Abstract
Several authors have shown that injections of the GABAA agonist muscimol into the medial shell region of the nucleus accumbens (AcbSh) result in large increases in food, but not water, intake. In previous studies we demonstrated that intra-AcbSh injections of either muscimol or of the indirect dopamine agonist amphetamine increase response output on a food-reinforced progressive ratio schedule. In the current experiment we extended these observations by examining the effects of muscimol and amphetamine injections on the performance of a water-reinforced progressive ratio task in mildly deprived animals. We found that muscimol did not affect the number of responses made in the water-reinforced task, even though a marked increase in responding was observed after amphetamine. Muscimol did, however, significantly increase food intake in these same animals. The results suggest that the enhancing effects of intra-AcbSh muscimol differ from those of amphetamine in that they are selective for food-reinforced behaviors.
Keywords: Feeding, Food intake, Ventral striatum, Drinking, Operant, Intracranial
1. Introduction
Injections of the GABAA agonist muscimol into the shell region of the nucleus accumbens (AcbSh) strongly potentiate food intake (Basso and Kelley 1999; Hanlon et al. 2004; Soderpalm and Berridge 2000; Stratford and Kelley 1997; Stratford and Kelley 1999; Stratford and Wirtshafter 2004; Taha et al. 2009). This effect appears to be specific to the medial AcbSh and similar injections in the accumbens core do not increase feeding (Reynolds and Berridge, 2001; Stratford and Kelley 1997). Furthermore, inactivation of the AcbSh fails to promote ingestion of either water or salt solutions (Basso and Kelley 1999; Stratford and Kelley 1997; Stratford et al. 1998; Stratford and Wirtshafter 2004). Although it has been suggested that these effects may reflect activation of a “feeding pattern generator,” (Baldo and Kelley 2007; Kelley et al. 2005; Meredith et al. 2008) it appears more likely that basic motivational processes are affected. For example, we have shown that intra-AcbSh muscimol injections are able to increase food-reinforced operant responding on a progressive ratio task (Wirtshafter and Stratford 2010).
In addition to GABA, dopamine within the nucleus accumbens plays an important role in motivated behaviors, although much remains to be learned about the relative importance of the shell and core regions. In general, dopaminergic manipulations in the accumbens tend to have more pronounced effects on appetitive behavior than they do on consummatory behaviors such as eating or drinking (Everitt 1990; Hanlon et al. 2004; Nowend et al. 2001). Furthermore, the effects of dopaminergic manipulations on appetitive behavior appear to be generalized, insofar as they can affect behaviors maintained by a number of different reinforcers (Everitt 1990; Gerber et al. 1981). It is also notable in this regard that dopamine release within the nucleus accumbens can be increased by presenting an animal in the appropriate state not only with food, but also with water, a salt solution, pups or a sexual partner (Afonso et al. 2009; Joseph and Datla 2003).
The available data thus suggest that GABAergic manipulations of the accumbens may influence primarily food-related behaviors, whereas the effects of dopaminergic manipulations may be much less selective. If correct, this conclusion would have important implications for the functional organization of the ventral striatum, but the available data are not sufficient to conclude with confidence that the two transmitter systems in the AcbSh actually differ in their effects on operant behavior. In particular, very little is known about the effects of AcbSh GABAA receptor stimulation on behaviors maintained by natural reinforcers other than food. It is quite possible, for example, that injections of muscimol into the AcbSh might promote responding for a variety of different reinforcers, even though the effects of these treatments on consummatory behaviors might be relatively specific for feeding. Along similar lines, many studies have investigated the behavioral role of dopamine in the accumbens, but relatively few of these have specifically examined the shell region of this structure. Thus, manipulations of dopamine in the AcbSh might produce behavioral effects that are more selective than those seen after treatments affecting the core.
In view of these considerations, the current study compared the effects of intra-AcbSh injections of muscimol and amphetamine on a water-reinforced progressive ratio (PR) task. We used methods closely modeled on those we employed in a previous study to demonstrate that intra-AcbSh injections of both muscimol and of amphetamine increase responding on a food-reinforced progressive ratio schedule (Wirtshafter and Stratford 2010). If the effects of muscimol in the AcbSh were specific to food-reinforced behaviors, one would expect that this drug would not increase responding in a water-reinforced task. On the other hand, if stimulation of GABAA receptors exerts a generalized potentiating effect on operant behavior, as is likely to occur after dopamine receptor stimulation, one would expect that both muscimol and amphetamine would enhance responding. We also examined the effects of muscimol injections on food intake, in order to confirm the ability of the drug to produce hyperphagia in the animals examined in this study.
2. Methods
2.1. Animals
Subjects were seven male Sprague – Dawley rats obtained from Charles River (Chicago, IL) weighing approximately 300 g at the time of surgery. Animals were individually housed in plastic cages with food (Wayne LabBlox) and water available ad libitum, except as noted below.
2.2. Surgery
Rats were anesthetized with sodium pentobarbital (60 mg/kg) and 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted bilaterally using standard, flat-skull stereotaxic techniques. The guide cannulae were aimed to terminate 2.0 mm dorsal to the AcbSh at coordinates of anteroposterior: 1.6, mediolateral: ±0.8, and dorsoventral: 6.1 (mm from bregma). The guide cannulae were held in place by stainless steel screws and denture lining material and stainless steel obturators were inserted into the lumen of each cannula to help maintain patency. Each rat was allowed to recover for at least seven days before deprivation and operant training began.
2.3. Apparatus
Animals were trained in standard twin lever operant chambers (Med-Associates, St. Albans, VT) housed within sound attenuating enclosures with ventilation and masking noise provided by an exhaust fan. Each chamber was equipped with a retractable drinking tube attached to lickometer circuitry (Med-Associates) which allowed for the recording of the times at which licks occurred. The chambers were also supplied with click generators to provide additional audible feedback at the time of water delivery.
2.4. Operant training
The methodology employed here was basically identical to that we have used in previous studies, except that water, instead of food, served as the reinforcer. After recovering from surgery, rats were placed on a restricted water access schedule, which continued for the remainder of the experiment. Animals were given access to water for 1 hour each day in their home cages; after the start of operant training, access to water began approximately two hours following operant testing. (Pilot data indicated that this level of water deprivation resulted in breaking points on the operant schedule being similar to those seen in our earlier study of food deprivation.) One day after starting on this deprivation schedule, animals were given a 30 min “magazine training session” in the operant boxes during which the water tubes were presented for five sec periods at 1-min intervals, with a “click” being generated at the time that water became accessible. Animals were then manually shaped to lever press over the next one or two days, after which they were placed on a continuous reinforcement schedule for three days. Rats were then given one 30-min session of training on an FR2 schedule followed the by two days on an FR4 schedule after which they were switched to progressive ratio 6 (PR6) schedule, which continued for the remainder of the experiment. On each day of PR6 training, rats were placed into the operant chambers with the house lights on and both levers extended; only one lever was associated with water access, although presses on both levers were recorded. The first response on the correct lever was followed by access to the water spout for five seconds, with insertion of the tube being paired with the operation of the clicker. The number of responses required to earn each subsequent water access was incremented by six after each reinforcement, so that seven responses were required to earn the second access, 13 to earn the third, and so on. The time of each lever press was recorded, as was the latency to the first lick and the number of licks per access. Each session continued until a pause in responding of 3 min duration occurred, a cut-off used previously by ourselves and other authors (Reilly 1999; Wirtshafter and Stratford 2010), or 60 min elapsed, at which time the house lights were turned off, the levers retracted, and the rats removed from the chambers. The breaking point was defined as the value of the final ratio completed in the session. Animals were run for five to six days per week and were given 16 daily training sessions on the PR6 schedule before the start of drug treatments.
2.5. Intracerebral injections
In order to make injections, rats were restrained gently, the obturators removed, and a 28-gauge stainless steel injection cannula, extending 2.0 mm beyond the tip of the guide, was inserted into each guide cannula. Rats then received simultaneous bilateral 0.50 μl infusions at a rate of 0.33 μl/min by means of a motor-driven microsyringe connected to the injection cannulae through a length of fluid-filled polyethylene tubing. After the infusions, the injection cannulae were left in place for an additional 60 s in order to minimize leakage up the cannula track after which they were removed and replaced with the obturators. Animals were then returned to their home cages for a period of 10 min, to allow for drug diffusion, at which time they were placed in the operant boxes for their daily run. Each subject received one saline injection several days prior to the start of drug injections to acclimate them to the procedure.
2.6. Operant testing procedure
Beginning on day 17 of training on the PR6 schedule, animals were tested on the PR6 schedule following injections, in a randomized order, of muscimol (50 ng/side, Tocris, Ellisville, MO), D-amphetamine (10 ug/side, Sigma Chemical Co, St. Louis, MO), or the sterile, isotonic saline vehicle. These doses were identical to those we have used in previous studies (Wirtshafter and Stratford 2010). Injections were separated from each other by at least 48 hr and animals were run in the operant boxes on at least one intervening day. Two days following the last injection of this series, two of the animals received an additional operant session following intra-AcbSh injections of muscimol at a dose of 100ng/side.
2.7 Food intake
Following the completion of operant studies, animals were given three days to recover from the water deprivation schedule, after which food intake was measured. Animals were tested in hanging mesh cages measuring 24 cm wide X 29 cm long X 20 cm high. In order to acclimate the animals these cages, each subject was placed in them three times before the start of testing; each acclimation session lasted one hour and food was available on the floor of the cage. Food intake in each animal was then measured after injections of saline and muscimol (50 ng/side), the order of testing being counterbalanced between subjects. During each session, animals were placed into the testing cages containing preweighed quantities of their standard chow 10 min following injections. One hr later, animals were removed from the test environments and the amounts of spillage and of food remaining in the test cages measured. The two tests were separated from each other by at least two days.
2.8. Perfusion and histology
At the completion of behavioral studies, animals were deeply anesthetized with sodium pentobarbital and then perfused transcardially with saline followed by 10% formalin. The brains were then removed and stored in formalin for several days after which cryostat sections were prepared through the injection sites at a thickness of 50 μm and subsequently stained with cresyl violet.
3. Results
3.1. Histology
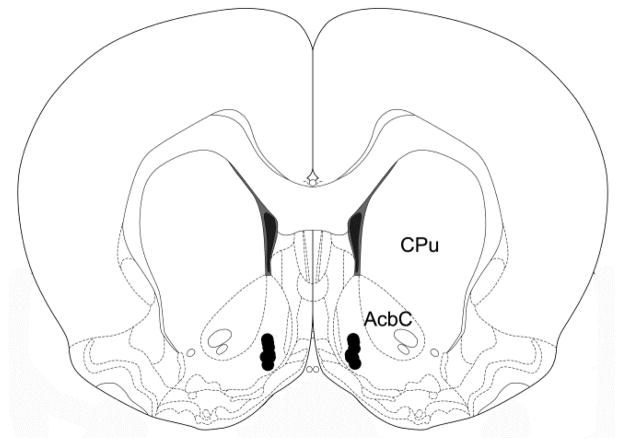
The locations of the cannula placements are shown in Fig. 1. All cannula tips terminated within the medial AcbSh at placements similar to those in our previous studies.
Figure 1.
Schematic representation of injection sites in the nucleus accumbens shell (modified from Paxinos and Watson, 2007). Cannula tips were located within 0.3 mm of the antero-posterior level depicted in the figure (1.6 mm rostral to bregma.) ( AcbC: nucleus accumbens core, CPu: caudate-putamen.
3.2. Operant behavior
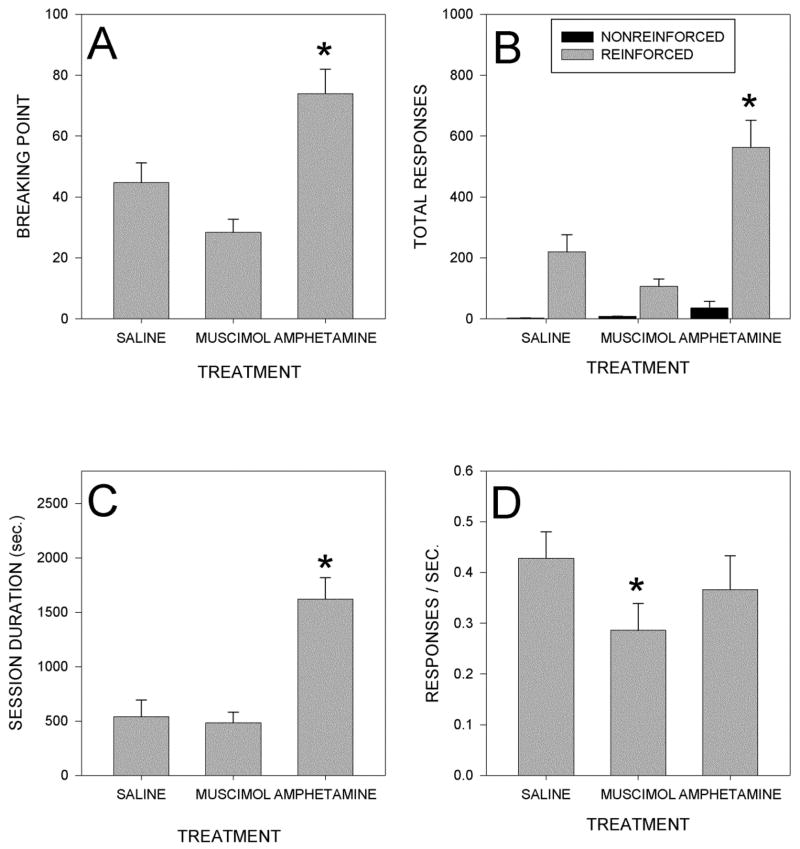
Several aspects of performance on the PR6 schedule are depicted in Fig. 2. Panel A displays breaking points, that is the values of the highest ratios completed in the test sessions. It can be seen that, relative to saline injections, breaking points were increased by amphetamine, but actually tended to be reduced by muscimol injections. Analysis of this data by means of a one-way repeated measures ANOVA indicated a significant effect of treatment (F(2,12)=18.4; p<0.001). Post hoc comparisons, adjusted for multiple testing using the Bonferroni technique, indicated that breaking points after amphetamine injections were significantly higher than those seen after saline (p<0.04) or muscimol injections (p<0.002), but that the values observed after muscimol and saline injections did not differ significantly (p>0.2).
Figure 2.
Panel A: Mean breaking points of rats after injections of saline, muscimol (50 ng/side) or amphetamine (10 ug/side) into the AcbSh.. Panel B: Mean total responses on the reinforced and non-reinforced levers after injections of saline, muscimol or amphetamine. Panel C: Mean session durations after injections of saline, muscimol or amphetamine into the AcbSh. Panel D: Mean response rates measured across the entire session after injections of saline, muscimol or amphetamine. *=p<.05 vs saline condition.
Data for total presses on the active and inactive levers are shown in Panel B. Analysis of the data on the active lever indicated a significant overall effect of treatment (F(2,12)=19.5; p<0.001) and post hoc tests indicated that more responses were made after amphetamine than after either saline (p<0.03) or muscimol (p<0.01), whereas responding after muscimol did not differ significantly from saline (p>0.2). Presses on the inactive lever did not differ significantly between groups (p>0.2), although there was a trend for an increase after amphetamine, which might reflect in part the longer session durations seen in these animals.
Increases in the total number of presses made could be the result of animals either pressing for a longer time or pressing at a higher rate. Panel C of Fig. 2 shows session durations (i.e., the time interval between the first and last press made in the session.) ANOVA indicated a significant effect of drug treatment (F(2,12)=21.2; p<0.01) and post hoc comparisons indicated that this resulted from amphetamine treated animals pressing for a longer period of time than either saline (p<0.02) or muscimol (p<0.001) treated rats, whereas muscimol injections did not significantly alter session duration (p>0.2).
Gross rates of pressing (i.e., total number of press divided by session duration) on the active lever are shown in Panel D, which shows that rates tended to be reduced by amphetamine, and, to a larger extent, by muscimol. An ANOVA indicated significant differences between groups (F(2,12)=7.3; p<0.01) and post hoc comparisons showed that the only significant difference was between muscimol and saline treated animals (p<0.01). Gross rates of pressing on the inactive lever (not shown) also failed to differ between groups (p>0.3), although a small trend for an increase after amphetamine was present.
In addition to the effects on lever pressing shown in Fig. 2, we also examined latencies from reinforcer presentation to the first lick and the mean numbers of licks made per reinforcer presentation. No significant effects were observed on these variables.
3.3. Food intake
One subject dislodged its cannula during the testing of ingestive behavior, so data are presented from the remaining six rats. Mean food intake in the 60 min following saline injections was 1.5±0.8g, as compared to 6.3±1.4g after muscimol (F(1,5)=33.8; p<0.002).
4. Discussion
The most important result of the current study is the finding that injections of muscimol into the AcbSh failed to increase responding on a PR6 schedule of water reinforcement. This result stands in marked contrast to the potentiation of responding we observed in a previous study using a food-reinforced PR6 schedule, where muscimol exerted an effect very similar to that of amphetamine (Wirtshafter and Stratford 2010). We attempted to match the methodologies of the two studies as closely as possible and the breaking points we obtained here after saline injections were indeed very similar to those we observed in our earlier study. Intra-AcbSh amphetamine in the current experiment produced increases in responding which were of similar magnitude to those we observed in the food-reinforced task, again suggesting that the two tasks did not differ in some fundamental way other than the difference in reinforcers. Although one must always be cautious in interpreting negative results, it is very unlikely that our failure to demonstrate an increase in responding was due to lack of statistical power, because there was not even a suggestion of increased responding in our data; in fact, muscimol treatments actually tended to decrease breaking points and total responses. It also seems unlikely that the lack of an enhancement was due to an inappropriate choice of doses as we employed here the same 50 ng/side dose that produced a clear increase in responding in the previous food-reinforced task. Indeed, in the current experiment, injection of this same dose of muscimol into the AcbSh of the same animals used in the operant study produced a pronounced and significant increase in food intake. Additionally, two of the animals in the current study received a further water-reinforced PR session after injections of muscimol at a higher dose (100ng/side), but, again, no trend for an increase was present (mean breaking point in these animals was 49.0 after injections of either saline or muscimol). Our findings demonstrate that a dose of muscimol in the AcbSh which is able to potentiate both feeding and food-reinforced PR responding, is unable to increase responding on a water-reinforced PR task. It seems likely that this finding is related to the fact that inactivation of the AcbSh is unable to increase water intake in either deprived or nondeprived animals (Basso and Kelley 1999; Stratford and Kelley 1997; Stratford et al. 1998; Stratford and Wirtshafter 2004) and is further evidence that muscimol injections in the AcbSh selectively influence behaviors related to feeding. Taken together, these data suggest that intra-AcbSh muscimol might not have direct actions on operant behavior but may only affect progressive ratio performance indirectly, as a consequence of selective alterations in the “reward” or “incentive” value of food or in the “drive” to obtain food. It is possible that some of the effects of intra-AcbSh muscimol which have been observed in more complex behavioral situations involving food reinforcement (Corbit and Balleine 2011; Floresco et al. 2008; Stopper and Floresco 2011) might also result, at least in part, from alterations in specific feeding-related mechanisms, rather than from more general motivational or cognitive effects.
In the current experiment, muscimol produced a small but significant decrease in the gross rate of lever pressing across the session as compared to saline, although the effect of this drug did not differ from that of amphetamine. Further studies will be required to evaluate the import of this effect, but it should be noted that, although not significant, a trend towards decreased gross response rates was also observed in our previous study of food reinforced PR6 performance (Wirtshafter and Stratford 2010).
In contrast to the effects observed with muscimol, we found that injections of amphetamine into the-AcbSh produced an increase in breaking point and session duration on the water-reinforced PR6 schedule that were very similar in magnitude to those we previously observed on the food-reinforced version of the task. Although some workers have suggested that dopamine in the AcbSh is selectively involved in behaviors maintained by drugs of abuse, but not by natural rewards (Bari and Pierce 2005), the current results, and a number of others (Nowend et al. 2001; Wirtshafter and Stratford 2010; Zhang et al. 2003), suggest that this distinction cannot be absolute. Other authors have observed effects of amphetamine given into the AcbSh (Hanlon et al. 2011; Wirtshafter and Stratford 2010; Zhang et al. 2003), and, sometimes, systemically (Poncelet et al. 1983; Smith et al. 1997; Thomas 1976; Thompson 1972) on food-reinforced PR tasks, but the current study is the first demonstration, to our knowledge, that stimulation of AcbSh dopamine receptors can promote responding on a water-reinforced task. These findings support the view that the effects of manipulations of AcbSh dopamine receptors do not depend upon the nature of the reinforcer. Such effects might occur if stimulation of dopamine receptors in the AcbSh were to increase the incentive value of all positively valenced stimuli, but it seems even simpler to propose that dopamine may influence some mechanism linked to response output, such as behavioral activation or the exertion of effort (Salamone et al. 2011). The effect of intra-AcbSh amphetamine on water intake does not appear to have been investigated, but given that amphetamine at this site does not alter food intake (Hanlon et al. 2004), it seems unlikely that drinking would be affected. This dissociation would further support the view that the effects of dopamine receptor stimulation in the AcbSh on operant behavior are not secondary to effects on consummatory behavior.
The current findings support other data in showing that drastically different behavioral effects can be produced by stimulation of dopamine and GABAA receptors within the AcbSh. Thus, intra-AcbSh muscimol increases feeding and potentiates responding reinforced by food, but not water. In contrast, amphetamine fails to influence food intake, but potentiates responding maintained by either food or water. Amphetamine in the shell also appears to have much more pronounced effects on locomotor activity than does muscimol (Heidbreder and Feldon 2011; Ikemoto 2002; Pothuizen et al. 2005; Schildein et al. 1999; Stratford and Kelley 1997). It does not seem likely that these two patterns of effects could result from disturbances in a single functional mechanism, nor is it clear what neural circuitry could underlie the ability of stimulation of different receptors within the AcbSh to produce such varied, nonreciprocal, patterns of behavior. One possibility is that some cells within the AcbSh are selectively sensitive to either GABA or dopamine agonists so that they are affected by only one of the two types of drugs. Alternatively, it is plausible that muscimol and amphetamine are associated with different patterns of activity within a single population of cells and that these different patterns are associated with distinctive behavioral effects. Any comprehensive theory of the functions of the AcbSh will have account for these different patterns of behavioral effects, and it is clear that doing so will provide a substantial challenge for future research.
Highlights.
Progressive ratio performance examined after drug injections in accumbens shell.
Amphetamine increased responding for water reward.
The amphetamine effect was due to increased duration of sessions.
Muscimol increased food intake, but did not increase responding for water.
We conclude that effects of muscimol, but not amphetamine, are food specific.
Acknowledgments
This publication is based upon work supported by grants 0641943 from the National Science Foundation, R01DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases, and R03DA020802 from the National Institute for Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health. The authors thank Beth Cowgill for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsivness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Hom Behav. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacol. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neurosci. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: Regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–326. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neurosci. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Sing J, Wise RA. Pimozide attenuates lever pressing for water reinforcement in rats. Physiol Behav. 1981;14:201–205. doi: 10.1016/0091-3057(81)90243-4. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacol. 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Benca RM, Baldo BA, Kelley AE. REM sleep deprivation produces a motivational deficit for food reward that is reversfed by intra-accumbens amphetamine in rats. Brain Res Bull. 2011;83:245–254. doi: 10.1016/j.brainresbull.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 2011;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neurosci. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Joseph MH, Datla KYAMJ. The interpretation of the measurment of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci. Biobehav Rev. 2003;27:527–541. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Strut Funct. 2008;231:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism the nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Chermat R, Soubrie PSP. The progressive ratio schedule as a model for studying the psychomotor stimulant action of drugs in the rat. Psychopharm. 1983;80:184–189. doi: 10.1007/BF00427967. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Fendon J. The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacol. 2005;30:683–696. doi: 10.1038/sj.npp.1300643. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S. Reinforcement value of gustatory stimuli determined by progressive ratio performance. Pharmacol Biochem Behav. 1999;36:301–311. doi: 10.1016/s0091-3057(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacol. 2011;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schildein S, Agmo A, Huston JP, Schwarting RKW. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1999;790:185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharm. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Berridge KC. Food intake after diazepam, morphine or muscimol microinjections in the nucleus accumbens shell. Pharmacol Biochem Behav. 2000;66:429–434. doi: 10.1016/s0091-3057(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Neurosci. 2011;11:97–112. doi: 10.3758/s13415-010-0015-9. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley AE. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. NeuroReport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- Taha SA, Katsuura Y, Noorvaash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neurosci. 2009;161:718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JR. Interaction between hyperbaric air and d-amphetamine effects on performance. Psychopharmacol. 1976;48:69–73. doi: 10.1007/BF00423308. [DOI] [PubMed] [Google Scholar]
- Thompson DM. Effects of d-amphetamine on the breaking point of progressive-ratio performance. Psychonomic Sci. 1972;29:282–284. [Google Scholar]
- Wirtshafter D, Stratford TR. Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol Biochem Behav. 2010;96:342–346. doi: 10.1016/j.pbb.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]