Abstract
In neonatal mouse skin, two types of dermal papilla (DP) are distinguished by Sox2 expression: CD133+Sox2+ DP are associated with guard/awl/auchene hairs, whereas CD133+Sox2− DP are associated with zigzag (ZZ) hairs. We describe a three-dimensional hydrogel culture system that supports clonal growth of CD133+Sox2+, CD133+Sox2−, and CD133−Sox2− (non-DP) neonatal dermal cells. All three cell populations formed spheres that expressed the DP markers alkaline phosphatase, α8 integrin, and CD133. Nevertheless, spheres formed by CD133− cells did not efficiently support hair follicle formation in skin reconstitution assays. In the presence of freshly isolated P2 dermal cells, CD133+Sox2+ and CD133+Sox2− spheres contributed to the DP of both AA and ZZ hairs. Hair type did not correlate with sphere size. Sox2 expression was maintained in culture, but not induced significantly in Sox2− cells in vitro or in vivo, suggesting that Sox2+ cells are a distinct cellular lineage. Although Sox2+ cells were least efficient at forming spheres, they had the greatest ability to contribute to DP and non-DP dermis in reconstituted skin. As the culture system supports clonal growth of DP cells and maintenance of distinct DP cell types, it will be useful for further analysis of intrinsic and extrinsic signals controlling DP function.
Introduction
The dermal papilla (DP) comprises a group of mesenchymal cells at the base of the hair follicle, which has a crucial role in hair follicle development and regulates the postnatal hair growth cycle (Yang and Cotsarelis, 2010; Driskell et al., 2011). DP cells have a distinct gene expression signature compared with non-DP dermal fibroblasts (Rendl et al., 2005, 2008). In addition, the DP cells of guard/awl/auchene (GAA) hair follicles in neonatal mouse skin express a distinct set of genes compared with those of zigzag (ZZ) hairs. GAA DP cells can be isolated on the basis of CD133 and Sox2 expression, whereas ZZ DP cells express CD133 and lack Sox2 (Driskell et al., 2009). DP cells that express Sox2 are the origin of skin-derived precursors, which exhibit multi-lineage differentiation potential in culture (Biernaskie et al., 2007, 2009; Jinno et al., 2010).
In view of the ability of DP cells to induce hair follicles in non-hair-bearing skin (Jahoda et al., 1984), there is considerable interest in therapeutic applications of DP cells to treat alopecia. A number of techniques for culturing DP cells have been reported, but hair-inductive ability tends to be lost during passaging. Various approaches to preventing loss of inductive potential have been described, including cultivation in the presence of bone morphogenic proteins (BMPs) and Wnts (Jahoda and Oliver, 1981; Kishimoto et al., 1999, 2000; Rendl et al., 2005, 2008). One notable characteristic of cultured DP cells is their tendency to aggregate (Jahoda and Oliver, 1981, 1984; Jahoda et al., 1984), and this has led to strategies for culturing DP cells as three-dimensional (3D) spheres, rather than adherent on tissue culture plastic (Osada et al., 2007; Higgins et al., 2010). Although there is some preservation of DP marker expression, a disadvantage of 3D cultures is the limited scope for expanding cell numbers (Young et al., 2008).
In the present study, we sought to develop a culture technique that would allow clonal expansion of DP cells as 3D spheres and subsequent engraftment in vivo. By using this hydrogel culture system, we have investigated the extent to which non-DP cells (CD133 negative; Ito et al., 2007) can be induced to express DP markers and whether Sox2 expression is an intrinsic property of a subpopulation of DP cells or is environmentally regulated.
Results
Expression of DP markers in hydrogel culture
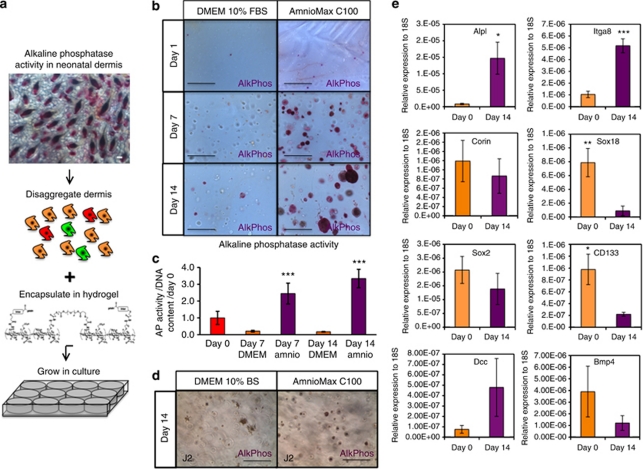
Disaggregated suspensions of dermal fibroblasts from postnatal day 2 (P2) mice were encapsulated in Extracel hydrogels, which consist of cross-linked gelatin and hyaluronic acid (Figure 1a). The cells were cultured for up to 14 days in DMEM containing 10% fetal bovine serum (FBS) or in AmnioMaxC100, which has previously been reported to maintain DP gene expression (Rendl et al., 2005, 2008). Alkaline phosphatase (AP) activity is a marker of DP cells in vivo (Handjiski et al., 1994; Figure 1a). After 1 day, single AP-positive (AP+) cells were readily detected in hydrogels cultured in DMEM+FBS or AmnioMax (Figure 1b).
Figure 1.
Dermal papilla (DP) markers are expressed in hydrogel culture. (a) Alkaline phosphatase (AP/AlkPhos) activity in DP of P2 dermis revealed by whole-mount labeling (top). Schematic showing isolation of heterogeneous dermal cells and hydrogel encapsulation (bottom). (b) AP activity of P2 dermal cells in hydrogel cultures. (c) Quantification of AP activity relative to cell number (DNA content). Day 0=1.0. Data are means±SEM of three biological replicates. (d) AP activity in hydrogel cultures of J2-3T3 cells. (e) Quantitative real-time PCR of AP (Alpl), Integrin α8 (Itga8), Corin, Sox18, Sox2, CD133 (Prom1), deleted in colorectal carcinoma (Dcc), and Bmp4 mRNA levels in freshly isolated P2 dermal cells (day 0) and following culture in hydrogels in AmnioMax medium. mRNA levels are expressed relative to 18S (n=3). Means±SEM are indicated. *P<0.05; **P<0.01; ***P<0.001, Student's t-test: comparison between DMEM and AmnioMax in c and between day 0 and day 14 in e. Bars=100 μm.
After 7 days in AmnioMax, AP+ spheres were observed, and sphere size and number increased through day 14 (Figure 1b). In AmnioMax, ∼2% of input cells had survived as single cells or formed spheres at day 14; cells and spheres were distributed evenly throughout the hydrogels, although a small number of cells settled at the bottom of the culture wells and grew as adherent cells. Sphere formation was lower in DMEM+FBS cultures, and only a few AP+ spheres were observed at day 14 (Figure 1b). Measurement of AP activity in cell lysates confirmed that whereas AP activity per cell increased with time in AmnioMax culture medium, activity per cell decreased in DMEM+FBS (Figure 1c). As a negative control, immortalized mouse embryonic J2-3T3 cells were cultured in hydrogels. J2-3T3 cells had reduced sphere-forming ability compared with P2 dermal cells and did not form AP+ spheres (Figure 1d). These observations suggest that culture in hydrogels using AmnioMax medium promotes the DP phenotype in freshly isolated neonatal dermal cells.
To investigate whether DP genes in addition to AP were expressed in AmnioMax hydrogel cultures, we performed quantitative reverse transcription-PCR on mRNA isolated from P2 dermal cells before plating and from cells that had been cultured for 14 days (Rendl et al., 2005, 2008; Enshell-Seijffers et al., 2008; Driskell et al., 2009). AP (alpl), Deleted in colorectal carcinoma (Dcc), and Itga8 mRNA expression increased in culture (Figure 1e). However, levels of five other DP markers, Corin, Sox18, Sox2, CD133, and Bmp4, decreased (Figure 1e; Driskell et al., 2009). Therefore, there is differential regulation of specific DP signature genes in culture.
Clonal origin of dermal spheres formed in hydrogels
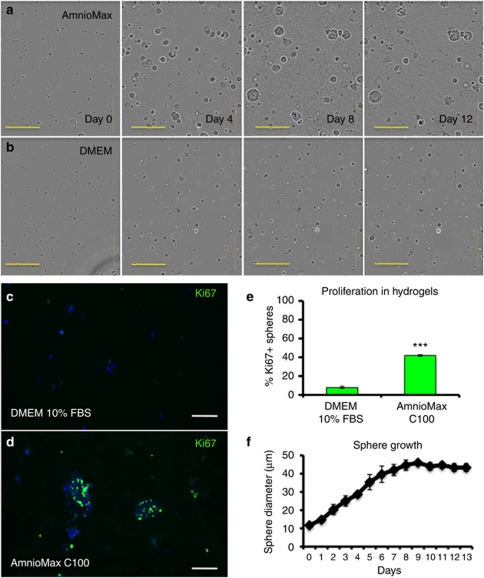
To investigate the mechanism of dermal sphere formation, we performed time-lapse imaging of hydrogels over a 13-day period. Encapsulated cells did not migrate within the gels, and therefore spheres did not form through aggregation (Figure 2a and b, Supplementary Movies online). Instead, cells cultured in AmnioMax formed spheres through a process of cell division, whereas cells in DMEM+FBS divided rarely. Proliferation within spheres cultured in AmnioMax C100 was confirmed by the presence of Ki67-positive cells (Figure 2c and d). The few spheres that formed in DMEM+FBS had 6-fold fewer Ki67-positive cells (Figure 2e). The mean diameter of spheres formed by cells in AmnioMax C100 increased during the first week of culture and reached a plateau thereafter (Figure 2f). We conclude that sphere formation occurs primarily via cell proliferation and not by cellular aggregation.
Figure 2.
Dermal spheres are clonal in origin. (a, b) Representative frames from time-lapse imaging of encapsulated P2 dermal cells cultured for up to 12 days in AmnioMax (a) or DMEM+fetal bovine serum (FBS) (b). (c, d) Frozen sections of 14-day dermal spheres grown in DMEM+10% bovine serum (c) or Amniomax C100 (d), and labeled with anti-Ki67 (green) with 4′-6-diamidino-2-phenylindole nuclear counterstain (blue). Bars=200 μm (a, b), 150 μm (c, d). (e) Quantification of percentage of Ki67-positive spheres at day 14. Means±SEM are indicated. ***P<0.001, Student's t-test. (f) Mean sphere diameter±SEM of unsorted cells grown in Amniomax C100.
DP heterogeneity is maintained in culture
We have previously reported that three distinct fibroblast populations can be isolated from P2 dermis. DP cells from GAA follicles are CD133+Sox2+, whereas DP cells from ZZ follicles are CD133+Sox2− and non-DP cells are CD133−Sox2− (Driskell et al., 2009). In skin reconstitution assays, Sox2+ cells are required for the formation of GAA follicles and CD133− cells do not induce hair follicle formation (Ito et al., 2007; Driskell et al., 2009).
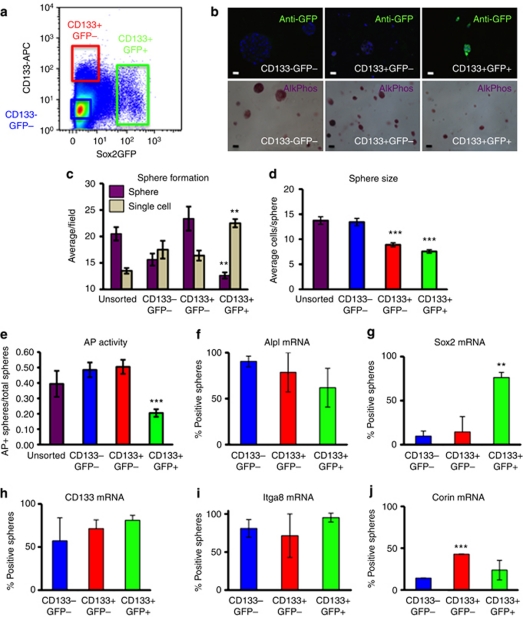
To compare the properties of the three dermal populations in culture, we isolated P2 dermal cells from mice in which green fluorescent protein (GFP) is expressed under the control of the Sox2 promoter (Sox2eGFP mice; Driskell et al., 2009). Cells were labeled with anti-CD133 and sorted on the basis of GFP and CD133 expression (Figure 3a). After cultivation in AmnioMax hydrogels for 2 weeks, AP–positive spheres were formed by all three cell populations, whereas GFP protein was only detected in cultures of CD133+GFP+ cells (Figure 3b).
Figure 3.
Dermal cell subpopulations exhibit different properties in hydrogel culture. (a) Flow sorting of Sox2eGFP P2 dermal cells labeled with anti-CD133. Gates used to select cells for hydrogel culture are shown. CD133−GFP− gate contains 30% of the total population; CD133+GFP− gate contains 4.25% and CD133+GFP+ gate contains 1.87%. (b) Green fluorescent protein (GFP) expression (top) and alkaline phosphatase (AP/AlkPhos) activity (bottom) of sorted populations after 14 days in hydrogels in AmnioMax. Bars=30 μm. (c) Sphere-forming efficiency was determined by comparing the number of spheres (purple) and single cells (beige) per field. Data are means±SEM from three biological replicates. The number of spheres formed by CD133+Sox2− cells is significantly higher than the number formed by CD133+Sox2+ cells, whereas the number of single cells is significantly lower (**P<0.01, Student's t-test). The number of spheres formed by CD133+Sox2− cells is also higher than that formed by CD133− cells (P<0.05). CD133− cells form significantly more spheres and have fewer single cells than CD133+Sox2+ cells (P<0.05). (d) The average number of cells per sphere in 14-day cultures, determined by scoring 4′-6-diamidino-2-phenylindole-labeled nuclei. Data are means±SEM from three biological replicates. The mean size of CD133+GFP− and CD133+GFP+ spheres is significantly less than the size of unsorted and CD133−GFP− spheres (***P<0.001). (e) Proportion of AP–positive spheres detected with a colorimetric assay of enzyme activity. Both GFP− populations are significantly different from GFP+ population (***P<0.001). Data are means±SEM. (f–j) mRNA was isolated from single spheres picked from hydrogels, and expression levels of DP genes was determined by quantitative real-time PCR (f: Alpl; g: Sox2; h: CD133; i: Itga8; j: Corin). A sphere was scored as positive if there was at least a 3-ΔCt value difference from negative control mRNA. Seven spheres in three biological replicates of each cell population were analyzed. Data are means±SEM. (g) Significant difference between CD133+Sox2− and CD133+Sox2+ (**P<0.01). (j) Significant difference between CD133− and CD133+Sox2− (***P<0.001).
By analyzing the ratio of spheres to single cells, we determined that Sox2+ cells formed spheres at a lower frequency than the other populations (Figure 3c); they also formed the smallest spheres (Figure 3d). CD133− cells formed larger spheres than CD133+ cells (Figure 3d), but their sphere-forming efficiency was lower (Figure 3c). A high proportion of Sox2− cells, whether or not they expressed CD133, founded spheres that exhibited AP activity (Figure 3e). The proportion of spheres with AP activity was lower in cultures of Sox2+ cells (Figure 3e).
We next analyzed gene expression in individual spheres by amplifying complementary DNA using a protocol developed for single-cell PCR (Figure 3f–j; Jensen and Watt, 2006). Seven individual spheres of each type were analyzed in each of three biological replicates. Expression of Alpl mRNA matched the trends in AP activity (Figure 3e and f). In agreement with GFP expression (Figure 3b), most spheres formed by Sox2+ cells maintained expression of Sox2, whereas Sox2 was barely expressed above background in spheres founded by Sox2− cells (Figure 3b and g). The low levels of Sox2 mRNA are consistent with the purity of sorted GFP− cells reported previously (Driskell et al., 2009). CD133 was expressed by the majority of spheres founded by CD133+ cells, and was also expressed in ∼50% of spheres that arose from CD133− cells (Figure 3h), although the average levels of CD133 in unfractionated cultures declined over time (Figure 1e). The majority of spheres expressed Itga8, regardless of the cell of origin (Figure 3i). The proportion of Corin-positive spheres was <50% in any group, but there were significantly more positive spheres in CD133+ spheres than CD133− spheres (Figure 3j).
We conclude that the three neonatal dermal populations we isolated behave differently in hydrogel culture. Although Sox2 expression is maintained, it is not induced to a significant extent in Sox2− cells. In contrast, CD133− cells form spheres that express CD133 and other DP markers.
Induction of new DP in adult skin
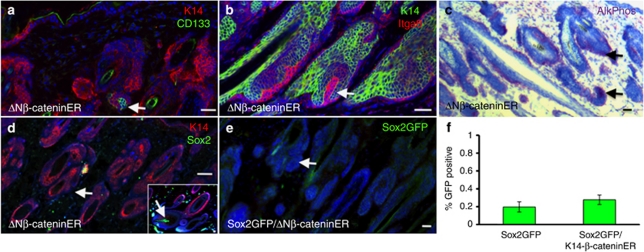
Although Sox2 was not significantly induced in Sox2− cells in culture, we tested whether Sox2 could be induced in vivo, in the context of de novo DP formation in adult back skin. It has previously been shown that expression of N-terminally truncated, stabilized β-catenin in the basal layer of adult epidermis, via a 4-hydroxy-tamoxifen (4OHT)-inducible transgene, results in the formation of ectopic hair follicles with associated DP (Lo Celso et al., 2004; Silva-Vargas et al., 2005). To investigate whether the new DP expressed Sox2, we crossed K14ΔNβ-cateninER transgenic mice (Lo Celso et al., 2004; Silva-Vargas et al., 2005) with Sox2eGFP mice and treated them with 4OHT to induce ectopic hair follicles. CD133, Itga8, and AP were expressed in the DP of all newly formed follicles (Figure 4a–c and data not shown). However, Sox2 was not expressed in the new DP, whether evaluated by labeling histological sections for GFP or Sox2 (Figure 4d and e and data not shown) or by quantifying the proportion of GFP+ cells in disaggregated dermis (Figure 4f). The failure to induce de novo Sox2 expression in culture or in adult skin is consistent with Sox2+ cells representing a distinct cell lineage that is specified during development.
Figure 4.
Sox2-positive dermal papillae are not induced in adult skin. Adult K14ΔNβ-cateninER × Sox2eGFP back skin was treated with 4-hydroxy-tamoxifen (4OHT) for 14 days to induce ectopic hair follicles. (a–e) Cryosections immunolabeled for K14 (a, d: red; b: green), CD133 (a: green), Itga8 (b: red), Sox2 (d: green), and green fluorescent protein (GFP; e: green) with 4′-6-diamidino-2-phenylindole nuclear counterstain (blue). (c) Section labeled for alkaline phosphatase (AlkPhos) activity (red) with hematoxylin counterstain (blue). Arrows (a–c, d main panel, e) indicate DP of ectopic follicles. Insert in d shows Sox2 expression in DP of preexisting guard/awl/auchene hair (arrow). Bars=100 μm. (f) Flow cytometric determination of % GFP-positive dermal cells in 4OHT-treated Sox2eGFP mice and Sox2eGFP × K14ΔNβ-cateninER bitransgenic mice. Data are means±SEM from three biological replicates. There is no significant difference between the two populations (Student's t-test).
Contribution of cultured dermal cells to DP in reconstituted skin
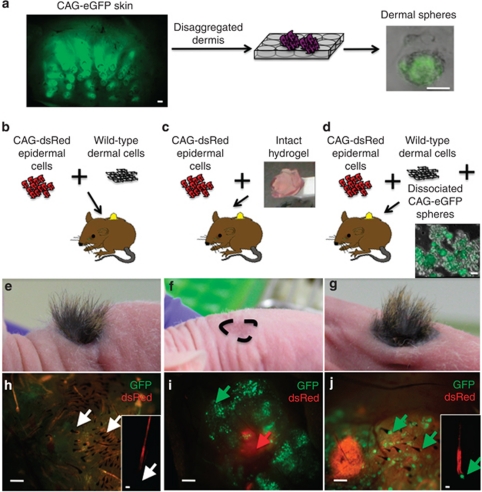
To investigate whether the dermal cell spheres formed in hydrogels had the ability to support de novo hair follicle formation, we performed skin reconstitution assays in nude mice (Figure 5). To distinguish the origins of dermal and epidermal cells, cultured dermal cells were obtained from mice expressing GFP under the control of the ubiquitously expressed CMV-β-actin promoter (CAG-eGFP mice; Figure 5a), and epidermal cells were isolated from mice expressing dsRed via the same promoter (CAG-dsRed mice). As shown in Figure 5a, GFP expression was readily detected in dermal cells cultured in AmnioMax hydrogels for 2 weeks. As a positive control, freshly isolated dsRed-positive epidermal cells and unlabeled dermal cells that had not been cultured were placed into chambers on the backs of mice (Figure 5b, e, and h). As reported previously (Driskell et al., 2009; Jensen et al., 2010), hair-bearing skin was evident 4–5 weeks after grafting in all three mice in the group, with dsRed expression confined to the hair follicles and interfollicular epidermis (Figure 5e and h).
Figure 5.
Cultured dermal spheres can contribute to dermal papillae (DP) in reconstituted skin. (a–d) Schematic representation of the hair reconstitution assays. P2 CAG-eGFP skin, visualized by whole-mount labeling in a, was disaggregated, and the dermal cells were cultured in hydrogels for 2 weeks in AmnioMax to generate green fluorescent protein (GFP)-positive spheres, illustrated in a. Hydrogel cultures were either grafted intact (c,f,i) or following enzymatic digestion (d,g,j) (illustrated) into chambers in combination with P2 epidermal cells from CAG-dsRed mice. Disaggregated hydrogels were grafted with ‘helper' dermal cells. As a positive control, some mice were grafted with freshly isolated P2 dermal cells from wild-type mice in combination with CAG-dsRed epidermal cells (b,e,h). (e–g) Macroscopic appearance of the grafts at 5 weeks. Position of the graft site in f is indicated. (h–j) Dermal side of grafts viewed under a fluorescence-dissecting microscope, with higher magnification insets showing individual hairs (h, j). Arrows indicate GFP-negative (h) and -positive (j) DP cells. In i, encapsulated spheres (green arrow) are visible underlying dsRed-positive epidermis (red arrow). Bars=60 μm (a, whole mount), 50 μm (a, sphere; h, j insets), 30 μm (d), 250 μm (h–j).
Mice were next grafted with disaggregated epidermis and either intact hydrogel cultures or cultures in which the hydrogels had been enzymatically disrupted to release the spheres. No hair follicles formed in any of the four mice grafted with intact hydrogels (Figure 5c, f, and i), although GFP-positive cell clusters and dsRed-positive epidermis were evident 5 weeks after grafting (Figure 5i). However, when spheres were combined with freshly isolated unlabeled dermal cells, hairs did form, and GFP-positive DP were readily detected in all four mice per group (Figure 5d, g, and j). In these grafts, the number of cells derived from hydrogel cultures was 10% of the number of unlabeled dermal cells (Figure 5b). We conclude that dermal cells cultured in AmnioMax hydrogels retain DP activity, and that the failure of hair follicle formation in the absence of unlabeled ‘helper' fibroblasts is either because the hydrogels had not been digested or because of the relatively low number of dermal cells in the gels (Ehama et al., 2007).
Preservation of DP heterogeneity in reconstituted skin
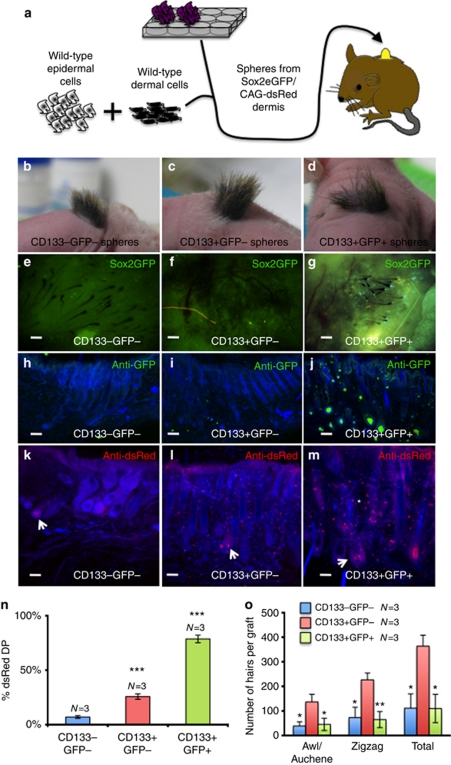
We next compared the hair-forming capacity of cultured CD133−Sox2−, CD133+Sox2−, and CD133+Sox2+ dermal cells by grafting disaggregated spheres in combination with an excess of uncultured, unfractionated P2 dermal cells (Figure 6a). For these experiments, dermal cells were isolated from Sox2eGFP/CAG-dsRed mice, labeled with anti-CD133, sorted, and cultured in AmnioMax hydrogels for 2 weeks. The helper P2 dermal cells and the epidermal cells were from wild-type mice and did not express GFP or dsRed (Figure 6a). Three grafts were performed with each type of sphere (CD133−Sox2−, CD133+Sox2−, and CD133+Sox2+).
Figure 6.
Sox2-positive dermal spheres contribute to dermal papilla (DP) and non-DP dermis. (a) Schematic representation of hair reconstitution assay using wild-type dermal ‘helper' cells. (b–d) Macroscopic appearance of grafts after 4 weeks. (e–g) Dermal side of grafts viewed under a fluorescence-dissecting microscope. Note that green fluorescent protein (GFP)-positive DP cells are only detected in g. (h–m) Thick cryosections of graft sites immunolabeled for GFP (h–j) or dsRed (k–m) with 4′-6-diamidino-2-phenylindole counterstain (blue). (k–m) dsRed-positive DP cells are indicated with arrows and dsRed-positive non-DP dermal cells with an asterisk. Bars=150 μm. (n) Percentage of DP derived from hydrogel cultures. Values are significantly higher for CD133+ than CD133− cultures (***P<0.001). (o) Number of hairs of each type formed per graft. Means±SEM are indicated. Values that differed significantly from CD133+GFP− are shown (*P<0.05; **P<0.01). (n, o) N=number of grafts analyzed.
Hair growth was evident within 5 weeks in all grafts (Figure 6b–d), at which time grafts were harvested and the contribution of the cultured dermal cells was assessed, both in intact grafts (Figure 6e–g) and following sectioning (Figure 6h–m). Cultures of CD133− cells contributed to fewer than 7% of DP, whereas Sox2−CD133+ cells contributed to ∼25% and Sox2+CD133+ cells contributed to almost 80% (Figure 6n). The total number of hair follicles per graft was significantly greater in grafts containing CD133+GFP− cells than those containing the other cell populations (Figure 6o).
GFP was detected only in grafts containing cultured Sox2+ cells (Figure 6e–j), indicating that Sox2 expression was not induced in Sox2− cells on grafting. CD133−Sox2− and CD133+Sox2− cultured cells were largely confined to the DP (Figure 6k and l). In contrast, CD133+Sox2+ cells were found both within the DP and throughout the dermis (Figure 6m; Biernaskie et al., 2009). The ratio of awl/auchene to ZZ hairs was the same in all grafts (Figure 6o), and cultured Sox2+CD133+ and Sox2−CD133+ cells contributed to both hair types. This indicates that the presence of a Sox2+ sphere within a DP does not inhibit ZZ hair formation. However, because of the presence of Sox2+ cells within the P2 dermal ‘helper' population, we cannot conclude that awl/auchene hairs can be induced by Sox2− spheres.
We conclude that our culture system has the ability to preserve the DP phenotype of CD133+ cells and intrinsic differences between Sox2+ and Sox2− DP cells. However, it does not confer significant hair follicle–inducing ability on CD133− cells, even though they express DP markers in culture.
Discussion
We have developed a 3D hydrogel culture system that supports clonal expansion of DP cells as spheroids and have shown that the spheroids can contribute to hair follicle–inducing activity in vivo. Our approach contrasts with previously reported 3D cultures, in which DP spheres are formed through aggregation (Osada et al., 2007; Higgins et al. 2010).
In vivo, cells within DP rarely proliferate (Tobin et al., 2003), and this is also the case in DP spheres formed through aggregation (Higgins et al., 2010). In contrast, cells within our DP spheres were capable of proliferating and yet retained hair follicle–inducing ability. In pilot experiments, we have found that cells within spheres can be disaggregated and will form spheres when replated in hydrogels (data not shown). However, further experiments are necessary to determine whether the secondary spheres are as efficient at contributing to DP in vivo as the primary spheres.
There was an inverse correlation between sphere size in vitro and trichogenicity in vivo at a population level, as CD133− spheres formed the largest spheres and largely failed to induce hair follicles in vivo, whereas Sox2+ cells were least proliferative in vitro and made the greatest contribution to the dermis in vivo. The inverse relationship between proliferation and trichogenicity may also contribute to the loss of DP activity when cells are grown in 2D cultures (Kishimoto et al., 2000; Rendl et al., 2008). Sphere size in vitro did not determine hair follicle type in vivo, at least in the presence of unfractionated P2 dermal cells, as the proportion of awl/auchene and ZZ hair follicles was similar in Sox2+CD133+ and Sox2−CD133+ grafts. We can conclude, however, that the presence of Sox2+ spheres is not inhibitory to ZZ hair formation.
In vivo, AP is expressed in the DP throughout the hair cycle (Handjiski et al., 1994), its level peaking during early anagen (Iida et al., 2007). In vitro, AP activity has been used as a marker for DP cells and AP levels decrease in culture as hair-inducing activity declines (McElwee et al., 2003; Rendl et al., 2008). Nevertheless, our data show that in vitro expression of AP is not a reliable indicator of in vivo DP activity. Two other DP markers, CD133 (Prominin-1; Ito et al., 2007) and α8 integrin (Driskell et al., 2009), are both expressed in all anagen follicles. Although spheres formed by CD133− cells were not only positive for AP but also for CD133 and α8 integrin, they did not induce follicles in skin reconstitution assays. It is therefore important to be cautious when interpreting the effects of different culture conditions on DP cells in the absence of in vivo data (Higgins et al., 2010).
Sox2+ cells continued to express Sox2 in culture. However, Sox2 was not induced to a significant extent in Sox2− cells, either in culture or following engraftment. In addition, Sox2 was not induced in new hair follicles that formed as a result of β-catenin activation in adult epidermis. These observations are consistent with Sox2+ and Sox2− cells representing distinct cell lineages that are specified during skin development (Driskell et al., 2009). Our findings are also consistent with the finding that Sox2 is a marker of multipotent dermal stem cells that reside in the DP and contribute extensively to the dermis following wounding (Biernaskie et al., 2009). Sox2+ cells represent only 1% of neonatal dermal cells (Driskell et al., 2009), and it has not, therefore, been possible to obtain sufficient cells to test their contribution to reconstituted skin in the absence of unfractionated, helper cells (Biernaskie et al., 2009; Driskell et al., 2009). One strategy that could potentially overcome this hurdle is to expand Sox2+ cells on tissue culture plastic before hydrogel encapsulation.
In conclusion, the system we have developed will be valuable for further studies of DP cells. It lends itself to high-throughput screens of agents that regulate DP proliferation and maintenance, and by seeding epidermal cells on top of the hydrogels it will be possible to examine reciprocal signaling between the DP and epidermis. Finally, by scaling up the system it may be possible to induce hair follicles from DP spheres without the need for helper dermal cells.
Materials and Methods
Transgenic mice
All procedures involving mice were performed under the terms of a UK Government Home Office project license and subject to institutional ethics approval. Sox2eGFP (D'Amour and Gage, 2003), CAG-eGFP, and CAG-dsRed mice were obtained as previously described (Driskell et al., 2009; Jensen et al., 2010). The K14ΔNβ-cateninER transgenic mice express a 4OHT-inducible, N-terminally truncated β-catenin construct under the control of the Keratin 14 promoter (Lo Celso et al., 2004; Silva-Vargas et al., 2005). Heterozygous K14ΔNβ-cateninER transgenic mice (line D2; Lo Celso et al., 2004; Silva-Vargas et al., 2005) were crossed with heterozygous Sox2eGFP mice, and mice expressing both transgenes were treated topically with 1.5 mg 4OHT (Sigma-Aldrich, St Louis, MO) dissolved in acetone every day for 2 weeks to induce ectopic hair follicles. Wild-type and transgenic mice were on the same F1 background (CBA × C57Bl6).
Isolation of neonatal mouse dermal and epidermal cells for grafting and flow cytometry
Analysis, isolation, and sorting of dermal cells were performed as previously described. Cells were labeled with a CD133 antibody conjugated to allophycocyanin (APC; eBiosciences, San Diego, CA) and sorted on a MoFlo high-speed sorter (Dako Cytomation, Glostrup, Denmark; Driskell et al., 2009).
Hydrogel culture and live-cell imaging
Cells were encapsulated at a density of 106 cells per ml in Extracel (Glycosan Biosystems, Salt Lake City, UT) according to the manufacturer's instructions. Cells in hydrogels were cultured in 96-well (BD Biosciences, Sparks, MD; 0.1 ml per well) or 24-well plates (Essen ImageLock, Basel, Switzerland; 0.5 ml per well). To retrieve cells, hydrogels were incubated for 2 hours at 37 °C in a 1 × solution of digestive enzymes, prepared by diluting 10 × collagenase/hyaluronidase (StemCell Technologies, Vancouver, BC, Canada) in basal AmnioMax solution to a final concentration of 300 U ml−1 collagenase and 100 U ml−1 hyaluronidase. Spheres and single cells were recovered by centrifugation at 500 × g for 5 minutes.
Hydrogel cultures were maintained in DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (PAA Laboratories, Pasching, Austria) and 1% Penicillin–Streptomycin (Invitrogen, Grand Island, NY), or AmnioMax C-100 with supplement (Gibco). Cultures were incubated at 37 °C in a 5% CO2 atmosphere and the medium was changed every 2 days.
For live-cell imaging, 24-well plates were placed in an IncuCyte (Essen Instruments, Basel, Switzerland) and imaged every 15 minutes. Representative fields were selected.
AP activity
Gels were placed in 1.5-ml Eppendorf tubes containing 800 μl of 1% Triton-X solution in PBS, frozen at −80 °C, freeze-thawed, mechanically disrupted, and centrifuged at 10,000 × g for 10 minutes. The supernatant was assayed for AP activity as described previously (Akcakaya et al., 2007) using the SpectraMax M2e (Molecular Devices, Sunnyvale, CA) spectrophotometer function at an absorbance of 405 nm. To normalize AP activity to cell number, the DNA content of the hydrogels was determined. DNA was precipitated and resuspended in 700 μl EDTA (pH 12.3; Ambion) and 50 μl 1 KH2PO4 (Sigma-Aldrich; Teixeira et al., 1995). A volume of 100 μl was placed in a black, clear-bottomed 96-well plate and 100 μl of 5 μg ml−1 Hoechst-33342 dye (Invitrogen) was added. Fluorescence was determined immediately using the SpectraMax M2e fluorometer function (excitation: 355 nm; emission: 465 nm).
Quantitative real-time PCR
Total RNA was isolated from cell populations using the Purelink RNA kit (Invitrogen) and reverse-transcribed to complementary DNA using the Superscript III kit (Invitrogen). PCR reactions were carried out with Taqman Gene Expression Assays for Sox2, CD133, Alpl, Dcc, Bmp4, Sox18 Corin, and Itga8 (Applied Biosystems, Foster City, CA), and all data were normalized to 18S expression. In some experiments, RNA was isolated from individual dermal spheres that had been picked following enzymatic digestion of hydrogels and placed directly into lysis buffer. Quantitative real-time PCR was performed essentially as described previously (Jensen and Watt, 2006).
Custom primers (Sigma-Aldrich) were as follows: Sox2: forward; 5′-ACTGGCAAGACCGTTTTCGTGGT-3′, reverse; 5′-ACCAACGATATCAACCTGCATGGACA-3′ CD133: forward; 5′-TCCCACTTGATGCCACTGCCAA-3′, reverse; 5′-ATTCCGCTCCCAGCTGAGCG-3′ IntA8: forward; 5′-GGGGCGACAAGACCAACACAGA-3′, reverse; 5′-GCCCTCCTGCACTTCTACAGTTGA-3′ Corin: forward; 5′-ACATCCGGTATTGCCATTTGCCTCA-3′, reverse; 5′-TCCCATAAAGTGGCCCAGTGCTT-3′ alkaline phosphatase: forward; 5′-CAGGTCCCACAAGCCCGCAA-3′, reverse; 5′-CCCGGTGGTGGGCCACAAAA-3.
Skin reconstitution assays
Skin was reconstituted in nude mice as previously described, except for the addition of cells cultured in hydrogels (Jensen et al., 2010). In the positive controls, 5 × 106 freshly isolated dermal cells and 8 × 106 epidermal cells were used per graft (Jensen et al., 2010). In some experimental grafts, mice received 8 × 106 epidermal cells and the intact hydrogel from one well of a 24-well plate. In other grafts, mice received 8 × 106 epidermal cells, 5 × 106 freshly isolated wild-type dermal cells, and 5 × 103 cells (spheres and single cells) released from hydrogels (corresponding to 1–3 wells of a 24-well plate). The number of cultured cells was calculated by disaggregating spheres from replicate wells using trypsin/EDTA. Grafts were analyzed after 4–5 weeks.
Histology, horizontal whole mounts, and immunostaining
AP activity was visualized using a kit purchased from Sigma-Aldrich. To analyze GFP and dsRed expression, excised grafts were fixed in 4% paraformaldehyde in a 50-ml conical flask (Falcon, Sparks, MD) for 10–12 minutes on a rocker, and then washed twice in PBS. The grafts were embedded in Optimum Cutting Temperature Medium (Leica, Milton Keynes, UK) and frozen. Sections (150 μm) were prepared and incubated in PBS to remove the Optimum Cutting Temperature Medium. Sections were stained using the same protocol as for whole mounts (Driskell et al., 2009), placed on coverslips in 100% glycerol, and mounted onto slides under a dissecting microscope to ensure correct orientation.
Dermal sphere quantification
Sphere-forming efficiency was determined by staining intact hydrogel cultures with 4′-6-diamidino-2-phenylindole and manually scoring single cells and spheres under a fluorescence microscope. Spheres were defined as containing at least four cells. At least eight representative fields were analyzed per culture. Sphere size was defined by the number of cells per sphere, which was assessed by counting 4′-6-diamidino-2-phenylindole-positive nuclei. All spheres in at least five representative fields were imaged and analyzed. A sphere was scored positive for AP if at least one cell within the sphere expressed AP.
Acknowledgments
We thank Kim B Jensen for help with epidermal cell isolation and Foad Rouhani for performing the initial hydrogel cultures. We thank the core facilities of the Wellcome Trust Centre for Stem Cell Research for superb technical assistance and Denny Cottle for useful discussions. VRJ was a fellow of The Winston Churchill Foundation of the United States. DWMT is an A* Scholar. KK holds a Medical Research Council (MRC) PhD studentship. This work was funded by the Wellcome Trust, the MRC, and EU FP7 TUMIC.
Glossary
- DP
dermal papilla
- GAA
guard/awl/auchene
- ZZ
zigzag
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Akcakaya H, Aroymak A, Gokce S. A quantitative colorimetric method of measuring alkaline phosphatase activity in eukaryotic cell membranes. Cell Biol Int. 2007;31:186–190. doi: 10.1016/j.cellbi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Paris M, Morozova O, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Sparling JS, Liu J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Gage FH. Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc Natl Acad Sci USA. 2003;100 (Suppl 1:11866–11872. doi: 10.1073/pnas.1834200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, et al. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, et al. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehama R, Ishimatsu-Tsuji Y, Iriyama S, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127:2106–2115. doi: 10.1038/sj.jid.5700823. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handjiski BK, Eichmuller S, Hofmann U, et al. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–310. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- Higgins CA, Richardson GD, Ferdinando D, et al. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19:546–548. doi: 10.1111/j.1600-0625.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007;49:185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hamazaki TS, Ohnuma K, et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- Jahoda C, Oliver RF. The growth of vibrissa dermal papilla cells in vitro. Br J Dermatol. 1981;105:623–627. doi: 10.1111/j.1365-2133.1981.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Oliver RF. Vibrissa dermal papilla cell aggregative behaviour in vivo and in vitro. J Embryol Exp Morphol. 1984;79:211–224. [PubMed] [Google Scholar]
- Jensen KB, Driskell RR, Watt FM. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc. 2010;5:898–911. doi: 10.1038/nprot.2010.39. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci USA. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno H, Morozova O, Jones KL, et al. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells. 2010;28:2027–2040. doi: 10.1002/stem.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Ehama R, Wu L, et al. Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Kissling S, Wenzel E, et al. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- Osada A, Iwabuchi T, Kishimoto J, et al. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–982. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Hatori M, Leboy PS, et al. A rapid and ultrasensitive method for measurement of DNA, calcium and protein content, and alkaline phosphatase activity of chondrocyte cultures. Calcif Tissue Int. 1995;56:252–256. doi: 10.1007/BF00298620. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Gunin A, Magerl M, et al. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TH, Lee CY, Chiu HC, et al. Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly(ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials. 2008;29:3521–3530. doi: 10.1016/j.biomaterials.2008.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.