Abstract
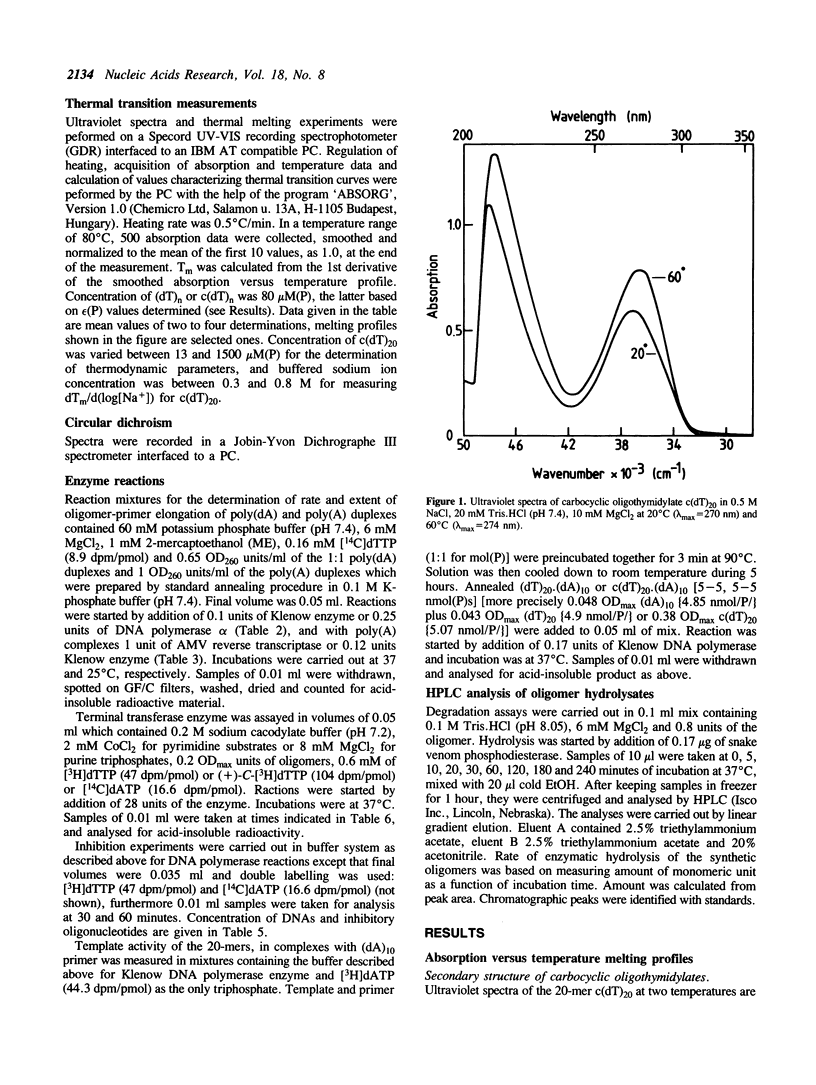
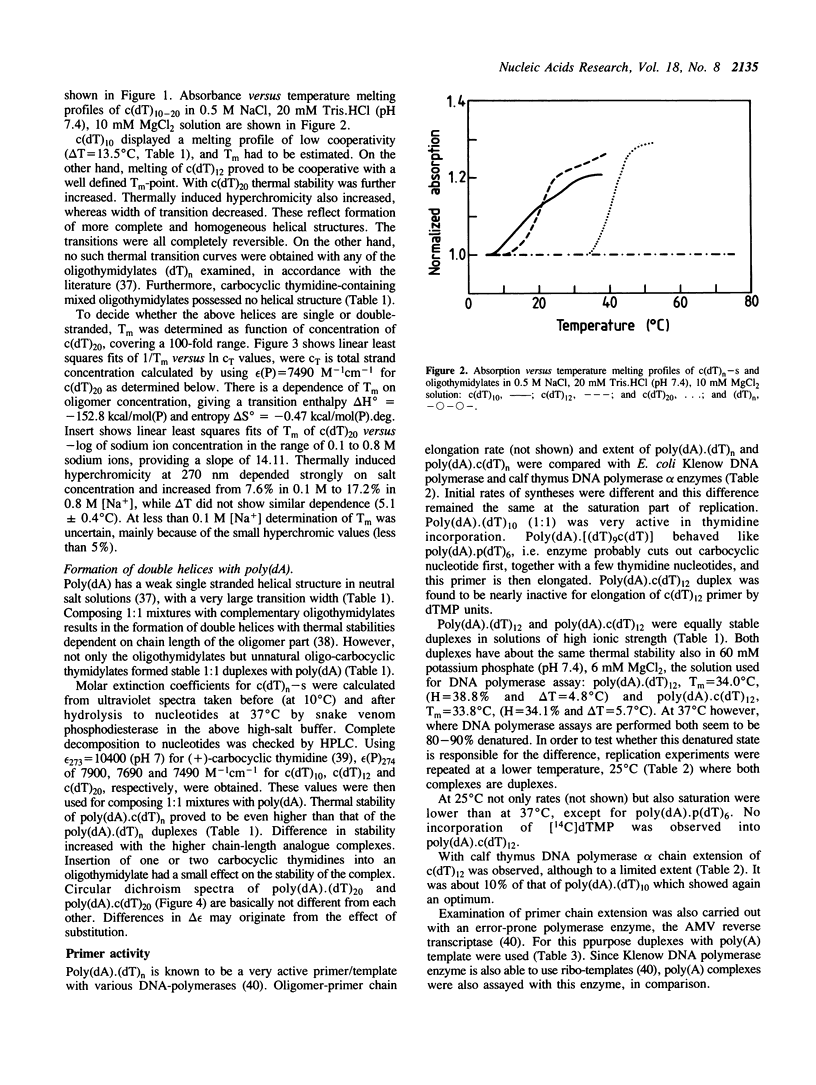
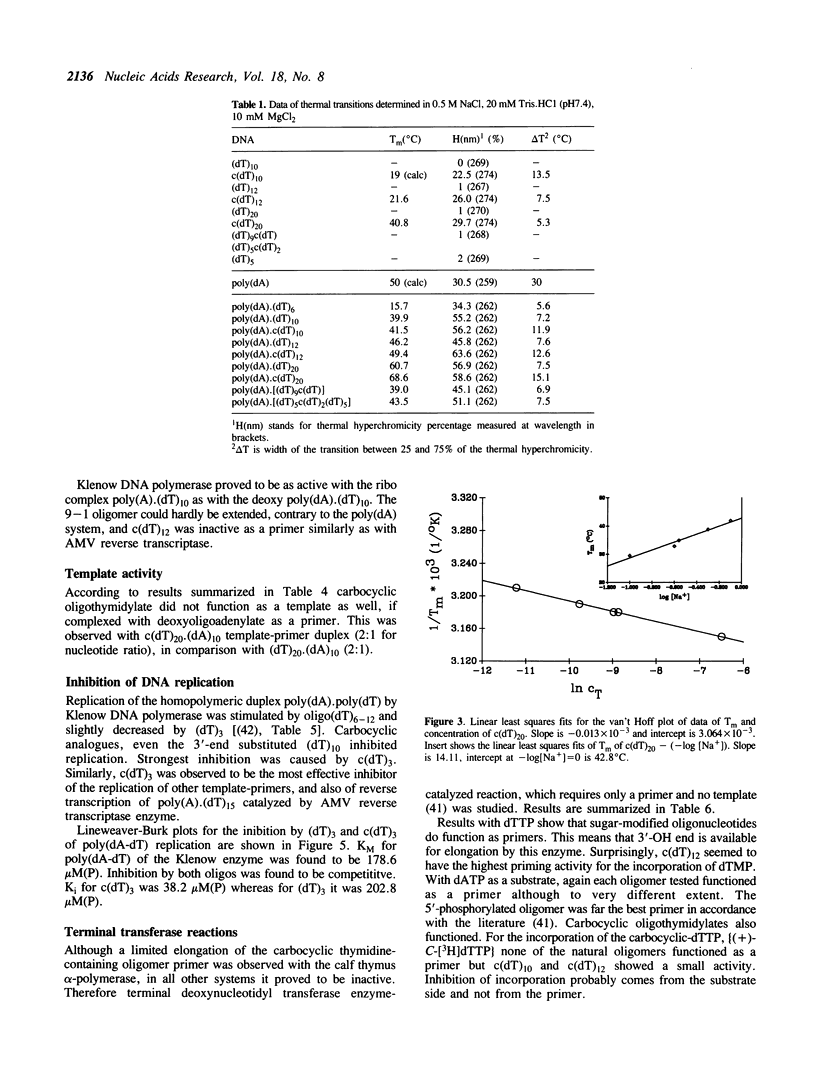
We report here spectroscopic and biochemical data of a novel series of sugar-modified oligodeoxy-nucleotides, the carbocyclic oligothymidylates, c(dT)3-20. In c(dT)n a methylene group has been substituted for the oxygen atom of the deoxyribose ring of the natural thymidylate unit. c(dT)10-20 form helical structures, in contrast with oligothymidylates or poly(dT), based on absorbance versus temperature melting profiles. Secondary structure of c(dT)n, where n greater than 10 is assumed to be double helix. In addition to this, c(dT)n forms as a stable duplex with complementary poly(dA) as does parent (dT)n. On the other hand, c(dT)n-containing oligo/poly duplex is nearly inactive either as a template or as a primer in various DNA polymerase systems, and c(dT)n inhibits DNA replication as well. c(dT)n can efficiently be extended by terminal transferase and shows an increased nuclease stability compared to (dT)n. Base-pairing ability and nuclease stability of c(dT)n suggest that (+)-carbocyclic nucleoside-containing oligomers could be new potential antisense oligodeoxynucleotides.
Full text
PDF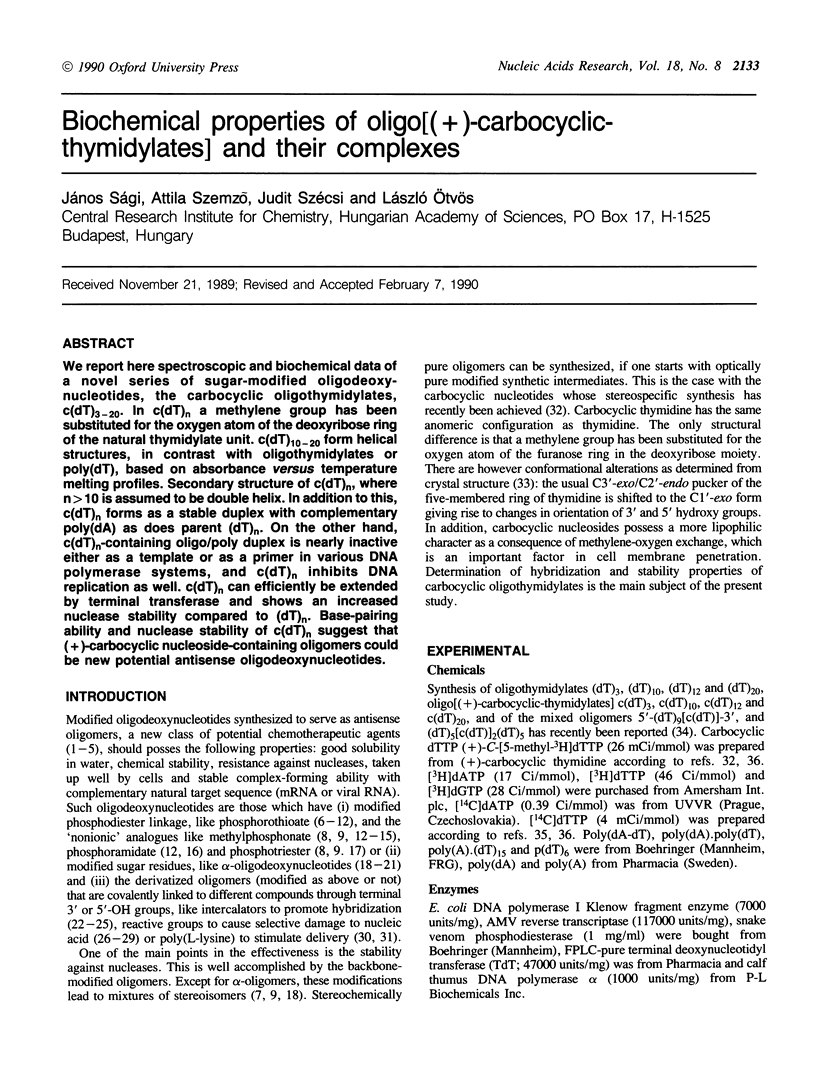
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris C. H., Blake K. R., Miller P. S., Reddy M. P., Ts'o P. O. Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Oct 7;25(20):6268–6275. doi: 10.1021/bi00368a065. [DOI] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani G. R., Bollum F. J. Oligodeoxythymidylate: polydeoxyadenylate and oligodeoxyadenylate: polydeoxythymidylate interactions. Biochemistry. 1969 Oct;8(10):3928–3936. doi: 10.1021/bi00838a008. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Cassani G. R., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. VII. Replication of homopolymers. J Biol Chem. 1972 Dec 10;247(23):7718–7723. [PubMed] [Google Scholar]
- Durand M., Maurizot J. C., Asseline U., Barbier C., Thuong N. T., Hélène C. Oligothymidylates covalently linked to an acridine derivative and with modified phosphodiester backbone: circular dichroism studies of their interactions with complementary sequences. Nucleic Acids Res. 1989 Mar 11;17(5):1823–1837. doi: 10.1093/nar/17.5.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Helene C. Sequence-targeted cleavage of single- and double-stranded DNA by oligothymidylates covalently linked to 1,10-phenanthroline. J Biol Chem. 1989 Apr 5;264(10):5891–5898. [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Fukui T. Polynucleotides. XVII. Effect of the diphosphates of aristeromycin and cycloadenosine on the polymerization of adenosine diphosphate by polynucleotide phosphorylase. J Biochem. 1973 May;73(5):945–950. doi: 10.1093/oxfordjournals.jbchem.a130177. [DOI] [PubMed] [Google Scholar]
- Kean J. M., Murakami A., Blake K. R., Cushman C. D., Miller P. S. Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry. 1988 Dec 27;27(26):9113–9121. doi: 10.1021/bi00426a008. [DOI] [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V., Zarytova V. F. Reactive oligonucleotide derivatives and sequence-specific modification of nucleic acids. Biochimie. 1985 Jul-Aug;67(7-8):785–789. doi: 10.1016/s0300-9084(85)80168-1. [DOI] [PubMed] [Google Scholar]
- Lavignon M., Bertrand J. R., Rayner B., Imbach J. L., Malvy C., Paoletti C. Inhibition of Moloney murine leukemia virus reverse transcriptase by alpha-anomeric oligonucleotides. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1184–1190. doi: 10.1016/0006-291x(89)91367-3. [DOI] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. B., Blake K. R., Miller P. S., Ts'o P. O. Use of EDTA derivatization to characterize interactions between oligodeoxyribonucleoside methylphosphonates and nucleic acids. Biochemistry. 1989 Feb 7;28(3):1054–1061. doi: 10.1021/bi00429a020. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell D. S. Antisense nucleic acids as a potential class of pharmaceutical agents. Trends Pharmacol Sci. 1988 Feb;9(2):45–47. doi: 10.1016/0165-6147(88)90112-5. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- Marquez V. E., Lim M. I. Carbocyclic nucleosides. Med Res Rev. 1986 Jan-Mar;6(1):1–40. doi: 10.1002/med.2610060102. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvös L., Sági J., Kovács T., Walker R. T. Substrate specificity of DNA polymerases. I. Enzyme-catalysed incorporation of 5-'1-alkenyl)-2'-deoxyuridines into DNA. Nucleic Acids Res. 1987 Feb 25;15(4):1763–1777. doi: 10.1093/nar/15.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter B. V., Romaniuk P. J., Eckstein F. Stereochemical course of DNA hydrolysis by nuclease S1. J Biol Chem. 1983 Feb 10;258(3):1758–1760. [PubMed] [Google Scholar]
- Quartin R. S., Brakel C. L., Wetmur J. G. Number and distribution of methylphosphonate linkages in oligodeoxynucleotides affect exo- and endonuclease sensitivity and ability to form RNase H substrates. Nucleic Acids Res. 1989 Sep 25;17(18):7253–7262. doi: 10.1093/nar/17.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Mukai S., Morisawa H., Nakashima H., Kobayashi S., Yamamoto N. Inhibition of human immunodeficiency virus (HIV-1) replication by synthetic oligo-RNA derivatives. Nucleic Acids Res. 1989 Jan 11;17(1):239–252. doi: 10.1093/nar/17.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sági J., De Clercq E., Szemzö A., Csárnyi A., Kovács T., Otvös L. Incorporation of the carbocyclic analogue of (E)-5-(2-bromovinyl)-2'-deoxyuridine 5'-triphosphate into a synthetic DNA. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1105–1112. doi: 10.1016/s0006-291x(87)80184-5. [DOI] [PubMed] [Google Scholar]
- Sági J., Szécsi J., Szemzó A., Sági G., Otvös L. Carbocyclic analogues of dTTP and UTP: properties in polymerase enzyme-catalyzed reactions. Nucleic Acids Symp Ser. 1987;(18):131–135. [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov V. V., Zarytova V. F., Kutyavin I. V., Mamaev S. V. Sequence-specific chemical modification of a hybrid bacteriophage M13 single-stranded DNA by alkylating oligonucleotide derivatives. FEBS Lett. 1988 Apr 25;231(2):352–354. doi: 10.1016/0014-5793(88)80848-2. [DOI] [PubMed] [Google Scholar]
- Westhof E. Water: an integral part of nucleic acid structure. Annu Rev Biophys Biophys Chem. 1988;17:125–144. doi: 10.1146/annurev.bb.17.060188.001013. [DOI] [PubMed] [Google Scholar]
- Zerial A., Thuong N. T., Hélène C. Selective inhibition of the cytopathic effect of type A influenza viruses by oligodeoxynucleotides covalently linked to an intercalating agent. Nucleic Acids Res. 1987 Dec 10;15(23):9909–9919. doi: 10.1093/nar/15.23.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]



