Abstract
Activation of CB1 receptors on axon terminals by exogenous cannabinoids (eg, Δ9-tetrahydrocannabinol) and by endogenous cannabinoids (endocannabinoids) released by postsynaptic neurons leads to presynaptic inhibition of neurotransmission. The aim of this study was to characterize the effect of cannabinoids on GABAergic synaptic transmission in the human neocortex. Brain slices were prepared from neocortical tissues surgically removed to eliminate epileptogenic foci. Spontaneous GABAergic inhibitory postsynaptic currents (sIPSCs) were recorded in putative pyramidal neurons using patch-clamp techniques. To enhance the activity of cannabinoid-sensitive presynaptic axons, muscarinic receptors were continuously stimulated by carbachol. The synthetic cannabinoid receptor agonist WIN55212-2 decreased the cumulative amplitude of sIPSCs. The CB1 antagonist rimonabant prevented this effect, verifying the involvement of CB1 receptors. WIN55212-2 decreased the frequency of miniature IPSCs (mIPSCs) recorded in the presence of tetrodotoxin, but did not change their amplitude, indicating that the neurotransmission was inhibited presynaptically. Depolarization of postsynaptic pyramidal neurons induced a suppression of sIPSCs. As rimonabant prevented this suppression, it is very likely that it was due to endocannabinods acting on CB1 receptors. This is the first demonstration that an exogenous cannabinoid inhibits synaptic transmission in the human neocortex and that endocannabinoids released by postsynaptic neurons suppress synaptic transmission in the human brain. Interferences of cannabinoid agonists and antagonists with synaptic transmission in the cortex may explain the cognitive and memory deficits elicited by these drugs.
Keywords: CB1 cannabinoid receptor, endocannabinoid, GABAergic synaptic transmission, human, neocortex, Δ9-tetrahydrocannabinol
INTRODUCTION
The Gαi/o protein-coupled CB1 cannabinoid receptor is probably the most abundant G protein-coupled receptor in the central nervous system. It is the primary neuronal target of the phytocannabinoid Δ9-tetrahydrocannabinol and endogenous cannabinoids (endocannabinoids) (Pertwee, 2005; Pertwee et al, 2010). Activation of CB1 receptors leads to presynaptic inhibition of synaptic transmission in many regions of the central and peripheral nervous system (Freund et al, 2003; Szabo and Schlicker, 2005).
Endocannabinoids and CB1 receptors have a physiological role in both short- and long-term regulation of the strength of synaptic transmission. As a form of retrograde signaling, endocannabinoids are released from postsynaptic neurons, diffuse to presynaptic axon terminals, and there they inhibit GABA or glutamate release by acting on presynaptic CB1 receptors (for review, see Alger (2002), Lovinger (2008), Heifets and Castillo (2009), and Kano et al (2009)).
Endocannabinoid production in postsynaptic neurons can be triggered in several ways. Depolarization of postsynaptic neurons activates voltage-gated calcium channels and the increase in intracellular calcium concentration is a trigger of endocannabinoid production (Ohno-Shosaku et al, 2001; Wilson and Nicoll, 2001; Wallmichrath and Szabo, 2002; Kim and Alger, 2004; Szabo et al, 2006). Endocannabinoid production can also be triggered by activation of certain Gαq/11 protein-coupled receptors on postsynaptic neurons (Maejima et al, 2001; Galante and Diana, 2004; Straiker and Mackie, 2007). A combination of depolarization-elicited calcium influx with activation of Gαq/11 protein-coupled receptors is an especially powerful trigger of endocannabinoid production and occurs physiologically during activation of glutamatergic synapses (Brown et al, 2003; Marcaggi and Attwell, 2005; Rancz and Häusser, 2006).
There are only a handful of publications on how activation of CB1 receptors affects synaptic function in the human brain (for review, see Raiteri, 2006). It has been shown in neurochemical experiments that exogenous cannabinoids inhibit the release of radiolabeled acetylcholine (Steffens et al, 2003b), GABA (Katona et al, 2000), noradrenaline (Schlicker et al, 1997), and dopamine (Steffens et al, 2004) from axon terminals in human brain slices. To our knowledge, there is only one study in which the effect of an exogenous cannabinoid on ‘synaptic transmission' (instead of chemically measured transmitter release) in the human brain has been examined: Nakatsuka et al (2003) have shown that an exogenous cannabinoid agonist inhibits GABAergic synaptic transmission in the hippocampus. Importantly, endocannabinoid-mediated retrograde signaling in the human brain has not yet been reported.
Therefore, the aim of this study was to determine how exogenous cannabinoids and endocannabinoids released by postsynaptic neurons affect GABAergic synaptic transmission in the human neocortex. Neuroanatomical and positron emission tomography studies have demonstrated that CB1 receptor mRNA and protein occur in most layers of the human neocortex (Westlake et al, 1994; Lopez de Jesus et al, 2006; van Laere et al, 2008; Eggan et al, 2010). We studied synaptic transmission using patch-clamp electrophysiological methods in cortical tissue removed during neurosurgery to eliminate epileptogenic foci.
MATERIALS AND METHODS
The experimental protocol was approved by the Ethics Committee of the University Hospital of the Albert-Ludwigs-Universität Freiburg (file no. 100020/09) and authorized in written form by every patient or his/her legal representative. Conformity with the Declaration of Helsinki (1964) was ensured. The methods were similar to those described previously (Steffens et al, 2003b; Freiman et al, 2006; Kovacs et al, 2011).
Brain Slices
Tissue was obtained from 18 patients (age range: 3–55 years) undergoing surgery because of pharmacoresistant epilepsy. After premedication with midazolam, anesthesia was performed with propofol plus fentanyl. Cisatracurium was used for muscle relaxation. To eliminate seizures, epileptic lesions (focal cortical dysplasia, hamartoma, or encephalocele) in non-eloquent brain areas were removed with a safety margin toward the non-affected neocortex. The blood vessels of the small cortical areas were preserved during the operation until the final removal of the specimen. After removal, the tissue was immediately immersed in ice-cold physiological saline and dissected: only tissue within the safety margin and appearing macroscopically unaffected by the underlying disease process was used. The tissue was then transported to the Department of Pharmacology in ice-cold artificial cerebrospinal fluid (ACSF) of the following composition (mM): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 5, CaCl2 1, NaHCO3 26, glucose 20, Na-lactate 4, pH 7.3–7.4 (after the solution was gassed with 95% O2/5% CO2). The same ACSF was used for cutting 250–300 μm-thick slices containing all neocortical layers using a Leica VT1000S vibrating tissue slicer (Wetzlar, Germany). After cutting, slices were stored in a Gibb chamber containing ACSF of the following composition (mM): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 1, CaCl2 2.5, NaHCO3 26, glucose 10, Na-lactate 4, pH 7.3–7.4. For patch clamping, brain slices were superfused with ACSF at 20–24 °C at a flow rate of 1.5 ml/min with ACSF of the following composition (mM): NaCl 126, NaH2PO4 1.2, KCl 3, MgCl2 1, CaCl2 2.5, NaHCO3 26, glucose 10, pH 7.3–7.4.
Three potential problems regarding the cortical tissue we used must be discussed: (1) The cortical tissue might have been pathologically affected. All neocortical specimens used in this study were obtained from patients with strictly localized epileptogenic lesions; tissues from patients with generalized epileptogenic lesions (eg, those requiring a hemispherotomy for seizure freedom) were not included. Moreover, the brain slices were always prepared from the presumably intact margin of the removed tissue. (2) The concentration of the endocannabinoid 2-arachidonoylglycerol in the brain greatly increases after death (see Buczynski and Parsons (2010) for review on the post mortem change in endocannabinoid levels). As mentioned, blood vessels to the small cortical areas were preserved during the operation until final removal of the tissue. As the ‘warm' ischemic period was very short, a major post mortem change in endocannabinoid levels is not expected. (3) Antiepileptic drugs and the drugs used for anesthesia (such as midazolam, propofol, fentanyl, cisatracurium) may interfere with components of the endocannabinoid system. For example, propofol inhibits the anandamide-metabolizing enzyme fatty acid amide hydrolase with an IC50=1.4 × 10−5 M (Patel et al, 2003), a concentration similar to the propofol plasma concentration during anesthesia (1.7–3.4 × 10−5 M). However, it is unlikely that these drugs affected our results. Thus, if applicable, antiepileptic drugs were withdrawn some days before surgery. Moreover, it is very likely that any drug remaining in the brain from the presurgical pharmacotherapy or from anesthesia was washed out of the brain slices during the >2 h preceding electrophysiological recordings. During this period, the incubation buffer was changed several times.
Patch Clamping
Neurons in slices were visualized with infrared video microscopy (Figure 1a), and patch-clamp recordings were obtained using an EPC-9 amplifier under the control of TIDA software (HEKA Elektronik, Lambrecht, Germany). Series resistance compensation of 50% was usually applied. Series resistance was measured before and after recordings and experiments with major changes in series resistance (>20%) were discarded. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at a holding potential of −70 mV with pipettes (2.5–5 MΩ) containing (mM): CsCl 147, MgCl2 1, HEPES 10, EGTA 1, ATP-Na2 4, GTP-Na 0.4, N-ethyl-lidocaine Cl2, pH 7.4. The superfusion ACSF contained the glutamate receptor antagonists DNQX (10−5 M) and DL-AP5 (2.5 × 10−5 M). Miniature IPSCs (mIPSCs) were recorded similarly, but tetrodotoxin (3 × 10−7 M) was included in the superfusion ACSF. sIPSCs and mIPSCs were detected using the MiniAnalysis software (version 6.0.3; Synaptosoft, Decatur, GA, USA). Amplitudes and recording times of sIPSCs and mIPSCs were transferred for further calculations from MiniAnalysis to SigmaPlot (version 10.0; Systat, San Jose, CA, USA). Cumulative sIPSC amplitudes were calculated by summating amplitudes of all events within 10–120 s periods.
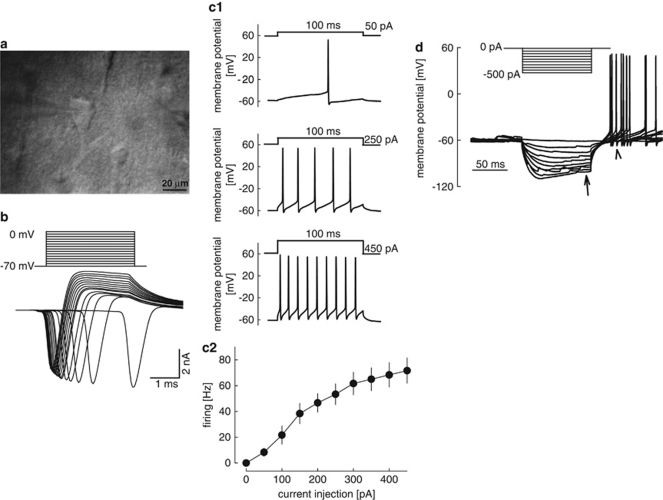
Figure 1.
Properties of pyramidal neurons in human neocortical slices. (a) Infrared video microscopic image of a neuron with the patch-clamp pipette. (b) Depolarizing steps elicited inward currents through voltage-gated sodium channels and subsequent outward potassium currents. (c1 and c2) Depolarizing current injections of increasing strength elicited action potentials at increasing frequencies. c1 shows original recordings, c2 the statistical analysis. Means±SEM of six experiments. (d) Response of a neuron to hyperpolarizing current injections. During strong hyperpolarizing current injections, slowly developing depolarizing potentials appear (depolarizing sags, arrow). After hyperpolarizing current injections, rebound action potentials can be observed (arrowhead). The recording represents seven recordings with similar results.
Protocols and Statistics
Recordings started 15–20 min after establishment of the whole-cell configuration. Cumulative sIPSC amplitudes and mIPSC amplitudes and frequencies were expressed as percentages of the initial reference values (PRE in the figures).
Several slices can be prepared from the cortical tissue of a patient, and several recordings can be performed on a patch-clamped neuron within a slice. Each statistical group within the figures contains data obtained on brain slices of 5–8 patients. In the experiments shown in Figures 1,2,3,4,5, only one recording per slice and neuron was performed. In the experiments shown in Figure 6, up to four recordings were performed on a neuron in a brain slice.
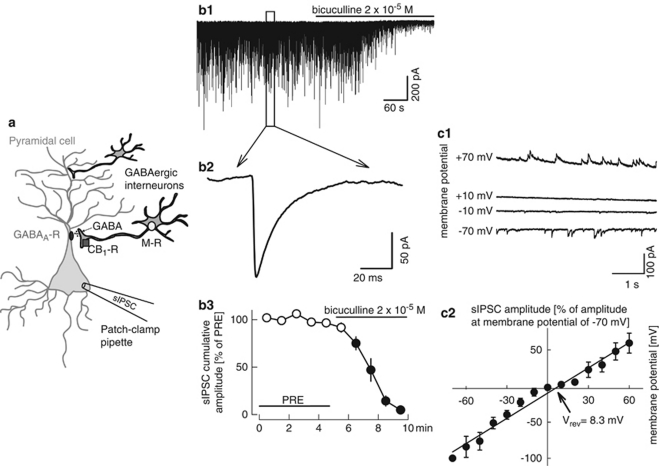
Figure 2.
Characterization of spontaneous inhibitory postsynaptic currents (sIPSCs) recorded in human cortical pyramidal neurons. (a) The scheme displays a pyramidal neuron with the recording patch-clamp pipette and two GABAergic interneurons. Only one of the interneurons possesses CB1 receptors (CB1-R) in the axon terminals and muscarinic receptors (M-R) in the somatodendritic region. (b1, b2, and b3) sIPSCs are observed in pyramidal neurons and are abolished by the GABAA receptor antagonist bicuculline. b1 shows an original recording, b2 the average of 100 sIPSCs, and b3 the statistical analysis. The cumulative amplitude of sIPSCs was calculated for 1-min periods, and values were expressed as percentages of the values determined during the initial reference period (PRE). Means±SEM of nine experiments. A filled symbol indicates significant difference (P<0.05; Wilcoxon's signed-rank test) from the initial reference value (PRE). (c1 and c2) Varying the membrane potential of the recorded neuron led to changes in sIPSC amplitude and polarity. c1 shows an original recording, c2 the statistical analysis. The reversal potential of sIPSCs (8.3 mV) was fairly near to the calculated chloride equilibrium potential (−0.6 mV). Means±SEM of six experiments.
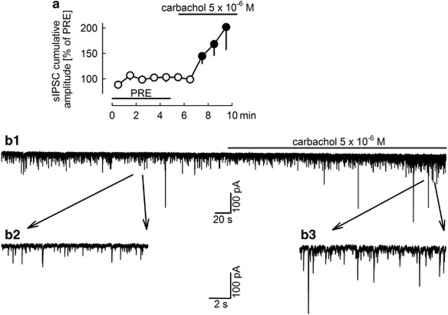
Figure 3.
The muscarinic receptor agonist carbachol increases the cumulative amplitude of sIPSCs. Carbachol was superfused as indicated. (a) The cumulative amplitude of sIPSCs was calculated for 1-min periods, and values were expressed as percentages of the values determined during the initial reference period (PRE). Means±SEM of 11 experiments. A filled symbol indicates significant difference (P<0.05; Wilcoxon's signed-rank test) from the initial reference value (PRE). (b1) Original tracing (b2 and b3 are magnified sections).
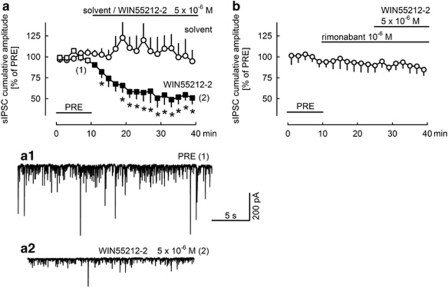
Figure 4.
The cannabinoid receptor agonist WIN55212-2 decreases the cumulative amplitude of sIPSCs, and this effect is prevented by the CB1 antagonist rimonabant. All experiments were performed in the presence of carbachol (5 × 10−6 M). Solvent, WIN55212-2, and rimonabant were superfused as indicated. The cumulative amplitude of sIPSCs was calculated for 2-min periods, and values were expressed as percentages of the values determined during the initial reference period (PRE). (a) Means±SEM of six (solvent) and five (WIN55212-2) experiments. A filled symbol indicates significant difference (P<0.05; Wilcoxon's signed-rank test) from the initial reference value (PRE); *indicates significant difference (P<0.05; Mann–Whitney test) vs solvent. (a1, a2) The original tracings were recorded at time points 1 and 2 indicated in panel a. (b) Means±SEM of six experiments.
Figure 5.
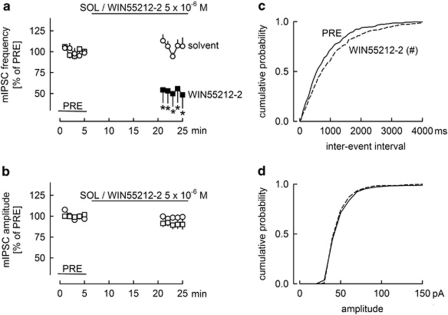
WIN55212-2 decreases the frequency of mIPSCs, but does not change their amplitude. mIPSCs were isolated by superfusion of tetrodotoxin (3 × 10−7 M). All experiments were performed in the presence of carbachol (5 × 10−6 M). (a, b) Solvent or the synthetic cannabinoid receptor agonist WIN55212-2 was superfused as indicated. The frequency and amplitude of mIPSCs were calculated for 1-min periods, and values were expressed as percentages of the values determined during the initial reference period (PRE). Means±SEM of seven (solvent) and six (WIN55212-2) experiments. A filled symbol indicates significant difference (P<0.05; Wilcoxon's signed-rank test) from the initial reference value (PRE); *indicates significant difference (P<0.05; Mann–Whitney test) vs solvent. (c, d) Cumulative probability distribution plots of mIPSC interevent intervals and amplitudes were constructed using 5-min periods preceding (PRE) and during WIN55212-2 application in one of the experiments shown in panels a and b. Bins for amplitudes and interevent intervals were multiples of 10 pA and 50 ms, respectively. #Indicates significant difference (P<0.05; Kolmogorov–Smirnov test) between the distribution plots.
Figure 6.
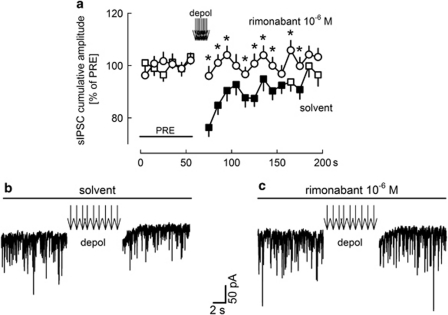
Depolarization leads to a CB1 cannabinoid receptor-dependent suppression of GABAergic inhibition. All experiments were performed in the presence of carbachol (5 × 10−6 M). (a) Cumulative amplitudes of sIPSCs were calculated for 10-s periods and expressed as percentages of the initial reference value (PRE). After PRE, 9 depolarizing pulses (from −70 to 0 mV for 100 ms) were applied at 1 Hz. In the presence of solvent, this depolarization led to suppression of the cumulative amplitude of sIPSCs, ie, depolarization-induced suppression of inhibition (DSI) occurred. No DSI occurred in the presence of rimonabant. Means±SEM of 63 (solvent) and 44 (rimonabant) experiments. A filled symbol indicates significant difference (P<0.05; Wilcoxon's signed-rank test) from the initial reference value (PRE) and *indicates significant difference (P<0.05; Mann–Whitney test) vs solvent. (b and c) Original tracings showing the effect of depolarization on sIPSCs in the presence of solvent and rimonabant.
Means±SEM are given throughout. Non-parametric statistical tests included in the statistical software SPSS/PASW (version 18.0.0; SPSS, Chicago, IL, USA) were used to identify significant differences. The two-tailed Mann–Whitney test was used for comparisons between groups; significant differences are indicated by *. The two-tailed Wilcoxon's signed-rank test and the Kolmogorov–Smirnov test were used for comparisons within groups (vs PRE); significant differences are indicated by filled symbols or by #. P<0.05 was considered the limit of statistical significance, and only this level is indicated, even if P was <0.01 or <0.001.
Drugs
Drugs were obtained from the following sources. Alamone Labs (Jerusalem, Israel): N-ethyl-lidocaine chloride (QX-314); Ascent Scientific (Weston, UK): 6,7-dinitroquinoxaline-2,3-dione (DNQX), DL-(-)-2-amino-5-phosphonopentanoic acid (DL-AP5), tetrodotoxin citrate; Sanofi-Aventis (Chilly-Mazarin, France): rimonabant (previously called SR141716A); Sigma-Aldrich (Deisenhof, Germany): bicuculline, carbachol chloride; Tocris Cookson (Bristol, England): R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2, 3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl)methanone mesylate (WIN55212-2).
Rimonabant, WIN55212-2, DNQX, and bicuculline were dissolved in dimethylsulphoxide (DMSO), and stock solutions were stored at −32 °C. Further dilutions were made with superfusion buffer; the final concentration of DMSO in the superfusion fluid was ⩽1 ml/l. Control solutions (‘solvent' in the figures) always contained the appropriate concentrations of DMSO.
RESULTS
Characterization of Human Neocortical Brain Slices
Neurons with pyramid-shaped somata were selected for patch clamping (Figure 1a). These neurons usually had a thick apical dendrite, and their smaller diameter was >10 μm. The electrophysiological properties of these putative pyramidal neurons were characterized using pipettes containing a potassium gluconate-based solution (Figure 1b–1d). In the voltage-clamp mode, depolarizing steps always elicited typical voltage-gated sodium channel-mediated currents (Figure 1b). The currents were inhibited by tetrodotoxin (3 × 10−7 M; not shown). When recorded in the current-clamp mode without additional current injection, most pyramidal neurons were silent. Injecting increasing depolarizing currents elicited action potentials at increasing frequencies (Figure 1c). The maximal rate of firing was ∼70 Hz (Figure 1c2). Similar to our observations, most human neocortical neurons were silent in previous studies using microelectrode recordings (Schwartzkroin et al, 1983; Koch et al, 2005). In most studied pyramidal neurons, hyperpolarizing current injections elicited depolarizing potential sags, and rebound action potentials appeared after the hyperpolarizing currents (Figure 1d). The depolarizing sags were most probably due to Ih currents through hyperpolarization-activated cyclic nucleotide-gated cation channels (HCNs). Thus, although the neurosurgical removal of human cortical tissue is far from optimal for patch-clamp electrophysiological experiments, the neurons in the brain slices appeared to be in good condition, firing action potentials at high frequencies and showing Ih currents.
When pyramidal neurons were patch clamped with pipettes containing a cesium chloride-based solution, and their membrane potential was clamped to −70 mV, spontaneous GABAergic inhibitory postsynaptic currents (sIPSCs) were observed (Figure 2b1 and b2). The cumulative amplitude of sIPSCs was 17.4±5.9 nA/60 s (n=11) at the beginning of the recordings. The GABAA receptor antagonist bicuculline abolished the sIPSCs (Figure 2b1 and b3). Varying the holding potential of pyramidal neurons led to changes in the amplitude and direction of sIPSCs (Figure 2c1). Statistical evaluation showed that the reversal potential of sIPSCs was at 8.3 mV (Figure 2c2). The fact that this value is fairly close to the calculated chloride equilibrium potential (−0.6 mV) indicates that chloride was the dominant charge carrier of sIPSCs. Thus, it is possible to record GABAA receptor-mediated synaptic events in pyramidal neurons of the human neocortex.
Effect of the Muscarinic Acetylcoline Receptor Agonist Carbachol on GABAergic Synaptic Transmission
Cortical pyramidal neurons receive GABAergic synaptic input from several classes of GABAergic interneurons, but only some classes of interneurons possess CB1 receptors (Trettel et al, 2004; Bodor et al, 2005; Hill et al, 2007; Petilla Interneuron Nomenclature Group (PING), 2008; Wedzony and Cochyk, 2009). It is believed that interneurons possessing CB1 receptors in their axon terminals also possess muscarinic acetylcholine receptors in their somatodendritic regions (Trettel et al, 2004; Hill et al, 2007). Figure 2a illustrates this situation by showing, for the sake of simplicity, only two kinds of interneurons. We wanted to increase the proportion of sIPSCs from those neurons which possess CB1 receptors, as has been done in previous experiments in mouse and rat brain slices (Trettel et al, 2004; Hill et al, 2007). For this purpose, we superfused the muscarinic agonist carbachol. Carbachol (5 × 10−6 M) strongly increased the frequency of sIPSCs (Figure 3). All further experiments, in which the effects of cannabinoids were studied, were carried out in the presence of carbachol (5 × 10−6 M).
Effect of the Exogenous Cannabinoid Agonist WIN55212-2 on GABAergic Synaptic Transmission
sIPSCs were recorded for 40 min. The cumulative amplitude of sIPSCs remained constant during superfusion of solvent (Figure 4a). The synthetic CB1/CB2 cannabinoid receptor agonist WIN55212-2 (5 × 10−6 M) decreased the cumulative amplitude of sIPSCs by ∼50% (Figure 4a). Maximal inhibition was reached after ∼20 min. It has been repeatedly observed in the past that the effects of cannabinoids develop slowly in brain slices, probably due to the high lipophilicity of cannabinoids (Szabo et al, 1998; Brown et al, 2004).
To identify the receptor activated by WIN55212-2, interaction with the CB1 receptor antagonist rimonabant was tested (Figure 4b). Rimonabant (10−6 M), when superfused alone, did not change the cumulative amplitude of sIPSCs (Figure 4b). This observation indicates that CB1 receptors influencing GABAergic synaptic transmission were not tonically activated by endocannabinoids. When WIN55212-2 was superfused in the presence of rimonabant, it no longer decreased the cumulative amplitude of sIPSCs (Figure 4b). Thus, it is very likely that WIN55212-2 inhibited GABAergic synaptic transmission (Figure 4a) by activating CB1 receptors.
During the next phase, we wanted to determine whether the cannabinoid agonist inhibited synaptic transmission by a presynaptic or a postsynaptic action. To this aim, we observed the effect of WIN55212-2 on mIPSCs. mIPSCs were isolated by including the voltage-gated sodium channel inhibitor tetrodotoxin in the superfusion buffer. During the initial reference period PRE, mIPSCs had a frequency of 2.13±0.40 Hz (n=13) and an amplitude of 50±2 pA (n=13). In slices superfused with solvent, the frequency and amplitude of mIPSCs remained constant (Figure 5a and b). Superfusion of WIN55212-2 decreased the frequency of mIPSCs, but did not change their amplitude (Figure 5a and b). WIN55212-2 caused a shift in the cumulative probability distribution plot of mIPSC interevent intervals (Figure 5c), confirming the lowering of the frequency. In contrast, the cumulative probability distribution plot of mIPSC amplitudes was not changed (Figure 5d). The lowering of the frequency of mIPSCs indicates that the cannabinoid acted at the presynaptic axon terminal. The lack of effect on the amplitude of mIPSCs means that the cannabinoid did not modify the effect of released GABA on the postsynaptic neuron.
Endocannabinoid-Mediated Suppression of GABAergic Synaptic Transmission
sIPSCs were recorded for 200 s (Figure 6). After 60 s, 9 depolarizing pulses (from −70 to 0 mV for 100 ms) were applied at 1 Hz. In the presence of solvent, this depolarization suppressed the cumulative amplitude of sIPSCs to 76% of PRE (Figure 6a and b). This means that depolarization-induced suppression of (GABAergic) inhibition (DSI) occurred. In the presence of the CB1 receptor antagonist rimonabant, depolarization did not induce suppression (Figure 6a and c), indicating involvement of endocannabinoids and CB1 receptors in the suppression.
Age Dependency of Effects
The age of patients varied within the wide range of 3–55 years. Conceivably, the effects of exogenous and endogenous cannabinoids may change with age. Therefore, we examined whether a correlation exists between age and the effects of cannabinoids. There was no significant correlation between age and the inhibition of sIPSCs by WIN55212-2 (R=0.19; P=0.76; n=5 patients; experiments shown in Figure 4a). Similarly, there was no significant correlation between age and the magnitude of endocannabinoid-mediated depolarization-induced suppression of inhibitory synaptic transmission (DSI) (R=0.38; P=0.35; n=8 patients; experiments shown in Figure 6a). Thus, the effects of an exogenous cannabinoid on synaptic activity and endocannabinoid-mediated synaptic plasticity do not greatly change with age.
DISCUSSION
The human neocortical tissue appeared to be suitable for electrophysiological studies. An exogenous cannabinoid agonist inhibited GABAergic synaptic transmission through CB1 cannabinoid receptors. Depolarization of postsynaptic neurons elicited endocannabinoid- and CB1 receptor-mediated retrograde synaptic signaling. To our knowledge, this is the first study, which shows the effect of a cannabinoid agonist on synaptic transmission in the human neocortex, and the first study which shows endocannabinoid-mediated synaptic plasticity in the human brain.
For patch-clamp studies on neurons in rat and mouse brain slices, usually young animals are used (ideally, younger than 20 days of age), the brain is quickly removed from the skull (ideally, within 1 min after decapitation), immediately placed in ice-cold ASCF, and handled mechanically very cautiously (Edwards and Konnerth, 1992; Stuart et al, 1993). Obviously, these requirements cannot be fulfilled during neurosurgical operations concentrating on removal of pathological structures. Despite this disadvantage, the cortical tissue becoming available for our electrophysiological study was in surprisingly good condition. Thus, many neurons had properly functioning voltage-gated sodium channels and HCN channels. The membrane potential of the neurons was also normal, and the neurons were able to fire action potentials at high rates. Moreover, spontaneous GABAergic synaptic input to patched-clamped neurons was also recordable. It is unlikely that levels of endocannabinoids artificially increased in the cortical pieces during their surgical preparation and removal, because rimonabant alone did not increase GABAergic transmission in the slices. However, one disadvantage of recording from human brain slices was evident: the visibility of neurons within human brain slices was clearly worse than in brain slices of young mice and rats. This hampered exact identification and localization of neurons within the human cortex. However, we are confident that, despite this poor visibility, most of the recorded neurons were pyramidal neurons. Another disadvantage of the human cortical tissue is its restricted availability, preventing large series of experiments. Accordingly, feasible studies on this tissue are mostly of translational character: they can test whether phenomena occurring in the brains of laboratory animals also occur in the human brain. Electrophysiological recordings from human neocortical slices—mostly with intracellular microelectrodes—have been successfully carried out in the past, and many of these studies focused on epileptogenic mechanisms (for review, see Avoli and Williamson (1996) and Köhling and Avoli (2006)).
The synthetic cannabinoid agonist WIN55212-2 decreased the spontaneous GABAergic synaptic input to cortical pyramidal neurons. WIN55212-2 is a mixed CB1/CB2 receptor agonist, without affinity for a long range of receptors and ion channels (Kuster et al, 1993; Pertwee, 2005). An involvement of CB1 receptors in the synaptic inhibition in our experiments is strongly supported by antagonism of the effects of WIN55212-2 by the CB1-selective antagonist rimonabant (Rinaldi-Carmona et al, 1994; Pertwee, 2005). Involvement of CB2 receptors in the effect of WIN55212-2 in these experiments is unlikely, because the density of CB2 receptors is generally very low in the brain, and CB2 receptors are mostly localized on microglial cells and astrocytes (Munro et al, 1993; Fernandez-Ruiz et al, 2007). However, it must be noted that neuronal CB2 receptors at low densities were observed in some brain regions recently (van Sickle et al, 2005; Gong et al, 2006; Brusco et al, 2008; Suarez et al, 2008; Lanciego et al, 2011).
Our study extends previous observations made on the mouse and rat cortex to the human cortex. Thus, it has been observed in mouse and rat brain slices that activation of CB1 cannabinoid receptors by exogenous agonists leads to inhibition of the GABAergic synaptic input to cortical layer II–III pyramidal neurons (Trettel and Levine, 2002; Bodor et al, 2005; Lemtiri-Chlieh and Levine, 2007; Hill et al, 2007; Chiu et al, 2010). Interestingly, the GABAergic input to layer V pyramidal neurons was less affected by cannabinoids (Bodor et al, 2005; Fortin and Levine, 2007).
Theoretically, WIN55212-2 can decrease the spontaneous GABAergic synaptic input to cortical pyramidal neurons by the following three mechanisms: (1) WIN55212-2 may decrease the firing rate of the afferent GABAergic cortical interneurons (somatodendritic effect). This mechanism is possible, because cannabinoids cause somatodendritic inhibition in some neurons (Kreitzer et al, 2002; Bacci et al, 2004). Remarkably, however, cannabinoids do not elicit somatodendritic inhibition in many CB1 receptor-bearing neurons (Szabo et al, 2004; Freiman and Szabo, 2005; Freiman et al, 2006). (2) WIN55212-2 may decrease GABA release from the axon terminals (presynaptic inhibition). Presynaptic inhibition in these experiments was verified by the observation that WIN55212-2 decreased mIPSC frequency. The result indicates that the vesicle release machinery in the presynaptic axon terminal was directly inhibited. (3) WIN55212-2 may interfere with the effect of released GABA on postsynaptic neurons (postsynaptic inhibition). The lack of effect of WIN55212-2 on mIPSC amplitude argues against a postsynaptic inhibition. The observed presynaptic inhibition is in line with the overwhelming evidence in the literature. Thus, to our knowledge, cannabinoids always inhibited synaptic transmission with a presynaptic mechanism (for review, see Szabo and Schlicker (2005)). The localization of CB1 receptors is compatible with a presynaptic action, namely several classes of afferent inhibitory neurons of cortical pyramidal neurons express CB1 receptors, and the CB1 receptors appear at the axon terminals of these interneurons (Marsicano and Lutz, 1999; Bodor et al, 2005; Hill et al, 2007; Wedzony and Cochyk, 2009).
Depolarization of human cortical pyramidal neurons elicited a suppression of the GABAergic input to these neurons, ie, DSI occurred. Very likely, endocannabinoids and CB1 receptors were involved, because DSI was abolished by the CB1 antagonist rimonabant. Endocannabinoid-mediated DSI has been observed in layer II–III pyramidal neurons in mouse and rat cortices (Trettel and Levine, 2003; Trettel et al, 2004; Bodor et al, 2005; Hill et al, 2007; Lemtiri-Chlieh and Levine, 2007; Galarreta et al, 2008). Interestingly, the GABAergic input to layer V pyramidal neurons was less prone to endocannabinoid-mediated suppression (Bodor et al, 2005; Fortin and Levine, 2007).
The identity of the endocannabinoid mediating DSI in the human neocortex in this study is not known. However, it has been shown previously with biochemical methods that anandamide is synthesized and degraded in the human neocortex (Steffens et al, 2003a; Steffens et al, 2005). It is also known that in the human hippocampus (‘archicortex,' the enzymes for synthesis and degradation of anandamide (N-acyl-phosphatidylethanolamine-hydrolyzing phospholipase D and fatty acid amide hydrolase) and 2-arachidonoylglycerol (diacylglycerol lipase, monoacylglycerol lipase, and α/β-hydrolase 6) are present (Ludanyi et al, 2008; Mulder et al, 2011).
All cannabinoid experiments were performed in the presence of the muscarinic receptor agonist carbachol (5 × 10−6 M), to increase the activity of those cortical GABAergic interneurons that possess CB1 receptors. It is believed that carbachol stimulates GABA release from these interneurons by acting on muscarinic receptors in their somatodendritic region (Kawaguchi, 1997; Kondo and Kawaguchi, 2001; Trettel et al, 2004). An involvement of nicotinic acetylcholine receptors is unlikely, because higher carbachol concentrations are necessary to activate these receptors (Koos and Tepper, 2002). Carbachol could have interfered with the effects of cannabinoids in two ways. First, it is likely that inhibition of the GABAergic input to pyramidal cells by exogenous and endogenous cannabinoids was enhanced, because carbachol increased the contribution of CB1 receptor-bearing GABAergic axons to the GABAergic input to pyramidal cells. Second, it is possible that carbachol, by acting on Gαq/11 protein-coupled M1 and M3 muscarinic receptors of postsynaptic pyramidal neurons potentiated DSI, as it was shown previously in the hippocampus and the striatum of rodents (Kim et al, 2002; Ohno-Shosaku et al, 2003; Narushima et al, 2007).
Rimonabant alone did not increase the GABAergic input to pyramidal neurons in these experiments, indicating that the GABAergic input is not tonically inhibited by endocannabinoids in the brain slice. In a previous study (Steffens et al, 2003b), rimonabant increased acetylcholine release in human neocortical brain slices under a certain condition (acetylcholine release was elicited by field stimulation using 26 electrical pulses at 0.1 Hz), pointing to tonic inhibition by endocannabinoids. It is very likely that endocannabinoid production was triggered by this specific electrical stimulation, because endocannabinoid-mediated tonic inhibition did not occur in experiments with different stimulation parameters (8 bursts at 0.02 Hz (a burst consisting of 4 electrical pulses at 100 Hz) (Steffens et al, 2003b).
The cortical tissue used in our study was neurosurgically removed to eliminate the epileptogenic focus, and the brain slices were prepared from the presumably intact, pathologically not affected margin of the removed tissue. Levels of mRNA for the CB1 receptor and the endocannabinoid-synthesizing enzyme diacylglycerol lipase-α are decreased in the epileptic human hippocampus (Ludanyi et al, 2008). If a similar decrease in the levels of these proteins also occurred in the cortex of epileptic patients in our study (which we cannot fully exclude), then the magnitude of synaptic inhibition by the exogenous cannabinoid and the magnitude of endocannabinoid-mediated retrograde signaling are underestimated in our study.
Fitzgerald et al (2009) determined short-interval cortical inhibition (SICI) of motor evoked potentials elicited by transcranial magnetic stimulation in awake humans. SICI was attenuated in cannabis users, and one of the interpretations was that GABAA receptor-mediated synaptic transmission was inhibited by cannabinoids in the cortex (Fitzgerald et al, 2009). Inhibition of GABAA receptor-mediated synaptic transmission by a cannabinoid in the human cortex in vitro, as demonstrated in our study at the monosynaptic level, may be the basis of the effect observed in vivo by Fitzgerald et al (2009).
Our observations are clinically relevant. The inhibition of synaptic transmission in the human cortex by cannabinoids shown in this study probably has a role in the impairment of perception and cognitive function occurring acutely after inhalation of cannabinoids (D'Souza et al, 2004; Ramaekers et al, 2006; for review, see Murray et al (2007)). We also demonstrated that the CB1 receptor antagonist rimonabant disrupts DSI in the human cortex, a form of short-term synaptic plasticity. Therefore, it is expected that rimonabant and other CB1 antagonists, considered to be useful for the treatment of obesity and type 2 diabetes mellitus, will interfere with cortical information processing. Remarkably, rimonabant elicits adverse psychiatric reactions (Christensen et al, 2007), and this contributed to its withdrawal from clinical use.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Sz 72/5-2 to Bela Szabo) and the Deutscher Akademischer Austausch-Dienst (DAAD) (A/07/91768 to Flora E Kovacs).
The authors declare no conflict of interest.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Avoli M, Williamson A. Functional and pharmacological properties of human neocortical neurons maintained in vitro. Prog Neurobiol. 1996;48:519–554. doi: 10.1016/0301-0082(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi E. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Brit J Pharmacol. 2010;160:423–442. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu Y-t, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsyhopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A.1992Patch-clamping cells in sliced tissue preparationsIn: Rudy B, Iverson LE (eds).Methods in Enzymology vol 207 Ion Channels Academic Press: San Diego; 208–222. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival. Trends Pharmacol Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Williams S, Daskalakis ZJ. A transcranial magnetic stimulation study of the effects of cannabis use on motor cortical inhibition and excitability. Neuropsychopharmacology. 2009;34:2368–2375. doi: 10.1038/npp.2009.71. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol (London) 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman I, Szabo B. Cannabinoids depress excitatory neurotransmission between the subthalamic nucleus and the globus pallidus. Neuroscience. 2005;133:305–313. doi: 10.1016/j.neuroscience.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron–Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex. 2008;18:2296–2305. doi: 10.1093/cercor/bhm253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J-P, Onaivi ES, Ishiguro H, Liu Q-R, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Köfalvi A, Czirjak S, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kawaguchi Y. Slow synchronized bursts of inhibitory postsynaptic currents (0.1–0.3 Hz) by cholinergic stimulation in the rat frontal cortex in vitro. Neuroscience. 2001;107:551–560. doi: 10.1016/s0306-4522(01)00388-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U-R, Mußhoff U, Pannek H-W, Ebner A, Wolf P, Speckmann E-J, et al. Intrinsic excitability, synaptic potentials, and short-term plasticity in human epileptic neocortex. J Neurosci Res. 2005;80:715–726. doi: 10.1002/jnr.20498. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the striatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R, Avoli M. Methodological approaches to exploring epileptic disorders in the human brain in vitro. J Neurosci Meth. 2006;155:1–19. doi: 10.1016/j.jneumeth.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Kovacs FE, Illes P, Szabo B. Purine receptor-mediated endocannabinoid production and retrograde synaptic signalling in the cerebellar cortex. Br J Pharmacol. 2011;162:974–988. doi: 10.1111/j.1476-5381.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Kuster JE, Stevenson JI, Ward SJ, D′Ambra TE, Haycock DA. Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids. J Pharmacol Exp Ther. 1993;264:1352–1363. [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2011;25:97–104. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. Lack of depolarization-induced suppression of inhibition (DSI) in layer 2/3 interneurons that receive cannabinoid-sensitive inhibitory inputs. J Neurophysiol. 2007;98:2517–2524. doi: 10.1152/jn.00817.2007. [DOI] [PubMed] [Google Scholar]
- Lopez de Jesus M, Salles J, Meana JJ, Callado LF. Characterization of CB1 cannabinoid receptor immnunoreactivity in postmortem human brain homogenates. Neuroscience. 2006;140:635–643. doi: 10.1016/j.neuroscience.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handbook of Experimental Pharmacology. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mulder J, Zilberter M, Pasquare SJ, Alpar A, Schulte G, Ferreira SG, et al. Molecular reorganization of endocannabinoid signalling in Alzheimer's disease. Brain. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Chen H-X, Roper SN, Gu JG. Cannabinoid receptor-1 activation suppresses inhibitory synaptic activity in human dentate gyrus. Neuropharmacology. 2003;45:116–121. doi: 10.1016/s0028-3908(03)00143-6. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, et al. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, et al. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry L-TJ, Kundu A, et al. The general anesthetic propofol increases brain N-arachidonylethanolamide (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. 2003;139:1005–1013. doi: 10.1038/sj.bjp.0705334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG.2005Pharmacological actions of cannabinoidsIn: Pertwee RG (eds).Handbook of Experimental Pharmacology vol 168 Cannabinoids Springer: Berlin; 1–51. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group (PING) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M. Functional pharmacology in human brain. Pharmacol Rev. 2006;58:162–193. doi: 10.1124/pr.58.2.5. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Rancz EA, Häusser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci. 2006;26:5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Timm J, Zentner J, Göthert M. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;356:583–589. doi: 10.1007/pl00005093. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Turner DA, Knowles WD, Wyler AK. Studies of human and monkey ‘pileptic'neocortex in the in vitro slice preparation. Ann Neurol. 1983;13:249–257. doi: 10.1002/ana.410130305. [DOI] [PubMed] [Google Scholar]
- Steffens M, Engler C, Zentner J, Feuerstein TJ. Cannabinoid CB1 receptor-mediated modulation of evoked dopamine release and of adenylyl cyclase activity in the human neocortex. Br J Pharmacol. 2004;141:1193–1203. doi: 10.1038/sj.bjp.0705706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Feuerstein TJ, van Velthoven V, Schnierle P, Knorle R. Quantitative measurement of depolarization-induced anandamide release in human and rat neocortex. Naunyn-Schmiedeberg's Arch Pharmacol. 2003a;368:432–436. doi: 10.1007/s00210-003-0817-1. [DOI] [PubMed] [Google Scholar]
- Steffens M, Schulze-Bonhage A, Surges R, Feuerstein TJ. Fatty acid amidohydrolase in human neocortex–Activity in epileptic and non-epileptic brain tissue and inhibition by putative endocannabinoids. Neurosci Lett. 2005;385:13–17. doi: 10.1016/j.neulet.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Steffens M, Szabo B, Klar M, Rominger A, Zentner J, Feuerstein TJ. Modulation of electrically evoked acetylcholine release through cannabinoid CB1 receptors: evidence for an endocannabinoid tone in the human neocortex. Neuroscience. 2003b;120:455–465. doi: 10.1016/s0306-4522(03)00318-x. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol (London) 2007;578:773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, et al. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Compar Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E.2005Effects of cannabinoids on neurotransmissionIn: Pertwee RG (eds).Handbook of Experimental Pharmacology vol 168, Cannabinoids Springer: Berlin; 327–365. [DOI] [PubMed] [Google Scholar]
- Szabo B, Than M, Thorn D, Wallmichrath I. Analysis of the effects of cannabinoids on synaptic transmission between basket and Purkinje cells in the cerebellar cortex of the rat. J Pharmacol Exp Ther. 2004;310:915–925. doi: 10.1124/jpet.104.066670. [DOI] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, et al. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol (London) 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol (London) 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol. 2002;88:534–539. doi: 10.1152/jn.2002.88.1.534. [DOI] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Endocannabinoids mediate rapid retrograde signaling at interneuron–pyramidal neuron synapses of the neocortex. J Neurophysiol. 2003;89:2334–2338. doi: 10.1152/jn.01037.2002. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, et al. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. Neuroimage. 2008;39:1533–1541. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Wallmichrath I, Szabo B. Cannabinoids inhibit striatonigral GABAergic neurotransmission in the mouse. Neuroscience. 2002;113:671–682. doi: 10.1016/s0306-4522(02)00109-4. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Cochyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep. 2009;61:1000–1007. doi: 10.1016/s1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer′s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]