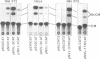Abstract
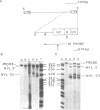
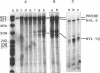
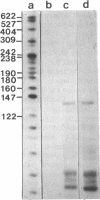
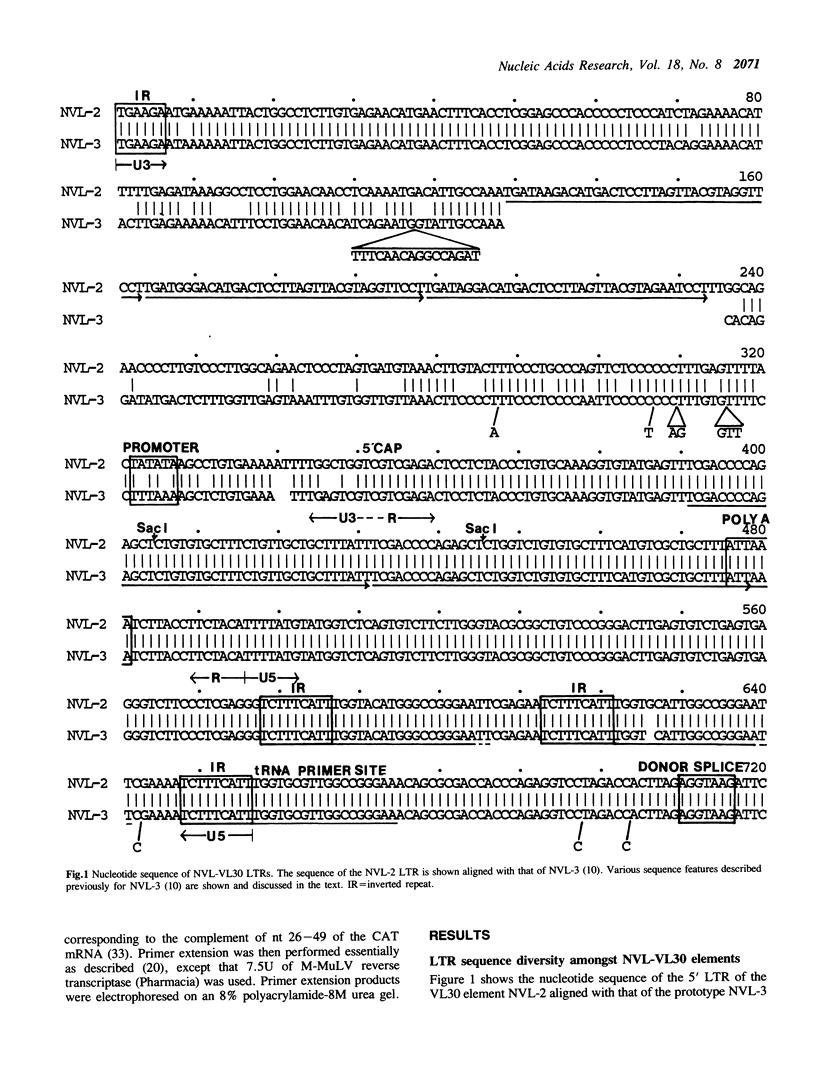
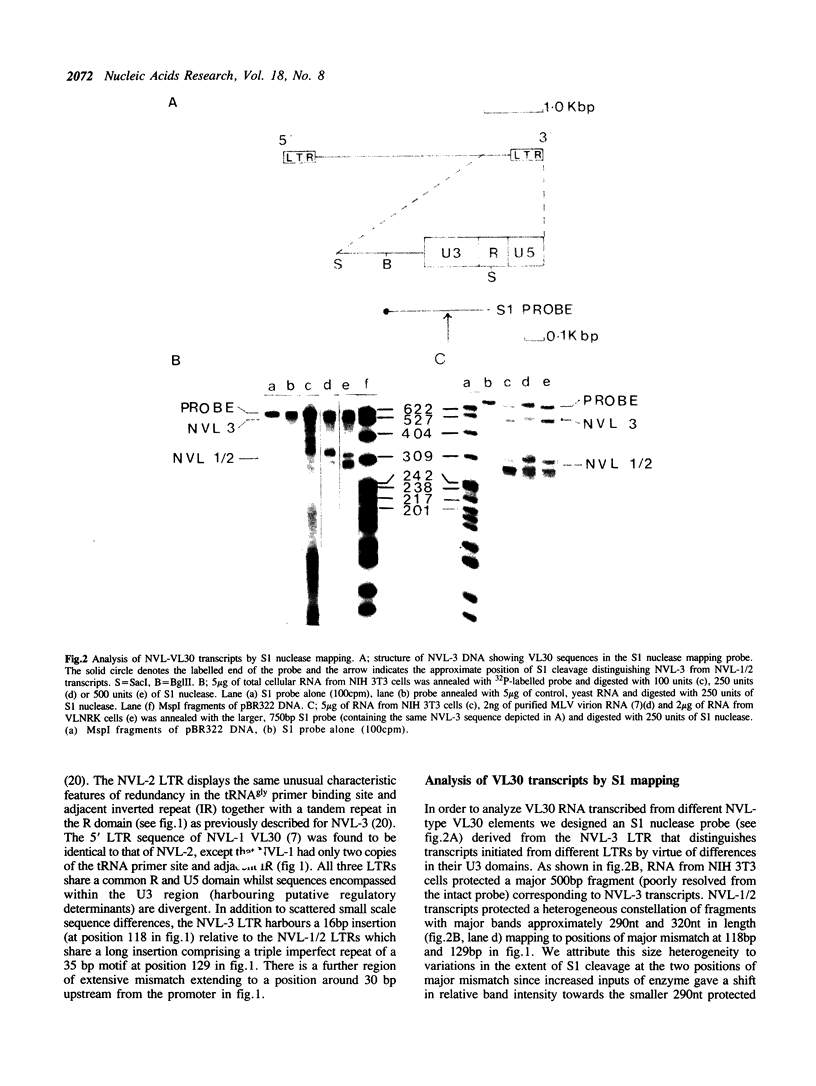
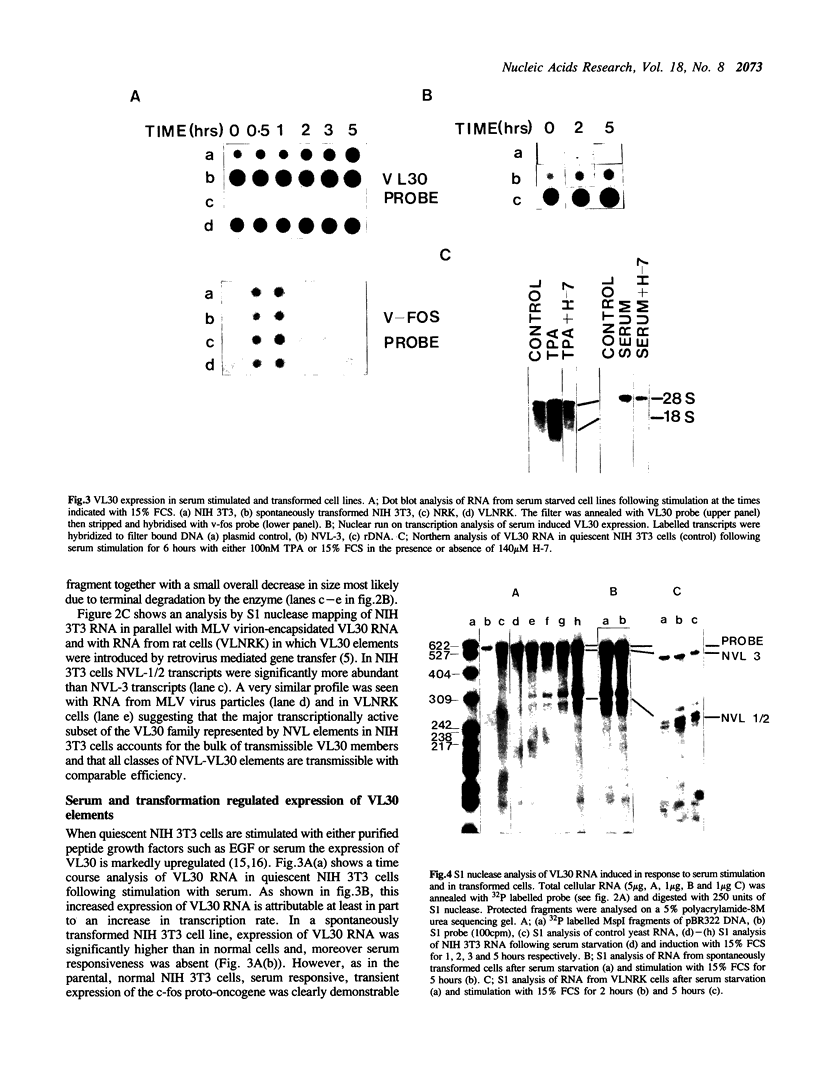
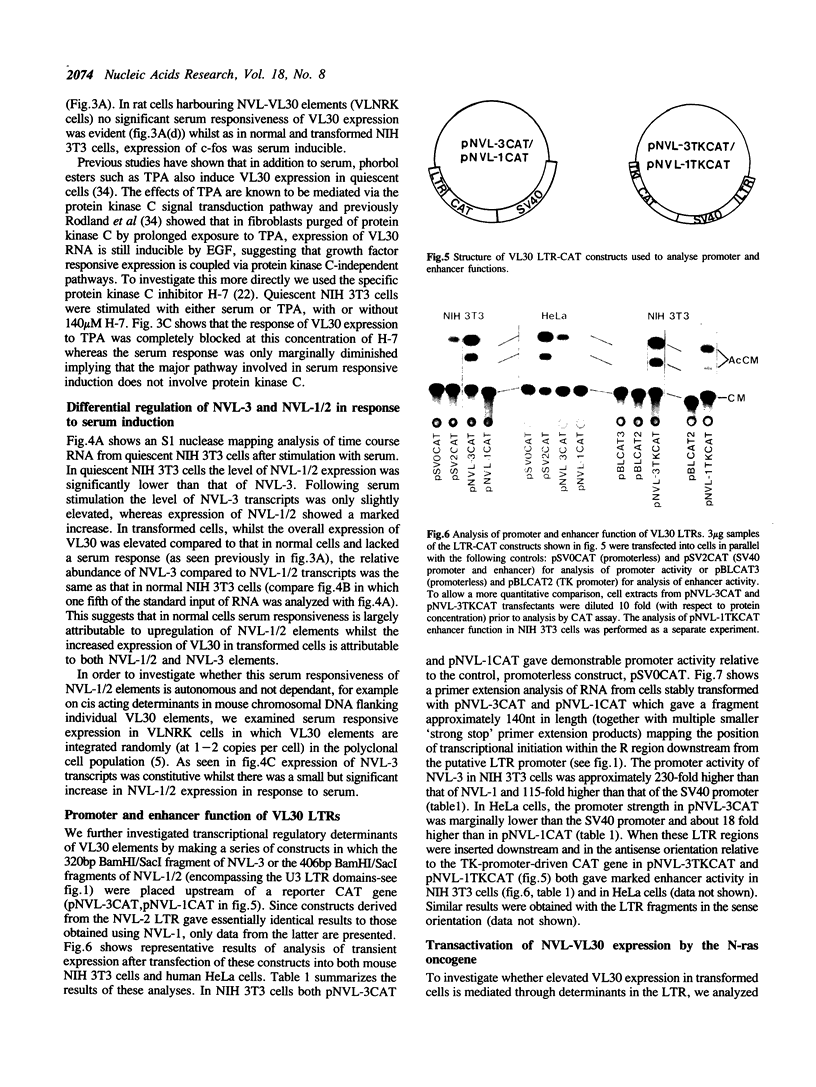
The nucleotide sequence of the long terminal repeats (LTRs) of retrovirus-transmissible mouse VL30 cDNA clones, NVL-1 and NVL-2 were determined and compared with that of the prototype NVL-3. Both shared the typical U3 R U5 structure together with unusual features of redundancy in the tRNAgly primer binding site and adjacent inverted repeat. NVL-1 and NVL-2 LTRs were almost identical and differed from the NVL-3 LTR in the U3 domain harbouring transcriptional regulatory determinants. S1 nuclease analysis of cellular and virus-encapsidated RNA suggested that NVL-1/2 and NVL-3 elements retrotranspose with comparable efficiency but that in contrast to transformation-regulated VL30 expression which affects all types of NVL element, only NVL-1/2 elements were found to be serum responsive. Both modes of VL30 regulation were found to be coupled through protein kinase C-independent pathways. Expression of N-ras transactivated U3 enhancer determinants in all classes of LTR. However the same region of NVL-1/2 LTR did not confer serum responsiveness implying that cis regulatory determinants of VL30 elements mediating growth factor responsiveness are at least in part dissociable from those responsible for cell transformation-regulated expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Avery R. J. A novel approach to cloning transcriptionally active retrovirus-like genetic elements from mouse cells. Nucleic Acids Res. 1983 Sep 24;11(18):6243–6254. doi: 10.1093/nar/11.18.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Avery R. J. The genomic DNA organisation and evolution of a retrovirus-transmissible family of mouse (VL30) genetic elements. Biochim Biophys Acta. 1988 Nov 10;951(1):130–138. doi: 10.1016/0167-4781(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Gibson Y., Avery R. J. Expression and transmission of a rodent retrovirus-like (VL30) gene family. J Mol Biol. 1986 Mar 5;188(1):105–108. doi: 10.1016/0022-2836(86)90485-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Courtney M. G., Schmidt L. J., Getz M. J. Organization and expression of endogenous virus-like (VL30) DNA sequences in nontransformed and chemically transformed mouse embryo cells in culture. Cancer Res. 1982 Feb;42(2):569–576. [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foster D. N., Schmidt L. J., Hodgson C. P., Moses H. L., Getz M. J. Polyadenylylated RNA complementary to a mouse retrovirus-like multigene family is rapidly and specifically induced by epidermal growth factor stimulation of quiescent cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7317–7321. doi: 10.1073/pnas.79.23.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harrigan M. T., Baughman G., Campbell N. F., Bourgeois S. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol Cell Biol. 1989 Aug;9(8):3438–3446. doi: 10.1128/mcb.9.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hodgson C. P., Elder P. K., Ono T., Foster D. N., Getz M. J. Structure and expression of mouse VL30 genes. Mol Cell Biol. 1983 Dec;3(12):2221–2231. doi: 10.1128/mcb.3.12.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Itin A. Patterns of genomic distribution and sequence heterogeneity of a murine "retrovirus-like" multigene family. J Virol. 1982 Jul;43(1):50–58. doi: 10.1128/jvi.43.1.50-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Keshet E., Shaul Y. Terminal direct repeats in a retrovirus-like repeated mouse gene family. Nature. 1981 Jan 1;289(5793):83–85. doi: 10.1038/289083a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Mordacq J. C. Transcriptional regulation of proliferin gene expression in response to serum in transfected mouse cells. EMBO J. 1987 Aug;6(8):2281–2288. doi: 10.1002/j.1460-2075.1987.tb02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. C., Paterson H. F., Morris J. D., Hall A., Marshall C. J. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989 Apr;8(4):1099–1104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A., Clegg C., Jones J., Rodgers B., Avery R. J. The isolation and characterization of a clonally related series of murine retrovirus-infected mouse cells. J Gen Virol. 1980 Jul;49(1):105–113. doi: 10.1099/0022-1317-49-1-105. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Norton J. D., Connor J., Avery R. J. Genesis of Kirsten murine sarcoma virus: sequence analysis reveals recombination points and potential leukaemogenic determinant on parental leukaemia virus genome. Nucleic Acids Res. 1984 Sep 11;12(17):6839–6852. doi: 10.1093/nar/12.17.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Connor J., Avery R. J. Unusual long terminal repeat sequence of a retrovirus transmissible mouse (VL 30) genetic element: identification of functional domains. Nucleic Acids Res. 1984 Apr 25;12(8):3445–3460. doi: 10.1093/nar/12.8.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Hogan B. L. Temporal and tissue-specific expression of distinct retrovirus-like (VL30) elements during mouse development. Dev Biol. 1988 Jan;125(1):226–228. doi: 10.1016/0012-1606(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. D., Ostrowski M. C. Rapid and selective alterations in the expression of cellular genes accompany conditional transcription of Ha-v-ras in NIH 3T3 cells. Mol Cell Biol. 1987 Jul;7(7):2512–2520. doi: 10.1128/mcb.7.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodland K. D., Jue S. F., Magun B. E. Regulation of VL30 gene expression by activators of protein kinase C. J Biol Chem. 1986 Apr 15;261(11):5029–5033. [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. Promoter and enhancer activities of long terminal repeats associated with cellular retrovirus-like (VL30) elements. Nucleic Acids Res. 1986 Jan 24;14(2):645–658. doi: 10.1093/nar/14.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Vass W. C., Howk R. S., Duesberg P. H. Defective retrovirus-like 30S RNA species of rat and mouse cells are infectious if packaged by type C helper virus. J Virol. 1979 Mar;29(3):964–972. doi: 10.1128/jvi.29.3.964-972.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Saragosti S., Botchan M. Isolation of cellular genes differentially expressed in mouse NIH 3T3 cells and a simian virus 40-transformed derivative: growth-specific expression of VL30 genes. Mol Cell Biol. 1985 Oct;5(10):2590–2598. doi: 10.1128/mcb.5.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]