Abstract
The nonmedical use of ‘designer' cathinone analogs, such as 4-methylmethcathinone (mephedrone) and 3,4-methylenedioxymethcathinone (methylone), is increasing worldwide, yet little information is available regarding the mechanism of action for these drugs. Here, we employed in vitro and in vivo methods to compare neurobiological effects of mephedrone and methylone with those produced by the structurally related compounds, 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine. In vitro release assays using rat brain synaptosomes revealed that mephedrone and methylone are nonselective substrates for plasma membrane monoamine transporters, similar to MDMA in potency and selectivity. In vivo microdialysis in rat nucleus accumbens showed that i.v. administration of 0.3 and 1.0 mg/kg of mephedrone or methylone produces dose-related increases in extracellular dopamine and serotonin (5-HT), with the magnitude of effect on 5-HT being greater. Both methcathinone analogs were weak motor stimulants when compared with methamphetamine. Repeated administrations of mephedrone or methylone (3.0 and 10.0 mg/kg, s.c., 3 doses) caused hyperthermia but no long-term change in cortical or striatal amines, whereas similar treatment with MDMA (2.5 and 7.5 mg/kg, s.c., 3 doses) evoked robust hyperthermia and persistent depletion of cortical and striatal 5-HT. Our data demonstrate that designer methcathinone analogs are substrates for monoamine transporters, with a profile of transmitter-releasing activity comparable to MDMA. Dopaminergic effects of mephedrone and methylone may contribute to their addictive potential, but this hypothesis awaits confirmation. Given the widespread use of mephedrone and methylone, determining the consequences of repeated drug exposure warrants further study.
Keywords: dopamine, MDMA, mesolimbic, microdialysis, serotonin (5-HT), transporters
INTRODUCTION
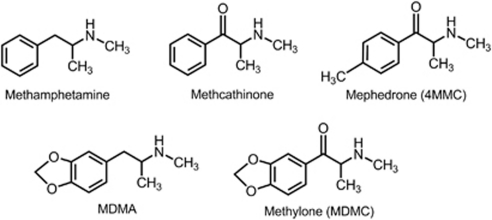
Cathinone (2-amino-1-phenylpropan-1-one) is a naturally occurring β-keto analog of amphetamine that has served as a structural template for the discovery of compounds with a range of pharmacological activities (Carroll et al, 2010; Damaj et al, 2004; Meltzer et al, 2006). For example, cathinone derivatives that display therapeutic properties include the antidepressant bupropion and the anorectic agent diethylpropion. Recently, a number of synthetic analogs of N-methylcathinone (ie, methcathinone) have become commercially available for purchase over the Internet and in retail shops (Bossong et al, 2005; Brandt et al, 2010; Karila and Reynaud, 2010; Vardakou et al, 2011; Winstock et al, 2011). These so-called ‘designer' methcathinones are used outside of medical settings for personal experimentation, mood elevation, and as ‘legal highs'. The European Monitoring Center for Drugs and Drug Addiction reported that in 2010 alone, 15 out of 40 newly encountered psychoactive substances were found to be cathinone derivatives or isomers (EMCDDA, 2010). Two of the most popular methcathinone derivatives are 4-methylmethcathinone (mephedrone) and 3,4-methylenedioxymethcathinone (methylone) (Figure 1). Reports describing serious cardiovascular and neurological side-effects of mephedrone have increased at an alarming rate (CDC, 2011; James et al, 2010; Wood et al, 2010, 2011), and deaths attributable to overdose from mephedrone alone or in combination with other drugs have been documented (Dickson et al, 2010; Lusthof et al, 2011; Maskell et al, 2011).
Figure 1.
Chemical structures of designer methcathinone analogs and related compounds.
Although it might be inferred from structural similarities with cathinone that designer analogs of methcathinone target monoamine transporters (Cozzi and Foley, 2003; Rothman et al, 2003) or nicotinic acetylcholine receptors (Carroll et al, 2010; Damaj et al, 2004), only limited information is available regarding the pharmacodynamic mechanisms of these drugs. Cozzi et al (1999) demonstrated that methylone blocks the reuptake of norepinephrine, dopamine and serotonin (5-HT) in vitro, whereas (Nagai et al, 2007) found that methylone evokes the release of radiolabeled monoamines from rat brain synaptosomes. The in vitro results indicate that methylone interacts with plasma membrane transporters, but the precise nature of this interaction requires clarification. Dal Cason et al (1997) used a drug discrimination paradigm to demonstrate that methylone substitutes for 3,4-methylenedioxymethamphetamine (MDMA) in rats trained to distinguish MDMA from saline, but fails to substitute for the psychedelic drug 2-amino-1-(2,5-dimethoxy-4-methylphenyl)propane (DOM) in rats trained to discriminate DOM from saline. A recent study by Kehr et al (2011) showed that mephedrone increases extracellular dopamine and 5-HT in rat nucleus accumbens, and these effects could be related to inhibition of monoamine uptake (Hadlock et al, 2011). Importantly, pharmacokinetic studies in rats and humans have shown that mephedrone and methylone are metabolized in a manner similar to ring-substituted amphetamines (Kamata et al, 2006; Meyer et al, 2010), suggesting the possibility that bioactive metabolites might be formed in vivo.
Given the lack of empirical data and the complex structure–activity relationships of cathinone analogs (Carroll et al, 2010; Dal Cason et al, 1997; Damaj et al, 2004; Nagai et al, 2007), we sought to examine the mechanism(s) of action for mephedrone and methylone. To this end, we used in vitro and in vivo methods to compare the neurobiological effects of methcathinone analogs with those produced by the structurally related compounds, MDMA and methamphetamine. Specifically, this study had three aims: (1) to determine the interactions of mephedrone, methylone, MDMA, and methamphetamine with monoamine transporters, using in vitro assays in rat brain synaptosomes (Rothman et al, 2001); (2) to examine the effects of these drugs on extracellular dopamine and 5-HT using in vivo microdialysis in awake, freely moving rats (Baumann et al, 2011); and (3) to evaluate the acute and long-term consequences of repeated high-dose drug administration (Baumann et al, 2008). Our findings indicate that mephedrone and methylone are potent substrates for monoamine transporters in rat brain, with a profile of dopamine- and 5-HT-releasing activity, which more closely resembles MDMA than methamphetamine. However, important differences were noted among the effects of mephedrone, methylone, and MDMA after high-dose administration. Further studies are warranted to elucidate the mechanistic distinctions among these various drugs.
MATERIALS AND METHODS
Drugs and Reagents
Mephedrone and methylone were synthesized in racemic form as HCl salts in our laboratories. Chemical and structural analysis included proton nuclear magnetic resonance, gas chromatography/mass spectrometry, thin layer chromatography, and melting point determination. All data confirmed the expected structures. (+)-3,4-Methylenedioxymethamphetamine HCl (MDMA) and (+)-methamphetamine HCl (methamphetamine) were obtained from the Pharmacy at the National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP) in Baltimore, MD. [3H]Serotonin ([3H]5-HT, specific activity=20 Ci/mmol) was purchased from Perkin Elmer (Shelton, CT) whereas [3H]1-methyl-4-phenylpyridinium ([3H]MPP+, specific activity=85 Ci/mmol) was purchased from American Radiolabeled Chemicals (St Louis, MO). All other chemicals and reagents used for the in vitro assays, microdialysis methods, and high-pressure liquid chromatography with electrochemical detection (HPLC-ECD) were acquired from Sigma-Aldrich (St Louis, MO).
Animals and Surgery
Male Sprague–Dawley rats weighing 300–350 g were housed under conditions of controlled temperature (22±2 °C) and humidity (45±5%) with food and water freely available. Rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the NIDA IRP. Lights were on from 0700 to 1900 hours and experiments were carried out between 0900 and 1400 hours. Rats were double-housed on receipt and allowed at least 2 weeks to acclimate to the vivarium conditions before being used in experiments. For in vitro assays, rats were euthanized with CO2 and decapitated. Brains were rapidly removed and tissue was dissected on ice. For in vivo microdialysis experiments, rats received sodium pentobarbital (60 mg/kg, i.p.) for surgical anesthesia. Each rat was fitted with an indwelling jugular catheter made of Silastic Medical Grade tubing (Dow Corning, Midland, MI). Immediately thereafter, an intracerebral guide cannula (CMA 12, CMA/Microdialysis, Acton, MA) was implanted above the nucleus accumbens, according to stereotaxic coordinates: 1.6 mm lateral and 1.6 mm anterior to bregma, and 6.0 mm below the surface of dura. Guide cannulae were secured to the skull using stainless steel anchor screws and dental acrylic. Animals were individually housed postoperatively and allowed 7–10 days for recovery. For the repeated dosing experiments, rats were individually housed 1 week before receiving the injection regimen and for 2 weeks thereafter.
In Vitro Transporter Assays
In our initial investigations, we tested the ability of mephedrone, methylone, MDMA, and methamphetamine to evoke the release of radiolabeled substrates in rat brain synaptosomes using modifications of published methods (Rothman et al, 2001). Briefly, in vitro release assays were conducted using [3H]MPP+ as the radiolabeled substrate for norepinephrine transporters (NET) and dopamine transporters (DAT), while using [3H]5-HT as the radiolabeled substrate for 5-HT transporters (SERT). Whole brain minus cerebellum (for NET and SERT assays) or striatum (for DAT assays) was homogenized in ice-cold 10% sucrose containing 1 μM reserpine. For the NET release assays, 100 nM GBR12935 and citalopram were added to the sucrose solution to block [3H]MPP+ uptake into DA and 5-HT terminals. For DAT release assays, 100 nM desipramine and citalopram were added to block [3H]MPP+ uptake into NE and 5-HT terminals. For SERT release assays, 100 nM nomifensine and GBR12935 were added to block uptake of [3H]5-HT into NE and DA terminals. After 12 strokes with a Potter–Elvehjem homogenizer, homogenates were centrifuged at 1000 × g for 10 min at 4 °C, and the supernatants (ie, synaptosomes) were retained on ice. Synaptosomes were incubated to steady state in a polypropylene beaker, with stirring at 25 °C, in Krebs-phosphate buffer (pH 7.4), which contained 1 μM reserpine and either 5 nM [3H]MPP+ or 5 nM [3H]5-HT. To commence the assay, 850 μl of preloaded synaptosomes were added to polystyrene test tubes or 96-well plates that contained 150 μl test drug in uptake buffer plus 1 mg/ml bovine serum albumin. After 30 min ([3H]MPP+ assays) or 5 min ([3H]5-HT), the release reaction was terminated by dilution with 4 ml wash buffer (10 mM Tris-HCl pH 7.4 containing 0.9% NaCl at 25 °C) followed by rapid vacuum filtration over Whatman GF/B filters using a Brandel cell harvester (Brandel, Gaithersburg, MD). Filters were rinsed twice with 4 ml wash buffer and dried under vacuum. The retained tritium was counted by a Trilux liquid scintillation counter (Micromedic Systems, Philadelphia, PA) at 40% efficiency after an overnight extraction in 0.6 ml scintillation cocktail.
In Vivo Microdialysis Experiments
In vivo microdialysis sampling was carried out as described, with minor modifications (Baumann et al, 2011). On the evening before an experiment, rats were moved to the testing room. A plastic collar was placed around the neck of each rat, a dialysis probe (CMA/12, CMA Microdialysis) was inserted into the guide cannula, and an extension tube was attached to the indwelling jugular catheter. The probe exchange surface was 2 × 0.5 mm. Each rat was placed into its own activity field arena (Coulbourn Instruments, Allentown, PA) and connected to a tethering system, which allowed motor activity within the container. Probes were perfused overnight with artificial cerebrospinal fluid pumped at a flow rate of 0.6 μl/min. On the next morning, dialysate samples were collected at 20-min intervals. Samples were immediately assayed for dopamine and 5-HT by HPLC-ECD as described below. Rats were randomly assigned to groups receiving either drug or saline injections. Once three stable baseline samples were obtained, rats received two sequential i.v. injections of drug, 0.3 mg/kg at time zero, followed by 1 mg/kg 60 min later. Control rats received sequential i.v. injections of saline (1 ml/kg) according to the same schedule. Microdialysis samples were collected every 20 min throughout the post-injection period for 120 min. At the end of the experiments, rats were euthanized with CO2 and decapitated. Brain sections were examined to verify placement of microdialysis probe tips within the nucleus accumbens. Only those rats with correct placements were included in data analyses.
Locomotor measures were obtained during microdialysis testing as previously described (Baumann et al, 2011). During the overnight acclimation period and while undergoing microdialysis, each rat was housed within a square Plexiglass arena (43 cm length × 43 cm width × 43 cm height) equipped with an activity monitoring system (Tru Scan, Coulbourn Instruments). A sensor ring lined with photobeams spaced 2.54 cm apart was positioned in the horizontal plane to allow for real-time monitoring of various motor parameters. Activity was monitored in 20-min bins during microdialysis testing, beginning 60 min before i.v. drug injections and continuing for 120 min thereafter. Horizontal locomotor activity and stereotypy were quantified separately; horizontal locomotor activity is defined as the total distance traveled in the horizontal plane (measured in cm), whereas stereotypy is defined as the number of photobeam breaks <±1.5 beam spaces and back to the original point, not exceeding 2 s apart (measured in number of moves).
HPLC-ECD analysis of dialysate samples was conducted as follows: aliquots of dialysate (5 μl) were injected onto a microbore C18 column that was coupled to an amperometric detector (Model LC-4C, Bioanalytical Systems, West Lafayette, IN) with a glassy carbon electrode set at a potential of +650 mV relative to Ag/AgCl reference. Mobile phase consisting of 150 mM monochloroacetic acid, 150 mM sodium hydroxide, 2.5 mM sodium octanesulfonic acid, 250 μM disodium EDTA, 6% methanol, and 6% acetonitrile per liter of water (pH=5.3) was pumped at 60 μl/min using a syringe pump (Model 260D, ISCO, Lincoln, NE). Chromatographic data were acquired on-line and exported to an Empower software system (Waters Associates, Milford, MA) for peak amplification, integration, and analysis. A monoamine standard mix containing dopamine, 5-HT and their respective acid metabolites was injected before and after dialysate samples to insure validity of constituent retention times. Peak heights of unknowns were compared with those of standards, and the lower limit of assay sensitivity (3 × baseline noise) was 50 fg/5 μl sample.
Repeated Dosing Experiments
The effects of repeated dosing with mephedrone, methylone, and MDMA were compared. Specifically, we assessed the effects of repeated high-dose administration of mephedrone, methylone, and MDMA on motor behavior, core temperature, and monoamine levels in post-mortem brain tissue. Groups of rats received a total of three s.c. injections of saline or mephedrone (3 and 10 mg/kg), saline or methylone (3 and 10 mg/kg), and saline or MDMA (2.5 and 7.5 mg/kg), one injection given every 2 h (Baumann et al, 2008). Injections were carried out in the vivarium, and rats were returned to their home cages immediately after each injection. Drug doses were determined from pilot studies and previously published behavioral data (Dal Cason et al, 1997). Rats were observed for 1 min every hour from the onset of injections until 6 h thereafter; after the 1-min observation period, rats were removed from their home cages and core temperatures were measured via insertion of a RET-2 probe (Physitemp Instruments, Clifton, NJ) into the rectum. At time zero, 2 and 4 h, rats received s.c. injections of drug or saline immediately after temperature measurements were recorded. At each viewing period, behaviors were scored using a graded scale with 0=absent, 1=equivocal, 2=present, and 3=intense. Specific behaviors included ambulation (four feet move), rearing (head raised, with forepaws off the floor), and forepaw treading, an element of the 5-HT behavioral syndrome.
Two weeks after dosing, rats were decapitated and brain tissue was dissected to obtain the frontal cortex and striatum as previously described (Baumann et al, 2008). Tissue samples were weighed, homogenized in 0.1 N HClO4 and centrifuged at 12 200 × g for 15 min. Concentrations of norepinephrine, dopamine and 5-HT were quantified in the supernatant using HPLC-ECD. Aliquots were injected onto an HPLC column linked to a coulometric detector (ESA Model Coulochem III, Dionex, Chelmsford, MA). Mobile phase consisting of 50 mM sodium phosphate monobasic, 250 μM Na2EDTA, 0.03% sodium octanesulfonic acid, and 25% methanol (pH=2.75) was recirculated at 0.9 ml/min. Data were acquired by an Empower software system, where peak heights of unknowns were compared with those of standards. The lower limit of assay sensitivity (3 × baseline noise) was 1 pg/20 μl sample.
Data Analyses and Statistics
For in vitro release assays, data are expressed as mean±SD, and EC50 values for releasing potency were calculated based on dose-response data using the MLAB computer program (Civilized Software, Silver Spring, MD). Data from microdialysis and repeated dosing experiments were analyzed using the Prism 4 computer program (GraphPad Software, La Jolla, CA). For in vivo microdialysis experiments, neurotransmitter and behavioral data from individual rats were normalized to percent control values (ie, % basal) using the averaged raw data from three preinjection time points as basal, or 100%. In this manner, each rat served as its own control. Normalized group data are expressed as mean±SEM, and were evaluated by a two-factor analysis of variance (ANOVA) (Drug, Time) where drug effects were compared with saline control. When significant main effects were noted, post hoc comparisons were carried out at each time point using the Bonferroni test. For the repeated dosing experiments, raw temperature data were evaluated by a two-factor ANOVA (Dose, Time) where drug effects were compared with saline control. When significant main effects were noted, one-way ANOVA (Dose) was carried at each time point followed by Newman–Keul's post hoc test to determine differences between group means. Maximal temperature increases were determined from time-course data by subtracting the mean preinjection temperature (time 0 min) from the mean peak temperature observed post-injection for a given treatment group. Behavioral and post-mortem neurotransmitter data are expressed as mean±SEM, and were analyzed by one-factor (Dose) ANOVA followed by Newman–Keul's post hoc test to determine differences between treatment groups. p<0.05 was chosen as the minimum level for statistical significance.
RESULTS
In Vitro Release Data
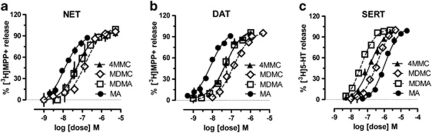
Figure 2 shows the dose-response effects of test drugs on the release of [3H]MPP+ and [3H]5-HT from rat brain synaptosomes, when assay conditions were optimized for NET, DAT, and SERT. Table 1 summarizes the dose-response data by reporting EC50 values and selectivity ratios for each compound. Mephedrone was a substrate for NET (EC50=62.7 nM) and DAT (EC50=49.1 nM), with slightly lower potency at SERT (EC50=118.3 nM). Methylone displayed a selectivity profile similar to mephedrone but was about half as potent. In general, the in vitro releasing capabilities of mephedrone and methylone resembled those of MDMA. By contrast, methamphetamine was more potent at NET (EC50=13.8 nM) and DAT (EC50=8.5 nM) when compared with the other drugs, and was also much less potent at SERT (EC50=1291.7 nM). With regard to selectivity ratios, mephedrone and methylone displayed NET/DAT ratios and DAT/SERT ratios close to unity, similar to MDMA. Methamphetamine displayed a NET/DAT ratio of 0.62 and a DAT/SERT ratio >150, confirming its high selectivity toward catecholamine transporters.
Figure 2.
Dose-response effects of test drugs on the release of [3H]MPP+ and [3H]5-HT from rat brain synaptosomes in vitro, under conditions optimized for NET (a), DAT (b), and SERT (c). Dose-response curves were constructed by incubating various concentrations of each test drug with synaptosomes that had been preloaded with tritiated substrate, either [3H]MPP+ or [3H]5-HT. Test drugs were mephedrone (4MMC), methylone (MDMC), 3,4-methylenedioxymethamphetamine (MDMA), and methamphetamine (MA). Data are mean±SD for N=6 (for NET assays) or N=3 (DAT and SERT assays) separate experiments.
Table 1. Effects of Test Drugs on Transporter-Mediated Release from Rat Brain Synaptosomes.
| Test drug | [3H]MPP+ release via NET EC50 (nM) | [3H]MPP+ release via DAT EC50 (nM) | [3H]5-HT release via SERT EC50 (nM) | NET/DAT ratio | DAT/SERT ratio |
|---|---|---|---|---|---|
| Mephedrone | 62.7±17.1 | 49.1±8.32 | 118.3±25.9 | 0.78 | 2.41 |
| Methylone | 152.3±33.2 | 133.0±11.2 | 242.1±48.3 | 0.87 | 1.82 |
| MDMA | 54.1±8.9 | 51.2±6.3 | 49.6±5.4 | 0.95 | 0.97 |
| Methamphetamine | 13.8±3.1 | 8.5±1.4 | 1291.7±241.6 | 0.62 | 152.0 |
Data are mean±SD for N=6 (NET) or N=3 (DAT and SERT) separate experiments. NET/DAT ratio=1/(NET EC50) divided by 1/(DAT EC50) for each drug; higher value indicates greater NET selectivity. DAT/SERT ratio=1/(DAT EC50) divided by 1/(SERT EC50) for each drug; higher value indicates greater DAT selectivity.
In Vivo Microdialysis Data
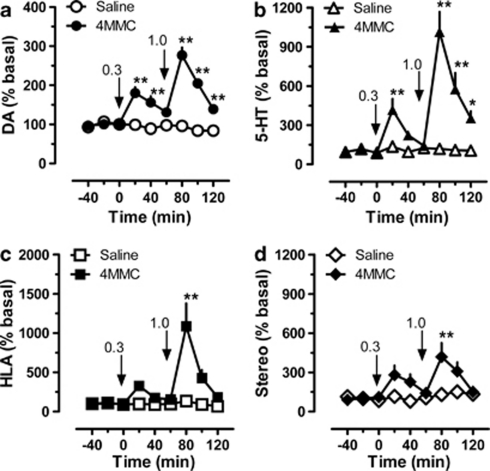
Figure 3 shows the effects of acute i.v. administration of mephedrone on neurochemistry and behavior in male rats (N=7 rats per group). Mephedrone produced significant main effects on extracellular concentrations of dopamine (Figure 3, panel a; F(1, 8)=190.4, p<0.0001) and 5-HT (Figure 3, panel b; F(1, 8)=68.8, p<0.0001) when compared with saline controls. Post hoc analysis demonstrated that mephedrone elevated dialysate dopamine at 20 and 40 min after 0.3 mg/kg (p<0.01), and at all-time points after 1.0 mg/kg (p<0.01). The peak magnitude of dopamine increase was 1.8-fold above baseline after 0.3 mg/kg and 2.9-fold after 1.0 mg/kg. Mephedrone elevated dialysate 5-HT at 20 min after 0.3 mg/kg (p<0.01), and at all-time points after 1.0 mg/kg (p<0.01). The peak effect on 5-HT was 4.2-fold above baseline after 0.3 mg/kg and 11.1-fold after 1.0 mg/kg. Mephedrone caused main effects on horizontal locomotor activity (Figure 3, panel c; F(1, 8)=24.3, p<0.0001) and stereotypy (Figure 3, panel d; F(1, 8)=17.8, p<0.0001) when compared with saline controls. Post hoc analysis revealed that mephedrone failed to significantly increase locomotion after 0.3 mg/kg but did significantly stimulate activity during the first 20-min period after 1.0 mg/kg (p<0.01). The peak effect of mephedrone on horizontal locomotor activity was 10.9-fold above baseline, though this effect was short-lived and returned to baseline by 1 h post-injection. Mephedrone elevated stereotypy to a significant extent at 20 min after 1.0 mg/kg (p<0.01), and the peak increase in stereotypic movements was 4.2-fold above baseline.
Figure 3.
Dose-response effects of mephedrone (4MMC) on neurochemistry and locomotor behavior in male rats undergoing in vivo microdialysis in nucleus accumbens. 4MMC-treated rats received i.v. injections of 0.3 mg/kg at time zero, followed by 1.0 mg/kg 60 min later. Control rats received i.v. saline injections (1 ml/kg) on the same schedule. Data are mean±SEM for N=7 rats per group, expressed as a percentage of preinjection baseline values (% basal) for dopamine (DA, panel a), serotonin (5-HT, panel b), horizontal locomotor activity (HLA, panel c), and stereotypic movements (Stereo, panel d). Basal concentrations of dialysate DA and 5-HT were 2.39±0.51 and 0.25±0.03 pg/5 μl, respectively. Basal levels of HLA and Stereo were 170±15 cm/20 min and 131±15 moves/20 min, respectively. **p<0.01 or *p<0.05 vs saline control at the corresponding time point (Bonferroni post hoc test).
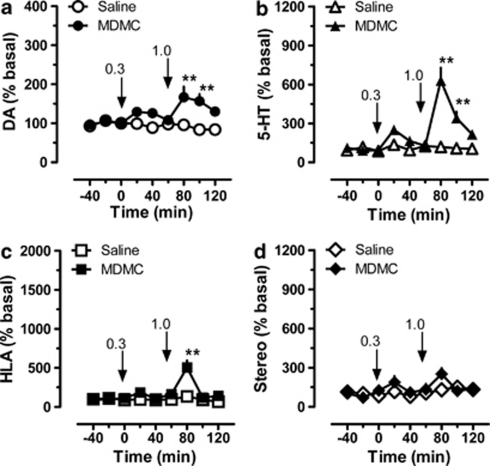
The data in Figure 4 depict the effects of methylone on neurochemistry and motor behavior (N=7 rats per group). Methylone produced significant main effects on extracellular levels of dopamine (Figure 4, panel a; F(1, 8)=36.8, p<0.0001) and 5-HT (Figure 4, panel b; F(1, 8)=54.1, p<0.0001) when compared with saline-treated controls. Post hoc analysis demonstrated that methylone failed to significantly influence dopamine at the 0.3 mg/kg dose, but elevated the transmitter at 20 and 40 min after 1.0 mg/kg (p<0.01). The peak rise in dopamine was 1.7-fold above baseline after 1.0 mg/kg. Similar to its effects on dopamine, methylone significantly elevated dialysate 5-HT only at 20 and 40 min after the high dose (p<0.01). The peak rise in 5-HT was 6.3-fold above baseline after 1.0 mg/kg. Methylone caused main effects on horizontal locomotor activity (Figure 4, panel c; F(1, 8)=21.7, p<0.0001) but did not alter stereotypy (Figure 4, panel d; F(1, 8)=2.6, p<0.1102) when compared with saline controls. Post hoc analysis revealed that methylone failed to significantly increase locomotion after 0.3 mg/kg, but did significantly stimulate activity during the first 20-min period after 1.0 mg/kg (p<0.01). The maximal effect of methylone on horizontal locomotor activity was 5.0-fold above baseline, though this increase was short-lived and returned to baseline by 40 min post-injection.
Figure 4.
Dose-response effects of methylone (MDMC) on neurochemistry and locomotor behavior in male rats undergoing in vivo microdialysis in nucleus accumbens. MDMC-treated rats received i.v. injections of 0.3 mg/kg at time zero, followed by 1.0 mg/kg 60 min later. Control rats received i.v. saline injections (1 ml/kg) on the same schedule. Data are mean±SEM for N=7 rats per group, expressed as a percentage of preinjection baseline values (% basal) for dopamine (DA, panel a), serotonin (5-HT, panel b), horizontal locomotor activity (HLA, panel c), and stereotypic movements (Stereo, panel d). Basal concentrations of dialysate DA and 5-HT were 2.63±0.49 and 0.36±0.09 pg/5 μl, respectively. Basal levels of HLA and Stereo were 181±18 cm/20 min and 132±17 moves/20 min, respectively. **p<0.01 vs saline control at the corresponding time point (Bonferroni post hoc test).
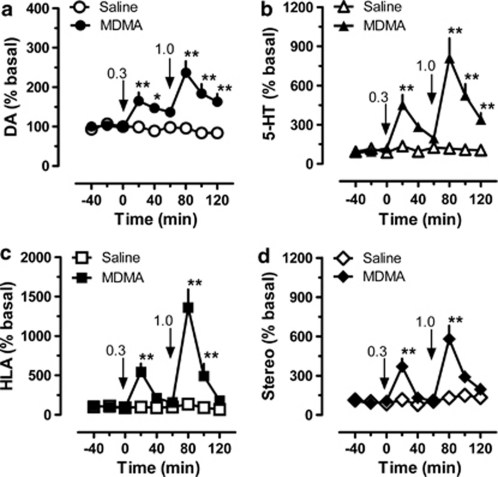
Figure 5 shows the effects of acute i.v. administration of MDMA on neurochemistry and behavior in male rats undergoing microdialysis (N=7 rats per group). As expected, MDMA produced significant main effects on extracellular concentrations of dopamine (Figure 5, panel a; F(1, 8)=71.7, p<0.0001) and 5-HT (Figure 5, panel b; F(1, 8)=77.2, p<0.0001) when compared with saline controls. Post hoc analysis demonstrated that MDMA elevated dialysate dopamine at 20 min (p<0.01) and 40 min (p<0.05) after 0.3 mg/kg, and at all-time points after 1.0 mg/kg (p<0.01). The peak magnitude of dopamine increase was 1.7-fold above baseline after 0.3 mg/kg and 2.3-fold after 1.0 mg/kg. MDMA elevated dialysate 5-HT at 20 min after 0.3 mg/kg (p<0.01), and at all-time points after 1.0 mg/kg (p<0.01). The peak magnitude of 5-HT increase was 4.5-fold above baseline after 0.3 mg/kg and 8.1-fold after 1.0 mg/kg. MDMA caused main effects on horizontal locomotor activity (Figure 5, panel c; F(1, 8) = 48.3, p<0.0001) and stereotypy (Figure 5, panel d; F(1, 8)=31.8, p<0.0001) when compared with saline controls. Post hoc analysis revealed that MDMA significantly increased locomotion at 20 min after 0.3 mg/kg (p<0.01), and at 20 and 40 min after 1.0 mg/kg (p<0.01). The peak effect of MDMA on horizontal locomotor activity was 5.4-fold above baseline after 3.0 mg/kg, and 13.6-fold after 1.0 mg/kg. MDMA increased stereotypy to a significant extent in the first 20-min period after 0.3 mg/kg (p<0.01) and the first 20-min period after 1.0 mg/kg (p<0.01). The peak magnitude of stereotypic movements was 3.4-fold and 5.1-fold above baseline after 0.3 and 1.0 mg/kg, respectively.
Figure 5.
Dose-response effects of 3,4-methylenedioxymethamphetamine (MDMA) on neurochemistry and locomotor behavior in male rats undergoing in vivo microdialysis in nucleus accumbens. MDMA-treated rats received i.v. injections of 0.3 mg/kg at time zero, followed by 1.0 mg/kg 60 min later. Control rats received i.v. saline injections (1 ml/kg) on the same schedule. Data are mean±SEM for N=7 rats per group, expressed as a percentage of preinjection baseline values (% basal) for dopamine (DA, panel a), serotonin (5-HT, panel b), horizontal locomotor activity (HLA, panel c), and stereotypic movements (Stereo, panel d). Basal concentrations of dialysate DA and 5-HT were 2.00±0.52 pg/5 μl and 0.44±0.09 pg/5 μl, respectively. Basal levels of HLA and Stereo were 134 ± 22 cm/20 min and 109±10 moves/20 min, respectively. **p<0.01 or *p<0.05 vs saline control at the corresponding time point (Bonferroni post hoc test).
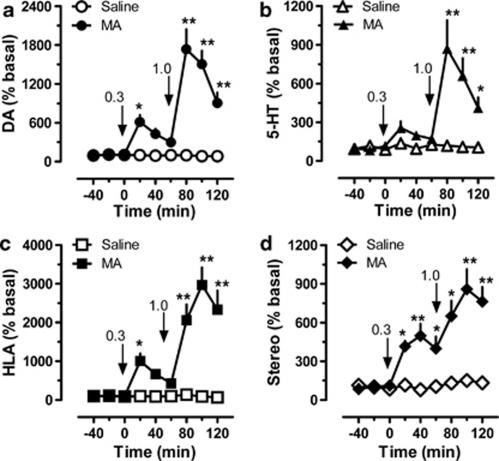
The results in Figure 6 depict the effects of methamphetamine on neurochemistry and motor behavior in rats undergoing microdialysis sampling (N=7 rats per group). Methamphetamine produced significant main effects on extracellular levels of dopamine (Figure 6, panel a; F(1, 8)=110.3, p<0.0001) and 5-HT (Figure 6, panel b; F(1, 8)=37.9, p<0.0001) when compared with saline-treated controls. Post hoc analysis demonstrated that methamphetamine significantly increased dopamine at 20 min after the 0.3 mg/kg dose (p<0.05), and at all-time points after the 1.0 mg/kg dose (p<0.01). The peak rise in dopamine was 5.1-fold above baseline after 0.3 mg/kg and 17.3-fold after 1.0 mg/kg. Methamphetamine failed to significantly alter 5-HT after the low dose, but increased 5-HT at all-time points after 1.0 mg/kg (p<0.01). The peak rise in 5-HT was 8.7-fold above baseline after 1.0 mg/kg. Methamphetamine caused robust main effects on horizontal locomotor activity (Figure 6, panel c; F(1, 8)=100.2, p<0.0001) and stereotypy (Figure 6, panel d; F(1, 8)=91.62, p<0.0001) when compared with saline controls. Post hoc analysis demonstrated that methamphetamine significantly increased locomotion during the first 20-min period after 0.3 mg/kg (p<0.05) and at all-time points after 1.0 mg/kg (p<0.01). The peak magnitude of horizontal locomotor activity was 10.0-fold and 30.0-fold above baseline after 0.3 and 1.0 mg/kg, respectively. Methamphetamine increased stereotypy at 20 (p<0.05), 40 (p<0.01), and 60 min (p<0.05) after 0.3 mg/kg, and at all-time points after 1.0 mg/kg (p<0.01). The maximal effect of methamphetamine on stereotypy was 4.8-fold after 0.3 mg/kg, and 8.6-fold after 1.0 mg/kg.
Figure 6.
Dose-response effects of methamphetamine (MA) on neurochemistry and locomotor behavior in male rats undergoing in vivo microdialysis in nucleus accumbens. MA-treated rats received i.v. injections of 0.3 mg/kg at time zero, followed by 1.0 mg/kg 60 min later. Control rats received i.v. saline injections (1 ml/kg) on the same schedule. Data are mean±SEM for N=7 rats per group, expressed as a percentage of preinjection baseline values (% basal) for dopamine (DA, panel a), serotonin (5-HT, panel b), horizontal locomotor activity (HLA, panel c), and stereotypic movements (Stereo, panel d). Basal concentrations of dialysate DA and 5-HT were 2.01±0.54 pg/5 μl and 0.31±0.05 pg/5 μl, respectively. Basal levels of HLA and Stereo were 151±29 cm/20 min and 129±29 moves/20 min, respectively. **p<0.01 or *p<0.05 vs saline control at the corresponding time point (Bonferroni post hoc test).
Repeated dosing data
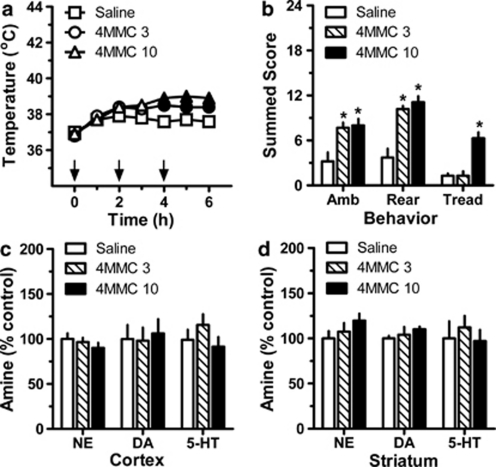
Figure 7 depicts the acute and long-term effects of repeated administration of mephedrone in single-housed male rats (N=6–7 rats per group). Mephedrone produced a main effect on core temperature (Figure 7, panel a; F(2, 6)=32.6, p<0.0001), with 3.0 mg/kg and 10.0 mg/kg doses causing significant hyperthermia at 4 h (F(2, 15)=14.8, p<0.001), 5 h (F(2, 15)=21.2, p<0.0001), and 6 h (F(2, 15)=10.7, p<0.001) post-injection when compared with saline-treated controls. The maximal rise in mean temperature was +1.8 °C after 3.0 mg/kg (ie, from 36.8 °C at time zero to 38.6 °C at 4 h), and +2.1 °C after 10.0 mg/kg (from 36.9 °C at time zero to 39.0 °C at 5 h). Mephedrone also caused significant effects on ambulation (Figure 7, panel b; F(2, 15)=7.9, p<0.005), rearing (F(2, 15)=21.8, p<0.0001), and forepaw treading (F(2, 15)=22.9, p<0.0001). Both doses of drug increased ambulation and rearing with respect to saline controls (p<0.05), whereas only 10.0 mg/kg increased forepaw treading (p<0.05). When examined 2 weeks after dosing, post-mortem tissue concentrations of monoamine transmitters in cortex and striatum were similar across all treatment groups (Figure 7, panels c and d).
Figure 7.
Acute and long-term effects of repeated administrations of mephedrone (4MMC) in single-housed male rats. 4MMC-treated rats received s.c. injections of 3.0 or 10.0 mg/kg at 0, 2, and 4 h (denoted by arrows in panel a). Control rats received s.c. saline injections (1 ml/kg) on the same schedule. Core temperature and motor behaviors were assessed every hour beginning at time zero, whereas post-mortem brain tissue amines were assessed 2 weeks after binge dosing. Data are mean±SEM for N=6–7 rats per group, expressed as core temperature in °C (panel a) and summed behavioral scores for ambulation (Amb), rearing (Rear), and forepaw treading (Tread) (panel b). Tissue amine concentrations in cortex (panel c) and striatum (panel d) are expressed as percentage of values for saline-treated rats (% control). Control amine levels in cortex were 241±15, 25±4, and 212±24 pg/mg for norepinephrine (NE), dopamine (DA), and serotonin (5-HT), respectively. Control amine levels in striatum were 840±68, 12691±363, and 254±48 pg/mg for NE, DA, and 5-HT, respectively. In panel (a), filled symbols indicate significant differences with respect to saline-treated controls at a given time point, p<0.05. In panels (b–d), *p<0.05 compared with saline control group (Newman–Keul's post hoc test).
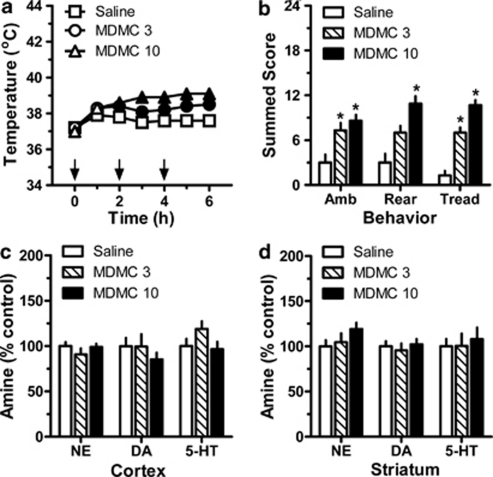
The data in Figure 8 show the effects of repeated administrations of methylone in male rats (N=6–7 rats per group). Methylone treatment had a main effect on core temperature (Figure 8, panel a; F(2, 6)=72.28, p<0.0001), with both drug doses causing significant hyperthermia from 2 h (F(2, 15)=17.3, p<0.0001) through 6 h (F(2, 15)=28.5, p<0.0001) post-injection. The peak increase in mean temperature was +1.4 °C in the 3.0 mg/kg group (ie, from 37.1 °C at time zero to 38.5 °C at 6 h), and +2.1 °C in the 10.0 mg/kg group (ie, from 37.0 °C at time zero to 39.1 °C at 5 h). Methylone also produced significant effects on summed scores for ambulation (Figure 8, panel b; F(2, 15)=9.0, p<0.003), rearing (F(2, 15)=14.4, p<0.001), and forepaw treading (F(2, 15)=50.2, p<0.0001). Both doses of methylone increased ambulation, rearing and forepaw treading (p<0.05) with respect to saline controls. When examined 2 weeks after dosing, post-mortem tissue concentrations of monoamine transmitters were similar across all treatment groups (Figure 8, panels c and d).
Figure 8.
Acute and long-term effects of repeated administrations of methylone (MDMC) in single-housed male rats. MDMC-treated rats received s.c. injections of 3.0 or 10.0 mg/kg at 0, 2, and 4 h (denoted by arrows in panel a). Control rats received s.c. saline injections (1 mL/kg) on the same schedule. Core temperature and motor behavior were assessed every hour beginning at time zero, whereas post-mortem brain tissue amines were assessed 2 weeks after binge dosing. Data are mean±SEM for N=6–7 rats per group, expressed as core temperature in °C (panel a) and summed behavioral scores for ambulation (Amb), rearing (Rear), and forepaw treading (Tread) (panel b). Tissue amine concentrations in cortex (panel c) and striatum (panel d) are expressed as percentage of values for saline-treated rats (% control). Control amine levels in cortex were 273±12, 29±3, and 284±23 pg/mg for norepinephrine (NE), dopamine (DA), and serotonin (5-HT), respectively. Control amine levels in striatum were 605±45, 11511±708, and 206±18 pg/mg for NE, DA and 5-HT, respectively. In panel (a), filled symbols indicate significant differences with respect to saline-treated control at a given time point, p<0.05. In panels (b–d), *p<0.05 compared with saline control group (Newman–Keul's post hoc test).
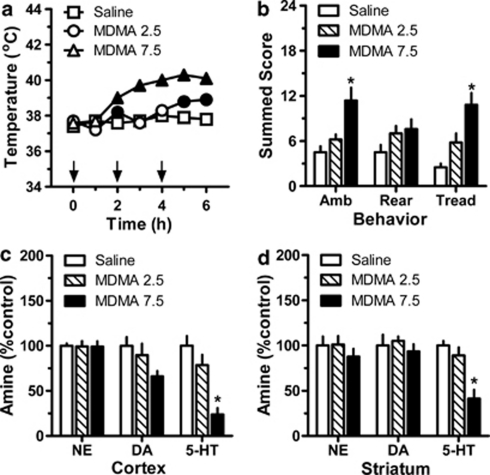
Figure 9 depicts the acute and long-term effects of repeated administration of MDMA in single-housed male rats (N=5–6 rats per group). MDMA exerted a main effect on core temperature (Figure 9, panel a; F(2, 6)=126.6, p<0.0001). Significant hyperthermia was evident in MDMA-treated rats from 2 h (F(2, 15)=24.7; p<0.0001) through 6 h (F(2, 15)=33.1; p<0.0001) post-injection. Specifically, in the 2.5 mg/kg group, hyperthermia was significant when compared with control values at 2, 5, and 6 h post-injection, whereas in the 7.5 mg/kg group, hyperthermia was significant from 2 h through 6 h. The maximal rise in mean temperature was +1.1 °C for the 2.5 mg/kg group (from 37.7 °C at time zero to 38.8 °C at 5 h), and +2.2 °C for the 7.5 mg/kg group (from 37.8 °C at time zero to 40.0 °C at 5 h). MDMA caused significant effects on ambulation (Figure 9, panel b; (2,15)=9.6, p<0.002) and forepaw treading (F(2, 15)=12.3, p<0.001), but had no influence on rearing. Only the 7.5 mg/kg MDMA dosing regimen increased ambulation and forepaw treading (p<0.05) when compared with saline-treated controls. When examined 2 weeks after dosing, post-mortem tissue concentrations of 5-HT were significantly depleted in the cortex (Figure 9, panel c; F(2, 15)=15.7, p<0.0002) and striatum (Figure 9, panel d; F(2, 15)=14.57, p<0.0003) of rats receiving 7.5 mg/kg MDMA, when compared with those receiving saline or 2.5 mg/kg. The extent of 5-HT reduction was down to 24% and 41% of control values in the cortex and striatum, respectively. Cortical dopamine was also reduced in the high-dose MDMA treatment group (66% of control values), but this effect did not reach statistical significance (F(2, 15)=3.1, p<0.07).
Figure 9.
Acute and long-term effects of repeated administrations of 3,4-methylenedioxymethamphetamine (MDMA) in single-housed male rats. MDMA-treated rats received s.c. injections of 2.5 or 7.5 mg/kg at 0, 2, and 4 h (denoted by arrows in panel a). Control rats received s.c. saline injections (1 ml/kg) on the same schedule. Core temperature and motor behavior were assessed every hour beginning at time zero, whereas post-mortem brain tissue amines were assessed 2 weeks after binge dosing. Data are mean±SEM for N=5–6 rats per group, expressed as core temperature in °C (panel a) and summed behavioral scores for ambulation (Amb), rearing (Rear), and forepaw treading (Tread) (panel b). Tissue amine concentrations in cortex (panel c) and striatum (panel d) are expressed as percentage of values for saline-treated rats (% control). Control amine levels in cortex were 275±7, 31±3, and 308±33 pg/mg for norepinephrine (NE), dopamine (DA), and serotonin (5-HT), respectively. Control amine levels in striatum were 753±75, 10 701±1335, and 313±20 pg/mg for NE, DA and 5-HT, respectively. In panel (a), filled symbols indicate significant differences with respect to saline-treated control at a given time point, p<0.05. In panels (b–d), *p<0.05 compared with saline control group (Newman–Keul's post hoc test).
DISCUSSION
A primary goal of this study was to determine the mechanism of action for the designer methcathinone analogs, mephedrone, and methylone. Despite widespread nonmedical use of these agents (Bossong et al, 2005; Karila and Reynaud, 2010; Vardakou et al, 2011; Winstock et al, 2011), few investigations have examined their neuropharmacological effects in laboratory animal models (see recent reports by Hadlock et al, 2011; Kehr et al, 2011). Here, we show that mephedrone and methylone are nonselective substrates for monoamine transporters in vitro, exhibiting potency and selectivity comparable to MDMA. Our microdialysis data reveal that mephedrone and methylone produce concurrent elevations in extracellular dopamine and 5-HT in vivo, with preferential effects on 5-HT. Repeated high-dose administration of either analog evokes hyperthermia and motor stimulation, but does not significantly alter brain tissue monoamines when examined 2 weeks after drug exposure. Collectively, our data demonstrate that mephedrone and methylone target monoamine transporters in a manner similar to MDMA, but there are important differences in the effects of the various drugs.
The in vitro data reported here show that mephedrone and methylone display nanomolar potency as substrates for NET, DAT, and SERT. More specifically, the drugs evoke transporter-mediated release of monoamines via reversal of normal transporter flux (Fleckenstein et al, 2007; Sitte and Freissmuth, 2010). Both methcathinone analogs are similar to MDMA in terms of transporter selectivity but differ from methamphetamine, which is a potent and selective substrate for NET and DAT (Figure 2 and Table 1) (Rothman et al, 2001; Rothman et al, 2003). Our release data are the first to demonstrate NET substrate activity for mephedrone, an action that may underlie the sympathomimetic effects of the drug in humans (James et al, 2010; Wood et al, 2010, 2011). The release data with methylone agree with previous reports showing the drug displays substrate activity at monoamine transporters in vitro (Nagai et al, 2007; Sogawa et al, 2011). It is noteworthy that we found methylone to be about half as potent as mephedrone at all transporters examined. Several studies have shown that mephedrone and methylone are capable of blocking uptake of [3H]DA and [3H]5-HT into cells expressing the plasma membrane monoamine transporters and in rat brain synaptosomes (Cozzi et al, 1999; Hadlock et al, 2011; Nagai et al, 2007; Sogawa et al, 2011). Such findings might seem incongruent with our release data, but it is well known that transporter substrates will ‘appear' to function as uptake blockers in traditional reuptake assays for two reasons: (1) substrates compete with radiolabeled transmitter for a finite number of transporters sites and (2) the releasing property of substrates will counteract accumulation of radiolabeled transmitter into tissue (Rothman et al, 2001). Using assay methods, which can discriminate releasers from uptake blockers, we demonstrate here that a fundamental mechanism of action for mephedrone and methylone is substrate activity at monoamine transporters.
The in vivo microdialysis data reveal that mephedrone and methylone produce simultaneous elevations in extracellular dopamine and 5-HT in rat nucleus accumbens, and the relative impact on dialysate 5-HT is greater for both drugs. Our in vivo data with mephedrone are consistent with the findings of Kehr et al (2011) who used microdialysis in rat nucleus accumbens to show that i.p. injection of 1 mg/kg mephedrone causes a threefold increase in dialysate dopamine and a sevenfold increase in dialysate 5-HT. Thus, the microdialysis results with i.p. mephedrone resemble our findings with i.v. dosing, although the time course of i.v. effects is more rapid (see Figure 3). Importantly, the present results and those of Kehr et al. show that acute neurochemical effects of mephedrone and MDMA are similar in rats given drug doses in the range of those self-administered by humans (ie, ∼1 mg/kg) (de la Torre et al, 2004; Vardakou et al, 2011; Winstock et al, 2011). Our microdialysis findings with methylone represent the first assessment of this drug's in vivo neurochemical actions. Methylone produces elevations in dialysate dopamine and 5-HT, which are qualitatively analogous to the effects of mephedrone and MDMA, but the drug is less potent, in agreement with in vitro results. A common neurochemical mechanism for methylone and MDMA is consistent with drug discrimination data showing methylone generalizes to the MDMA stimulus cue (Dal Cason et al, 1997). Finally, it seems pertinent to point out that the in vivo neurochemical effects of mephedrone, methylone, and MDMA are in marked contrast to the effects of methamphetamine, which causes preferential increases in dialysate DA rather than 5-HT (Figure 6) (Baumann et al, 2002; Zolkowska et al, 2009).
In the microdialysis experiments, we observed that mephedrone and methylone are weak psychomotor stimulants when compared with methamphetamine. After administration of a 1 mg/kg i.v. dose, methamphetamine elicits a sustained increase in horizontal locomotor motor activity that peaks at 30-fold above baseline, while mephedrone and methylone produce transient increases that peak at 10- and 5-fold above baseline, respectively (Figures 3, 4 and 6). We speculate that greater motor stimulation produced by methamphetamine is due to its much larger dopaminergic effects when compared with the other drugs. Indeed, recent in vivo experiments have shown robust correlations between dialysate dopamine in rat nucleus accumbens and the extent of motor activation produced by various stimulants (Baumann et al, 2011; Zolkowska et al, 2009). Extracellular dopamine in the nucleus accumbens is also critically linked to the reinforcing effects of abused substances (Willuhn et al, 2010; Wise, 2008). Owing to the modest dopaminergic effects of mephedrone and methylone, it might be predicted that these drugs would be less reinforcing than methamphetamine. However, a recent report by Hadlock et al (2011) showed that mephedrone is self-administered at a greater rate than methamphetamine when both drugs are given at 0.24 mg per i.v. infusion. Based on the self-administration data alone it might be inferred that mephedrone is more reinforcing than methamphetamine, but as shown by microdialysis data presented here, equivalent doses of these drugs do not produce equivalent neurochemical effects. Our microdialysis data reveal that 0.3 mg/kg, i.v., mephedrone increases extracellular dopamine about twofold above baseline, whereas the same dose of methamphetamine increases dopamine sixfold above baseline. Thus, 0.24 mg mephedrone may engender greater lever pressing than the same dose of methamphetamine because the former drug is less reinforcing (ie, produces less DA release) than the latter. Until full dose-response curves for self-administration behavior are investigated to compare the effects of mephedrone and methylone with other stimulants, the reinforcing effects of these designer drugs remain unclear.
Our i.v. dosing experiments show that acute locomotor effects of mephedrone and methylone are analogous to those produced by MDMA, but subtle differences in behavioral responsiveness emerge when the drugs are administered repeatedly at high doses. For example, repeated administration of mephedrone and methylone produces dose-related increases in rearing behavior (Figures 7 and 8), whereas MDMA does not elicit this effect (Figure 9). High-dose administration of MDMA to rats is known to produce flattened body posture, a hallmark feature of the 5-HT behavioral syndrome, which tends to suppress rearing behavior (Shankaran and Gudelsky, 1999; Spanos and Yamamoto, 1989). It seems possible that mephedrone and methylone are able to increase rearing because of the absence of robust flat body posture. Additionally, mephedrone tends to elicit less forepaw treading, another sign of the 5-HT syndrome. Despite the similar 5-HT-releasing action of mephedrone, methylone, and MDMA reported here and elsewhere (Kehr et al, 2011), it appears that mephedrone and methylone do not induce all of the features of the 5-HT syndrome. More investigation is needed to explore the mechanisms responsible for the unique behavioral effects produced by substituted methcathinone analogs.
On the basis of the 5-HT-releasing capability of mephedrone and methylone, we postulated that the drugs would cause persistent depletion of brain tissue 5-HT, analogous to the effects of SERT substrates like MDMA (Baumann et al, 2008; Commins et al, 1987; Malberg and Seiden, 1998). Surprisingly, we found that repeated high-dose administration of mephedrone and methylone produce acute hyperthermia and motor stimulation, but no lasting changes in brain tissue monoamines (Figures 7 and 8). In a parallel group of rats, high-dose MDMA caused marked depletion of 5-HT in the frontal cortex and striatum when examined 2 weeks after treatment (Figure 9). Our data suggest that mephedrone and methylone are less apt to cause 5-HT deficits when compared with other SERT substrates, and this notion is supported by findings with the parent drug, methcathinone. Sparago et al (1996) reported that the ED50 of (+)-methcathinone for increasing motor activity in rats is 0.7 mg/kg, i.p., whereas a cumulative dose of 100 mg/kg, i.p., per day, for 4 consecutive days, is required to cause significant depletions of brain tissue 5-HT. Thus, the difference between an efficacious pharmacological dose of (+)-methcathinone and a noxious 5-HT-depleting dose is >100-fold. The difference between pharmacological vs toxic doses of MDMA is certainly much smaller (Battaglia et al, 1988; Baumann et al, 2008; O'Shea et al, 1998).
One feasible explanation for the lack of 5-HT depletion associated with methcathinone analogs could be that our repeated dosing regimen did not induce a sufficient level of hyperthermia. It is well established that elevated core temperature is an important contributor to 5-HT deficits produced by MDMA (Baumann et al, 2008; Green et al, 2004; Malberg and Seiden, 1998). Careful inspection of the temperature data presented here reveals that the highest doses of mephedrone, methylone, and MDMA increase hyperthermia to the same extent, about 2 °C above preinjection values (Figures 7, 8, 9). However, the absolute temperature maximum produced by MDMA (ie, 40 °C) is somewhat greater than that produced by the substituted methcathinones (ie, 39 °C). Another possible reason why mephedrone and methylone fail to deplete 5-HT might be related to their low potency at the vesicular monoamine transporter 2 (VMAT2) when compared with other amphetamine-type agents (Cozzi et al, 1999). Cozzi et al. have postulated that SERT substrates are able to induce 5-HT deficits by interacting with VMAT2 and disrupting normal vesicular function, and this proposal warrants further investigation. Our high-dose administration data with mephedrone seem at odds with the report by Hadlock et al (2011) who demonstrated that four sequential doses of 10–25 mg/kg, i.p., mephedrone produce significant depletion of striatal 5-HT when examined 1 week later. There are methodological differences between our experiments and those of Hadlock et al., with the most obvious distinction being that we gave less mephedrone by a different route (ie, we administered three sequential doses of 3 or 10 mg/kg, s.c.). Perhaps more importantly, Hadlock et al. carried out their injection regimen at elevated ambient temperature (>27 °C) in group-housed rats (four rats per cage), conditions that are known to impede heat dissipation and exacerbate the deleterious effects of amphetamines (Green et al, 2004; Malberg and Seiden, 1998). It must be noted that Hadlock et al. did not include groups of rats given MDMA or methamphetamine under the same conditions as those given mephedrone, so the relative risk of mephedrone to produce central 5-HT deficits remains unclear.
In conclusion, we have shown that mephedrone and methylone are transporter substrates capable of increasing extracellular dopamine and 5-HT in a manner analogous to MDMA. Although convergent lines of evidence agree that designer methcathinone analogs target monoamine transporters, the abuse liability and toxic potential of these drugs remain uncertain. It is essential that future studies compare in vivo effects of methcathinone analogs with those produced by structurally related compounds under identical experimental conditions. To this end, the dose-response effects of mephedrone and methylone should be evaluated in assays measuring drug reinforcement (eg, self-administration studies) and persistent 5-HT deficits (eg, high-dose administration studies).
Acknowledgments
This work was generously supported by the National Institute on Drug Abuse (NIDA), Intramural Research Program (MHB, MAA, JSP, JRS and RBR), NIDA Grants DA017675 (NVC) and DA027191 (AER), and the Retina Research Foundation/UW Eye Research Institute Edwin and Dorothy Gamewell Professorship (AER). We thank Ava Cozzi and Lisa Ehrlicher for helpful discussions.
The authors declare no conflict to interest.
References
- Battaglia G, Yeh SY, De Souza EB. MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav. 1988;29:269–274. doi: 10.1016/0091-3057(88)90155-4. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Sharpe LG, Lewis DB, Rice KC, Rothman RB. Persistent antagonism of methamphetamine-induced dopamine release in rats pretreated with GBR12909 decanoate. J Pharmacol Exp Ther. 2002;301:1190–1197. doi: 10.1124/jpet.301.3.1190. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Van Dijk JP, Niesink RJ. Methylone and mCPP, two new drugs of abuse. Addict Biol. 2005;10:321–323. doi: 10.1080/13556210500350794. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs' in the UK: initial findings. Drug Test Anal. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Mascarella SW, Navarro HA, Eaton JB, Lukas RJ, et al. Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for smoking cessation. J Med Chem. 2010;53:2204–2214. doi: 10.1021/jm9017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Emergency department visits after use of a drug sold as ‘bath salts'---Michigan, November 13, 2010--March 31, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:624–627. [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Cozzi NV, Foley KF. Methcathinone is a substrate for the serotonin uptake transporter. Pharmacol Toxicol. 2003;93:219–225. doi: 10.1046/j.1600-0773.2003.pto930504.x. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Dickson AJ, Vorce SP, Levine B, Past MR. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J Anal Toxicol. 2010;34:162–168. doi: 10.1093/jat/34.3.162. [DOI] [PubMed] [Google Scholar]
- EMCDDA . Annual Report on the sSate of Drug Problems in Europe. European Monitoring Centre for Drugs and Drug Addiction: Lisbon; 2010. [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol. 2004;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J. 2010;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, et al. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36:709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- Karila L, Reynaud M.2010GHB and synthetic cathinones: clinical effects and potential consequences Drug Test Anale-pub ahead of print 2 December 2010. [DOI] [PubMed]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. 2011Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats Br J Pharmacole-pub ahead of print 21 November 2011. [DOI] [PMC free article] [PubMed]
- Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in the Netherlands. Forensic Sci Int. 2011;206:e93–e95. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397:1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Granados R, Esteban B, Colado MI, Green AR. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy') Neuropharmacology. 1998;37:919–926. doi: 10.1016/s0028-3908(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology (Berl) 1999;147:66–72. doi: 10.1007/s002130051143. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters--why amphetamines take two to tango. J Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa C, Sogawa N, Ohyama K, Kikura-Hanajiri R, Goda Y, Sora I, et al. Methylone and monoamine transporters: correlation with toxicity. Curr Neuropharmacology. 2011;9:58–62. doi: 10.2174/157015911795017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos LJ, Yamamoto BK. Acute and subchronic effects of methylenedioxymethamphetamine [(+/−)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacol Biochem Behav. 1989;32:835–840. doi: 10.1016/0091-3057(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, et al. Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J Pharmacol Exp Ther. 1996;279:1043–1052. [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop. Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010;6:327–330. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]