Abstract
Alzheimer’s disease is the most common form of senile dementia. Although the amyloid-β peptide was identified in 1984 as the major constituent of the senile plaques that characterize the disease, accumulating evidence indicates that the plaque density does not correspond well to the concurrent disease state. In order to resolve this disconnect, a number of recent studies have shifted away from the senile plaque and classical fibrillar forms of amyloid toward a less well structured species as the proximate neurotoxic factor underlying cognitive failure in Alzheimer’s disease: soluble amyloid-β peptide oligomer (also known as the amyloid-β peptide–derived diffusible ligand). Paradoxically, several studies in the last 2 years have shown that picomolar levels of amyloid-β peptide have neutral activity or perhaps even an essential role in learning and memory. Here we highlight some of the key observations underlying the growing focus on the amyloid-β peptide oligomer.
Keywords: Alzheimer’s disease, amyloid-β peptide, dementia, oligomer
Alzheimer’s disease (AD) is the most common cause of senile dementia. Clinically, AD patients exhibit progressive cognitive failure, including a loss of the ability to form and retrieve new memories, changes in personality, and a loss of the ability to navigate even the most familiar environments. Ultimately, all cortical function is lost, and death occurs as a complication of the terminal bed-bound state (ie, pneumonia or sepsis).
Pathologically, AD is defined by a characteristic loss of hippocampal and cerebrocortical neurons, especially those involving the diffuse cholinergic projection from the basal forebrain to the cerebral cortex. The neurodegenerative process in AD is also characterized by the accumulation of structural pathology in the form of interstitial and cerebrovascular deposits of amyloid and intraneuronal neurofibrillary tangles due to collapse of the cytoskeleton. The cerebral amyloid in AD, unlike that of other organs, is deposited not as a mass of fibrils but rather as miliary structures known as plaques, which primarily consist of the amyloid-β peptide (Aβ). During proteolytic processing of the Aβ parent or amyloid precursor protein (APP), the Aβ domain is excised by successive cleavage of APP by enzymes known as β-secretase and γ-secretase (discussed in more detail later). γ-Secretase cleavage generates multiple Aβ species, including soluble monomeric peptides of 40 amino acids and more insoluble peptides of 42 amino acids.
The neurodegenerative process in Alzheimer’s disease (AD) is also characterized by the accumulation of structural pathology in the form of interstitial and cerebrovascular deposits of amyloid and intraneuronal neurofibrillary tangles due to collapse of the cytoskeleton.
In AD, Aβ42 monomers will aggregate into progressively larger polymers (dimers, trimers, etc.) generically known as oligomers. A popular current concept regarding the pathogenesis of cognitive failure in AD is that Aβ oligomers are especially important neurotoxic conformers of Aβ.1 This formulation contrasts with the traditional concept in which the key toxins are the highly structured Aβ fibrils that compose amyloid plaques. Furthermore, the current formulation is that the oligomer pathway and the amyloid fibril pathway are separate and distinct.
Initially, clinicopathological correlation studies brought the role of the amyloid plaque into question,2–5 and more recently, the advent of amyloid imaging with Pittsburgh compound B has confirmed that many healthy adults accumulate numerous Aβ deposits with few signs of dementia.2–5 The most compelling lines of evidence linking Aβ to AD are genetic because rare mutations either in APP or in 1 of 2 enzymes that act upon it (γ-secretase and α-secretase) can cause hereditary forms of AD that are clinically and pathologically indistinguishable from the more common, so-called sporadic form of the disease. These mutations apparently exert their actions by modulating APP processing (for a review, see Gandy6).
AMYLOID PRECURSOR PROTEIN PROCESSING
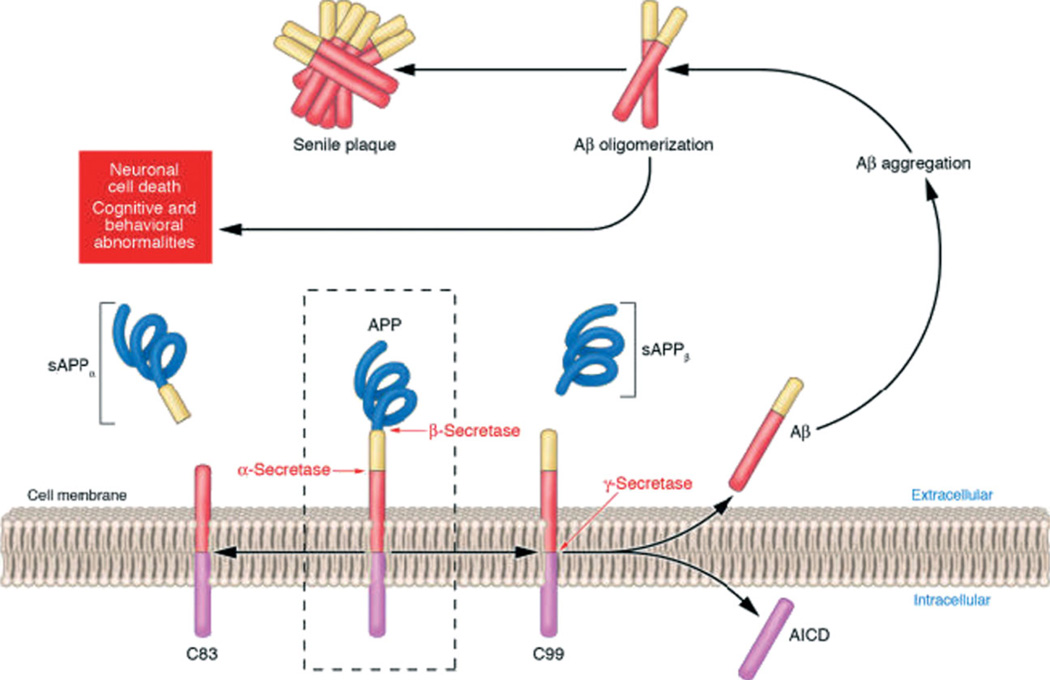
The formation of Aβ occurs though the sequential proteolysis of APP. APP is a type I, single-pass transmembrane protein that contains 3 potential sites for cleavage by various proteinases, which are designated α, β, and γ. α-Secretase cleavage by a disintegrin and metalloproteinase 10 (ADAM10) and ADAM17/transforming growth factor α–converting enzyme (or tumor necrosis factor α–converting enzyme), within the Aβ domain of APP, is the predominant and nonamyloidogenic pathway producing soluble amyloid precursor protein α (sAPPα) and the carboxyl terminal fragment C83. The β- and γ-cleavage pathway of APP produces sAPPβ and Aβ through successive cleavage by β-secretase (also known as BACE for β-amyloid precursor protein site cleaving enzyme) and the γ-secretase complex (Figure 1). The γ-secretase complex is made up of several substituents (presenilin 1, presenilin 2, presenilin enhancer 2, anterior pharynx-defective 1, and nicastrin).7 All 4 proteins must be present in order for catalysis to proceed.
Fig 1.
APP processing and Aβ accumulation. Mature APP (center and inside the dashed box) is metabolized by 2 competing pathways: the α-secretase pathway, which generates sAPPα and C83 (also known as C-terminal fragment α; left), and the β-secretase pathway, which generates sAPPβ and C99 (right). Some β-secretase cleavage is displaced by 10–amino acid residues and generates sAPPβ′ and C89. All carboxy-terminal fragments (C83, C99, and C89) are substrates for γ-secretase, which generates AICD, and the secreted peptides p3 (not shown), Aβ (right), and Glu11 Aβ. Aβ aggregates into small multimers (dimers, trimers, etc.) known as oligomers. Oligomers appear to be the most potent neurotoxins, whereas the end-stage senile plaque is relatively inert. Abbreviations: Aβ, amyloid-β peptide; AICD, amyloid precursor protein intracellular domain; APP, amyloid precursor protein; sAPP, soluble amyloid precursor protein. Reprinted with permission from Journal of Clinical Investigation.6 Copyright 2005, American Society for Clinical Investigation.
The amyloidogenic pathway produces Aβ of several different lengths ranging from 37 to 43 amino acids. The predominantly produced species is Aβ40. A trace amount of Aβ42 is produced in a ratio of approximately 99 to 1. Aβ42 spontaneously aggregates, and this makes it more likely to form oligomers; it is consequently considered to be the more toxic species. In familial Alzheimer’s disease (FAD), there are mutations in APP, presenilin 1, presenilin 2, or ADAM10. Data gathered from inherited forms of AD show that genetic defects in any of these genes are associated with the enhanced accumulation of the more toxic Aβ42 species. Some mutations cause quantitative or qualitative changes in the cleavages that generate Aβ. Other mutations are localized within the Aβ sequence of APP and cause the generation of Aβ peptides that bear an enhanced inherent predisposition toward oligomerization,8 and this provides further support for the oligomer hypothesis of Aβ neurotoxicity.
OLIGOMERIC AMYLOID-β PEPTIDE IN ALZHEIMER’S DISEASE
Several approaches have converged to implicate soluble oligomeric Aβ in AD. One such approach has employed a well-known electrophysiological correlate to learning and memory known as hippocampal long-term potentiation (LTP). Several studies have shown that hippocampal LTP can be inhibited by both synthetic and naturally secreted human Aβ oligomers. Some investigators have reported that the oligomeric Aβ levels needed to disrupt LTP correlate well with the amount observed in cerebrospinal fluid in AD patients. Furthermore, the effects of Aβ oligomers on LTP can be reduced through the application of anti-Aβ antibodies in vivo.9–12
Several studies have shown that hippocampal long-term potentiation, which is correlated with learning and memory, can be inhibited by both synthetic and naturally secreted human amyloid beta oligomers.
Although LTP correlates with enhanced learning and memory, the opposite can be said for long-term synaptic depression (LTD). Therefore, LTD in the hippocampus is a negative regulator of learning and memory. Using Aβ oligomers from several sources (cell cultures and synthetic and human brain extracts) on mouse hippocampal slices, Li et al.11 demonstrated that oligomeric Aβ enhanced LTD. The enhancement of LTD could be blocked by the addition of a glutamate scavenger, and this suggests that oligomeric Aβ might facilitate LTD through a glutamate-dependent system.11 Thus, oligomeric Aβ may lead to a disruption in learning and memory by inhibiting prosynaptic LTP and enhancing the silencing of LTD, and this appears to occur via a glutamatergic mechanism.
In addition to LTP, behavioral studies have been performed in which human Aβ oligomers were infused into the hippocampus of living wild-type rats. The direct application of oligomeric Aβ allows for the immediate study of the effects of acute treatment. In one study by Cleary et al.,12 human Aβ oligomers were generated in Chinese hamster ovary cells and secreted into a conditioned medium from which these Aβ oligomers were collected. Rats were studied before and after treatment with intrahippocampal injections of Aβ and then placed in the Morris water maze, in which they had been pretrained to find a hidden platform in a pool of opaque liquid. These animals developed a deficit in learning and memory that was readily observable in the Morris water maze after the administration of Aβ42. This deficit was reversible upon washout or the application of an anti-Aβ antibody. Thus, the in vitro LTP and LDP results appeared to be relevant in vivo as well.
Rats developed a deficit in learning and memory that was readily observable in the Morris water maze after the administration of Aβ42.
Other brain regions are also involved in transgenic mouse models of AD. In a recent study, España et al.,13 using the inhibitory avoidance/fear conditioning paradigm, found an enhanced level of freezing behavior in APP Indiana, APP Sweden/Indiana, and triple mutant (3xTg-AD) mouse models of AD. Using immunocytochemistry, they observed that these mutant mice had both extracellular amyloid deposits and intraneuronal Aβ-like immunoreactivity in neurons of the basolateral amygdala, a region of the brain known to modulate the acquisition of fear and fear-related behaviors. The observed increase in freezing behaviors by the mutant mice was ameliorated by the administration of several anxiolytics, and this suggests that the behavioral abnormalities were caused by an increase in anxiety. These findings suggest that Aβ accumulation in the basolateral amygdala contributes to an increase in anxiety in the mouse model. The data also raise the possibility that intraneuronal Aβ may play a role in Aβ toxicity; this notion has been raised in the past and remains unresolved.
PHYSIOLOGICAL ROLES FOR AMYLOID-β PEPTIDE OLIGOMERS
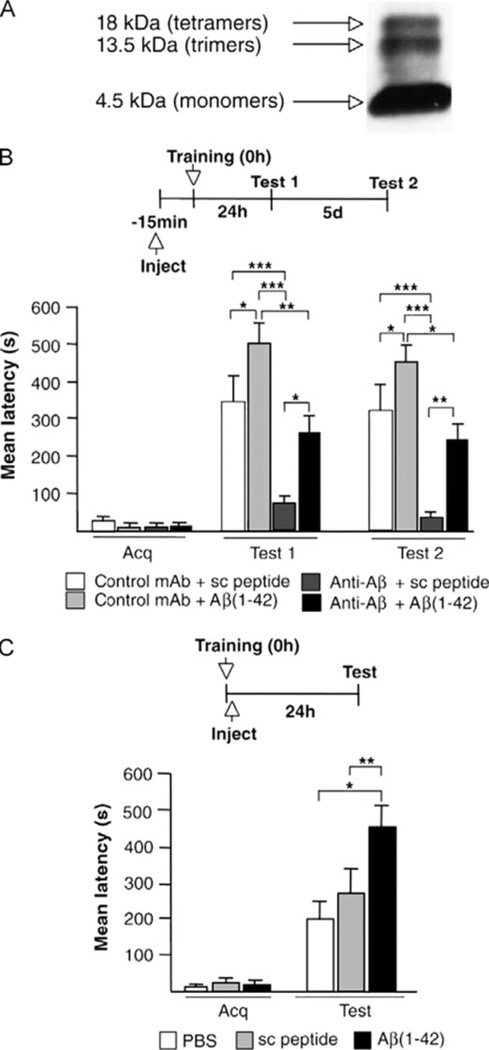
Although it is clear that high levels of oligomeric Aβ in mammalian models impair LTP, new evidence is emerging that Aβ oligomers in normal physiological concentrations can positively regulate learning and/or memory. Garcia-Osta and Alberini14 performed a series of inhibitory avoidance/fear conditioning experiments in which a rat was trained by the application of a painful foot shock in association with a given area of the test apparatus. The animal’s aversion was then measured either 1 hour after training (short-term memory) or 24 hours after training (long-term memory). In one experiment, a monoclonal antibody specific to Aβ (4G8) or a control antibody was administered 15 minutes prior to training. The results demonstrated a deficit in both the short-term and long-term memory tests after treatment with anti-Aβ but not after treatment with the control antibody (Figure 2).14 Alternatively, when picomolar amounts of exogenous human Aβ42 were added in combination with the anti-Aβ antibody, a significant rescue of the amnesia was observed. In this instance, the interpretation is that the anti-Aβ antibody bound endogenous Aβ that was putatively involved in physiological learning and memory processing.
New evidence is emerging that Aβ oligomers in normal physiological concentrations can positively regulate learning and/or memory.
Fig 2.
Memory impairment produced by the depletion of endogenous Aβ(1–42) is rescued by exogenous oligomeric human Aβ(1–42). (A) An oligomeric/monomeric preparation of Aβ42 was examined via 4% to 12% tris-tricine nondenaturing polyacrylamide gel electrophoresis/western blotting with the anti-Aβ monoclonal antibody 6E10 (Covance Research; 1:1000). Bands corresponding to tetramers, trimers, and monomers were detected. (B) Acq and retention, expressed as the mean latency ± SEM (seconds), of rats that received intrahippocampal injections of either anti-Aβ or a control monoclonal antibody combined with either sc peptide or Aβ(1–42) 15 minutes before IA training. *P < 0.05, **P < 0.01, and ***P < 0.001. Test 1 occurred 24 hours after training; test 2 occurred 5 days after training. (C) Acq and retention, expressed as the mean latency ± SEM (seconds), of rats that received intrahippocampal injections of PBS, sc peptide, or Aβ(1–42) immediately after IA training. The administration of Aβ(1–42) immediately after IA training enhanced memory retention 24 hours after training. *P < 0.05 and **P < 0.01. Abbreviations: Aβ, amyloid-β peptide; Acq, memory acquisition; IA, inhibitory avoidance; PBS, phosphate-buffered saline; sc, scrambled; SEM, standard error of the mean. Reprinted with permission from Learning & Memory.14 Copyright 2009, Cold Spring Harbor Press.
Similar experiments were performed on mice by Puzzo et al.15 Using an experimental system similar to the one that Cleary et al.12 employed to demonstrate the detrimental effects of high levels of oligomeric Aβ, Puzzo et al. were able to show a learning and memory benefit when picomolar quantities of oligomeric Aβ were used. Then, using brain sections, these same investigators demonstrated that LTP was increased in mice that received picomolar quantities of Aβ, but when levels of Aβ over 200 pM were applied, a deficit developed. This was reinforced by behavioral studies in which those mice that were treated with picomolar Aβ had an enhanced Morris water maze performance and better fear memory in inhibitory avoidance/fear conditioning tests.15 These observations suggest that oligomeric Aβ has an inverted U-shaped dose-response curve, which is consistent with the proposal that this peptide has mnemonoactive properties.
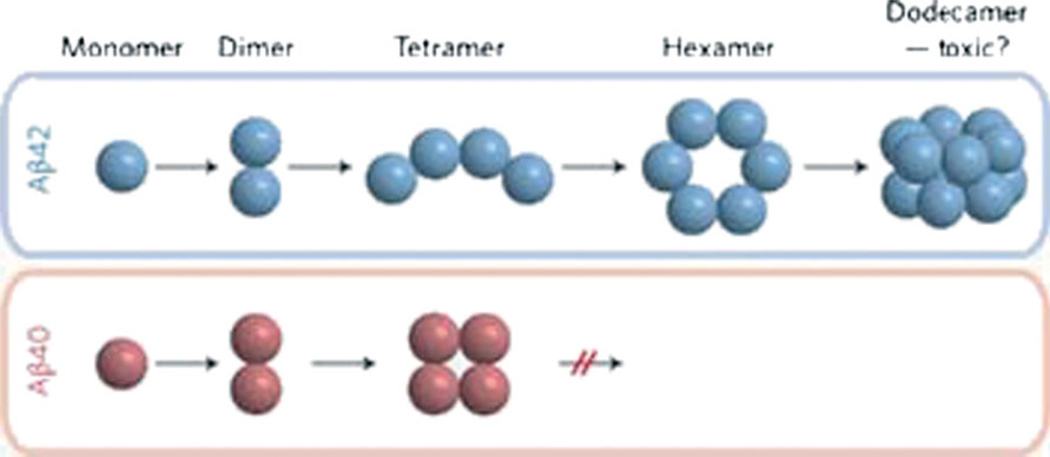
In addition to the Aβ concentration, however, current data suggest that the different conformations and species of oligomeric Aβ are functionally distinct. To address this issue, Murray et al.16 used mass spectrometry coupled with ion mobility spectrometry to determine the number of Aβ peptides per aggregate in a series of experimental solutions.
Solutions containing Aβ40 only, Aβ42 only, or a mixture of the two were analyzed for the relative quantities of the differently sized oligomers (ie, dimers, tetramers, hexamers, etc.). Aβ40 oligomers reached a maximum size at the tetramer level, whereas Aβ42 was capable of producing much larger oligomers (ie, up to dodecamers; Figure 3). However, when Aβ40 and Aβ42 were combined in solution, the largest resulting oligomer was only a tetramer. Therefore, Aβ40 is capable of suppressing the formation of the larger oligomers seen with Aβ42 alone.16 In addition to suggesting new pharmaceutical targets, this finding adds strength to the theory that the ratio of Aβ40 to Aβ42 is a key determinant of risk for AD.
Fig 3.
Relationship linking the Aβ peptide species to the oligomerization state and neurotoxicity. Abbreviation: Aβ, amyloid-β peptide. Reprinted with permission from Nature Chemistry.20 Copyright 2009, Nature Publishing Group.
Another possible explanation for the Aβ benefit/deficit paradox invokes a role for monomeric Aβ. Guiffida et al.17 demonstrated that cultured neurons, under conditions of insulin deprivation, appeared to benefit when monomeric Aβ42 was administered. This benefit correlated with an observed increase in the phosphorylation of Akt, and this suggests that the rescue of the neurons occurred through the insulin-responsive phosphatidylinositol-3-kinase pathway. Monomers of Aβ42 were also able to protect against an N-methyl-d-aspartic acid–induced excitotoxic death, the opposite of which is observed in the presence of aggregated Aβ. Interestingly, there was no neuronal protection when the monomeric Aβ42 contained the Arctic (E22G) substitution, a rare FAD mutation that leads to rapid Aβ oligomerization. These results suggest that a lack of monomeric Aβ can be detrimental. Therefore, the increased formation of oligomers that occurs in association with many FAD mutations might cause neurotoxicity, at least in part by depleting the supply of beneficial Aβ monomers while simultaneously increasing the levels of potentially neurotoxic Aβ oligomers.17
Another recent study sheds more light on the toxicity of Aβ42. In the amino acid sequence of Aβ42, there is a hydrophobic GxxxG motif that is vital for dimerization. Substitution of Gly33 for the more hydrophobic residues of either alanine or isoleucine within the dimerization motif resulted in a marked increase in the formation of higher order oligomers (16- to 20-mers). Surprisingly, even with larger aggregates, the Gly33 substitution proved to be nontoxic in comparison with normal Aβ42 in both neuronal cells and the Drosophila eye photoreceptor assay. Furthermore, it was found that substitution of Gly33 abolished the deleterious effect that Aβ42 has on LTP, and this indicates that Gly33 is necessary for Aβ42 toxicity and, more importantly, that toxicity is not directly correlated with all higher order oligomers because dimers, trimers, and tetramers were the most toxic in this experimental paradigm.18
The issue of what happens when extracellular oligomeric Aβ binds to neurons was recently addressed by Laurén et al.19 These researchers suggested that cellular prion protein (PrPc) might act as a neuronal receptor for oligomeric Aβ. As previously shown, oligomeric Aβ inhibits LTP in the hippocampus of wild-type mice. In Laurén et al.’s study, mice lacking PrPc exhibited little or no inhibition of LTP after treatment with oligomeric Aβ. Furthermore, blocking PrPc with antibodies was also sufficient to ameliorate the detrimental effects of oligomeric Aβ. However, PrPc accounted for only 50% of the total neuronal binding of oligomeric Aβ.19 Additional binding of Aβ is presumably due to other receptors, perhaps including low-density lipoprotein receptors, which have been shown to bind several ligands, including Aβ. Alternatively, there could be as yet unknown factors that are responsible for binding oligomeric Aβ. More studies are required to confirm and elucidate this potential role for PrP in Aβ neurotoxicity.
CONCLUSIONS
Recent advances initially demonstrated that soluble oligomers of Aβ may be key to the pathogenesis of AD. However, with the dissection of the concentration dependence and assembly state dependence, soluble oligomeric Aβ species are now believed to play vital physiological functions in learning and memory. The discovery of PrPc as a functional receptor of Aβ oligomers may enhance our understanding of how Aβ leads to cognitive defects. However, the binding of Aβ by PrPc is responsible for only 50% of the observed neuronal oligomer binding capacity, and this raises the question of what other receptors for oligomeric Aβ might exist. The evolving concepts of amyloid plaques, amyloid fibrils, and amyloid oligomers point to the necessity of reflection and continual re-evaluation of our concepts of physiology and pathogenesis in normal brain function and in AD.
ACKNOWLEDGMENT
This work was supported by NIA P01 AG10491 (to Sam Gandy), P50 AG05138 (to Mary Sano), and the Cure Alzheimer’s Fund.
Footnotes
DISCLOSURES
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 3.Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 4.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haass C. Take five–BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J. 2004;23:483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 9.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar GM, Bloodgood BL, Townsend M, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Hong S, Shepardson NE, et al. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 13.España J, Giménez-Llort L, Valero J, et al. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol Psychiatry. doi: 10.1016/j.biopsych.2009.06.015. Available at: http://www.sciencedirect.com. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puzzo D, Privitera L, Leznik E, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray MM, Bernstein SL, Nyugen V, et al. Amyloid beta protein: Abeta40 inhibits Abeta42 oligomerization. J Am Chem Soc. 2009;131:6316–6317. doi: 10.1021/ja8092604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuffrida ML, Caraci F, Pignataro B, et al. β-Amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmeier A, Wozny C, Rost BR, et al. Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity. J Neurosci. 2009;29:7582–7590. doi: 10.1523/JNEUROSCI.1336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurén J, Gimbel DA, Nygaard HB, et al. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein SL, Dupuis NF, Lazo ND, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]