].
The activity of Harvey Ras (HRas), a major subtype of Ras, during LTP has been imaged with 2pFLIM [76, 77] (Box1). When LTP was induced in a single spine, Ras activity increased at the stimulated spine in ~1 min, and spread along its parent dendritic shaft over ~10 μm [77]. Ras activity in surrounding spines was ~70% as high as that of the stimulated spines, but still was not sufficient for inducing plasticity. The activation of HRas was partially inhibited by the CaMKII inhibitor KN-62, confirming that HRas is downstream of CaMKII [73, 77]. The link between CaMKII and Ras is not clear, but it has been suggested that CaMKII regulates activity of some GEFs and GAPs [78]. Overexpression of dominant negative HRas or inhibition of downstream extracellular signal-regulated kinase (ERK) inhibits LTP and spine enlargement [73, 77]. Thus, these studies suggest that the Ras-ERK pathway is required, but not sufficient, for inducing morphological plasticity (Figure 2).
The spatiotemporal pattern of HRas activity resembles that of activity-dependent AMPAR exocytosis [38, 39, 77]. Further, the activity-dependent increase of AMPAR exocytosis was found to be dependent on the Ras-ERK pathway [39]. Thus, the spreading of Ras signaling seems to be important for producing a similar pattern of exocytosis events (Figure 2). In addition, the spreading of Ras was found to be important for the priming of LTP: when LTP is induced in a spine, LTP can be induced in neighboring spines with weak stimuli that usually do not induce LTP [77] (Figure 2).
Rho and Cdc42
The Rho family GTPases, including Ras homolog (Rho), Cell division cycle 42 (Cdc42) and Ras-related C3 botulinum toxin substrate (Rac), are close relatives of Ras, and are key players in regulating the actin cytoskeleton [74, 79, 80]. Like Ras, the activity of Rho proteins is regulated by a GTP-GDP cycle caused by GEFs and GAPs [74]. Rho is also regulated by Rho GDP-dissociation inhibitor (GDI), which binds to inactive Rho GTPases and controls the interaction of Rho with membranes [74]. The function of these proteins for actin-mediated cell morphological changes [81], migration [82] and polarization [80] has been well characterized in non-neuronal cells. In neurons, Rho GTPases are known to be important for regulating spine morphology: in general it is thought that Rho activation causes spine loss and shrinkage by inhibiting actin polymerization, while Cdc42 and Rac activation increases the number of spines by promoting actin polymerization [83].
To image Rho activity in single dendritic spines undergoing structural plasticity, FRET-FLIM sensors for RhoA, a major subtype of Rho in neurons, and Cdc42 have been developed using a design similar to the Ras sensor (Box 1) [84]. Upon single-spine stimulation with 2-photon glutamate uncaging, the activity of RhoA and Cdc42 increased rapidly in the stimulated spines within ~1 min, and decayed over 3–5 min (Figure 1) [84]. This transient activity was followed by a sustained activation lasting more than ~30 min (Figure 2). Although RhoA and Cdc42 were similarly mobile, the spatial patterns of RhoA and Cdc42 signaling were different: RhoA activity diffused out of stimulated spines, and spread along their parent dendritic shafts over ~5 μm, while Cdc42 activity was restricted to the stimulated spines (Figure 2) [84, 85]. Like Ras, both Cdc42 and RhoA activation were partially inhibited by inhibiting CaMKII signaling, suggesting that these molecules are also downstream of CaMKII [84].
Activation of Cdc42 and RhoA during LTP is perhaps important for regulating actin organization [83]. Inhibition of Rho or its downstream Rho kinase (ROCK) preferentially inhibited the transient phase of structural plasticity, while inhibition of Cdc42 or downstream p21-activated kinase (PAK) inhibits the maintenance of spine enlargement [84] (Figure 2). The activation and requirement of Rho signaling in the initial phase of the spine enlargement may be surprising, as previous studies have suggested that Rho activation caused spine loss and shrinkage (reviewed in [83]). However, considering the extreme stability of the basal actin structure, Rho activation may be important for disassembling the actin network, allowing the later growth and restabilization of the network in a larger form.
The sustained activation of Cdc42 and RhoA (Figure 1c) suggests that actin polymerization is continuously regulated during the sustained phase of LTP (Figure 2). Consistent with this hypothesis, partial inhibition of actin polymerization with low concentrations of Latrunculin A or cytochalasin B/D inhibits the maintenance of morphological plasticity [7] and LTP [86, 87]. Also, FRET imaging of the F-/G-actin ratio shows a long-term shift in the equilibration toward F-actin [8].
Spatiotemporal signal integration during morphological plasticity
Aligning various signals on multiple time scales (Figure 1b–d), we can now visualize how the short Ca2+ signaling, which lasts only ~0.1 s, can be relayed to long-lasting changes in spines. First, the initial Ca2+ signal is integrated by CaMKII activation over seconds to ~1 min. Subsequently, small GTPase proteins including Cdc42 and RhoA are activated by CaMKII, which expands the time scale to tens of minutes. Following the peak of small GTPase activity, spine enlargement peaks at ~2 min. RhoA and Cdc42 display sustained activity similarly to the spine volume change (Figure 1b–c).
CaMKII, RhoA, Cdc42, and HRas during these processes also display various length constants in their activity profile (Figure 1, 2) [85]. While Ca2+ elevation and CaMKII and Cdc42 activities are compartmentalized in the stimulated spine, HRas and RhoA activities spread out of the stimulated spine over ~5 μm, invading the adjacent dendrites and spines. In particular, Cdc42 activity is spine-specific and lasts more than 30 min, suggesting that Ca2+-CaMKII-Cdc42 signaling constitutes spine-specific signaling spanning from 0.1 s to more than 30 min (green in Figure 2). Other signaling molecules, such as activated RhoA and HRas, spread into the dendrite from the stimulated spine. These spreading signals are important for heterosynaptic metaplasticity, such as priming of LTP in adjacent spines [18, 77, 88] and spine formation in the adjacent dendrite [89].
The mechanisms to produce localized activity of signaling proteins in dendrites have been reviewed elsewhere in detail [85, 90]. Basically, the degree of compartmentalization can be determined by the distance a molecule can diffuse before it is inactivated. In particular, small GTPase proteins HRas, RhoA and Cdc42 have similar structure and mobility, yet they have very different spatial patterns (Figure 2), providing the basis to mathematically model the spatial spreading of molecules. Using a simple model in which a molecule is activated in a spine, and inactivated by GAPs homogeneously distributed in the dendritic shaft, the spatial profile of small GTPase proteins was reproduced in silico (Box 2). This local excitation-global inhibition mechanism was also proposed as the mechanism for producing the spatial gradient of intracellular signaling in other systems such as chemotaxis of the amoeba Dictyostelium, as well as chemotaxis of neutrophils in the mammalian immune system (reviewed in [91]).
Box 2 Figure I. Spatial spreading of small GTPase activity upon single-spine stimulation.
Upon single-spine stimulation with 2-photon glutamate uncaging (indicated by the orange circle at the tip of spine), Cell division cycle 42 (Cdc42) is activated and localized in the spine, whereas the activity of Ras homolog A (RhoA) and Harvey Rat sarcoma (HRas) diffuse into the dendrite (top).The spatial profile of Cdc42, RhoA, and HRas activities measured with 2-photon fluorescence lifetime imaging microscopy were plotted as a function of the contour distance along the dendrite from the stimulated spines (bottom; at the stimulated spine, distance = 0). The curves were obtained by fitting the data with Eq. 3. For each protein, the activity in the stimulated spine was normalized to 1. Figure is adapted, with permission, from [85].
Conclusions
Recent studies that have utilized a variety of new imaging techniques have provided us with a more detailed understanding of the molecular processes of synaptic plasticity. In particular, imaging signal transduction with 2pFLIM has enabled the visualization of how signaling cascades temporally integrate signals in single dendritic spines from ~0.1 s to several tens of minutes (Figure 2), as well as how spatially distinct events are orchestrated (Figure 1 and 2). Since actual signaling networks contain hundreds of components, the monitoring of more nodes of the networks and the simultaneous observations of the activity of a few kind of molecules, will achieve a more complete view of signal transduction in spines (Box 3). In addition to imaging methods, the manipulation of a signaling node with photo-activatable proteins [92–95] will also provide more information of the function of signaling networks in single dendritic spines. A better understanding of single spine dynamics may ultimately help to understand how memories are encoded at the cellular level.
Box 1. Visualizing molecular interactions with 2-photon fluorescence lifetime imaging microscopy (2pFLIM)
Fluorescence resonance energy transfer (FRET) and fluorescence lifetime imaging (FLIM)
FRET is the distance/orientation-sensitive phenomenon that occurs between two fluorophores due to dipole-dipole interaction. Since FRET efficiency decays steeply as the distance between fluorophores (donor and acceptor) increases over nanometers, it can be used as a molecular-ruler to detect the conformational changes or interaction between proteins tagged with fluorophores [96–98]. Fluorescence lifetime, the time elapsed between fluorophore excitation and photon emission, is a sensitive and quantitative measure of FRET [96]. The fluorescence lifetime can be measured as the time constant of fluorescence decay (nanoseconds) after excitation with a short laser pulse (< 0.1 ns). Usually, free donor shows mono-exponential decay, and this decay rate is accelerated by FRET. When multiple populations, for example non-FRET and FRET populations, co-exist, the fluorescence lifetime decays in a multi-exponential manner. Thus, one can deconvolve the fraction of donor interacting with acceptor. Compared to other readouts based on the wavelength shift (for example, ratiometric FRET imaging), the obtained value is more robust against the local concentration change in the donor-to-acceptor ratio or wavelength-dependent light scattering [96, 99] (Figure Ia).
Overview of FRET sensor optimized for FLIM
The FRET sensor for FLIM needs to be optimized in a manner different from other imaging techniques. First, a combination of the bright donor and dim accepter provides better signal-to-noise ratio, as FLIM uses only donor fluorescence. Second, donor with mono-exponential fluorescence decay is preferable for calculation of the fraction of donor bound to acceptor. Although the enhanced cyan fluorescent protein(ECFP)-enhanced yellow fluorescent protein (EYFP) pair is the most popular pair for the ratiometric FRET imaging, they are not an optimum pair for FLIM [98, 100]. This is because CFP-YFP is a dim donor-bright acceptor pair and CFP fluorescence decays in a multi-exponential manner. The enhanced green fluorescent protein (EGFP)-monomeric red fluorescent protein (mRFP), EGFP-monomeric Cherry (mCherry) or EGFP-super resonance energy-accepting chromoprotein (sREACh; non-radiative YFP) pairs provide much better and robust signal for FLIM, as GFP is much brighter than CFP, and shows mono-exponential decay [76, 101, 102].
FRET sensor for CaMKII
Since many kinases, such as CaMKII, change their conformation when they are activated, a FRET probe sensing the conformational change of a kinase can serve as an indicator of the activity of the kinase. An example is a CaMKII FRET sensor named Camui [36, 103], which senses the conformational change of CaMKII by FRET between fluorophores attached to the both ends of the molecule (Figure Ib).
FRET sensors for small GTPase proteins
Activity of a small GTPase protein including Ras, Rho and Cdc42 can be monitored by measuring the interaction between the small GTPase protein fused to a donor fluorophore and small GTPase protein binding domain (Ras binding domain or RBD; chosen from its effectors) fused to acceptor [77, 84]. As RBD binds selectively to the active protein, activation of the small GTPase protein leads to increase in FRET (Figure Ic).
Box 2: Modeling the spatial profile of small GTPase proteins.
The spatial profile of small GTPase activity along the dendrite, CD(x) (x is the contour distance from the neck of the stimulated spine along the dendritic shaft), relative to activity in the head of the stimulated spine, Chead, can be mathematically formulated using a diffusion-reaction model [85]. Assuming that small GTPase is activated in the stimulated spine, diffuses out of the spine, and is inactivated by GAP homogeneously distributed along the dendrite, the distribution of the activity on the dendritic shaft (CD) as a function of time (t) and the distance along the dendrite (x) is described as:
| (3) |
where αneck is the gradient of activity at the spine neck and λ is the length constant of the decay along the dendrite. αneck and λ are given by:
| (4) |
where rD is the radius of the dendrite (~ 0.4 μm) [5], Shead is the surface area of the spine head (~4 μm2 during spine enlargement), D is the diffusion coefficient (~ 0.5 μm2/s) [84]. The equation fits well to the measured spatial profile, when one free parameter τD is obtained by fitting [85] (Figure I).
Box 3: Outstanding questions
Is morphological plasticity of spines required for LTP and learning and memory?
How is the molecular composition of the spine different after induction of LTP? Does the size of the PSD grow during LTP?
How can the dynamic actin cytoskeleton maintain the stable spine structure? Is the cytoskeleton destabilized before extension during LTP?
Among over one hundred GEFs and GAPs, which molecules are responsible for activation of small GTPase proteins during LTP?
Can imaging techniques be scaled-up to allow for the visualization of hundreds of signaling proteins in spines?
Acknowledgements
We thank Drs. S. Soderling, J. Lisman, M. Ehlers and N. Hedrick for critical reading and discussion. The work done in the lab of R.Y. is supported by the National Institutes of Health and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris KM, et al. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svoboda K, et al. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- 3.Bloodgood BL, Sabatini BL. Neuronal Activity Regulates Diffusion Across the Neck of Dendritic Spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini BL, et al. The life cycle of Ca2+ ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi J, et al. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki M, et al. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto K, et al. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature neuroscience. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 9.Park M, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Derkach VA, et al. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 12.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 13.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakharenko SS, et al. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nature neuroscience. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- 15.Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- 16.Enoki R, et al. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron. 2009;62:242–253. doi: 10.1016/j.neuron.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Sutton MA, Schuman EM. Dendritic Protein Synthesis, Synaptic Plasticity, and Memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Govindarajan A, et al. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, et al. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka J, et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy MB, et al. Integration of biochemical signalling in spines. Nat. Rev. Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 22.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 23.Star EN, et al. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature neuroscience. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 24.Honkura N, et al. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Tatavarty V, et al. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS One. 2009;4:e7724. doi: 10.1371/journal.pone.0007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost NA, et al. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, et al. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, et al. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani A, et al. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanpied TA, et al. Structural plasticity with preserved topology in the postsynaptic protein network. Proc Natl Acad Sci U S A. 2008;105:12587–12592. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner P, et al. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otmakhov N, et al. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YP, et al. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, et al. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopec CD, et al. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson MA, et al. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okabe S, et al. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci. 2001;21:6105–6114. doi: 10.1523/JNEUROSCI.21-16-06105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Roo M, et al. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex. 2008;18:151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- 43.Knott GW, et al. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature neuroscience. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- 44.Woods GF, et al. Loss of PSD-95 Enrichment Is Not a Prerequisite for Spine Retraction. J Neurosci. 2011;31:12129–12138. doi: 10.1523/JNEUROSCI.6662-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai J, Sheetz MP. Axon membrane flows from the growth cone to the cell body. Cell. 1995;83:693–701. doi: 10.1016/0092-8674(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 46.Park M, et al. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 47.Lledo PM, et al. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy MJ, et al. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miesenbock G, et al. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 50.Yudowski GA, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaskolski F, et al. Dynamin-dependent membrane drift recruits AMPA receptors to dendritic spines. J Biol Chem. 2009;284:12491–12503. doi: 10.1074/jbc.M808401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin DT, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nature neuroscience. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Opazo P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Tomita S, et al. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Sumioka A, et al. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown TC, et al. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correia SS, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nature neuroscience. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Sobczyk A, et al. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature neuroscience. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng D, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- 63.Lisman J, et al. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 64.Chao LH, et al. A Mechanism for Tunable Autoinhibition in the Structure of a Human Ca2+/Calmodulin- Dependent Kinase II Holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang Y, et al. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukunaga K, et al. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- 67.Zhabotinsky AM. Bistability in the Ca2+/calmodulin-dependent protein kinase-phosphatase system. Biophys J. 2000;79:2211–2221. doi: 10.1016/S0006-3495(00)76469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayer KU, et al. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 69.Bayer KU, et al. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sumioka A, et al. PDZ binding of TARPgamma-8 controls synaptic transmission but not synaptic plasticity. Nature neuroscience. 2011;14:1410–1412. doi: 10.1038/nn.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamoto K, et al. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borgesius NZ, et al. {beta}CaMKII Plays a Nonenzymatic Role in Hippocampal Synaptic Plasticity and Learning by Targeting {alpha}CaMKII to Synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu JJ, et al. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- 74.Takai Y, et al. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 75.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 76.Yasuda R, et al. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nature neuroscience. 2006;9:283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- 77.Harvey CD, et al. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stornetta RL, Zhu JJ. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2011;17:54–78. doi: 10.1177/1073858410365562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 80.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 81.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 82.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 83.Saneyoshi T, et al. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr Opin Neurobiol. 2009;20:108–115. doi: 10.1016/j.conb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murakoshi H, et al. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yasuda R, Murakoshi H. The mechanisms underlying the spatial spreading of signaling activity. Curr Opin Neurobiol. 2011;21:313–321. doi: 10.1016/j.conb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krucker T, et al. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SJ, Yasuda R. Spatiotemporal Regulation of Signaling In and Out of Dendritic Spines: CaMKII and Ras. Open Neurosci J. 2010;3:117–127. doi: 10.2174/1874082000903020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iglesias PA, Devreotes PN. Navigating through models of chemotaxis. Curr Opin Cell Biol. 2008;20:35–40. doi: 10.1016/j.ceb.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 92.Levskaya A, et al. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yazawa M, et al. Induction of protein-protein interactions in live cells using light. Nat Biotechnol. 2009;27:941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- 94.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy MJ, et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lakowicz JR. Principles of Fluorescence Spectroscopy. Plenum; NY, USA: 2006. [Google Scholar]
- 97.Miyawaki A. Development of probes for cellular functions using fluorescent proteins and fluorescence resonance energy transfer. Annu Rev Biochem. 2011;80:357–373. doi: 10.1146/annurev-biochem-072909-094736. [DOI] [PubMed] [Google Scholar]
- 98.Kiyokawa E, et al. Spatiotemporal regulation of small GTPases as revealed by probes based on the principle of Forster Resonance Energy Transfer (FRET): Implications for signaling and pharmacology. Annu Rev Pharmacol Toxicol. 2011;51:337–358. doi: 10.1146/annurev-pharmtox-010510-100234. [DOI] [PubMed] [Google Scholar]
- 99.Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol. 2006;16:551–561. doi: 10.1016/j.conb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 100.Pertz O, Hahn KM. Designing biosensors for Rho family proteins--deciphering the dynamics of Rho family GTPase activation in living cells. J Cell Sci. 2004;117:1313–1318. doi: 10.1242/jcs.01117. [DOI] [PubMed] [Google Scholar]
- 101.Ganesan S, et al. A dark yellow fluorescent protein (YFP)-based Resonance Energy-Accepting Chromoprotein (REACh) for Forster resonance energy transfer with GFP. Proc Natl Acad Sci U S A. 2006;103:4089–4094. doi: 10.1073/pnas.0509922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murakoshi H, et al. Highly sensitive and quantitative FRET-FLIM imaging in single dendritic spines using improved non-radiative YFP. Brain Cell Biol. 2008;36:31–42. doi: 10.1007/s11068-008-9024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takao K, et al. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]