Abstract
The noradrenergic system plays a critical role in the ‘consolidation' of emotional memory. If we are to target ‘reconsolidation' in patients with anxiety disorders, the noradrenergic strengthening of fear memory should not impair the disruption of reconsolidation. In Experiment I, we addressed this issue using a differential fear conditioning procedure allowing selective reactivation of one of two fear associations. First, we strengthened fear memory by administering an α2-adrenergic receptor antagonist (ie, yohimbine HCl; double-blind placebo-controlled study) 30 min before acquisition (time for peak value yohimbine HCl <1 h). Next, the reconsolidation of one of the fear associations was manipulated by administering a β-adrenergic receptor antagonist (ie, propranolol HCl) 90 min before its selective reactivation (time for peak value propranolol HCl <2 h). In Experiment II, we administered propranolol HCl after reactivation of the memory to rule out a possible effect of the pharmacological manipulation on the memory retrieval itself. The excessive release of noradrenaline during memory formation not only delayed the process of extinction 48 h later, but also triggered broader fear generalization. Yet, the β-adrenergic receptor blocker during reconsolidation selectively ‘neutralized' the fear-arousing aspects of the noradrenergic-strengthened memory and undermined the generalization of fear. We observed a similar reduction in fear responding when propranolol HCl was administered after reactivation of the memory. The present findings demonstrate the involvement of noradrenergic modulation in the formation as well as generalization of human fear memory. Given that the noradrenergic strengthening of fear memory impaired extinction learning but not the disruption of reconsolidation, our findings may have implications for the treatment of anxiety disorders.
Keywords: fear memory, reconsolidation, extinction, generalization, noradrenergic modulation, anxiety disorders
INTRODUCTION
The process of reconsolidation, the protein-synthesis-dependent restabilization of a memory upon retrieval, enables the modification of memory representation (Nader et al, 2000). We previously demonstrated that disrupting reconsolidation by administering the β-adrenergic receptor antagonist propranolol HCl before reactivation selectively ‘deleted' the emotional expression of a fear memory in humans (ie, startle fear responding) (Kindt et al, 2009; Soeter and Kindt, 2010, 2011a). Importantly, the fear-erasing effects following the pharmacological manipulation were not restricted to the feared cue itself, but instead generalized to category-related information (Soeter and Kindt, 2011a). Given that fear generalization lies at the heart of many anxiety disorders (Lissek et al, 2008), targeting the process of reconsolidation may provide a novel therapeutic strategy in the treatment of (for instance) post-traumatic stress disorder. However, there are a number of conditions that may prevent reconsolidation from occurring, such as the strength of memories (Suzuki et al, 2004; Wang et al, 2009). If we are to target reconsolidation in patients suffering from post-traumatic stress disorder, strong fear memory should evidently not act as a constraint on reconsolidation.
To date, a substantial body of evidence supports the noradrenergic modulation in the formation of emotional memory (McGaugh and Roozendaal, 2009). Recently, we demonstrated that stimulation of the noradrenergic system during memory formation strengthened the emotional expression of human associative fear memory (Soeter and Kindt, 2011b). That is, the excessive release of noradrenaline during memory formation not only delayed the ‘process of extinction' 48 h later, but also generated a superior recovery of fear following re-exposure to the original stressor. These findings thus suggest that noradrenaline may play an important role in the etiology and maintenance of anxiety disorders. However, the effect of noradrenaline on fear generalization (ie, a main characteristic of anxiety disorders) currently remains unknown. The generalization of fear seems to be dependent on the strength of the memory as operationalized by training intensity (Laxmi et al, 2003). Hence, an interesting question is whether the noradrenaline-induced strengthening of associative fear memory also promotes the generalization of fear responding. At the same time, the stimulation of the noradrenergic system during memory formation should not impair the disruption of reconsolidation if we are to target reconsolidation clinically.
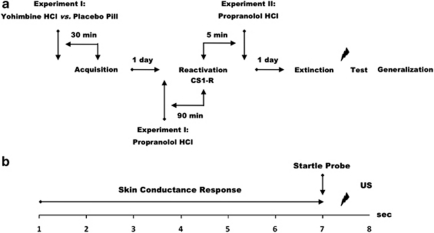
Here, we addressed these issues by using a differential fear conditioning procedure allowing selective reactivation of one of two categorically distinct fear associations sharing the same aversive outcome and a test of fear generalization. Testing included different phases across three consecutive days, each separated by 24 h (see Figure 1a). During acquisition (day 1), two fear-relevant stimuli (CS1Spider–CS2Gun) were repeatedly paired with an aversive electric stimulus (US), whereas a fear-irrelevant stimulus was not (CS3Mug) (Supplementary Figure S1). Furthermore—in Experiment I—the formation of fear memory was manipulated by the systemic administration of yohimbine HCl (day 1), an α2-adrenergic receptor antagonist supposed to stimulate central noradrenergic activity by blocking the α2-adrenergic autoreceptor (Charney et al, 1987; Peskind et al, 1995; Soeter and Kindt, 2011b). To reach peak plasma levels upon completion of the acquisition phase, participants (n=30) received, in a double-blind manner, an oral dose of either 20 mg of yohimbine HCl or placebo pill 30 min before fear learning (day 1) (time for peak value yohimbine HCl <1 h; Grasing et al, 1996). On day 2, the reconsolidation of one of the fear associations (CS1) was manipulated by the systemic administration of propranolol HCl—a β-adrenergic receptor antagonist that indirectly targets the protein synthesis required for reconsolidation by inhibiting the noradrenaline-stimulated cAMP response element binding (CREB) phosphorylation (Thonberg et al, 2002). In view of the peak plasma concentrations of propranolol HCl (Gilman and Goodman, 1996), all of the participants received, in a single-blind manner, an oral dose of 40 mg of propranolol HCl 90 min before the selective reactivation of the CS1 memory (day 2). (In line with animal studies (eg, Debiec and LeDoux, 2004), we previously demonstrated that (1) a reactivation trial in combination with placebo pill and (2) the omission of memory reactivation after propranolol HCl intake yielded intact fear responding (Kindt et al, 2009; Soeter and Kindt, 2010). Taken together, these findings indicate that both the oral administration of propranolol HCl and the reactivation of the fear memory are necessary for the observed fear-erasing effects.). Administering pills before reactivation does not—however—rule out a possible effect of the pharmacological manipulation on the retrieval of the fear memory itself. Therefore, the participants in Experiment II (n=10) received, in a single-blind manner, an oral dose of propranolol HCl subsequent to memory reactivation. Memory retention was tested 24 h later (ie, first test trial day 3), followed by an extinction procedure, reminder shock, and a test of fear generalization. Expression of fear was measured using startle fear potentiation (Hamm and Weike, 2005). We obtained skin conductance responding and retrospective US expectancy ratings to assess the anticipation of threat (Weike et al, 2007). Here, the US expectancy was measured retrospectively instead of ‘online' (Soeter and Kindt, 2010, 2011a, 2011b) to prevent the expectancy ratings from interfering with the measurement of electrodermal activity. Salivary α-amylase and blood pressure levels were determined to ensure that the drug manipulations exerted its intended physiological effect (Stegeren et al, 2006, 2009). See Supplementary Materials and methods for a detailed description of the apparatus and materials. We hypothesized that the yohimbine HCl manipulation during acquisition would strengthen the fear memory and thereby (1) act as a constraint on the reconsolidation of the CS1 fear association, (2) delay the process of extinction learning, and (3) promote the generalization of fear responding, relative to placebo pill.
Figure 1.
Schematic of the experimental design (a) and the CS1+ or CS2+ conditioning trial (b). In the CS1−, CS2−, CS3−, and CS1-R trials, no US was delivered. CS, conditioned stimuli.
MATERIALS AND METHODS
Participants
In all, 40 undergraduate students (10 men, 30 women) from the University of Amsterdam ranging in the age of 18–26 years (mean±SD age, 20.7±1.9 years) participated in the study. Participants were assessed to be free from any current or previous medical or psychiatric condition that would contraindicate taking a single dose of yohimbine HCl (20 mg) and propranolol HCl (40 mg) (ie, pregnancy; seizure disorder; respiratory disorder; cardiovascular disease; blood pressure ≤90/60 or ≥140/90 mmHg; diabetes; liver/kidney disorder; depression; or psychosis). To eliminate individuals who might have difficulty with any temporary symptoms induced by either drug manipulation, an additional exclusion criterion contained a score ≥26 on the Anxiety Sensitivity Index (ASI) (Peterson and Reiss, 1992). All participants who were enrolled completed the study. The participants in Experiment I were randomly assigned to one of two conditions, with the restriction that conditions were matched on Trait Anxiety (STAI-T) (Spielberger et al, 1970), Spider Phobic Questionnaire (SPQ) (Klorman et al, 1974), and ASI scores as close as possible (see Table 1). Participants received either partial course credits or were paid a small amount (€42, –) for their participation in one of the experiments. The ethical committee of the University of Amsterdam approved the study and informed consent was obtained from all participants.
Table 1. Mean Values (SD) of the Reported Spider Fear, Trait Anxiety, Anxiety Sensitivity, Shock Intensity, and US Evaluation for the Yohimbine HCl and Placebo Pill Condition and the Propranolol after Reactivation Group.
| Yohimbine HCl propranolol | Placebo pill propranolol | Propranolol after reactivation | T-test | |
|---|---|---|---|---|
| Spider fear | 4.6 (3.3) | 6.3 (5.3) | 4.2 (4.5) | ts28<1.04 |
| Trait anxiety | 37.5 (8.0) | 36.3 (7.9) | 32.0 (10.1) | ts28<1.53 |
| Anxiety sensitivity | 9.2 (5.4) | 8.5 (5.0) | 7.8 (5.6) | ts28<1 |
| Shock intensity | 16.4 (6.9) | 14.5 (4.7) | 17.3 (5.6) | ts28<1.35 |
| US evaluation | −2.7 (1.0) | −2.8 (0.6) | −2.4 (0.9) | ts28<1 |
Experimental Procedure
Participants were subjected to a differential fear conditioning procedure, with three pictures serving as conditioned stimuli (CS1Spider–CS2Gun–CS3Mug). The two fear-relevant pictures (CS1, CS2) were paired with an electric stimulus, whereas the fear-irrelevant picture (CS3) was not. We employed fear-relevant stimuli because they lead to a superior conditioning of aversive associations and are especially resistant to extinction learning compared with fear-irrelevant cues (Mineka and Öhman, 2002; Lang et al, 2005). Moreover, given that most anxiety disorders are associated with these categories of stimuli (Mineka and Öhman, 2002), we are specifically interested in targeting stronger fear memory. One of the fear-relevant stimuli (CS2) served as control for the other fear-relevant stimulus (CS1). However, since fear-relevant stimuli are known to have an innate prepotency to elicit fear responses (Lovibond et al, 1994), we employed an additional fear-irrelevant control stimulus (CS3) to verify whether the procedure was capable of neutralizing the acquired fear responding. Testing included several phases across three subsequent days, each separated by 24 h. During each session, participants sat behind a table with a computer monitor at a distance of 50 cm in a sound-attenuated room. Each session began with a 1-min acclimation period consisting of 70 dB broadband noise, which continued throughout the session as background noise, followed by a habituation phase consisting of 10 startle probes to reduce initial startle reactivity. Characteristics of the CSs, trial order, ITIs, and startle probes during memory reactivation (day 2) and extinction, testing (day 3) were similar to acquisition (day 1). Assignment of the pictures as CS1+ and CS2+ was counterbalanced across participants.
Acquisition—day 1
Before participants subscribed to the study, they were already informed about the pill administrations and the electric stimulus. Upon arrival at the first testing day, details of the various study procedures (eg, EMG electrodes, electric stimulus, side effects of the pharmacological treatments, medical screening) were explained and possible questions were answered. Participants who agreed to enroll in the study were interviewed with regard to their health and any medical or psychiatric conditions that would contraindicate taking a single dose of yohimbine HCl (20 mg) and propranolol HCl (40 mg). In addition, blood pressure was measured. Once a participant was medically cleared, written informed consent was obtained and the ASI, SPQ, and STAI were administered. Furthermore, saliva samples were collected. To this end, participants were instructed just to place the swab in their mouths for 3 min.
After attachment of the startle, skin conductance, and shock electrodes, the intensity of the US was determined. Starting at an intensity of 1 mA, the level of a 2-ms aversive electric stimulus delivered to the wrist of the non-preferred hand was gradually increased. The intensity of shock was individually set at a level defined by the participant as ‘uncomfortable, but not painful' and remained set to this intensity throughout the following days. To test for the potential effects of yohimbine HCl on the fear-potentiated startle reflex (Davis et al, 1993), 10 baseline startle probes (noise alone; NA) were presented before pill intake. Afterwards, participants were detached from the experimental setup and received, in a double-blind manner, an oral dose of either 20 mg of yohimbine HCl or placebo pill. To reach peak plasma levels upon completion of the acquisition phase (Grasing et al, 1996), a resting period of 30 min was inserted. Participants were offered magazines to read. Before acquisition, participants were attached to the experimental apparatus and were informed with regard to the CSs. They were instructed that two of the pictures would be followed by an electric stimulus in most of the cases, whereas the third picture would never be followed by the US. They were told to learn to predict whether an electric stimulus would occur or not on the basis of the three pictures.
In the acquisition phase, the CS1+, CS2+, and CS3− were presented five times for 8 s. The startle probe was presented 7 s after CS onset and was followed by the US 500 ms later (CS1+ and CS2+) (see Figure 1b). To reduce the possibility that the reactivation trial on day 2 resulted in extinction learning, the first presentation of both the CS1+ and CS2+ was unreinforced (LaBar et al, 1998). Five baseline startle probes were presented alone (NA). Order of trial type was randomized within blocks (ie, CS1+, CS2+, CS3−, and NA). Intertrial intervals (ITI) varied between 15, 20, and 25 s, with a mean of 20 s.
The STAI-S was filled out both before and upon completion of the acquisition phase. In addition, blood pressure as well as saliva samples were collected. At the conclusion of the experiment, participants were asked to evaluate the pleasantness of the US. Furthermore, they were explicitly instructed to remember what they had learned during acquisition. These instructions were included to enhance retention of the CS–US contingency on the following days (Norrholm et al, 2006) and to prevent participants from erroneously expecting a different contingency scheme during subsequent testing. The procedure in Experiment II, Propranolol after Reactivation, was similar to Experiment I, except for pill administration and resting period.
Memory reactivation—day 2
To substantiate consolidation of the fear memory, a break of 24 h after acquisition was inserted. All of the participants received, in a single-blind manner, an oral dose of 40 mg of propranolol HCl 90 min before memory reactivation (CS1-R)—even though they were informed that they could also receive a placebo pill. The STAI-S was filled out both before pill administration and upon completion of the experiment. In addition, at these time points, blood pressure and saliva samples were collected.
After electrode attachment, participants were told that the same three pictures would be presented and they were asked to remember what they had learned during acquisition. They were again instructed that two of the pictures would be followed by an electric stimulus in most of the cases, whereas the third picture would never be followed by the US. In the memory reactivation phase, a single unreinforced CS1-R was presented for 8 s, followed by a startle probe presented alone. The procedure in Experiment II, Propranolol after Reactivation, was similar to Experiment I, except for pill administration, which occurred 5 min after reactivation of the memory.
Extinction, testing—day 3
Upon arriving at the experimental site, blood pressure and saliva samples were again collected. In addition, the STAI-S was completed. After attachment of the electrodes, the participants were informed that the same three pictures provided during acquisition would be presented. No further instructions were given. In the extinction phase, participants were exposed to the three pictures (CS1−, CS2−, and CS3−) for 10 times without the electric stimulus (US). Startle probes were again presented 7 s after CS onset. Furthermore, 10 startle probes were presented alone (NA). After the extinction procedure, we presented an unsignaled reminder shock to reinstate the expression of the original fear memory. Evidence for a reinstatement effect is indicated by an increase of the differential conditioned response from the last extinction trial to the first trial at test. We predicted that the unsignaled reminder shock would not result in a return of fear to the reactivated stimulus (CS1) given that propranolol HCl is supposed to disrupt the reconsolidation of the reactivated fear memory (CS1–US). We further predicted that yohimbine HCl would enhance the return of fear (CS2) following the unsignaled reminder shock (US) (see also, Soeter and Kindt, 2011b). Therefore, we presented only one as opposed to the traditional procedure of three unsignaled USs to avoid a ceiling effect in the return of fear for the participants who received a placebo pill during fear acquisition (day 1) (Norrholm et al, 2006; Kindt et al, 2009; Soeter and Kindt, 2010). The time between the last extinction trial and the reinstating US was 19 s. Following the unsignaled US, participants were again presented with 1 CS1−, CS2−, CS3−, and NA trial (ie, reinstatement testing). The time between the reinstating USs and reinstatement testing was 18 s. Next, generalization testing took place. That is, participants were exposed to category-related pictures (GpCS1, GpCS2, and GpCS3) and category-related words (GwCS1, GwCS2, and GwCS3) (see Supplementary Figure S1). The order of generalization stimuli (ie, pictures vs words) was counterbalanced across participants. At the conclusion of the experiment, participants completed the STAI-S and judged the pleasantness of the US. In addition, participants were asked to indicate for each phase (beginning vs end) of the experiment to what extent they had expected the US after each of the CSs. The procedure in Experiment II, Propranolol after Reactivation, was similar to Experiment I.
Statistical Analysis
State anxiety, salivary α-amylase, and systolic as well as diastolic blood pressure were subjected to a 2 (condition: yohimbine HCl vs placebo pill) × 2 (moment: before vs after pill intake) mixed analysis of variance (ANOVA). Startle responses, electrodermal activity, and US expectancy ratings were analyzed by means of a mixed ANOVA for repeated measures with condition (ie, yohimbine HCl vs placebo pill) as between-subjects factor and stimulus (ie, simple contrasts: CS1 vs CS3 and CS2 vs CS3) and trial (ie, stimulus presentation) as within-subjects factors. The differential response (CS1 vs CS3 and CS2 vs CS3) was compared over testing phases, respectively (first trial vs last trial). To determine the speed of extinction learning, a 2 (condition: yohimbine HCl vs placebo pill) × 2 (stimulus: CS1 vs CS3 and CS2 vs CS3) × 5 (trials: averaging over each two consecutive extinction trials) mixed ANOVA was performed. To test whether the two conditioned stimuli (ie, spider and gun) were equally affected by the drug manipulation, we performed a 2 (trial: stimulus presentation) × 2 (stimulus: CS1 vs CS3) × 2 (stimulus category: spider vs gun) mixed ANOVA. Planned comparisons between the CS1 and CS2 stimuli were performed separately. Missing data due to artifacts (ie, 1.13% of the trials) were not replaced and hence excluded from the analyses. Significance was set at p<0.05.
RESULTS
Manipulation Check Drug Administration
Analysis of the effect of the yohimbine HCl manipulation on blood pressure and sAA level during fear acquisition (day 1) revealed the expected increase in both systolic and diastolic blood pressure (moment × condition, F1,28=26.58, p<0.001, ηp2=0.49; F1,28=12.24, p<0.01, ηp2=0.30, respectively), as well as salivary α-amylase (Stegeren et al, 2009) (moment × condition, F1,23=6.50, p<0.05, ηp2=0.22) in comparison to placebo pill. However, the yohimbine HCl manipulation during fear acquisition did not affect the reported state anxiety that was assessed before and upon completion of the acquisition phase (moment × condition, F1,28<1.45) (see also, Table 2).
Table 2. Mean Values (SD) of the Systolic and Diastolic Blood Pressure (in mmHg) and Amylase Level (in U/ml) Pre- and Post-Pill Intake during Fear Acquisition for the Yohimbine HCl and Placebo Pill Condition (ie, Experiment I).
| Fear acquisition yohimbine HCl | Pre-pill intake | Post-pill intake | T-test: two-tailed |
|---|---|---|---|
| Yohimbine condition | |||
| Systolic BP | 126.1 (SD=10.1) | 138.0 (SD=11.9) | t14=−4.84, p<0.001 |
| Diastolic BP | 72.5 (SD=6.3) | 77.7 (SD=8.3) | t14=−2.72, p<0.05 |
| sAA Level | 91.4 (SD=127.1) | 168.9 (SD=233.9) | t11=−2.36, p<0.05 |
| Placebo pill condition | |||
| Systolic BP | 125.3 (SD=14.7) | 120.8 (SD=8.9) | t14=2.22, p<0.05 |
| Diastolic BP | 73.0 (SD=7.5) | 70.3 (SD=7.0) | t14=2.25, p<0.05 |
| sAA Level | 103.1 (SD=105.2) | 75.7 (SD=62.9) | t12<1 |
The propranolol HCl manipulation during memory reactivation (day 2) did not differentially affect the BP and sAA between conditions (moment × condition, F1,28<1.92). In both the yohimbine and placebo pill group, we observed a significant decrease in systolic and diastolic BP (moment, F1,28=92.22, p<0.001, ηp2=0.77; F1,28=39.81, p<0.001, ηp2=0.59, respectively), as well as in the amylase level (Stegeren et al, 2006) (moment, F1,22=7.95, p=0.01, ηp2=0.27) following propranolol HCl administration, indicating that the pill manipulation exerted its intended physiological effect. Consistent with other studies (Grillon et al, 2004), the propranolol HCl manipulation during memory retrieval did not affect the reported state anxiety that was assessed before and upon completion of the reactivation phase (moment, F1,28<1; moment × condition, F1,28<1). The decrease in BP, sAA, and reported state anxiety during memory reactivation (day 2) in Experiment II, Propranolol after Reactivation, did not differ from both the yohimbine HCl and placebo pill condition (moment × condition, F1,28<1.98) (see also, Table 3).
Table 3. Mean Values (SD) of the Systolic and Diastolic Blood Pressure (in mmHg) and Amylase Level (in U/ml) Pre- and Post Propranolol HCl Administration during Memory Reactivation for the Yohimbine HCl and Placebo Pill Condition (ie, Experiment I) and the Propranolol after Reactivation Group (ie, Experiment II).
| Memory reactivation propranolol HCl | Pre-pill intake | Post-pill intake | T-test: two-tailed |
|---|---|---|---|
| Yohimbine condition | |||
| Systolic BP | 130.3 (SD=11.2) | 113.3 (SD=11.7) | t14=6.52, p<0.001 |
| Diastolic BP | 75.0 (SD=7.6) | 67.8 (SD=6.7) | t14=4.56, p<0.001 |
| sAA Level | 193.0 (SD=261.4) | 32.1 (SD=38.4) | t11=2.21, p<0.05 |
| Placebo pill condition | |||
| Systolic BP | 125.5 (SD=9.2) | 110.3 (SD=7.8) | t14=7.53, p<0.001 |
| Diastolic BP | 70.1 (SD=5.6) | 65.8 (SD=5.5) | t14=4.72, p<0.05 |
| sAA Level | 96.1 (SD=105.6) | 41.2 (SD=38.0) | t11=2.32, p<0.05 |
| Propranolol after reactivation | |||
| Systolic BP | 121.3 (SD=4.9) | 107.1 (SD=7.0) | t9=5.94, p<0.001 |
| Diastolic BP | 73.2 (SD=8.7) | 67.8 (SD=8.1) | t9=3.28, p<0.05 |
| sAA Level | 56.9 (SD=41.8) | 30.1 (SD=36.2) | t9=2.55, p<0.05 |
Experiment I, Yohimbine HCl vs Placebo Pill
Startle fear responding
Acquisition—day 1 ANOVA showed fear conditioning on day 1 by a significant increase of the differential startle response (ie, simple contrasts: CS1Spider vs CS3Mug–CS2Gun vs CS3Mug) from trials 1 to 5 (stimulus × trial, F1,28=21.73, p<0.001, ηp2=0.44; F1,28=19.85, p<0.001, ηp2=0.42, respectively). We observed no difference in responses to the first trial of acquisition (CS1Spider vs CS2Gun vs CS3Mug) (stimulus, F2,27<1), indicating that the fear relevancy of the pictures did not affect startle fear responding in the absence of associative learning (see also, Soeter and Kindt, 2011a). Moreover, fear responses to the reinforced pictures (CS1Spider vs CS2Gun) were equally acquired (stimulus × trial, F1,28<1; Figure 2a and b). Furthermore, the administration of yohimbine HCl did not directly affect the fear learning given that we observed no difference during acquisition between the two conditions (stimulus × trial × condition, F1,28<2.09). Yohimbine HCl did also not affect the startle response per se as we found no effect on the habituation trials that were presented before and after pill administration (moment × trial × condition, F9,20<1.06).
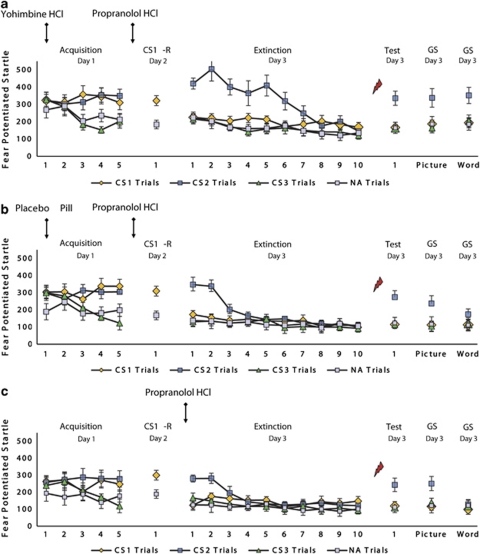
Figure 2.
Mean startle potentiation to the fear-conditioned stimuli (CS1 and CS2), the control stimulus (CS3), and noise alone (NA) trials during acquisition, memory reactivation, extinction, test, and generalization for the (a) yohimbine HCl–propranolol HCl and (b) placebo pill–propranolol HCl condition (ie, Experiment I), and the (c) propranolol HCl after reactivation group (ie, Experiment II). Error bars represent SEM.
Memory reactivation—day 2 The two groups (yohimbine HCl vs placebo pill) expressed similar levels of differential startle potentiation (CS1-R vs NA) during memory reactivation (day 2) (stimulus × condition, F1,28<1). Since the NA trial always followed the reactivation trial (CS1-R) on day 2, we further compared the CS1-R with the last trial of habituation (ie, an NA trial). This analysis also revealed a similar level of startle potentiation during memory reactivation for both conditions (stimulus × condition, F1,28<1.46). Furthermore, the absence of a significant change in startle fear responding (CS1 vs NA) from the last trial of acquisition to memory reactivation (stimulus × trial × condition, F1,28<1) demonstrates that the acquired fear memory was equally well consolidated in the two groups.
Memory retention—day 3 In both the yohimbine and placebo pill group, the administration of propranolol HCl significantly decreased startle potentiation to the reactivated CS1 from the last trial of acquisition to the first extinction trial 48 h later (CS1 vs CS3; stimulus × trial, F1,28=15.98, p<0.001, ηp2=0.36), irrespective of the reactivated stimulus (ie, spider vs gun) (stimulus × trial × category, F1,26<1.16). We even no longer observed differential startle responding to the reactivated CS1 on the first trial of extinction learning (CS1 vs CS3; t14<1.25; Figure 2a and b), indicating that the yohimbine HCl manipulation did not prevent the disruption of reconsolidation 24 h later. Conversely, startle responses to the non-reactivated CS2 remained stable from acquisition to extinction learning 48 h later in the yohimbine as well as placebo pill condition (CS2 vs CS3; stimulus × trial, F1,28<1.12).
Extinction learning—day 3 Given that responses to the reactivated CS1 were already eliminated on the first trial of fear extinction (day 3), we observed no extinction learning to the CS1 stimulus (CS1 vs CS3) in both the yohimbine and placebo pill group (trial 1 vs trial 10; stimulus × trial, F1,28<1). In contrast, startle responses to the non-reactivated CS2 decreased similarly from the first extinction trial to the last trial of extinction learning in the yohimbine and placebo pill condition (CS2 vs CS3; stimulus × trial, F1,28=53.52, p<0.001, ηp2=0.66; stimulus × trial × condition, F1,28<1). However, we observed a significant difference in the speed of the extinction learning process between the two groups (CS2 vs CS3 and CS1 vs CS2; stimulus × trial × condition, F1,28=21.49, p<0.001, ηp2=0.43; F1,28=23,41, p<0.001, ηp2=0.46, respectively), irrespective of the non-reactivated stimulus (ie, spider vs gun) (stimulus × trial × condition × category, F1,26<1). Bonferroni-adjusted pairwise comparisons indeed showed that the differential startle response to the non-reactivated stimulus (CS2 vs CS3) only reached significance on the first trials of extinction learning (trials 1 and 2) in the placebo pill condition (p<0.001; Figure 2b). Conversely, the differential startle response to the non-reactivated stimulus (CS2 vs CS3) remained significant up to trials 5 and 6 of extinction learning in the yohimbine group (all p's<0.001; Figure 2a), indicating that the α2-adrenergic drug strongly delayed the extinction learning process (see also, Soeter and Kindt, 2011b).
Reinstatement testing—day 3 Contrary to our expectations, the administration of yohimbine HCl relative to placebo pill did not result in a superior recovery of fear to the non-reactivated CS2 following the reminder shock (CS2 vs CS3; stimulus × trial × condition, F1,28<1). However, in both the yohimbine and placebo pill condition, the differential startle response to the non-reactivated CS2 significantly increased from the last trial of extinction to the first trial at test (stimulus × trial, F1,28=26.56, p<0.001, ηp2=0.49; Figure 2a and b). Most importantly, the reminder shock following extinction learning did not unveil a recovery of fear to the reactivated CS1 in the yohimbine as well as placebo pill condition (CS1 vs CS3; stimulus × trial, F1,28<1.24; CS1 vs CS3; stimulus × trial, F1,28=36.88, p<0.001, ηp2=0.57). This finding indicates that propranolol HCl effectively disrupted the reconsolidation of the reactivated fear memory and that the administration of yohimbine HCl before fear learning did not prevent this process 24 h later.
Generalization testing—day 3 We observed a significant generalization of fear to the category-related picture of the non-reactivated CS2 in both the yohimbine and placebo pill group (GpCS2 vs GpCS3 and GpCS1 vs GpCS2; main effect of stimulus, F1,28=31.29, p<0.001, ηp2=0.53; main effect of stimulus, F1,28=25.55, p<0.001, ηp2=0.48, respectively). Conversely, the category-related picture of the reactivated CS1 did not reveal any fear response in the yohimbine and placebo pill condition (GpCS1 vs GpCS3; main effect of stimulus, F1,28<1.16; Figure 2a and b). Notably, a significant difference in fear generalization for the word cue of the non-reactivated CS2 was observed between the two groups (G2CS2 vs G2CS3 and G2CS1 vs G2CS2; stimulus × condition, F1,28=4.51, p<0.05, ηp2=0.14; stimulus × condition, F1,28=7.21, p<0.05, ηp2=0.21, respectively). That is, we observed a significant generalization of the startle fear response to the word cue of the non-reactivated CS2 in the yohimbine condition (G2CS2 vs G2CS3 and G2CS1 vs G2CS2; stimulus, F1,14=22.59, p<0.001, ηp2=0.62; stimulus, F1,14=46.86, p<0.001, ηp2=0.77, respectively) but not in the placebo pill group (stimulus, F1,14<3.19). Hence, the yohimbine HCl manipulation triggered fear generalization to the more abstract word stimulus. The word cue of the reactivated CS1 did not uncover any fear response in both the yohimbine and placebo pill condition (G2CS1 vs G2CS3; stimulus, F1,28<1). Taken together, these findings demonstrate that the administration of yohimbine HCl during acquisition resulted in a broader generalization of fear 48 h later, while this generalization effect was eliminated by the administration of propranolol HCl during memory reactivation.
Analysis of the startle response to NA showed no significant differences between the two conditions during acquisition and extinction learning (condition, F1,28<2.85) nor during memory reactivation, reinstatement testing, and generalization (t28<1.47).
Skin conductance responding
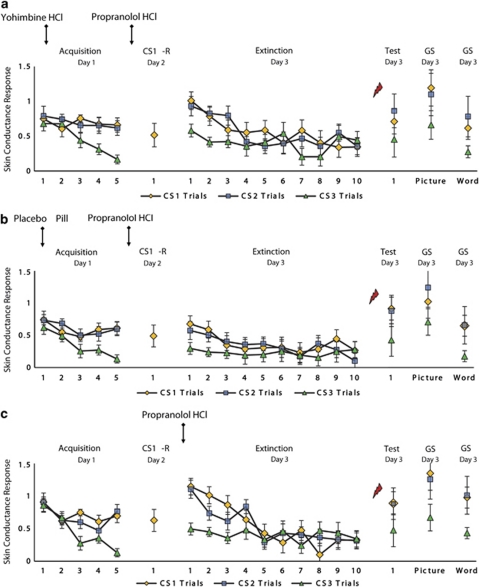
Overall analysis of electrodermal responding revealed no fear conditioning during acquisition (CS1Spider vs CS3Mug–CS2Gun vs CS3Mug) (stimulus × trial, F1,28<1). When fear responses are not successfully acquired, one cannot assess the return of fear. Therefore, only subjects showing successful levels of fear acquisition (ie, trial 5 CS1 or CS2>CS3) were included in the analyses. Five subjects were eliminated. That is, two subjects from the yohimbine condition and three subjects from the placebo pill group.
Subsequent analyses of variance showed no effects of the yohimbine HCl and propranolol HCl manipulation on skin conductance responding. We observed a significant increase in electrodermal activity during acquisition (trial 1 vs trial 5—day 1) in both conditions (CS1 vs CS3, CS2 vs CS3; stimulus × trial, F1,23=11.51, p<0.01, ηp2=0.33; F1,23=7.53, p<0.05, ηp2=0.25, respectively; stimulus × trial × condition, F1,23<1; Figure 3a and b). Furthermore, the two groups showed similar levels of electrodermal responding during the reactivation trial on day 2 (CS1-R; t23<1). Moreover, in both the yohimbine and placebo pill group, the differential skin conductance response (CS1 vs CS3, CS2 vs CS3) obtained during acquisition (ie, trial 5—day 1) remained stable 48 h later (ie, trial 1—day 3) (stimulus × trial, stimulus × trial × condition, F1,23<1.52). In both groups, a significant decrease in electrodermal responding was observed during extinction learning (trial 1 vs trial 10—day 3) (CS1 vs CS3, CS2 vs CS3; stimulus × trial, F1,23=11.83, p<0.01, ηp2=0.34; F1,23=11.03, p<0.01, ηp2=0.32, respectively; stimulus × trial × condition, F1,23<1). Analysis of the reinstatement effect further revealed a recovery in electrodermal responding from the last trial of extinction to the first trial at test in the yohimbine as well as placebo pill condition (CS1 vs CS3, CS2 vs CS3; stimulus × trial, F1,23=4.77, p<0.05, ηp2=0.17; F1,23=4.35, p<0.05, ηp2=0.16, respectively; stimulus × trial × condition, F1,23<1). In addition, the two groups showed a generalization of the skin conductance response to the category-related picture (GpCS1 vs GpCS3, GpCS2 vs GpCS3; main effect of stimulus, F1,23=5.31, p<0.05, ηp2=0.19; F1,23=4.81, p<0.05, ηp2=0.17, respectively; stimulus × condition, F1,23<1) as well as word cue (GwCS1 vs GwCS3, GwCS2 vs GwCS3; main effect of stimulus, F1,23=5.08, p<0.05, ηp2=0.17; F1,23=5.56, p<0.05, ηp2=0.20, respectively; stimulus × condition, F1,23<1). Note that analyses over the entire sample also revealed no differences between the yohimbine and placebo pill condition (CS1 vs CS3, CS2 vs CS3; stimulus × trial × condition, F1,28<1.07).
Figure 3.
Mean skin conductance responses to the CS1, CS2, and CS3 trials during acquisition, memory reactivation, extinction, test, and generalization for the (a) yohimbine HCl–propranolol HCl and (b) placebo pill–propranolol HCl condition (ie, Experiment I), and the (c) propranolol HCl after reactivation group (ie, Experiment II). Error bars represent SEM.
Retrospective US expectancy ratings
ANOVA revealed no effects of either drug manipulations on the retrospective US expectancy ratings. In both the yohimbine and placebo pill group, we observed a significant differential increase in expectancy ratings during acquisition (trial 1 vs trial 5; day 1) (CS1 vs CS3, CS2 vs CS3; stimulus × trial, F1,28=405.18, p<0.001, ηp2=0.94; F1,28=547.74, p<0.001, ηp2=0.95, respectively; stimulus × trial × condition, F1,28<1.23; see Supplementary Figure S2a and b). Furthermore, the two groups did not differ in their US expectancy during memory reactivation (day 2) (CS1-R; t28<1). Moreover, in both the yohimbine and placebo pill condition, the expectancy ratings remained stable from the last acquisition trial (day 1) to the first extinction trial 48 h later (day 3) (stimulus × trial, stimulus × trial × condition, F1,28<1.84). ANOVA showed extinction learning on day 3 by a significant differential decrease in US expectancy from the first extinction trial to the last trial of extinction learning in both conditions (CS1 vs CS3, CS2 vs CS3; stimulus × trial, F1,28=208.45, p<0.001, ηp2=0.88; F1,28=165.67, p<0.001, ηp2=0.86, respectively; stimulus × trial × condition, F1,28<1.56). We further observed a significant reinstatement effect from the last trial of extinction to the first trial at test in the yohimbine as well as placebo pill group (CS1 vs CS3, CS2 vs CS3, stimulus × trial, F1,28=107.67, p<0.001, ηp2=0.79; F1,28=89.90, p<0.001, ηp2=0.76, respectively; stimulus × trial × condition, F1,28<1.21). Moreover, in both groups, we observed a generalization of the US expectancy ratings to the category-related picture (GpCS1 vs GpCS3, GpCS2 vs GpCS3; main effect of stimulus, F1,28=142.73, p<0.001, ηp2=0.84; F1,28=137.87, p<0.001, ηp2=0.83, respectively; stimulus × condition, F1,28<1.45) as well as word cue (GwCS1 vs GwCS3, GwCS2 vs GwCS3; main effect of stimulus, F1,28=138.07, p<0.001, ηp2=0.83; F1,28=140.63, p<0.001, ηp2=0.83, respectively; stimulus × condition, F1,28<1.12).
Experiment II, Propranolol after Reactivation
To rule out the effect of the propranolol HCl manipulation on the retrieval of the fear memory itself, participants in Experiment II received an oral dose of propranolol HCl directly after reactivation. Resembling our previous findings (Kindt et al, 2009; Soeter and Kindt, 2010, 2011a), the administration of pills following memory reactivation also selectively eliminated the startle fear response 48 h after fear acquisition (CS1 vs CS3; stimulus × trial, F1,9=12.11, p<0.01, ηp2=0.57; Figure 2c), without affecting skin conductance discrimination and the retrospective US expectancy ratings (Figure 3c; see also Supplementary Results and Supplementary Figure S2c).
DISCUSSION
The present findings demonstrate that stimulation of the noradrenergic system during memory formation delayed the process of fear extinction without impairing the disruption of reconsolidation (ie, startle fear responding). The competition between the original excitatory fear association and the newly formed inhibitory memory trace determines the behavioral outcome of extinction learning (Myers and Davis, 2002). Given that yohimbine HCl was administered during fear acquisition—and not during fear extinction 48 h later—the α2-adrenergic drug apparently delayed the process of extinction by strengthening the original excitatory fear association (Soeter and Kindt, 2011b). Yet, the yohimbine HCl manipulation did not directly augment the differential startle fear responding either 24 or 48 h after fear learning. That is, in both the placebo pill and yohimbine HCl group, the fear responding obtained during acquisition (day 1) remained stable during memory reactivation (day 2—reactivated fear association) as well as retention testing 48 h later (non-reactivated fear association). Our finding that yohimbine HCl strengthened the original fear association without directly augmenting its behavioral expression may suggest a ceiling effect in startle fear conditioning (day 1) (see also, Soeter and Kindt, 2011b). Note that the β-blocker (propranolol HCl) during memory retrieval (day 2) may also have suppressed a potential fear-enhancing effect of the yohimbine HCl manipulation (day 1). In any case, whereas the α2-adrenergic drug delayed the process of extinction learning and triggered broader fear generalization 48 h later, the β-adrenergic receptor blocker (ie, propranolol HCl) during reconsolidation (day 2) selectively diminished the startle fear responding to the reactivated fear association along with its category-related information (day 3). Moreover, we observed a similar reduction in startle fear responding when the β-adrenergic receptor antagonist was administered after reactivation of the memory (ie, Experiment II), suggesting that the propranolol HCl manipulation before reactivation also affected the processes mediating reconsolidation (ie, Experiment I) (Nader et al, 2000). The present study employed stimuli of different ‘valence' categories (fear-relevant vs fear-irrelevant) to verify whether the propranolol HCl manipulation was capable of neutralizing fear responding. That is, since fear-relevant stimuli are known to have an innate prepotency to elicit fear responses (Lovibond et al, 1994), we employed a fear-irrelevant cue as an additional control stimulus. The downside of this procedure is that the differential responding observed during acquisition (day 1) may simply be due to the emotional ‘valence' of the stimuli employed (fear-relevant vs fear-irrelevant) rather than associative learning. Previously, we demonstrated in a fear conditioning paradigm that fear relevancy does not affect fear responding in the absence of associative learning (ie, first acquisition trial; Soeter and Kindt, 2011a). Again, we did not observe any difference in fear responding to the first trial of acquisition, indicating that the differential fear responding observed at the end of fear conditioning (day 1) was due to associative learning instead of the ‘valence' of the cues. Thus, taken together, given that we observed a similar level of startle fear responding to the reactivated fear association and the fear-irrelevant control stimulus at retention testing (day 3), the β-adrenergic receptor blocker during reconsolidation apparently ‘neutralized' the fear-arousing aspects of the ‘associative' fear memory along with its category-related information (ie, generalization testing).
In line with our preceding studies, the α2-adrenergic (Soeter and Kindt, 2011b) as well as β-adrenergic drug (Kindt et al, 2009; Soeter and Kindt, 2010, 2011b) did not affect the skin conductance responding and retrospective US expectancy ratings. Considering that online ratings direct the attention towards the CS–US relation (Baeyens et al, 1990; Lovibond and Shanks, 2002) and skin conductance responding is highly sensitive to attentional processes (Filion et al, 1991), the use of online ratings may previously have interfered with the measurement of electrodermal activity (Soeter and Kindt, 2010, 2011a, 2011b). The current observation that the omission of online ratings does not differentially affect skin conductance responding corresponds to the view that electrodermal activity may primarily reflect the more cognitive level of contingency learning (ie, declarative knowledge) (Weike et al, 2007; but see, Schultz and Helmstetter, 2010). Studying human fear conditioning allows for the independent evaluation of both declarative knowledge and the fear response. In most fear conditioning studies, the conscious anticipation of an aversive stimulus (US) is associated with an increase in startle potentiation (Grillon and Davis, 1995; Lovibond and Shanks, 2002). However, there are also several observations showing that unawareness of a CS–US contingency does not preclude a startle fear response (eg, Weike et al, 2007). This indicates that the anticipation of an aversive stimulus is not a necessary condition to observe fear-potentiated startle responses. Our findings show that the anticipation of an US is also not a sufficient condition to generate fear-potentiated startle responses. As such, our results emphasize the concept of multiple memory systems and suggest a double dissociation between the emotional and cognitive representation of fear (Squire, 2005; LaBar and Cabeza, 2006; Weike et al, 2005, 2007). It should be noted that these findings do not imply that reconsolidation is restricted to the emotional expression of fear memory (ie, startle fear responding). In view of the hypothesized adaptive function of memory reconsolidation (Dudai, 2006, 2009; Lee, 2009), there is no a priori reason for assuming that some memory systems would not be sensitive to disrupting reconsolidation (Lee, 2009).
In our previous work (Kindt et al, 2009; Soeter and Kindt, 2010), the erasure of the fear response could also have resulted from a more diffuse effect of the propranolol HCl manipulation by reducing the fear-provoking aspects of the aversive consequence itself (US). In considering clinical implications, disrupting reconsolidation should not radically alter functional reactions to potentially dangerous situations (US), but selectively weaken the underlying maladaptive fear association (CS1–US). Our current observation that the non-reactivated stimulus (CS2) potentiated the startle fear responding at test (day 3) indicates that the participants still feared the US, but no longer the reactivated conditioned stimulus (CS1) (see also, Soeter and Kindt, 2011a). Apparently, the propranolol HCl manipulation selectively disrupted the CS1–US association at the emotional level given that the participants also expected the US when presented with the reactivated stimulus (CS1) at test (day 3). Taken together, these findings show that there is no causal link between the ‘actual knowledge' of a fear association and its fear response, even though they often operate in parallel. Note that the fear-erasing effects were not restricted to the feared cue itself (CS1), but instead generalized to category-related information not previously associated with the originally feared stimulus. The generalization of fear has been demonstrated to be dependent on the training-induced strength of the memory (Laxmi et al, 2003). Here, the strengthening of the fear memory trace by the α2-adrenergic manipulation indeed triggered broader fear generalization (category-related word cues). Conversely, the β-adrenergic interference with reconsolidation left the memory trace too weak to yield a generalized fear response (Soeter and Kindt, 2011a). Several findings have implicated CREB phosphorylation in the formation (Josselyn et al, 2001; Davies et al, 2004) as well as generalization of fear memory (Han et al, 2008). Whereas yohimbine HCl is known to induce pCREB activation (Sun et al, 2010), the β-adrenergic receptor antagonist propranolol has been shown to inhibit noradrenaline-stimulated CREB phosphorylation (Thonberg et al, 2002). The present findings show the involvement of noradrenergic modulation in the generalization of human associative fear memory. Corroborating our previous study, the findings further demonstrate that the assimilation of individual memory items into a generalized schema may be dissociable for semantic and affective knowledge (Soeter and Kindt, 2011a). That is, upon exposure to the category-related information of the reactivated fear memory, the participants again predicted danger without a concomitant fear response.
While fear responses are very common in the aftermath of a traumatic event, the intensity of this initial fear responding is generally a poor indicator of symptom development or PTSD diagnosis (Rothbaum et al, 1992; Brewin et al, 2000; Murray et al, 2002). Rather, the impairment in the ‘unlearning' of fear-related behavior as well as the generalization of fear to intrinsically safe stimuli (Blechert et al, 2007; Lissek and Grillon, 2010) has a strong relevance to the development of anxiety disorders such as PTSD. Hence, our data indicate that the noradrenaline level during or shortly after a traumatic experience may contribute to the etiology of post-traumatic stress disorder. At the same time, the present findings may have valuable therapeutic implications given that the strength of the fear memory did not act as a constraint on reconsolidation. It may be possible that the stimulation of the noradrenergic system during memory formation was not strong enough to prevent the disruption of reconsolidation (Suzuki et al, 2004; Wang et al, 2009). On the other hand, the yohimbine HCl manipulation yielded a resistance to fear extinction 48 h later and triggered broader fear generalization, indicating the former strengthening of the excitatory fear memory (Myers and Davis, 2002; Soeter and Kindt, 2011b). Moreover, preliminary evidence in trauma patients also revealed diminished trauma-relevant physiological responding following the β-adrenergic interference with reconsolidation (Brunet et al, 2008). The current observation that propranolol HCl can well be administered after reactivation of the fear memory (ie, Experiment II) further has implications in light of the demarcation between ‘extinction' and ‘reconsolidation' (Eisenberg et al, 2003; Pedreira and Maldonado, 2003; Suzuki et al, 2004). That is, when memory retrieval initiates extinction learning instead of reconsolidation, the propranolol HCl manipulation should be omitted to avoid interference with the consolidation of extinction training (Ouyang and Thomas, 2005; Mueller et al, 2008).
The ‘process of extinction' and ‘disrupting reconsolidation' are two approaches to diminish fear-related behavior. The extinction of fear not only leaves the original fear memory intact—thereby explaining the return of fear after apparently successful fear reduction (Bouton, 1993)—but may also be impaired by noradrenergic stimulation during the formation of the excitatory fear memory. Conversely, β-adrenergic receptor blockade during reconsolidation selectively ‘deletes' the fear-arousing aspect of noradrenergic strengthened fear memory and—on top of that—undermines the generalization of fear responding. Given that fear generalization lies at the heart of many anxiety disorders, disrupting reconsolidation points to a promising strategy in reducing excessive fear responding.
Acknowledgments
We thank B Molenkamp for technical assistance. This work was supported by a Vici grant (Merel Kindt) from the Netherlands Organization for Scientific Research.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Baeyens F, Eelen P, van den Berg O. Contingency awareness in evaluative conditioning: a case for unaware affective-evaluative learning. Cogn Emot. 1990;4:3–18. [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigm of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Cons Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiological responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psych Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodam WK, Heninger GR. Neurobiological mechanisms of panic-anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. Am J Psych. 1987;44:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- Davies MF, Tsui J, Flannery JA, Li X, DeLorey TM, Hoffman BB. Activation of α2 adrenergic receptors suppresses fear conditioning: expression of c-Fos and phosphorylated CREB in mouse amygdala. Neuropsychopharmacology. 2004;29:229–239. doi: 10.1038/sj.npp.1300324. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Predicting not to predict too much: how the cellular machinery of memory anticipates the uncertain future. Philoso Trans R Soc B. 2009;364:1255–1262. doi: 10.1098/rstb.2008.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM, Hazlett EA. The relationship between skin conductance orienting and the allocation of processing resources. Psychophysiology. 1991;28:410–424. doi: 10.1111/j.1469-8986.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Goodman LS. Goodman and Gilman's the Pharmacological Basis of Therapeutics. McGraw-Hill: New York, NY; 1996. [Google Scholar]
- Grasing K, Sturgill MG, Rosen RC, Trout JR, Thomas TJ, Kulkarni GD, et al. Effects of yohimbine on autonomic measures are determined by individual valued for area under the concentration–time curve. J Clin Pharmacol. 1996;36:814–822. doi: 10.1002/j.1552-4604.1996.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, Chaney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology. 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Acoustic startle and anticipatory anxiety in humans: effects of monaural right and left ear stimulation. Psychophysiology. 1995;32:155–161. doi: 10.1111/j.1469-8986.1995.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. Int J Psychophysiol. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Han J-H, Yiu AP, Cole CJ, Hsiang H-L, Neve RL, Josselyn SA. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem. 2008;15:443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Chanjun S, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed GBG, Lang PJ. Psychometric description of some specific fear questionnaires. Behav Ther. 1974;5:401–409. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BM. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. University of Florida: Gainesville, FL; 2005. [Google Scholar]
- Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Grillon C. Overgeneralization of conditioned fear in the anxiety disorders. J Psychol. 2010;218:146–148. [Google Scholar]
- Lovibond PF, Hanna SK, Siddle DAT, Bond NW. Electrodermal and subjective reactions to fear-relevant stimuli under threat of shock. Aus J Psychol. 1994;46:73–80. [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol. 2002;28:3–26. [PubMed] [Google Scholar]
- McGaugh ML, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2009;202:1–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Mineka S, Öhman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psych. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Ehlers A, Mayou RA. Dissociation and posttraumatic stress disorder: two prospective studies of road accident survivors. Br J Psych. 2002;180:363–368. doi: 10.1192/bjp.180.4.363. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:722–726. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers K, Davis M, Rothbaum BO, et al. Conditioned fear extinction and reinstatement in a human fear potentiated startle paradigm. Learn Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Wingerson D, Murray S, Pascualy M, Dobie DJ, Le Corre P, et al. Effects of Alzheimer's disease and normal aging on cerebrospinal fluid norepinephrine responses to yohimbine and clonidine. Arch Gen Psych. 1995;52:774–782. doi: 10.1001/archpsyc.1995.03950210068012. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Manual. International Diagnostic System: Worthington, OH; 1992. [Google Scholar]
- Rothbaum BO, Foa EB, Riggs DS, Murdock T, Walsh W. A prospective examination of post-traumatic stress disorder in rape victims. J Traum Stress. 1992;5:455–475. [Google Scholar]
- Schultz DH, Helmstetter FJ. Classical conditioning of autonomic fear responses is independent of contingency awareness. J Exp Psychol. 2010;36:495–500. doi: 10.1037/a0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011a;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiol Learn Mem. 2011b;96:263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lusthene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA; 1970. [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2005;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stegeren AH, Rohleder B, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: effect of beta blockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2009;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Sun HS, Green TA, Theobald DEH, Laali S, Shrikhande G, Birnbaum S, et al. The pharmacological stressor yohimbine increases impulsivity through activation of CREB in the orbitofrontal cortex. Biol Psych. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonberg H, Fredriksson JM, Nedergaard J, Cannon B. A novel pathway for adrenergic stimulation of cAMP-response-element-binding protein (CREB) phosphorylation: mediation via α1-adrenoceptors and protein kinase C activation. Biochem J. 2002;264:73–79. doi: 10.1042/bj3640073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, Oliveira Alvares L, Nader K. Cellular and systems mechanism of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:906–913. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HWS, Kessler C. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J Neurosci. 2005;25:11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.