Abstract
Available evidence strongly suggests that the γ-aminobutyric acid type A (GABAA) receptor has a crucial role in memory retrieval. However, the signaling mechanisms underlying the role of GABAA receptor modulation in memory retrieval are unclear. We conducted one-trial passive avoidance task with pre-retention trial drug administration in the hippocampus to test the effects of GABAA receptor modulation on memory retrieval. We further tested the co-involvement of signaling molecules: extracellular signal-regulated kinase (ERK), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and cAMP responsive element-binding protein (CREB). First, we observed that the phosphorylation of hippocampal ERK was required for memory retrieval during the task. Accordingly, to investigate whether GABAA receptor activation or inhibition induces ERK phosphorylation during memory retrieval, drugs that target the GABAA receptor were administered into the hippocampus before the retention trial. Muscimol, a GABAA receptor agonist, and diazepam, an agonist to benzodiazepine-binding site of GABAA receptor, blocked retention trial-induced ERK phosphorylation and impaired memory retrieval. Furthermore, co-treatment with sub-effective dose of U0126, a mitogen-activated protein kinase inhibitor, blocked the upregulation of ERK phosphorylation and impaired memory retrieval, and bicuculline methiodide (BMI), a GABAA receptor antagonist, increased ERK phosphorylation induced by the retention trial and facilitated memory retrieval. Finally, the effects of BMI were blocked by the co-application of a sub-effective dose of U0126. These results suggest that GABAA receptor-mediated memory retrieval is closely related to ERK activity.
Keywords: memory retrieval, GABAA receptor, extracellular signal-regulated kinase, one-trial passive avoidance task
INTRODUCTION
Many pharmacological aspects of the GABAergic neurotransmitter system have been elucidated, including its anxiolytic, anti-convulsive and anesthetic activities (Skolnick and Paul, 1981; Brailowsky and García, 2009; Christmas et al, 2008; Henschel et al, 2008). In addition, the involvement of the GABAergic neurotransmitter system in learning and memory is supported by a substantial body of evidence (Makkar et al, 2010), although there is controversy (Castellano et al, 1996). For example, the γ-aminobutyric acid type A (GABAA) receptor antagonists including bicuculline (GABA-binding site) and flumazenil (benzodiazepine-binding site) enhance (Brioni and McGaugh, 1988; Castellano and Pavone, 1988; Herzog et al, 1996; Luft et al, 2004; Matsuyama et al, 2008) or impair (Nabeshima et al, 1988; Chrobak and Napier, 1991) memory formation depending on the study. GABAA receptor agonists, including muscimol (GABA-binding site) and diazepam (benzodiazepine-binding site), impair learning (Chrobak et al, 1989) and memory (Castellano and McGaugh, 1989, 1990; Shah and Parent, 2003; Krebs-Kraft et al, 2007; Kim et al, 2009). These findings suggest that the GABAA receptor has an important role in learning and memory. However, comparatively little work has been conducted regarding the intracellular molecular mechanisms through which GABAA receptor modulation affects memory processes.
Previous reports have suggested a possible interaction between the GABAA receptor and extracellular signal-regulated kinase (ERK) signaling (Kalluri and Ticku, 2002; Igaz et al, 2006; Zheng et al, 2007). The ERK subfamily of mitogen-activated protein kinase (MAPK) contributes to crucial signal transduction systems that regulate cAMP response element-binding protein (CREB) activity, which has a key role in long-term memory formation (Ahi et al, 2004). The early activation of ERK signaling in the hippocampus is necessary for the establishment of long-term memory (Giovannini, 2006). Memory formation can be blocked by the administration of U0126, an inhibitor of MAPK/ERK-kinase (MEK), into the CA1 region of the hippocampus (Sananbenesi et al, 2003; Trifilieff et al, 2006). It has also been reported that MAPK/ERK are involved in various types of memory retrieval and extinction of step-down inhibitory avoidance and auditory fear conditioning (Barros et al, 2000; Herry et al, 2006; Bonini et al, 2011). Although the GABAA receptor is known to be strongly involved in learning and memory processes, the signaling cascades associated with memory retrieval have not yet been elucidated. Hall et al., (2001) suggested that the molecular signaling underlying gene expression and protein synthesis during memory acquisition are reactivated during memory retrieval. Thus, it is possible that muscimol-induced memory deficits and concomitant decreases in hippocampal ERK phosphorylation impair the memory retrieval process. Therefore, we hypothesized that GABAA receptor modulation is involved in the memory retrieval process through the modulation of hippocampal ERK–CREB signaling. To test this hypothesis, we first confirmed the importance of this signaling pathway in memory retrieval using KN62, a selective and cell-permeable inhibitor of CaMKII, which is an alternative pathway involved in CREB signaling (Ahi et al, 2004), and U0126, a MAPK inhibitor. Next, we examined the effect of GABAA receptor agonists (muscimol and diazepam) or antagonists (bicuculline methiodide (BMI) and flumazenil) on memory retrieval and concomitant ERK–CREB signaling changes in the mouse hippocampus using a one-trial passive avoidance task.
MATERIALS AND METHODS
Animals
Male ICR mice (25–30 g, 7 weeks old) were purchased from the Orient, a branch of Charles River Laboratories (Seoul, Korea). Mice were housed five per cage, provided with food and water ad libitum, and kept under a 12-h light/dark cycle (light on 0730 hours to 1930 hours) at constant temperature (23±2 °C). Animal treatment and maintenance were performed in accordance with the Animal Care and Use Guidelines issued by Kyung Hee University, Korea. We used a total of 550 mice; different mice were used in each experiment (Supplementary Table 1). All efforts were made to minimize the number of animals as well as their suffering.
Materials
Muscimol, BMI, flumazenil, KN63 and U0126 were purchased from Sigma Chemical (St. Louis, MO). Diazepam was from the Daewon Pharmaceutical Company (Seoul, Korea). Anti-CaMKII, anti-phosphorylated CaMKII (pCaMKII), anti-ERK, anti-CREB, anti-phosphorylated ERK (pERK), and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphorylated CREB (pCREB) antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Vecta stain ABC kits were purchased from Vector Laboratories (Burlingame, CA). All other materials were of the highest grade available and were obtained from normal commercial sources.
Microinfusion of Drugs
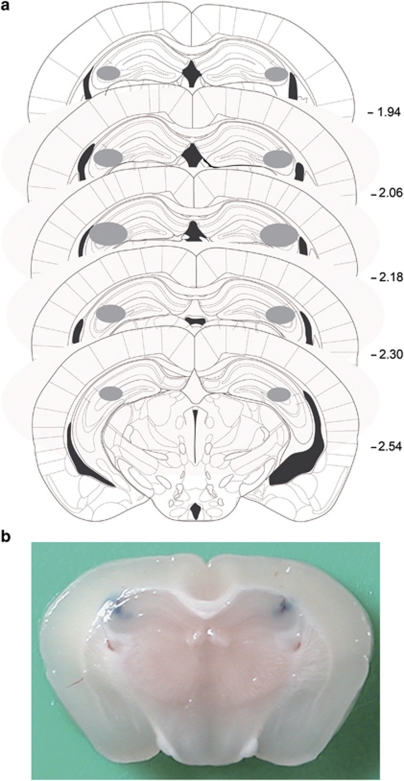
Mice were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) under Zoletil 50 anesthesia (10 mg/kg, i.m.), and guide cannulae (26 G) were aimed at the hippocampal CA3 region (AP, −2.10 mm from bregma; ML, ±2.50 mm from midline; DV, −1.50 mm from the dura) using an atlas of the mouse brain (Paxinos and Franklin, 2001). The guide cannulae were fixed to the skull with dental cement and covered with dummy cannulae. Following surgery, mice were allowed to recover for 7 days. BMI and muscimol were dissolved in artificial cerebrospinal fluid; flumazenil, U0126 and KN62 were dissolved in 0.1% DMSO in saline (0.9% NaCl) before infusion. Fifteen minutes before the retention trial, mice were carefully restrained by hand and bilaterally infused with BMI (0.25, 0.5, or 1 μg/μl/side, Luft et al, 2004), flumazenil (0.5, 1, or 2 μg/μl/side, Gonzalez et al, 1998), muscimol (0.25, 0.5, or 1 μg/μl/side, Holt and Maren, 1999), diazepam (10, 20, or 40 μg/μl/side, Wesołowska et al, 2006), U0126 (0.25, 0.5, or 1 nmol/μl/side, Kim et al, 2009), KN62 (1 μg/μl/side, Ahi et al, 2004) or vehicle through injector cannulae (30 G) extended 1.0 mm beyond the tips of guide cannulae. The infusion volume was 1 μl/side, and the infusion rate was 0.5 μl/min. In the case of KN62, the dose was chosen to fully inhibit CaMKII activity (Ou and Gean, 2007). After the infusion, the infusion needle was left in the guide cannula for 1 min to ensure proper delivery of the reagents. At the end of the experiment, the position of the cannulae was verified by injection of 0.1 μl of 1% Evans Blue solution in saline (Figure 1). A total of 48 mice (out of 598) were excluded from further analyses on the basis of these histological findings.
Figure 1.
The site of intra-hippocampal microinjections. (a) Summary of the site of microinjection in the hippocampal CA3 region. Mice in which the cannulae were found to be misplaced were excluded. (b) Drug administration site confirmed by the infusion of 0.1 μl of 1% Evans Blue into the hippocampus after behavioral testing (coordinates: anteroposterior −2.10 mm from bregma; mediolateral ±2.50 mm from midline; and dorsoventral −2.00 mm from dura).
Passive Avoidance Task
The acquisition and retention of passive avoidance behaviors were examined using identical illuminated and non-illuminated (20 × 20 × 20 cm3) boxes separated by a guillotine door (5 × 5 cm2), as described elsewhere (Kim et al, 2008). The animals underwent two separate trials. First, in the acquisition trial, a mouse was initially placed in the light compartment, and the door between the two compartments was opened 10 s later. When the mouse entered the dark compartment, the guillotine door automatically closed, and an electrical foot shock (0.25 mA, 3 s, Kim et al, 2007) was delivered through the floor. The latency time before crossing into the dark chamber was recorded. Only the mice that entered the dark chamber within 60 s were subjected to a retention trial. For the retention trial, the mouse was again placed in the light compartment and the latency time before crossing into the dark compartment was recorded (up to 600 s). The control group underwent normal acquisition but did not undergo a retention trial, and control animals were killed at the time point equivalent to other groups. To examine the effect of drugs on pain threshold, electrical foot shock (same as the acquisition trial) was delivered and the responses to foot shock was checked when mice entered dark compartment in the retention trial. Responses to electric shock were recorded as described elsewhere (Wang et al, 2001, 2004). The following scores were awarded based on the responses to electric shock: 3, jumping: 2, vocalization: 1, flinching: 0, no response. This was conducted 15 min after drug infusion into the hippocampus. We found that no drugs that used in the present study affected pain threshold when they were injected into the hippocampus (Supplementary Figure S2).
Spontaneous Locomotor Behavior
To investigate the motoric effect of the indicated dose of GABAergic drugs during the passive avoidance task, spontaneous locomotor behavior was measured as described previously (Jung et al, 2006). Briefly, the mice were placed in the center of a horizontal locomotor activity box (40 × 40 × 40 cm3), and locomotor activity was measured 15 min after drug injection and analyzed using the video-based Ethovision System (Noldus, Wageningen, The Netherlands). Horizontal locomotor activity was converted to total ambulatory distance.
Immunohistochemistry
The mice were anesthetized with Zoletil 50 (10 mg/kg, i.m.) immediately after the retention trial and rapidly perfused transcardially with 0.1 M phosphate buffer (pH 7.4) followed by ice-cold 4% paraformaldehyde. Brains were removed and postfixed overnight in a phosphate buffer (0.05 M, pH 7.4) containing 4% paraformaldehyde. Frozen brains were coronally sectioned at 30 μm thickness on a cryostat and stored in storage solution at 4 °C. Immunohistochemistry was performed using polyclonal anti-pCREB (1 : 1000 dilution), anti-pERK (1 : 1000 dilution) or anti-pCaMKII (1 : 1000 dilution) antibodies as described previously (Kim et al, 2006).
Western Blot Analysis
Mice were killed immediately after the retention trial. Isolated hippocampal tissue was homogenized in ice-chilled Tris-HCl buffer (20 mM, pH 7.4) containing 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 μg/ml aprotinin, 15 μg/ml leupeptin, 10 μg/ml bacitracin, 10 μg/ml pepstatin, 15 μg/ml trypsin inhibitor, 50 mM NaF and 1 mM sodium orthovanadate. The samples of homogenates (20 μg of protein) were then subjected to SDS-PAGE (8%) under reducing conditions. Proteins were then transferred to PVDF membranes using transfer buffer (25 mM Tris-HCl, pH 7.4, containing 192 mM glycine and 20% v/v methanol) and separated at 100 V for 2 h at 4°C. Blots were then incubated for 2 h in blocking solution (5% skim milk) at 4 °C overnight with 1 : 1000 dilutions of anti-pCREB, anti-pERK, or anti-pCaMKII antibodies, incubated with a 1 : 5000 dilution of horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, and then developed by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL). The blots were then stripped with stripping buffer (100 mM ß-mercapto-ethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.8) and incubated with anti-CREB, anti-ERK or anti-CaMKII antibodies (1 : 5000). Film densitometry was performed using Quantity One Image Analysis System version 4.6.3 (Bio-Rad Laboratories, CA). The levels of phosphorylated CREB, ERK, and CaMKII were determined by calculating the ratios of phosphorylated protein to the corresponding total protein on the same membranes. These mean values were normalized to the same ratios obtained from the control animals. In the cases of pERK and ERK, we quantified total density of two bands.
Experiment 1: The Involvement of ERK Phosphorylation in Memory Retrieval
Our target molecules are all known to be involved in learning and memory. To test which molecule(s) are required for memory retrieval, the mice were divided into control and retrieval groups, and killed immediately after the retention trial (control group was killed at a time point equivalent to their respective experimental group). Next, mice were treated with KN62 or U0126 15 min before the retention trial, and killed immediately after the retention trial. Using the same procedure in another group of mice, we observed a dose-dependent effect of U0126 on memory retrieval.
Experiment 2: GABAA Receptor Activation Impairs Memory Retrieval
To investigate whether GABAA receptor agonists affect memory retrieval, mice with previous exposure to the acquisition trial were treated with muscimol or diazepam, underwent a retention trial and were killed. Because of any result from the passive avoidance task may be affected by state-dependency, the mice were treated with muscimol or diazepam 15 min before the acquisition and the retention trials. To investigate the interactive effects of GABAA receptor agonists and U0126 on memory retrieval, mice were co-administered muscimol or diazepam and U0126 15 min before the retention trial.
Experiment 3: GABAA Receptor Blockade Enhances Memory Retrieval
To test whether GABAA receptor antagonists affect memory retrieval, mice were treated with BMI or flumazenil 15 min before the retention trial and killed immediately after the retention trial. To test state-dependency, the mice were treated with BMI 15 min before acquisition and retention trials. To investigate the interactive effects of BMI and U0126 on memory retrieval, the mice were co-administered BMI with U0126 15 min before the retention trial.
Statistics
The results of the passive avoidance test, locomotor activity and western blot were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test for multiple comparisons. For the antagonism study, interactions between GABAA receptor ligands and U0126 were separately analyzed by two-way ANOVA followed by Bonferroni's post-hoc test for multiple comparisons. Student's t-test was used to compare control and retrieval groups in Figure 2 and state-dependency test. Statistical significance was set at p<0.05.
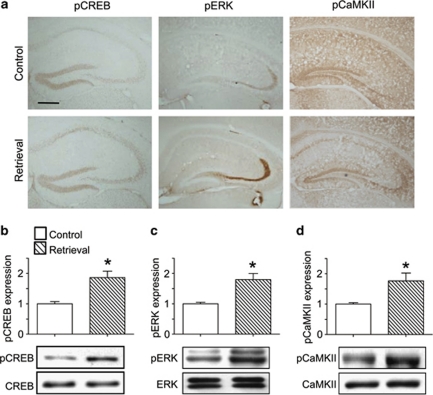
Figure 2.
Memory retrieval-induced changes in the expression of memory-related signaling molecules in hippocampal tissues. (a) Photographs of immunohistochemical results showing memory retrieval-induced changes in the immunoreactivity of anti-phosphorylated CREB (pCREB), anti-phosphorylated ERK (pERK), or anti-phosphorylated CaMKII (pCaMKII) in the hippocampus. (b–d) Western blot analysis of pCREB (b), pERK (c), and pCaMKII (d). Mice were killed immediately after the retention trial of a one-trial passive avoidance task. Data are presented as the means±SEM (n=4). *p<0.05, compared with the control group. The control group was not subjected to a retention trial. The retrieval group was subjected to a retention trial. Magnification: × 50. Bar=200 μm.
RESULTS
Experiment 1: Memory Retrieval Requires ERK Phosphorylation
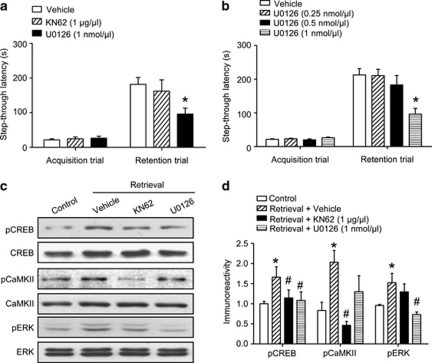
The expression of several memory-related molecules in the hippocampal region, including pCREB, pCaMKII, and pERK, was examined following the retention trial of a one-trial passive avoidance task and compared against expression in mice that were not exposed to the retention trial (Figure 2a–d). The immunoreactivities of pCREB (t(6)=3.888, p<0.05; Figure 2b), pERK (t(6)=3.881, p<0.05; Figure 2c) and pCaMKII (t(6)=2.903, p<0.001; Figure 2d) were significantly increased in the hippocampus immediately after the retention trial. To determine whether pCREB, pERK, or pCaMKII have key roles in memory retrieval, we injected KN62, a CaMKII inhibitor, or U0126, a MEK inhibitor, into the hippocampus 15 min before the retention trial.
Intrahippocampal injection of KN62 15 min before the retention trial significantly inhibited the upregulation of pCaMKII (F(2, 9)=14.480, p<0.001; Figure 3c and d) and pCREB (F(2, 9)=7.373, p<0.001; Figure 3c and d), but had no effect on the immunoreactivity of pERK (F(2, 9)=6.086, p=0.448; Figure 3c and d) or memory retrieval (t(14)=0.5164, p>0.05, Figure 3a). By contrast, U0126 reduced upregulation of pERK (F(2, 9)=16.910, p<0.05; Figure 3c and d) and pCREB (F(2, 9)=7.376, p<0.001; Figure 3c and d) while impairing memory retrieval, with no effect on the expression of pCaMKII (F(2, 9)=4.995, p=0.45; Figure 3c and d). Moreover, injection of U0126 significantly impaired memory retrieval in a dose-dependent manner (F(3, 36)=6.768, p<0.05; Figure 3b) without affecting foot shock response (Supplementary Figure S2). We used the sub-effective dose of U0126 (0.25 nmol/μl/side) for further blocking studies.
Figure 3.
Blockade of extracellular signal-regulated kinase (ERK) phosphorylation inhibits memory retrieval. (a) Latency times measured during the retention trial were reduced by the administration of U0126 but not KN62. (b), Dose-dependent effects of U0126 on memory retrieval. Fifteen minutes before the retention trial, KN62 (1 μg/μl/side) or U0126 (0.25, 0.5, or 1 nmol/μl/side) were administered bilaterally into the hippocampus. Data are presented as the means±SEM (n=8–10 per group). *p<0.05, compared with the vehicle-treated group. (c, d), Immunoblotting (c) and quantitative analysis (d) of pCREB, pCaMKII, and pERK levels in hippocampal tissues after the administration of KN62 or U0126 before the retention trial. Fifteen minutes before the retention trial, KN62 (1 μg/μl/side) or U0126 (1 nmol/μl/side) were administered bilaterally into the hippocampus. The mice were immediately killed after the retention trial. Data are presented as the means±SEM (n=4 per group). *p<0.05, compared with the control group; #p<0.05, compared with the vehicle-treated group. Control, animals that did not undergo a retention trial; Vehicle, animals that underwent a retention trial but were treated only with vehicle; KN62, animals treated with KN62 that underwent a retention trial; U0126, animals treated with U0126 that underwent a retention trial.
Experiment 2: GABAA Receptor Activation Impairs Memory Retrieval by Inhibiting ERK Phosphorylation
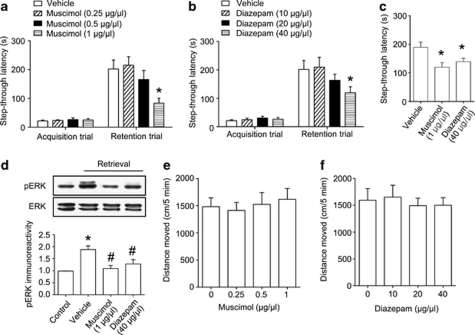
To investigate the effect of GABAA receptor agonists on memory recall, we utilized the one-trial passive avoidance task with pre-retention trial drug administration. Both the muscimol and diazepam impaired memory retrieval in a dose-dependent manner as demonstrated by shortened latencies to cross into the dark compartment (muscimol, F(3, 28)=4.467, p<0.05, Figure 4a; diazepam, F(3, 28)=5.149, p<0.05, Figure 4b) without affecting foot shock response (Supplementary Fig. S2). There were no significant differences in the latencies during the acquisition trial (Figure 4a and b). Drug administration before both the acquisition and the retention trials also decreased the latency time, suggesting that there was no state-dependency (muscimol, t(18)=2.918, p<0.05; diazepam, t(18)=2.413, p<0.05) (Figure 4c). Moreover, memory-impairing doses of muscimol and diazepam also blocked retrieval-induced ERK phosphorylation in the hippocampus (muscimol, F(2, 9)=17.73, p<0.05; diazepam, F(2, 9)=11.18, p<0.05) (Figure 4d). Notably, intra-hippocampal injection of these drugs did not affect spontaneous locomotor activity (muscimol, F(3, 36)=0.2192, p>0.05, Figure 4e; diazepam, F(3, 36)=0.1712, p>0.05) (Figure 4f).
Figure 4.
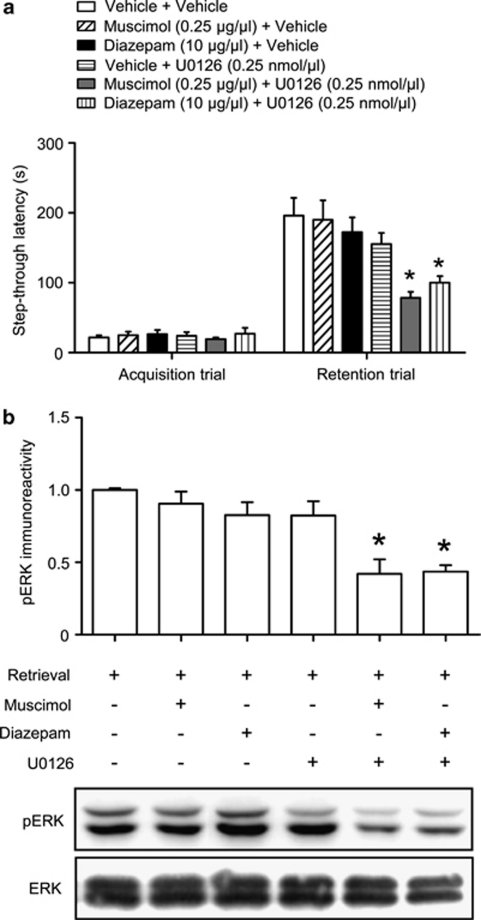
γ-Aminobutyric acid type A (GABAA) receptor activation detrimentally affects memory retrieval. (a, b) Fifteen minutes before the retention trial, (a) muscimol (0.25, 0.5, or 1 μg/μl/side) or (b) diazepam (10, 20, or 40 μg/μl/side) were administered bilaterally into the hippocampus. Latency times measured during the retention trial were reduced by muscimol or diazepam administration in a dose-dependent manner. (c) To test state-dependency of GABAergic drugs, muscimol (1 μg/μl/side) or diazepam (40 μg/μl/side) was administered bilaterally 15 min before both acquisition and retention trial. Data are presented as the means±SEM (n=8–10 per group). *p<0.05, compared with the vehicle-treated control group. (d) The effect of muscimol (1 μg/μl/side) or diazepam (40 μg/μl/side) on memory retrieval-induced ERK activation. The mice were killed immediately after the retention trial of passive avoidance task. Data are presented as the means±SEM (n=4 per group). *p<0.05, compared with the control group; #p<0.05, compared with the vehicle-treated retrieval group. Control, animals exposed to acquisition but not retention trial. (e, f) The motoric effects of muscimol (0.25, 0.5, or 1 μg/μl/side, (e) or diazepam (10, 20, or 40 μg/μl/side, f) in the open field test. Drugs were injected 15 min before the test, and the distance that each mouse moved was recorded for 5 min. Data are presented as the means±SEM (n=10 per group).
To determine whether the memory retrieval impairments caused by GABAA receptor agonists were correlated with the inhibition of ERK phosphorylation, we studied the effects of co-administering sub-effective doses of muscimol (0.25 μg/μl/side) or diazepam (10 μg/μl/side) with a sub-effective dose of U0126 (0.25 nmol/μl/side) on memory retrieval (Figure 5a) and the expression of pERK in the hippocampus (Figure 5b). ANOVA analysis revealed significant treatment effects for the co-administration of muscimol with U0126 and diazepam with U0126 on memory retrieval (F(5, 36)=7.380, p<0.05; Figure 5a). Moreover, significant interactions between muscimol and U0126 (F(1, 28)=4.596, p<0.05) and between diazepam and U0126 (F(1, 28)=7.489, p<0.05) on latency time were observed (Figure 5a). In addition, ANOVA analysis revealed that pERK levels were significantly affected by the co-administration of muscimol and U0126 or diazepam and U0126 in the hippocampus (F(5, 18)=10.036, p<0.03; Figure 5b). Moreover, significant interactions were found between muscimol and U0126 (F(1, 12)=6.334, p<0.05) and between diazepam and U0126 (F(1, 12)=13.124, p<0.05) on pERK levels (Figure 5b). These results indicate that the hippocampal GABAA receptor activation-induced impairment of memory retrieval correlates with ERK phosphorylation.
Figure 5.
γ-Aminobutyric acid type A (GABAA) receptor activation-induced memory retrieval impairment correlates with extracellular signal-regulated kinase (ERK) phosphorylation. Fifteen minutes before the retention trial, muscimol (0.25 μg/μl/side) with U0126 (0.25 nmol/μl/side) or diazepam (10 μg/μl/side) with U0126 (0.25 nmol/μl/side) was administered bilaterally into the hippocampus. (a), Latency times measured during the retention trial were reduced by muscimol and U0126 or diazepam and U0126 administration. Data are presented as the means±SEM (n=8 per group). *p<0.05, compared with the vehicle-treated control group. (b), Immediately after the retention trial, the mice were killed for western blotting to measure pERK level in the hippocampus. Data are presented as the means±SEM (n=4 per group). *p<0.05, compared with the vehicle only-treated retrieval group.
Experiment 3: GABAA Receptor Blockade Enhances Memory Retrieval by ERK Phosphorylation
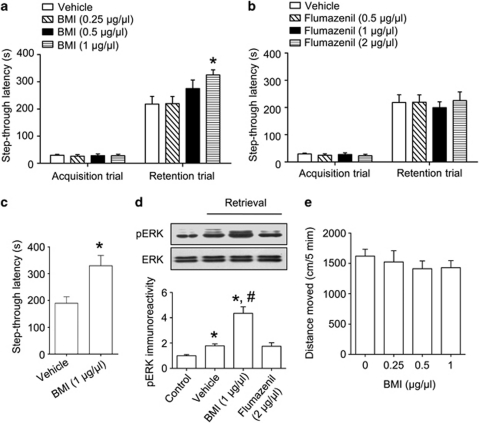
To investigate the effect of GABAA receptor antagonists on memory recall, we used a one-trial passive avoidance task and administered drugs before the retention trial. The injection of BMI increased latency time in a dose-dependent manner (F(3, 28)=3.651, p<0.05, Figure 6a) without affecting foot shock response (Supplementary Fig. S2), and the latency time was significantly increased at 1 nmol/side. However, flumazenil failed to affect memory retrieval (Figure 6b). Drug administration before both acquisition and retention trials also increased latency time, suggesting that BMI did not induce state-dependency (t(18)=3.093, p<0.05, Figure 6c). Memory-enhancing doses of BMI also significantly increased retrieval-induced ERK phosphorylation in the hippocampus (F(2, 9)=31.17, p<0.05, Figure 6d). However, intra-hippocampal injections of these drugs did not affect spontaneous locomotor activity (F(3, 35)=0.4543, p>0.05, Figure 6e).
Figure 6.
γ-Aminobutyric acid type A (GABAA) receptor blockade facilitates memory retrieval. (a, b), Fifteen minutes before the retention trial, bicuculline methiodide (BMI: 0.25, 0.5, or 1 μg/μl/side, a) or flumazenil (0.5, 1, or 2 μg/μl/side, b) was administered bilaterally into the hippocampus. The latency times that were measured during retention trial were increased by BMI administration in a dose-dependent manner. (c), To test state-dependency of BMI (1 μg/μl/side), the mice were treated with BMI bilaterally 15 min before both the acquisition and the retention trial. Data are presented as the means±SEM (n=8–10 per group). *p<0.05, compared with the vehicle-treated group. (d), The effect of BMI (1 μg/μl/side) on memory retrieval-induced ERK activation. The mice were killed immediately after the retention trial. Data are presented as means±SEM (n=4 per group). *p<0.05, compared with the control group; #p<0.05, compared with the vehicle-treated group. Control, animals exposed to the acquisition but not the retention trial. (e), The motoric effect of BMI (0.25, 0.5, or 1 μg/μl/side) in the open field test. Drugs were injected 15 min before the test and the distance each mouse moved was recorded for 5 min. Data are presented as means±SEM (n=10 per group).
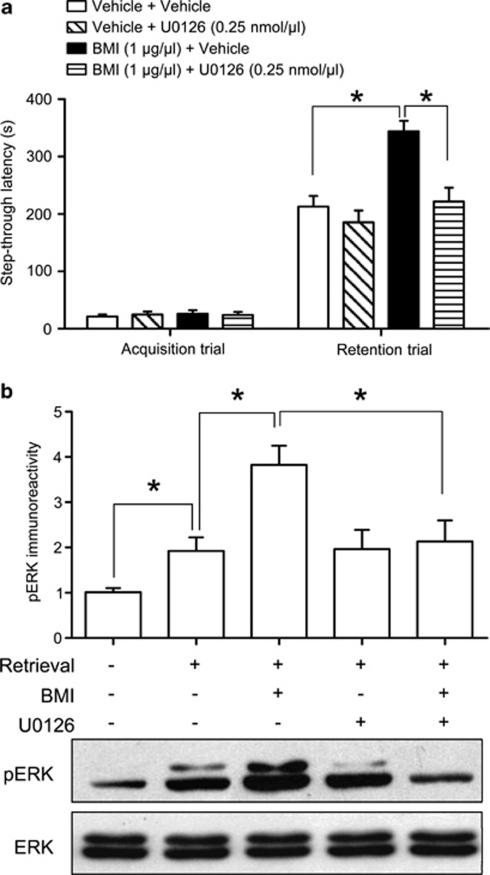
To determine whether memory retrieval enhancement by BMI is correlated with ERK phosphorylation, we studied the effects of the co-administration of BMI (1 μg/μl/side) and the sub-effective dose of U0126 (0.25 nmol/μl/side) on memory retrieval (Figure 7a) and the immunoreactivity of pERK in the hippocampus (Figure 7b). ANOVA analysis revealed significant treatment effects for the co-administration of BMI and U0126 on memory retrieval (F(3, 28)=11.76, p<0.05, Figure 7a). Moreover, a significant interaction between BMI and U0126 on latency was observed (F(1, 28)=5.349, p<0.05, Figure 7a). In addition, ANOVA analysis revealed that pERK levels were significantly affected by the co-administration of BMI and U0126 in the hippocampus (F(4, 15)=6.648, p<0.05, Figure 7b), and significant interactions were found between BMI and U0126 on pERK level (F(1, 12)=5.586, p<0.05, Figure 7b). Together, these results indicate that the enhancement of memory retrieval caused by the blockade of hippocampal GABAA receptor correlates with ERK phosphorylation.
Figure 7.
γ-Aminobutyric acid type A (GABAA) receptor blockade-induced memory retrieval enhancement correlates with ERK phosphorylation. Fifteen minutes before the retention trial, BMI (1 μg/μl/side) with U0126 (0.25 nmol/μl/side) was administered bilaterally into the hippocampus. (a) BMI-induced increases in the latency time during the retention trial were reduced by U0126 administration. Data are presented as the means±SEM (n=8 per group). *p<0.05. (b) Immediately after the retention trial, the mice were killed for Western blotting to measure pERK levels in the hippocampus. Data are presented as the means±SEM (n=4 per group). *p<0.05.
DISCUSSION
Memory retrieval is an essential component of memory processing and is the first aspect of memory function to decline in early Alzheimer's disease (Nakazawa et al, 2002). Although various molecules, including ERK and CREB, are involved in memory retrieval in the several brain regions examined (Abel and Lattal, 2001; Nader, 2003; Suzuki et al, 2004; Viosca et al, 2007), it is widely accepted that the ERK signaling pathway is crucial for memory retrieval (Barros et al, 2000, 2003; Izquierdo et al, 2001; Leon et al, 2010). Our present findings indicate that GABAA receptor activation impairs memory retrieval and reduces retrieval-associated ERK phosphorylation. The findings also indicate that blockade of GABAA receptor facilitates memory retrieval and increases retrieval-associated ERK phosphorylation.
In the present study, the phosphorylation of CREB, ERK, and CaMKII in the hippocampus were all increased during the retention trial of the passive avoidance task. These signaling molecules are all known to have key roles in learning and memory processes. In particular, the transcription factor CREB is required for hippocampus-dependent LTP (Balschun et al, 2003). The activation of CREB by phosphorylation requires the activation of protein kinase A, ERKs, or CaMKs, which suggests that several memory-associated signaling molecules are related to the phosphorylation of CREB (Dubynina and Dolotov, 2009). In addition, the possibility that retrieval is in some way similar to memory consolidation or encoding has been suggested (Smith and Spear, 1984; Szapiro et al, 2000; Hall et al, 2001). In line with these reports, we found that several markers of memory consolidation or acquisition (ie, phosphorylated CaMKII, ERK, and CREB) were increased after the retention trial. Furthermore, it has been reported that memory retrieval requires various molecules, such as MAPK and neurotransmitter receptors, including metabotropic glutamate receptors (Bianchin et al, 1994; Micheau and Riedel, 1999; Izquierdo et al, 2000; Mikami et al, 2007; Leon et al, 2010). Interestingly, although pCaMKII expression was found to be positively associated with retrieval performance, KN62 failed to impair memory retrieval. KN62 decreased the retrieval-induced expression of both pCaMKII and pCREB. Previously, Kida et al (2002) reported that CREB-mediated transcription and de novo protein synthesis are not required for the retrieval of long-term conditioned fear memory. In addition, CaMKII is reported to be required for memory reconsolidation, but not retrieval (Sakurai et al, 2007). Collectively, these results indicate that although pCREB and pCaMKII were increased by memory retrieval performance, they are not critically required for memory retrieval. Previous work by another group also supports this finding (Szapiro et al, 2000).
Hippocampal MAPK/ERK has a central role in several different memory types, including fear memory in contextual fear conditioning and inhibitory passive avoidance (Sweatt, 2004; Davis and Laroche, 2006; Giovannini, 2006; Leon et al, 2010). Moreover, PD098059, a MEK inhibitor, which was bilaterally infused into hippocampal CA1 region or the cerebral cortex before retention testing, blocked the retrieval of long-term memories (Barros et al, 2000; Izquierdo et al, 2000). Szapiro et al (2000) have also suggested that the activity of the MAPK pathway in hippocampal CA1 is important for memory retrieval. In agreement with these previous reports, we observed increased ERK phosphorylation in the hippocampus of mice following the retention trial of a passive avoidance task. Furthermore, mice treated with U0126 exhibited impaired memory retrieval, but mice treated with KN62 did not. We conclude that although memory retrieval induces the upregulation of several memory-related molecules, only a subset of these molecules, such as ERK, is involved in memory retrieval.
GABAergic neurotransmission has been implicated in learning and memory processes, including memory acquisition, consolidation, and retrieval. It is generally accepted that GABAA receptor agonists impair and antagonists facilitate memory function (Brioni and McGaugh, 1988; Castellano et al, 1989; Castellano and McGaugh, 1989; McGaugh et al, 1990; Kim et al, 2009). However, the mechanism underlying the effects of GABAergic drugs on memory retrieval have not been fully elucidated. Recently, Boyle et al (2009) reported that flurazepam, an agonist of the benzodiazepine-binding site of GABAA receptor, impairs the memory acquisition process. It has also been reported that flurazepam inhibits ERK phosphorylation in the cerebral cortex (Kalluri and Ticku, 2002), which suggests that the memory impairments mediated by GABAA receptor agonists may be associated with the inhibition of ERK phosphorylation. The increases in pERK levels induced by acute restraint stress have also been reported to be inhibited by muscimol, an agonist of the GABAA receptor, and augmented by bicuculline, an antagonist of the GABAA receptor (Zheng et al, 2007). As the GABAA receptor modulates ERK phosphorylation during acquisition or memory consolidation, ERK phosphorylation or the ERK signaling pathway should have a crucial role in memory retrieval during manipulation of GABAA receptor. More directly, Igaz et al (2006) reported that pre-training muscimol administration produced deficits in short-term memory retention and decrease in training-induced ERK2 activation. Although these previous findings investigated the role of both the GABAA receptor and intracellular molecular events in memory process or hippocampal ERK activation, they did not specifically demonstrate the role of direct interactions between the GABAA receptor and ERK signaling in long-term memory retrieval. However, from those works, it can be speculated that hippocampal ERK activity is required for GABAA receptor ligand-induced memory retrieval. We investigated this speculation and found that muscimol and diazepam inhibited memory retrieval-induced increases in ERK phosphorylation and impaired memory retrieval. Moreover, co-administration of sub-effective doses (doses that did not affect memory retrieval) of muscimol plus U0126 or diazepam plus U0126 blocked the increases in ERK phosphorylation and impaired memory retrieval. These findings suggest that the ERK pathway is involved in GABAA receptor-mediated memory retrieval impairments. In addition, the failure of muscimol and diazepam to affect basal pERK levels indicates that ERK phosphorylation may be primarily responsible for GABAA receptor signaling during memory retrieval (Supplementary Figure S1).
Blockade of the GABAA receptor with BMI increased basal pERK levels. Hardingham et al (2001) reported that bicuculline induced the phosphorylation of ERK in untreated hippocampal neuronal cultures. Previously, we observed that BMI enhanced memory consolidation and increased ERK and CREB phosphorylation levels (Supplementary Figure S3). These findings suggest that BMI may enhance memory retrieval. In the present study, BMI further facilitated the retrieval-induced increase in ERK phosphorylation and enhanced memory retrieval. Moreover, a sub-effective dose of U0126 blocked BMI-induced memory retrieval enhancement and increased ERK phosphorylation. Thus, we conclude that ERK signaling has a role in GABAA receptor blockade-induced facilitation of memory retrieval. Celik et al (1999) reported that flumazenil had no effect on acquisition performance. However, several previous studies have reported that a pre-training injection of flumazenil enhanced learning and memory (Lal and Forster, 1990; Prather et al, 1992) and antagonized diazepam-induced memory impairment (Venault et al, 1987). In the present study, flumazenil did not modify memory retrieval. These results suggest that flumazenil does not directly modify memory retrieval or ERK phosphorylation. Until now, it has been unclear exactly where the relevant GABAA receptors are located. In previous reports, hippocampal noradrenergic activation has been reported to induce ERK activation at the CA3–CA1 synapses (Gelinas and Nguyen, 2005; Scheiderer et al, 2008). Bonanno and Raiteri (1987) also reported that release-regulating GABAA receptors are present on noradrenergic nerve terminals in the hippocampus. Although we do not have direct evidence, we speculate that the effect of GABAA receptor ligands may be mediated by GABAA receptors on noradrenergic neurons (Makkar et al, 2010). However, the possibility that those ligands act directly on receptors on the membranes of excitatory neurons of the hippocampus to affect ERK signaling in those neurons cannot be ruled out. Further studies will be needed to clarify these issues.
In summary, GABAA receptor agonists appear to decrease memory retrieval by inhibiting the increase of ERK phosphorylation that is normally induced by memory retrieval. Furthermore, GABAA receptor antagonists appear to facilitate memory retrieval by augmenting the increase of ERK phosphorylation that is induced by memory retrieval. Based on these results, we suggest that the GABAA receptor modulates memory retrieval by modulating the ERK phosphorylation that is induced during this process.
Acknowledgments
This work was supported by the Korean Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-313-E00123).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schütz G, et al. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory. J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros DM, Izquierdo LA, Medina JH, Izquierdo I. Pharmacological findings contribute to the understanding of the main physiological mechanisms of memory retrieval. Curr Drug Targets CNS Neurol Disord. 2003;2:81–94. doi: 10.2174/1568007033482931. [DOI] [PubMed] [Google Scholar]
- Barros DM, Izquierdo LA, Mello e Souza T, Ardenghi PG, Pereira P, Medina JH, et al. Molecular signalling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res. 2000;114:183–192. doi: 10.1016/s0166-4328(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Bianchin M, Da Silva RC, Schmitz PK, Medina JH, Izquierdo I. Memory of inhibitory avoidance in the rat is regulated by glutamate metabotropic receptors in the hippocampus. Behav Pharmacol. 1994;5:356–359. doi: 10.1097/00008877-199406000-00014. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Raiteri M. Release-regulating GABAA receptors are present on noradrenergic nerve terminals in selective areas of the rat brain. Synapse. 1987;1:254–257. doi: 10.1002/syn.890010306. [DOI] [PubMed] [Google Scholar]
- Bonini JS, Da Silva WC, Da Silveira CK, Köhler CA, Izquierdo I, Cammarota M. Histamine facilitates consolidation of fear extinction. Int J Neuropsychopharmacol. 2011;7:1–9. doi: 10.1017/S1461145710001501. [DOI] [PubMed] [Google Scholar]
- Boyle J, Wolford D, Gargano C, McCrea J, Cummings C, Cerchio K, et al. Next-day residual effects of gaboxadol and flurazepam administered at bedtime: a randomized double-blind study in healthy elderly subjects. Hum Psychopharmacol. 2009;24:61–71. doi: 10.1002/hup.986. [DOI] [PubMed] [Google Scholar]
- Brailowsky S, García O. Ethanol, GABA and epilepsy. Arch Med Res. 2009;30:3–9. doi: 10.1016/s0188-0128(98)00013-x. [DOI] [PubMed] [Google Scholar]
- Brioni JD, McGaugh JL. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology (Berl) 1988;96:505–510. doi: 10.1007/BF02180032. [DOI] [PubMed] [Google Scholar]
- Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–179. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cabib S, Puglisi-Allegra S. Psychopharmacology of memory modulation: Evidence for multiple interaction among neurotransmitters hormones. Behav Brain Res. 1996;77:1–21. doi: 10.1016/0166-4328(96)00200-8. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Retention enhancement with post-training picrotoxin: lack of state dependency. Behav Neural Biol. 1989;51:165–170. doi: 10.1016/s0163-1047(89)90797-8. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Effects of post-training bicuculline and muscimol on retention: lack of state dependency. Behav Neural Biol. 1990;54:156–164. doi: 10.1016/0163-1047(90)91352-c. [DOI] [PubMed] [Google Scholar]
- Castellano C, Pavone F. Effects of ethanol on passive avoidance behavior in the mouse: involvement of GABAergic mechanisms. Pharmacol Biochem Behav. 1988;29:321–324. doi: 10.1016/0091-3057(88)90163-3. [DOI] [PubMed] [Google Scholar]
- Celik T, Deniz G, Uzbay IT, Palaoǧlu O, Ayhan IH. The effects of flumazenil on two way active avoidance and locomotor activity in diazepam-treated rats. Eur Neuropsychopharmacol. 1999;9:45–50. doi: 10.1016/s0924-977x(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Christmas D, Hood S, Nutt D. Potential novel anxiolytic drugs. Curr Pharm Des. 2008;14:3534–3546. doi: 10.2174/138161208786848775. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Intraseptal administration of bicuculline produces working memory impairments in the rat. Behav Neural Biol. 1991;55:247–254. doi: 10.1016/0163-1047(91)80142-2. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Stackman RW, Walsh TJ. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav Neural Biol. 1989;52:357–369. doi: 10.1016/s0163-1047(89)90472-x. [DOI] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav. 2006;5:61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- Dubynina EV, Dolotov OV. The CREB transcription factor and processes of memory formation. Neurochem J. 2009;26:181–190. [Google Scholar]
- Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG. The role of the extracellular signal-regulated kinase pathway in memory encoding. Rev Neurosci. 2006;17:619–634. doi: 10.1515/revneuro.2006.17.6.619. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Ouagazzal AM, File SE. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median raphé reveals differential GABAergic control in two animal tests of anxiety. Eur J Neurosci. 1998;10:3673–3680. doi: 10.1046/j.1460-9568.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Henschel O, Gipson KE, Bordey A. GABAA receptors, anesthetics and anticonvulsants in brain development. CNS Neurol Disord Drug Targets. 2008;7:211–224. doi: 10.2174/187152708784083812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Stackman RW, Walsh TJ. Intraseptal flumazenil enhances, while diazepam binding inhibitor impairs, performance in a working memory task. Neurobiol Learn Mem. 1996;66:341–352. doi: 10.1006/nlme.1996.0074. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Winograd M, Cammarota M, Izquierdo LA, Alonso M, Izquierdo I, et al. Early activation of extracellular signal-regulated kinase signaling pathway in the hippocampus is required for short-term memory formation of a fear-motivated learning. Cell Mol Neurobiol. 2006;26:989–1002. doi: 10.1007/s10571-006-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Ardenghi PG, Pereira P, Rodrigues C, Choi H, et al. Different hippocampal molecular requirements for short- and long-term retrieval of one-trial avoidance learning. Behav Brain Res. 2000;111:93–98. doi: 10.1016/s0166-4328(00)00137-6. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Viola H, Barros DM, Alonso M, Vianna MR, Furman M, et al. Novelty enhances retrieval: molecular mechanisms involved in rat hippocampus. Eur J Neurosci. 2001;13:1464–1467. doi: 10.1046/j.0953-816x.2001.01530.x. [DOI] [PubMed] [Google Scholar]
- Jung JW, Yoon BH, Oh HR, Ahn JH, Kim SY, Park SY, et al. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol Pharm Bull. 2006;29:261–265. doi: 10.1248/bpb.29.261. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Ticku MK. Role of GABAA receptors in the ethanol-mediated inhibition of extracellular signal-regulated kinase. Eur J Pharmacol. 2002;451:51–54. doi: 10.1016/s0014-2999(02)02100-3. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jeon SJ, Son KH, Jung JW, Lee S, Yoon BH, et al. Effect of the flavonoid, oroxylin A, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharmacol Biochem Behav. 2006;85:658–668. doi: 10.1016/j.pbb.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jeon SJ, Son KH, Jung JW, Lee S, Yoon BH, et al. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol Learn Mem. 2007;87:536–546. doi: 10.1016/j.nlm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim S, Jeon SJ, Son KH, Lee S, Yoon BH, et al. The effects of acute and repeated oroxylin A treatments on Aβ25−35-induced memory impairment in mice. Neuropharmacology. 2008;55:639–647. doi: 10.1016/j.neuropharm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim S, Jeon SJ, Son KH, Lee S, Yoon BH, et al. Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathway. Br J Pharmacol. 2009;158:1131–1142. doi: 10.1111/j.1476-5381.2009.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Wheeler MG, Parent MB. The memory-impairing effects of septal GABA receptor activation involve GABAergic septo-hippocampal projection neurons. Learn Mem. 2007;14:833–841. doi: 10.1101/lm.809407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Forster MJ. Flumazenil improves active avoidance performance in aging NZB/BlNJ and C57BL/6NNia mice. Pharmacol Biochem Behav. 1990;35:747–750. doi: 10.1016/0091-3057(90)90319-d. [DOI] [PubMed] [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- Luft T, Pereira GS, Cammarota M, Izquierdo I. Different time course for the memory facilitating effect of bicuculline in hippocampus, entorhinal cortex, and posterior parietal cortex of rats. Neurobiol Learn Mem. 2004;82:52–56. doi: 10.1016/j.nlm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Taniguchi T, Kadoyama K, Matsumoto A. Long-term potentiation-like facilitation through GABAA receptor blockade in the mouse dentate gyrus in vivo. Neuroreport. 2008;19:1809–1813. doi: 10.1097/WNR.0b013e328319ab94. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci. 1990;104:264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G. Protein kinases: which one is the memory molecule. Cell Mol Life Sci. 1999;55:534–548. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A, Masuoka T, Yasuda M, Yamamoto Y, Kamei C. Participation of cholinergic system in memory deficits induced by blockade of hippocampal mGlu1 receptors. Eur J Pharmacol. 2007;575:82–86. doi: 10.1016/j.ejphar.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Noda Y, Kameyama T. GABAergic modulation of memory with regard to passive avoidance and conditioned suppression task in mice. Psychopharmacology (Berl) 1988;94:69–73. doi: 10.1007/BF00735883. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.2001The Mouse Brain in Stereotaxic Coordinates2nd Edition. Academic Press : San Diego [Google Scholar]
- Prather PL, Forster MJ, Lal H. Learning and memory-enhancing effects of Ro 15-4513: a comparison with flumazenil. Neuropharmacology. 1992;31:299–306. doi: 10.1016/0028-3908(92)90180-w. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, et al. Coactivation of M1 muscarinic and alpha1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J Neurosci. 2008;28:5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Parent MB. Septal infusions of glucose or pyruvate, but not fructose, produce avoidance deficits when co-infused with the GABA agonist muscimol. Neurobiol Learn Mem. 2003;79:243–251. doi: 10.1016/s1074-7427(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Paul SM. The mechanism(s) of action of the benzodiazepines. Med Res Rev. 1981;1:3–22. doi: 10.1002/med.2610010103. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Spear NE. Analysis of treatments to alleviate forgetting in rats. Am J Psychol. 1984;97:475–491. [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Izquierdo LA, Alonso M, Barros D, Paratcha G, Ardenghi P, et al. Participation of hippocampal metabotropic glutamate receptors, protein kinase A and mitogen-activated protein kinases in memory retrieval. Neuroscience. 2000;99:1–5. doi: 10.1016/s0306-4522(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, et al. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venault P, Chapouthier G, Simiand J, Dodd RH, Rossier J. Enhancement of performance by methyl β-carboline-3-carboxylate, in learning and memory tasks. Brain Res Bull. 1987;19:365–370. doi: 10.1016/0361-9230(87)90105-5. [DOI] [PubMed] [Google Scholar]
- Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, et al. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn Mem. 2007;16:198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Chou CJ, Liao JF, Chen CF. Dehydroevodiamine attenuates β-amyloid peptide-induced amnesia in mice. Eur J Pharmacol. 2001;413:221–225. doi: 10.1016/s0014-2999(00)00913-4. [DOI] [PubMed] [Google Scholar]
- Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on β-amyloid peptide-(25–35)-induced amnesia in mice. Eur J Pharmacol. 2004;506:55–61. doi: 10.1016/j.ejphar.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol. 2006;553:185–190. doi: 10.1016/j.ejphar.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Zheng G, Zhang X, Chen Y, Zhang Y, Luo W, Chen J. Evidence for a role of GABAA receptor in the acute restraint stress-induced enhancement of spatial memory. Brain Res. 2007;1181:61–73. doi: 10.1016/j.brainres.2007.08.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.