Abstract
Individuals diagnosed with schizophrenia have an exceptionally high risk for tobacco dependence. Postmortem studies show that these individuals have significant reductions in α7 nicotinic acetylcholine receptors (nAChRs) in several brain areas. Decreased α7-mediated function might not only be linked to schizophrenia but also to increased tobacco consumption. The purpose of this study was to determine whether pharmacological blockade of α7 nAChRs would increase motivation of rats to intravenously self-administer nicotine (NIC) during a progressive ratio schedule of reinforcement (PR). Before PR, rats received local infusions of 0, 10, or 20 pmol of a selective α7 nAChR antagonist, α-conotoxin ArIB [V11L,V16D] (ArIB) into the nucleus accumbens (NAc) shell or the anterior cingulate cortex, brain areas that contribute to motivation for drug reward. We additionally sought to determine whether local infusion of 0, 10, or 40 nmol of a selective α7 nAChR agonist, PNU 282987, into these brain areas would decrease motivation for NIC use. Infusion of ArIB into the NAc shell and anterior cingulate cortex resulted in a significant increase in active lever pressing, breakpoints, and NIC intake, suggesting that a decrease in α7 nAChR function increases motivation to work for NIC. In contrast, PNU 282987 infusion resulted in reductions in these measures when administered into the NAc shell, but had no effect after administration into the anterior cingulate cortex. These data identify reduction of α7 nAChR function as a potential mechanism for elevated tobacco use in schizophrenia and also identify activation of α7 nAChRs as a potential strategy for tobacco cessation therapy.
Keywords: α7, motivation, nicotine, smoking, tobacco, schizophrenia
INTRODUCTION
Individuals with schizophrenia have a high risk for tobacco dependence. Epidemiological studies estimate that as many as 80% of individuals diagnosed with schizophrenia smoke cigarettes and clinical reports indicate that those with schizophrenia are particularly heavy smokers (Hughes et al, 1986; Glassman, 1993; Olincy et al, 1997; Kalman et al, 2005; Tidey et al, 2005; Williams et al, 2005, 2007; McKee et al, 2009). In support of a self-medication hypothesis, some studies have shown that smoking enhances cognition, improves sensory-gating deficits, and relieves side effects of neuroleptic therapeutics (Leonard et al, 1998, 2007; Sacco et al, 2005; Levin and Rezvani, 2007; D'Souza and Markou, 2011). Another equally plausible hypothesis is that these individuals have a shared vulnerability for schizophrenia and tobacco dependence.
An accumulation of genetic reports have identified polymorphisms linked to the α7 nicotinic acetylcholine receptor (nAChR) gene with diagnosis of schizophrenia (Leonard et al, 1996; Freedman et al, 1997; Stassen et al, 2000; Stephens et al, 2009; Mexal et al, 2010). Recent reports suggest that genetic variations in CHRNA7 may be associated with tobacco dependence as well (De Luca et al, 2004; Saccone et al, 2010). Nicotine (NIC), a major psychoactive ingredient in tobacco, binds to these ion channel receptors that are activated endogenously by the neurotransmitter, acetylcholine (ACh). Postmortem studies indicate that individuals with schizophrenia have marked reductions of α7 nAChRs in several brain areas, including the hippocampus and cingulate cortex (Freedman et al, 1995; Leonard et al, 1996, 1998, 2000; Guan et al, 1999; Court et al, 2000; Marutle et al, 2001; Mexal et al, 2010). Several CHRNA7 polymorphisms may contribute to a smoking phenotype in otherwise healthy subjects (Saccone et al, 2010), and independent data sets have revealed CHRNA7 polymorphisms associated with increased vulnerability for a tobacco dependence phenotype in those with schizophrenia diagnosis (De Luca et al, 2004). α7 Polymorphisms are linked to sensory-gating deficits (Freedman et al, 1997), a phenomenon commonly observed with schizophrenia diagnosis. Rodent studies have indicated that sensory-gating deficits may be due to a reduction in α7 nAChR function (Luntz-Leybman et al, 1992; Stevens et al, 1996, 1998; Hajos et al, 2005). Although no studies to date have correlated genetic analyses with α7 nAChR expression in humans, in vitro studies show that point mutations in the CHRNA7 promoter region and in an α7-duplicated gene is sufficient to alter α7 nAChR function (Leonard et al, 2002; Araud et al, 2011; de Lucas-Cerrillo et al, 2011).
The purpose of this preclinical study was to determine whether reductions of the α7 nAChR function constitute a biological mechanism for increased motivation for tobacco use as measured using a progressive ratio schedule of reinforcement (PR) during NIC self-administration in rats. Rats, like humans, readily self-administer NIC and there is 93% sequence homology in the rat and human α7 nAChRs (as determined using the Basic Local Alignment Search Tool for accession numbers AAB25224-rat and AAB40114-human) (Séguéla et al, 1993; Elliott et al, 1996). During PR, rats must work increasingly hard for a single delivery of NIC until they give up responding (ie, reach their breakpoint). We tested the hypothesis that reduced activity of α7 nAChRs in areas that regulate motivation for drug use would result in an increase in active lever pressing for NIC and breakpoints during PR. Such an effect might contribute to increased tobacco addiction in those with schizophrenia. The selective α7 nAChR antagonist, α-conotoxin ArIB [V11L,V16D] (ArIB) (Whiteaker et al, 2007) was infused into the nucleus accumbens (NAc) shell or anterior cingulate cortex immediately before PR. This study also questioned whether local infusion of a selective agonist of α7 nAChRs, PNU 282987 (Bodnar et al, 2005), into the NAc shell and anterior cingulate cortex would lead to reductions in responding maintained by NIC during PR. The α7 agonists are currently being explored as therapeutics to improve cognition, working memory, and sensory-gating deficits in schizophrenia (Bodnar et al, 2005; Olincy et al, 2006; Bitner et al, 2007; Freedman et al, 2008; Rezvani et al, 2009; Thomsen et al, 2009; Hajos and Rogers, 2010; Castner et al, 2011; Marquis et al, 2011). A positive finding would have implications for smoking cessation therapies in general.
MATERIALS AND METHODS
Animals
A total of 34, adult, male, Long-Evans rats (Harlan Laboratories, Dublin, VA) were used for these studies. Rats were individually housed in a temperature- and humidity-controlled vivarium under a 12/12 h light/dark cycle (lights on at 0600 hours). Behavioral testing took place between 1300 and 1900 hours. Rats weighed ∼300 g upon arrival and began testing at 320 g. This body weight was maintained by daily food rations throughout behavioral testing. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the Virginia Commonwealth University and were in accordance with the Guidelines for the Care and Use of Laboratory Animals, as set forth by the National Institutes of Health.
Drug Dosing and Administration
During self-administration procedures, rats received 0.03 mg/kg/i.v. infusion of NIC (by weight of freebase) in 0.0533 ml delivered over 1 s. NIC hydrogen tartrate salt was dissolved in 0.9% sterile saline and stored in the dark to prevent degradation. α-CTX ArIB [V11L,V16D] was synthesized as described previously (Whiteaker et al, 2007) (Institute for Behavioral Genetics, Boulder, CO) and dissolved in 0.9% sterile saline. PNU 282987 was obtained commercially (Tocris, Ellisville, MO) and dissolved according to the supplier's recommendations in 100 mM HCl sterile saline. Aliquots were stored at −20 °C and thawed immediately before use. Immediately before PR testing, intra-accumbens shell or intra-anterior cingulate infusions of 0, 10, or 20 pmol/hemisphere of ArIB, or 0, 10 or 40 nmol/hemisphere PNU 282987 were administered at a volume of 0.5–1.0 μl at a rate no greater than 0.5 μl/min using a within-subject, Latin-square design across days. Vehicle infusions were administered intermittently to assure drug clearance. Animals either received infusions into the anterior cingulate cortex or the NAc, and separate animals were used in experiments that assessed the effects of α7 nAChR antagonist and agonist.
Intra-Cranial Guide Cannula Implantations
All surgeries were performed using aseptic procedures under isoflurane anesthesia (induced at 3.5 l/min of oxygen and 3.5% isoflurane gas and maintained at ∼2.5 l/min of oxygen and 2.0–2.75% isoflurane). During surgery, rats received 5 mg/kg i.p. carprofen for preemptive analgesia. Surgical areas were shaved and cleaned with 7.5% povidone-iodine and 70% reagent alcohol. Rats were placed in a stereotaxic device with the bregma and lambda leveled to within 0.05 mm. Animals were implanted with 22-G bilateral guide cannula (Plastics One, Roanoke, VA) targeting either the NAc shell (+1.6 mm anterior, ±0.75 mm from midline, −6.5 mm ventral from the bregma) or the anterior cingulate cortex (+1.8 anterior, ±0.75 from midline, −2.75 ventral from the bregma). Guide cannulae were held in place with dental cement anchored with jeweler's screws, and dummy cannulae were inserted into the guides to maintain patency. Rats received 64 mg acetaminophen mixed in wet chow for 3 days after surgery. After behavioral procedures, brains were harvested to assess cannulae placement.
Intra-Jugular Catheter Implantation
Animals were anesthetized, prepped for surgery, and received analgesia as described above. A polyurethane catheter (3.5 French, Access Technologies) was implanted in the right jugular vein above the atrium and passed subcutaneously to the rat's back where it was connected to a cannula connector pedestal (Plastics One) implanted posterior to the rat's scapulae. To prevent infection, all rats received s.c. injection of 75 000 Units of penicillin G and 0.1 ml intra-catheter injection of 0.031 mg/ml ticarcillin/clavulanate in a 25% glycerol/heparinized saline solution (catheter lock). Rats were allowed to recover for at least 5 days before self-administration training. Before and after training sessions, catheters were irrigated with 0.9% sterile saline. Catheter patency was defined as a rapid loss of consciousness after 1.6 mg i.v. ketamine infusion. In the case of catheter failure, the left jugular vein was catheterized and the animal was returned to the study.
NIC Self-Administration
Self-administration procedures were as described previously (Brunzell et al, 2010). All self-administration procedures occurred in MED Associates operant chambers located within sound-attenuating boxes (St Albans, VT). Rats were tethered to a stainless steel-encased infusion tubing that was suspended from the chamber ceiling (Plastics One) to enable them to move freely about the chamber during each 2 h self-administration session. Levers were extended and a 5-w house light remained illuminated during all behavioral procedures. For a period of at least 10 days, rats were reinforced under a fixed ratio 1 (FR1) schedule of reinforcement maintained by NIC. NIC infusions were delivered using a Model PHS-100 syringe pump located on the outside of each sound-attenuating box. A panel light above the active (right-side) lever and a Sonalert tone generator at the rear of the chamber operated as cues. For NIC rats, depression of the ‘active' lever resulted in delivery of a 1 s, 0.03 mg/kg/i.v. NIC bolus plus a 20 s light+tone. No further NIC was delivered during this time-out period. To control for potential locomotor effects of the α7 nAChR ligands and for the primary reinforcing properties of the cues, a separate group of rats received the same cues without NIC infusion upon depression of the active lever (CUEonly). Depressions of the ‘inactive' left lever were recorded but were without scheduled consequences for NIC and CUEonly rats. Rats were trained for at least 10 days and until they reached a criterion of 3 consecutive days of >70% active:total lever presses. Behavioral programs and data collection were controlled by MED-PC IV software (MED Associates).
PR Responding Maintained by NIC
After FR training, rats were reinforced using a PR schedule. This phase of training required that rats depress the lever an increasing number of times to obtain a single NIC infusion and/or cue reinforcement (eg, 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95) (Arnold and Roberts, 1997). Sessions lasted for 2h or until a rat failed to respond for 20 consecutive minutes. Active and inactive lever responses, number of infusions, and the highest lever depression criterion achieved (breakpoint) were recorded.
Local Infusions of α7 Agonists and Antagonists into the Anterior Cingulate and NAc Shell
Rats received 2–3 days of 0.9% sterile saline infusions and intermittent infusions of vehicle to assure a stable level of PR responding. Independent groups of rats received daily infusions of either ArIB (0, 10, 20 pmol/hemisphere; n=18) or PNU 282987 (0, 10, 40 nmol/hemisphere; n=16) using a within-subject, Latin-square counterbalanced delivery of doses. Implanted guide cannulae targeting the NAc shell or anterior cingulate cortex assured that ligands did not diffuse to brain areas dorsal to the desired brain regions (Brunzell et al, 2009, 2010). Infusions were made using a micro infusion pump with Hamilton syringes attached to PE 20 tubing (Braintree Scientific, Braintree, MA). Infusion volumes were no greater than 1 μl and were delivered at a rate no faster than 0.5 μl/min through internal cannulae that extended 0.5 mm beyond the guides. Infusions were followed by a 2 min wait period to allow for drug diffusion and to prevent backflow of ArIB or PNU 282987 through the guide cannula.
Statistical Analysis
Within-subject, repeated-measures ANOVAs were used to analyze effects of vehicle and various concentrations of ArIB or PNU 282987 on behavioral measures for NIC and CUE-only rats during PR. Significant interactions of NIC condition with drug infusion were followed up with post hoc t-tests that compared behavioral data after drug infusion to data collected after infusion of vehicle into the brain areas studied.
RESULTS
Antagonism of NAc shell α7 nAChRs resulted in a dose-dependent increase in motivation to self-administer NIC (Figure 1). There was a significant interaction of NIC condition with the ArIB dose for measures of breakpoint (F1,7=8.26, p=0.02) and active lever pressing (F1,7=9.56, p=0.02) revealing that this effect was specific to NIC animals. There were main effects of NIC treatment (F1,7=92.077, p<0.01) and ArIB dosage (F1,7=5.53, p=0.05) for infusions/CUE presentations earned, but the interaction of these factors failed to reach significance. Post hoc t-tests showed that the 20 pmol/hemisphere infusion led to significant increases in how hard animals were willing to work for NIC (Figure 1a and b) and in the amount of NIC infusions earned (Table 1) (p's <0.05). Previous studies have shown that rodents will press for a visual stimulus reinforcer and that this behavior, like drug reinforcement, is dopamine mediated (Caggiula et al, 2002; Olsen and Winder, 2009). Infusion of ArIB into the NAc shell had no effect on lever pressing or breakpoints in CUEonly rats, suggesting that this effect did not generalize to other reinforcers. There was also no effect of drug exposure on response accuracy (active lever presses/(active+inactive lever presses)) after infusions of ArIB into the NAc shell (F<1.0); response accuracy remained high regardless of ArIB dose (Figure 1c), indicating that ArIB-associated increases in lever pressing were directed toward the lever that was reinforced with NIC infusions and not due to a non-specific increase in lever pressing activity.
Figure 1.
Antagonism of α7 nAChRs in the NAc shell increases motivation to self-administer nicotine. Local NAc shell infusion of ArIB led to a dose-dependent increase in (a) breakpoints and (b) active lever pressing maintained by nicotine during a progressive ratio schedule of reinforcement (NIC; n=4). There was no effect of ArIB infusion on breakpoint or active lever pressing in rats reinforced with light+tone cues but no nicotine (CUEonly; n=4). (c) Response accuracy as measured by % active lever pressing was not affected by NAc shell infusion of ArIB in NIC or CUEonly rats. *Significantly different from NIC vehicle infusion (p<0.05).
Table 1. Nicotine Intake after NAc Shell or Anterior Cingulate Infusion of α7 nAChR Antagonist or Agonist.
|
α-Conotoxin ArIB [V11L, V16D] |
PNU 282987 |
||||||
|---|---|---|---|---|---|---|---|
| pmol/hemisphere: | 0 | 10 | 20 | 0 | 10 | 40 | |
| NAc shell | Nicotine infusions | 7.4±0.92 | 9.4±0.75a | 10.20±0.37a | 7.0±0.41 | 2.75±0.63a | 4.0±1.22a |
| Intake mg/kg | 0.21±0.03 | 0.31±0.02a | 0.34±0.01a | 0.23±0.01 | 0.09±0.02a | 0.13±0.04a | |
| Anterior cingulate cortex | Nicotine infusions | 5.2±1.02 | 8.4±1.17a | 8.2±1.16a | 5.8±0.74 | 5.4±0.97 | 5.75±1.49 |
| Intake mg/kg | 0.16±0.03 | 0.25±0.03a | 0.25±0.03a | 0.19±0.03 | 0.16±0.03 | 0.23±0.03 | |
Number of i.v. nicotine infusions and total nicotine intake achieved during a progressive ratio schedule of reinforcement after local infusion of either α-conotoxin ArIB [V11L, V16D] (0, 10, or 40 pmol/hemisphere) or PNU 282987 (0, 10, or 40 nmol/hemisphere) into the nucleus accumbens (NAc) shell or into the anterior cingulate cortex of rats. Antagonism of α7 nicotinic acetylcholine receptors (nAChRs) with α-conotoxin ArIB[V11L, V16D] resulted in a significant increase in nicotine intake when administered into the NAc shell or the anterior cingulate cortex. In contrast, local infusion of an agonist of nAChRs,
PNU 282987, led to significant decreases in nicotine intake, but only when administered into the NAc shell.
Indicates significantly different from vehicle infusion (p<0.05; n=4–5 per group).
Antagonism of α7 nAChRs in the anterior cingulate cortex of an independent group of rats resulted in similar increases in motivation to self-administer NIC. There was a significant interaction of NIC condition with ArIB dose for breakpoint (F1,8=10.77, p=0.01) and active lever pressing (F1,8=8.53, p=0.02) and NIC infusions/CUE presentations (F1,8=18.71, p<0.01). In comparison to vehicle infusion, local infusion of 10 or 20 pmol/hemisphere of ArIB into the anterior cingulate cortex led to a significant increase in active lever pressing, breakpoints (Figure 2a and b), and NIC/CUE delivery (Table 1) in NIC (p's <0.05) but not CUEonly subjects. As with the NAc shell, there was no effect of ArIB infusion into the anterior cingulate on response accuracy (Figure 2c, F<1.0), suggesting that α7 nAChRs in this region do not modulate non-specific lever pressing activity or motivation to work for cue reinforcement. These findings support the hypothesis that reductions of the α7 nAChR function in the NAc shell and anterior cingulate cortex significantly increase motivation for NIC use.
Figure 2.
Antagonism of α7 nAChRs in the anterior cingulate cortex increases motivation to self-administer nicotine. Local anterior cingulate infusion of ArIB led to an increase in (a) breakpoints and (b) active lever pressing maintained by nicotine during a progressive ratio schedule of reinforcement (NIC; n=5). There was no effect of ArIB infusion on breakpoint or active lever pressing in rats reinforced with light+tone cues but no nicotine (CUEonly; n=5). (c) Response accuracy as measured by % active lever pressing was not affected by anterior cingulate infusion of ArIB in NIC or CUEonly rats. *Significantly different from NIC vehicle infusion (p<0.05).
The next series of experiments tested whether activation of α7 nAChRs in these brain regions would attenuate responding maintained by NIC during PR. Local infusion of the selective α7 nAChR agonist PNU 282987 into the NAc shell resulted in a significant interaction of NIC condition and drug dosage for breakpoint (F1,6=13.80, p=0.01) for active lever pressing (F1,6=33.19, p<0.01) and number of infusions/CUE presentations attained (F1,6=6.82, p=0.04). In contrast to increases in active lever presses and breakpoints observed in rats after antagonism of the NAc shell α7 nAChRs, rats that received local activation of these nAChRs through infusion of PNU 282987 showed reductions in active lever presses and breakpoints during PR (Figure 3). NIC rats showed a significant reduction in active lever presses and breakpoints after intra-accumbens shell infusion of 10 or 40 nmol/hemisphere of the PNU compound (p's <0.05). As before, this effect was specific to NIC rats and not observed in CUEonly animals. There was also no effect of NIC condition or drug infusion on response accuracy (F<1.0).
Figure 3.
Agonism of α7 nAChRs in the NAc shell decreases motivation to self-administer nicotine. Local NAc shell infusion of PNU 282987 led to a significant decrease in (a) breakpoints and (b) active lever pressing maintained by nicotine during a progressive ratio schedule of reinforcement (NIC; n=4). There was no effect of PNU 282987 infusion on breakpoint or active lever pressing in rats reinforced with light+tone cues but no nicotine (CUEonly; n=4). (c) Response accuracy as measured by % active lever pressing was not affected by NAc shell infusion of ArIB in NIC or CUEonly rats. *Significantly different from NIC vehicle infusion (p<0.05).
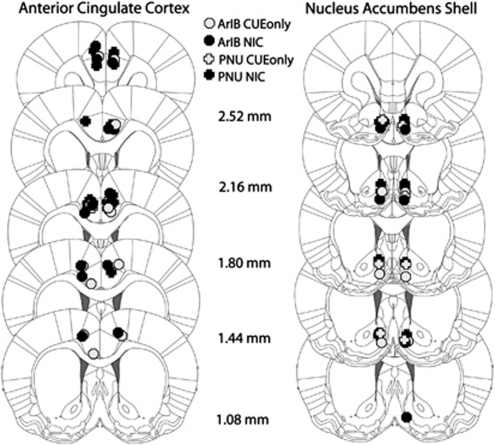
In contrast to pretreatment with the α7 antagonist, ArIB, effects of PNU 282987 were specific to the NAc shell. There was no interaction of NIC condition with PNU 282987 concentration observed for measures of breakpoint, active lever presses, or number of NIC infusions (F<1.0) after anterior cingulate infusion of this selective agonist of α7 nAChRs. These results were specific to drug infusion and not due to behavioral variation between NAc shell- and anterior cingulate-infused rats. On the last day of FR1 before PR testing, NIC rats earned a similar number of NIC infusions regardless of whether they had cannulae implanted into the NAc shell (mean=20.57±3.03) or the anterior cingulate cortex (mean=20.13±3.35). Rats also showed similar levels of NIC intake when infused with vehicle during PR (Table 1). Neuroanatomical reconstructions of guide cannula placement are shown in Figure 4.
Figure 4.
Neuroanatomical reconstructions of guide cannulae placement in the NAc shell and anterior cingulate cortex.
DISCUSSION
This study provides functional evidence that reductions in α7 nAChR activity support motivation for NIC self-administration. Selective antagonism of α7 nAChRs significantly increased the impetus of rats to work for NIC during PR. This effect was observed after drug infusion into the NAc shell, a brain area well-known for its contributions to motivational valence for drugs and natural rewards, as well as after antagonist infusion into the anterior cingulate cortex, a brain area that contributes to addiction, supports behavioral inhibition, and is morphologically compromised in individuals with schizophrenia (Goldman-Rakic, 1994; Guan et al, 1999; Chambers et al, 2001; Marutle et al, 2001; Moss et al, 2009). In contrast, local infusion of a selective α7 nAChR agonist into the NAc shell significantly reduced how hard rats were willing to work for a single infusion of NIC, suggesting that activation of these receptors may promote tobacco cessation. This effect was specific to the NAc shell and not observed in the anterior cingulate cortex. These data suggest that α7 nAChRs are not required for NIC self-administration but rather have a critical role in modulating NIC use.
Together with genetic and postmortem human studies (Freedman et al, 1995, 1997; Guan et al, 1999; Marutle et al, 2001; De Luca et al, 2004; Mexal et al, 2010), these findings suggest that some individuals may have a shared vulnerability for tobacco dependence and schizophrenia phenotype. An accumulation of data indicates that α7 nAChR expression is compromised in several brain areas of those diagnosed with schizophrenia (Freedman et al, 1997, 2000; Guan et al, 1999; Marutle et al, 2001). This phenotype seems to be due to faulty receptor assembly or trafficking as cigarette smoking results in elevated α7 nAChR message and protein yet surface α7 nAChRs are decreased as measured by α-bungarotoxin binding (Mexal et al, 2010). This could be due to post-translational changes such as reduced palmitoylation of the α7 nAChRs (Drisdel et al, 2004; Alexander et al, 2010) and further exacerbated in individuals with a truncated, duplicate α7 nAChR gene polymorphism that seems to lead to the formation of faulty α7 nAChRs (Araud et al, 2011; de Lucas-Cerrillo et al, 2011). Other studies suggest that a CHRNA7 intron dinucleotide repeat is positively associated with a significant risk for smoking behavior in those with schizophrenia (De Luca et al, 2004) with evidence for marginal associations for smoking risk in ‘healthy' individuals with other point mutations within the CHRNA7 gene (Saccone et al, 2010). These studies in rodents give biological credence to these genetic studies, demonstrating that reductions in α7 nAChR function result in significant elevations in motivation to self-administer NIC.
Although there may be some neuroanatomical overlap with α7 nAChR-associated sensory-gating abnormalities, it is likely that α7 nAChR deficits regulate smoking and schizophrenia phenotype through different neuroanatomical pathways. The α7 nAChRs are enriched in deep layers of the cortex that are connected to the sensory thalamus, perhaps accounting for the sensory-gating deficits that are associated with this genotype (Clarke et al, 1985; Luntz-Leybman et al, 1992; Stevens et al, 1996; Breese et al, 1997; Freedman et al, 1997; Bodnar et al, 2005; Hajos et al, 2005; Leonard et al, 2007). α-Bungarotoxin-binding studies show that α7 nAChR expression is higher in the cingulate and more ubiquitously expressed across layers than in other cortical regions (Marutle et al, 2001). Individuals diagnosed with schizophrenia have a 50% reduction in cingulate expression of α7 nAChRs (Marutle et al, 2001). We observed that antagonism of α7 nAChRs in the anterior cingulate cortex was sufficient to increase motivation to self-administer NIC. Human studies have revealed that deficits in prefrontal cortex function are also correlated with failed quit attempts in schizophrenic smokers (Moss et al, 2009). We saw a similar effect of blocking α7 nAChRs in the NAc shell; local administration of an α7 nAChR antagonist significantly increased NIC self-administration during PR. In contrast, application of an α7 nAChR agonist effectively reduced NIC administration but only when administered into the NAc shell, perhaps due to the unique influence of dopamine signaling on α7 nAChR activity at this locus vs the predominant basal ganglia innervation that supports activation of α7 nAChRs in the anterior cingulate cortex (Thomsen et al, 2010). These findings warrant further study into the expression of α7 nAChRs in the NAc shell of smokers and in individuals diagnosed with schizophrenia.
Previous preclinical studies exploring the role of α7 nAChRs in NIC reinforcement have returned equivocal results. Systemic administration of the α7 antagonist methylcoconitine (MLA) resulted in significant reductions in self-administration of NIC during an FR1 schedule of reinforcement (Markou and Paterson, 2001) at 3.8 and 7.9 mg/kg MLA, whereas others have reported no effect of a similar dosing regimen on self-administration when rats had a 2 day wash-out period between MLA dosings (Grottick et al, 2000). Although it is not clear what concentrations would be achieved in the brain at these doses, in vitro studies have shown that higher concentrations of MLA antagonize α6*nAChRs (Mogg et al, 2002), as well as α7 nAChRs. Recent reports show that activation of α6*nAChRs in the VTA and NAc shell are critical for acquisition and maintenance of NIC self-administration (Pons et al, 2008; Brunzell et al, 2010; Gotti et al, 2010); thus, effects with high doses of MLA may have been due to off-target antagonism of α6*nAChRs. The ArIB compound used in this study has >500-fold selectivity for α7 nAChRs over other nAChR subtypes (Whiteaker et al, 2008). In contrast, α-conotoxin MII is an antagonist of α6β2* nAChRs. NAc infusion of MII using the same paradigm as that used in this study shows the reverse effect of ArIB. That is, blockade of α6β2*nAChRs by α-conotoxin MII dose dependently decreased breakpoints and number of infusions earned (Brunzell et al, 2010), whereas antagonism of α7 nAChRs by ArIB increased NIC self-administration during PR, a schedule of reinforcement that is considered to measure motivation to self-administer drugs of abuse (Arnold and Roberts, 1997). Moreover, in contrast to effects of ArIB, we further observed that local administration of a selective α7 nAChR agonist into the NAc shell decreased NIC self-administration during PR. Thus, α6β2*nAChRs and α7 nAChRs in the NAc shell seem to have an opposing effect on NIC self-administration. Activation of α6β2*nAChRs supports NIC self-administration, whereas activation of α7 nAChRs impedes motivation to self-administer the drug.
Rather than having an essential role in NIC self-administration, our findings suggest that α7 nAChRs modulate NIC administration behavior. This hypothesis is supported by studies that show that mice with α7 subunit null mutations show normal NIC reward as measured by NIC conditioned place preference and acquisition of tail vein NIC administration (Walters et al, 2006; Pons et al, 2008). Other reports suggest that α7 may contribute to NIC intake after repeated exposure as measured by a reduction in NIC:water ratio during a 2-bottle choice paradigm (Levin et al, 2009). However, it is not clear in these latter studies if mice favor less NIC over time, or if over time, α7 knockout mice need less drug to achieve the desired effects of NIC. Our studies performed during PR indicate that local antagonism of α7 nAChRs in the NAc shell and anterior cingulate cortex increased the motivation of rats to work for an intravenous NIC reinforcer. Given the low affinity of the α7 nAChRs for NIC and the limited number of NIC infusions achieved during the demanding PR schedule of reinforcement, it is likely that the effect of ArIB was due to blockade of an endogenous cholinergic signal and not due to blockade of NIC action at α7 nAChRs (McGehee and Role, 1995; Mansvelder et al, 2002; Wooltorton et al, 2003; Papke et al, 2010). Unlike the high-affinity β2*nAChRs, activation of α7 nAChRs on DA terminals is not critical for NIC-stimulated striatal DA release (Champtiaux and Changeux, 2004; Salminen et al, 2004), and a recent microdialysis study showed that systemic administration of NIC results in elevated, persistent release of DA in the NAc shell of α7 nAChR knockout mice compared with wild-type mice on the same background (Besson et al, 2011). Hence, ACh activity at α7 nAChRs in the NAc shell and anterior cingulate cortex may curb NIC self-administration behavior through modulation of DA release. These findings have implications for individuals with schizophrenia who have a poverty of α7 nAChRs in the cingulate cortex (Marutle et al, 2001) that may render them vulnerable to heavy tobacco use. Whereas these findings resemble recent reports showing that α5 nAChR subunit knockout mice administer more NIC than their wild-type counterparts during FR schedules of reinforcement (Fowler et al, 2011), we make a distinction that the present observations do not reflect a decrease in the aversive effects of NIC after α7 nAChR antagonism. In general, NIC becomes aversive at high doses. During PR, rats in this study received approximately one-third of the NIC that they demonstrated they were willing to ingest during the FR1 schedule of reinforcement (Table 1).
It appears from our studies that activation of α7 nAChRs by the endogenous neurotransmitter ACh counters the behavioral effects of NIC at the high-affinity nAChRs. It is not clear whether most smokers achieve brain levels of NIC that are sufficient to activate the lower-affinity α7 nAChRs, but recent work using a 11C NIC tracer estimates that heavy smokers, such as those with schizophrenia (de Leon, 1996), may achieve as high as 700 nM NIC in the brain after a smoking episode and that brain levels of NIC accumulate during the day (Rose et al, 2010). In vitro studies suggest that 1–10 μM NIC is sufficient to bind and activate α7 nAChRs and quickly after activation by NIC, nAChRs undergo a period of desensitization (Lester and Dani, 1995; McGehee et al, 1995; Mansvelder et al, 2002; Uteshev et al, 2002; Wooltorton et al, 2003; Papke et al, 2009). These findings suggest that motivation to self-administer NIC could be significantly elevated by desensitization of α7 nAChRs in the NAc shell or anterior cingulate cortex. This effect could be exaggerated in schizophrenic smokers who already have a deficit of α7 nAChRs in the cingulate cortex (Marutle et al, 2001).
Recent studies have identified α7 nAChRs as therapeutic targets for improving cognition, memory, and gating deficits in schizophrenia (Bodnar et al, 2005; Olincy et al, 2006; Bitner et al, 2007; Freedman et al, 2008; Rezvani et al, 2009; Hajos and Rogers, 2010; Marquis et al, 2011). This study in rats suggests that activation of α7 nAChRs may have the added benefit of curbing motivation to smoke cigarettes. It is of interest to note that the US Food and Drug Administration approved smoking cessation drug, varenicline, although marketed as an α4β2 nAChR partial agonist, also has full agonist properties at α7 nAChRs (Mihalak et al, 2006). The findings of this study suggest that varenicline may exert some of its therapeutic effects through activation of α7 nAChRs.
In summary, these data demonstrate that reductions in α7 nAChR function promote NIC use. These findings expand on previous data which suggest that the CHRNA7 genotype is associated with tobacco dependence and identify low expression of α7 nAChRs as a potential mechanism by which individuals express a shared vulnerability to tobacco use and schizophrenia. These findings further identify activation of α7 nAChRs as a strategy that should be further explored for treatment of tobacco addiction.
Acknowledgments
This study was supported by a Virginia Foundation for Healthy Youth grant 8520667 and NIH grants DA031289 and DA023114 to DHB and MH53631 and GM48677 to JMM. We thank Karen Boschen, William Renzulli, Jennifer Lee, and Lauren Thompson for their technical assistance with these studies.
Virginia Commonwealth University holds an invention patent with DH Brunzell listed as an inventor ‘Alpha7 nicotinic acetylcholine receptor (nAChR) agonism to promote smoking cessation' VCU BRU-11-008F, which is based on the findings presented within this paper. JM McIntosh has received funding from Targacept for projects unrelated to this work.
References
- Alexander JK, Govind AP, Drisdel RC, Blanton MP, Vallejo Y, Lam TT, et al. Palmitoylation of nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:12–20. doi: 10.1007/s12031-009-9246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, et al. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function. Biochem Pharmacol. 2011;82:904–914. doi: 10.1016/j.bcp.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Baudonnat M, Cazala P, Guilloux JP, Reperant C, et al. 2011Alpha7-nicotinic receptors modulate nicotine-induced reinforcement and extracellular dopamine outflow in the mesolimbic system in mice Psychopharmacology (Berl)e-pub ahead of print 8 September 2011. [DOI] [PubMed]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, et al. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, et al. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem. 2005;48:905–908. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, et al. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Castner SA, Smagin GN, Piser TM, Wang Y, Smith JS, Christian EP, et al. Immediate and sustained improvements in working memory after selective stimulation of alpha7 nicotinic acetylcholine receptors. Biol Psychiatry. 2011;69:12–18. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Changeux JP. Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Prog Brain Res. 2004;145:235–251. doi: 10.1016/s0079-6123(03)45016-4. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat. 2000;20:281–298. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2011. [DOI] [PMC free article] [PubMed]
- de Leon J. Smoking and vulnerability for schizophrenia. Schizophr Bull. 1996;22:405–409. doi: 10.1093/schbul/22.3.405. [DOI] [PubMed] [Google Scholar]
- De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology. 2004;29:1522–1526. doi: 10.1038/sj.npp.1300466. [DOI] [PubMed] [Google Scholar]
- de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, et al. Function of partially duplicated human alpha7 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606. doi: 10.1074/jbc.M110.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Manzana E, Green WN. The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J Neurosci. 2004;24:10502–10510. doi: 10.1523/JNEUROSCI.3315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KJ, Ellis SB, Berckhan KJ, Urrutia A, Chavez-Noriega LE, Johnson EC, et al. Comparative structure of human neuronal alpha 2-alpha 7 and beta 2-beta 4 nicotinic acetylcholine receptor subunits and functional expression of the alpha 2, alpha 3, alpha 4, alpha 7, beta 2, and beta 4 subunits. J Mol Neurosci. 1996;7:217–228. doi: 10.1007/BF02736842. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat. 2000;20:299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: implications for psychiatric illness. Am J Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, et al. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Hajos M, Rogers BN. Targeting alpha7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr Pharm Des. 2010;16:538–554. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, et al. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, genetics and schizophrenia: evidence for self medication. J Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, et al. Nicotinic receptor function in schizophrenia. Schizophr Bull. 1996;22:431–445. doi: 10.1093/schbul/22.3.431. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, et al. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Adams C, Breese CR, Rollins Y, Adler LE, et al. Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci. 1998;12:195–201. [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Comery TA, Jow F, Navarra RL, Grauer SM, Pulicicchio C, et al. Preclinical assessment of an adjunctive treatment approach for cognitive impairment associated with schizophrenia using the alpha7 nicotinic acetylcholine receptor agonist WYE-103914/SEN34625. Psychopharmacology (Berl) 2011;218:635–647. doi: 10.1007/s00213-011-2357-6. [DOI] [PubMed] [Google Scholar]
- Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Harrison EL, Coppola S, George TP. Effects of the nicotinic receptor antagonist mecamylamine on ad-lib smoking behavior, topography, and nicotine levels in smokers with and without schizophrenia: a preliminary study. Schizophr Res. 2009;115:317–324. doi: 10.1016/j.schres.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci. 2010;40:185–195. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- Moss TG, Sacco KA, Allen TM, Weinberger AH, Vessicchio JC, George TP. Prefrontal cognitive dysfunction is associated with tobacco dependence treatment failure in smokers with schizophrenia. Drug Alcohol Depend. 2009;104:94–99. doi: 10.1016/j.drugalcdep.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, Lopez-Hernandez GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333:501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, Behm FM, et al. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci USA. 2010;107:5190–5195. doi: 10.1073/pnas.0909184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen HH, Bridler R, Hagele S, Hergersberg M, Mehmann B, Schinzel A, et al. Schizophrenia and smoking: evidence for a common neurobiological basis. Am J Med Genet. 2000;96:173–177. [PubMed] [Google Scholar]
- Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, et al. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Christensen DZ, Hansen HH, Redrobe JP, Mikkelsen JD. Alpha(7) nicotinic acetylcholine receptor activation prevents behavioral and molecular changes induced by repeated phencyclidine treatment. Neuropharmacology. 2009;56:1001–1009. doi: 10.1016/j.neuropharm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hay-Schmidt A, Hansen HH, Mikkelsen JD. Distinct neural pathways mediate alpha7 nicotinic acetylcholine receptor-dependent activation of the forebrain. Cereb Cortex. 2010;20:2092–2102. doi: 10.1093/cercor/bhp283. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Marks MJ, Christensen S, Dowell C, Collins AC, McIntosh JM. Synthesis and characterization of 125I-alpha-conotoxin ArIB[V11 L;V16A], a selective alpha7 nicotinic acetylcholine receptor antagonist. J Pharmacol Exp Ther. 2008;325:910–919. doi: 10.1124/jpet.108.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Christensen S, Yoshikami D, Dowell C, Watkins M, Gulyas J, et al. Discovery, synthesis, and structure activity of a highly selective alpha7 nicotinic acetylcholine receptor antagonist. Biochemistry. 2007;46:6628–6638. doi: 10.1021/bi7004202. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res. 2007;9:873–881. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]