Abstract
Background It is unclear whether the incidence of first episode psychoses is in decline. We had the opportunity to determine whether incidence had changed over a 20-year period in a single setting, and test whether this could be explained by demographic or clinical changes.
Methods The entire population at-risk aged 16–54 in Nottingham over three time periods (1978–80, 1993–95 and 1997–99) were followed up. All participants presenting with an ICD-9/10 first episode psychosis were included. The remainder of the population at-risk formed the denominator. Standardized incidence rates were calculated at each time period with possible change over time assessed via Poisson regression. We studied six outcomes: substance-induced psychoses, schizophrenia, other non-affective psychoses, manic psychoses, depressive psychoses and all psychotic disorders combined.
Results Three hundred and forty-seven participants with a first episode psychosis during 1.2 million person-years of follow-up over three time periods were identified. The incidence of non-affective or affective psychoses had not changed over time following standardization for age, sex and ethnicity. We observed a linear increase in the incidence of substance-induced psychosis, per annum, over time (incidence rate ratios: 1.15; 95% CI 1.05–1.25). This could not be explained by longitudinal changes in the age, sex and ethnic structure of the population at-risk.
Conclusions Our findings suggest psychotic disorders are not in decline, though there has been a change in the syndromal presentation of non-affective disorders, away from schizophrenia towards other non-affective psychoses. The incidence of substance-induced psychosis has increased, consistent with increases in substance toxicity over time, rather than changes in the prevalence or vulnerability to substance misuse. Increased clinical and popular awareness of substance misuse could also not be excluded.
Keywords: Psychotic disorder, incidence, time, epidemiology, schizophrenia, demographic factors
Introduction
There has been much debate about whether the incidence of psychotic disorders has changed over time, with several studies during the 1980s and 1990s reporting a decline in the incidence of schizophrenia in Western countries.1–5 It remains unclear whether such declines are attributable to methodological constraints.6,7 Many studies were based on administrative hospital records that may have underestimated true incidence in the population, since modern day mental health services do not admit all individuals with psychotic presentations.
The reorganization of mental healthcare in the UK during the 1980s and 1990s towards outpatient rather than inpatient care may further explain apparent declines,8 though not all commentators agree.2 More recently, it has been suggested that changes in diagnostic criteria may be important.2,3,5 Brewin et al.9 have suggested that the apparent decline in schizophrenia in Nottingham between 1978 and 1994 may be attributable to an increase in the proportion of participants meeting diagnostic criteria for substance-induced, or other non-affective psychoses.
The evidence base does not support a ubiquitous decline in the incidence of psychotic disorder and there have been reports of an increase in the incidence of schizophrenia in some parts of the UK.10,11 Increased rates of schizophrenia were observed in Southeast London over a 30-year period,11 though this may have been attributable to increases in black and minority ethnic (BME) populations,12 where the incidence of psychoses is elevated.13 Findings for the affective psychoses are also contradictory. Two studies reported a rise in the incidence of mania,3,14 with one also reporting a decline in depressive psychoses.3
We had the opportunity to estimate the incidence of several psychotic disorders in Nottingham from studies with similar aims and methodologies, conducted at three time periods over two decades. We sought to determine: (i) whether the incidence of psychoses varied over time, independent of age and sex; (ii) whether the reported decline in schizophrenia could be ascribed to increases in the incidence of other psychotic disorders and; (iii) whether any change in incidence over time could be ascribed to changes in the proportion of BME populations.
Methodology
Time periods
We obtained data from three methodologically robust and highly similar studies conducted in a geographically defined catchment area of Nottingham, UK, between 1978 and 1999. Briefly, these studies were the Nottingham centre of the World Health Organisation (WHO) 10-country study (1978–80), the Schizophrenia in Nottingham (SIN) study (1992–94) and the Aetiology and Ethnicity in Schizophrenia and Other Psychoses (AESOP) study (1997–99). Full methodologies of the WHO,15,16 SIN9 and AESOP17 studies have previously been given. Henceforth these studies are referred to as time periods one to three (TP1-3), respectively.
Case ascertainment (TP1)
Participants were obtained prospectively as part of the WHO 10-country Determinants of Outcome of Severe Mental Disorders study between 1978 and 1980. The study population at-risk was defined by the catchment area of the Mapperley Hospital, covering the City of Nottingham. An over-inclusive screening schedule was used to identify patients (i) aged 15–55 years, (ii) resident in the catchment area, (iii) no previous contact, (iv) presence of hallucinations, delusions, thought disorder, bizarre or disturbed behaviour, which may have indicated a psychotic illness, (v) absence of an organic cause or severe learning disability. Participants who passed the screen were assessed using the Present State Examination.18 The Personal and Psychiatric History Schedule (PPHS) and a social disability schedule were completed with a close relative or key informant. A leakage study was conducted to identify any participants who may have been missed by the screening process.16 A similar methodology was used to obtain participants in studies TP2 and TP3.
Case ascertainment (TP2 and TP3)
Potential participants with a first onset psychosis were screened using the above WHO psychosis screen.9,15 Inclusion criteria were identical to those in TP1, except the age range was broadened to 16–64 years. Service bases (Community Mental Health Teams) were regularly contacted to ensure all potential contacts were followed-up. Secondary psychiatric services were regularly monitored by telephone or personal contact. All potential contacts were reviewed with appropriate staff. As for TP1, a leakage study was conducted to ensure comprehensive ascertainment of all first onset psychoses.16
Participants who had passed the screen underwent an extensive battery of assessments including the Schedule for Clinical Assessment in Neuropsychiatry (SCAN);19 the Schedule for the Assessment of Negative Symptoms (SANS);20 a modified PPHS19 and a schedule developed to record sociodemographic data.
Comparison between time periods
At each time period, participants were classified by consensus diagnosis i.e. the agreement of a panel of clinicians, including the researcher who conducted the individual assessments. The researcher presented the clinical information to members of the diagnostic panel. Diagnoses were made according to the International Classification of Diseases, either 9th21 (TP1) or 10th edition21 (TP2 and TP3), using all other information from the case notes, item ratings in SCAN and collateral histories.
To enable comparisons between time periods it was necessary to align the diagnostic criteria across studies. Thus, all diagnoses at TP1 were re-diagnosed under ICD-10, by clinicians at TP2 (J.B.K., I.M., C.G., G.H.) as described in Brewin et al.9 using all case information originally available at TP1. Inter-rater reliability kappas exceeded 0.79. At each time point we included incidence data on participants aged 16- to 54-years-old. Participants were excluded if they did not reside in the catchment area as defined at TP1.
Data on ethnicity were available for both the numerator (participants) and denominator (population at-risk) at only TP2 and TP3. Therefore, analyses related to objective (iii) were restricted to these two time periods.
Population at-risk
The population at-risk at each time point was estimated from the closest Census of Great Britain to the time period under study (1981, 1991 and 2001, respectively). From each census we obtained an estimate of the denominator population by age (16–54 years: 16–19, and subsequent 5-year age bands) and sex, residing in the Mapperley Hospital catchment area. We applied Office for National Statistics (ONS) correction factors22 to adjust 1991 census estimates for likely under-enumeration of key demographic groups. The ONS has not had to publish such correction factors for other censuses. At each time period, we doubled the census estimate to take into account the 2-year case ascertainment period.
Ethnicity was defined according to the categories used in the SIN study (TP2) for both the numerator and denominator populations. Therefore, for analyses relating to objective (iii), data at TP2 and TP3 were stratified using the following seven ethnicity categories: white (White British, White Irish and White Other), Black Caribbean, Black African, Black Other, Indian, Pakistani and all other ethnic groups. The BME group was defined as all non-white ethnicities.
Statistical analyses
We considered four principal outcomes in this study; all psychoses (ICD-10 F10–33); substance-induced psychosis (F10–19); non-affective psychoses (F20–29) and the affective psychoses (F30–33). We also considered specific diagnoses separately within the non-affective and affective psychoses: schizophrenia (F20); other non-affective psychoses (F21–29), manic psychoses (F30–31) and the depressive psychoses (F32–33). Standardized incidence rates at each time period were reported, using direct standardization to the stratified population of England from the most recent census (2001) to control for age, sex and, where possible, ethnicity differences over time.
We used Poisson regression to address the three main objectives of this study. The Poisson distribution is appropriate for modelling the number of events (for example, cases of first episode psychoses) which occur over a given time period. We assumed that these events were independent of each other and occurred randomly over time. For a given outcome, Poisson regression models estimate incidence rate ratios (IRR) for a given level of exposure in relation to another, with covariates optionally entered into the model to control for confounding. Here, we used Poisson regression to estimate changes in incidence rates over time. Poisson regression is analogous to logistic regression such that models are fitted on a log scale, with the results anti-logged to obtain IRR and 95% confidence intervals (95% CI). The natural logarithm of the count of cases of psychoses provides the outcome variable, with the natural logarithm of person-years at risk treated as an offset variable in the model. The data are stratified across the exposure variable(s) included in the model.
To address our objectives, age and sex (and where relevant, their interaction) were entered into a Poisson regression model as categorical variables. This model was then compared—via Likelihood Ratio Test (LR test)—with the same model, but with time period entered as a continuous variable to examine possible linear change in incidence over time. Time period was treated as a continuous variable, using the mid-point of each time period (i.e. TP1 = 1978, TP2 = 1993 and TP3 = 1998), to take into account the unequal intervals between the three study periods. Thus, the IRR for our continuous time period variable could be interpreted as the change in incidence over a 1-year period. To assess for possible non-linear changes in incidence over time, we compared via LR test, the previous model with an alternate model where time period was fitted as a categorical variable. We repeated the above analysis, stratified by sex, to assess whether our findings were consistent for men and women separately. Although our data were cross-sectional, and we could therefore not truly disentangle age, period and cohort effects, we tested whether there was an interaction between age category and time period, which may have indicated the presence of cohort effects (i.e. period effects conditional upon age). All analyses were performed in Stata (version 9) (2005) (StataCorp, College Station, TX, USA).
Results
Three hundred and forty-seven occurrences of clinically relevant first onset psychoses were identified over the three 2-year periods from an estimated denominator population of 1.2 million person-years at-risk. The number of participants identified at TP3 was greater than at either two of the preceding time periods (n = 128), but the estimated denominator population also increased over this period (Table 1).
Table 1.
First onset psychosis by diagnosis and time period
| Diagnosis (ICD-10) | TP1 (1978–80)an (%) | TP2 (1992–94)bn (%) | TP3 (1997–99) n (%) | Total n | χ2 test P-valuec |
|---|---|---|---|---|---|
| All psychoses (F10–33) | 97 (100) | 122 (100) | 128 (100) | 347 | – |
| Non-affective psychoses (F20–29) | 70 (72.2) | 80 (65.6) | 78 (60.9) | 228 | 0.21 |
| Of which: | |||||
| Schizophrenia (F20) | 55 (56.7) | 39 (31.2) | 43 (33.6) | 137 | <0.01 |
| Other non-affective psychoses (F21–29) | 15 (15.5) | 41 (33.6) | 35 (27.3) | 91 | 0.01 |
| Substance-induced psychoses (F10–19) | 1 (1.0) | 10 (8.2) | 19 (14.8) | 30 | <0.01d |
| Affective psychoses (F30–33) | 26 (26.8) | 32 (26.2) | 31 (24.2) | 89 | 0.89 |
| Of which | |||||
| Manic psychoses (F30-31) | 9 (9.3) | 15 (12.3) | 14 (10.9) | 38 | 0.77 |
| Depressive psychoses (F32-33) | 17 (17.5) | 17 (13.9) | 17 (13.3) | 51 | 0.64 |
| Denominator (1 year) | 195 616 | 208 069 (100) | 215 479 (100) | 619 164 | – |
| Of which | |||||
| BME populatione | – | 17 175 (8.3) | 25 604 (11.9) | – | <0.01 |
aThe total number of participants reported here is two less than previously reported.9 One participant was excluded because of the narrower age range used in the current study, while one participant was excluded because of a primary ICD-10 diagnosis of personality disorder (F60.1).
bThe total number of participants reported at TP2 was one less than previously reported because of the narrower age range used here.9
cTest for difference over time in proportion of given diagnosis vs all others. χ2 test on 1 df.
dFisher's exact test instead of χ2 due to small numbers in cells.
eUnknown in 1981 because ethnicity was not recorded in 1981 census.
Diagnostic differences over time
Overall, there was no evidence the proportion of participants diagnosed with a non-affective psychoses changed over time. However, within this category, a greater proportion of participants were diagnosed with schizophrenia (F20) at TP1 than at later time points (57% vs ∼32%; χ2 P < 0.01). Conversely, the proportion of the sample diagnosed with another non-affective psychoses (F21–29) at TP1 was smaller than at later time periods (16% vs ∼30%; χ2 P = 0.01). Furthermore, there was a notable increase in the proportion of participants diagnosed with substance-induced psychosis (TP1 = 1% to TP3 = 15%). The proportion of participants receiving a diagnosis of affective psychoses was 25% at each time period. Within this category, there was no evidence that the proportion of participants diagnosed with either manic or depressive psychoses differed over time.
Incidence rates over time
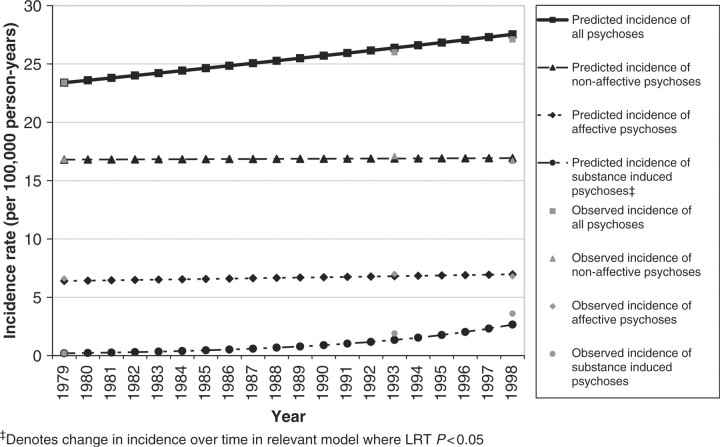
There was no evidence of an increase in the age and sex standardized incidence of all clinically relevant psychoses over time (Table 2), despite a small increase in the point estimate of incidence from 23.4 cases per 100 000 person-years at TP1 (95% CI 18.6–28.2) to 27.1/100 000 (95% CI 22.3–31.9) at TP3 (LR test P = 0.19). Further, there was no evidence that the overall incidence of non-affective (F20–29) or affective psychoses (F30–33) changed over time after adjustment for age and sex (Figure 1).
Table 2.
Age and sex standardized incidence of psychotic syndromes over three time periods
| Diagnosis (ICD-10) | TP1 (1978–80) Standardized incidence ratea (95% CI) | TP2 (1992–94) Standardized incidence ratea (95% CI) | TP3 (1997–99) Standardized incidence ratea (95% CI) | IRR for linear change in incidence per year (95% CI) | LRT test for change in incidence rate over time |
|---|---|---|---|---|---|
| All psychoses (F10–33) | 23.4 (18.6–28.2) | 26.0 (21.2–30.8) | 27.1 (22.3–31.9) | 1.01 (0.99–1.02) | 0.19 |
| Non-affective psychoses (F20-29) | 16.8 (12.8–20.9) | 17.1 (13.2–21.0) | 16.7 (12.9–20.5) | 1.00 (0.98–1.02) | 0.96 |
| Of which | |||||
| Schizophrenia (F20) | 13.2 (9.6–16.8) | 8.2 (5.5–10.8) | 8.9 (6.2–11.6) | 0.98 (0.96–1.00) | 0.04 |
| Other non-affective psychoses (F21–29) | 3.6 (1.7–5.5) | 9.0 (6.1–11.8) | 7.8 (5.2–10.4) | 1.04 (1.01–1.07) | 0.01 |
| Substance-induced psychoses (F10–19) | 0.2 (0.0–0.5) | 1.9 (0.7–3.1) | 3.6 (1.9–5.2) | 1.15 (1.05–1.25) | <0.01 |
| Affective psychoses (F30–33) | 6.4 (3.9–8.9) | 7.0 (4.5–9.4) | 6.8 (4.4–9.3) | 1.00 (0.98–1.03) | 0.73 |
| Of which | |||||
| Manic psychoses (F30–31) | 2.2 (0.7–3.8) | 3.3 (1.6–5.1) | 3.0 (1.4–4.6) | 1.02 (0.98–1.06) | 0.35 |
| Depressive psychoses (F32–33) | 4.2 (2.1–6.2) | 3.6 (1.8–5.4) | 3.8 (2.0–5.7) | 0.99 (0.96–1.02) | 0.74 |
aDirectly standardized to the age and sex stratified population of England and Wales from 2001 census. Rates expressed per 100 000 person-years.
Figure 1.
Observed and interpolated incidence of principal psychotic outcomes, 1979–98. (Interpolated incidence rate based on IRR for continuous time period variable in each model, adjusted for age and sex.)
The incidence of substance-induced psychosis increased linearly over time (IRR: 1.15; 95% CI 1.05–1.25) after adjustment for age and sex. The interpretation of the IRR being that the incidence of substance-induced psychoses increased by ∼15% per annum. Furthermore, the broad non-affective outcome appeared to mask variation in the incidence of specific outcomes over time (Figure 2). For example, there was a small decline in the incidence of schizophrenia over time (IRR 0.98; 95% CI 0.96–1.00; LR test P = 0.04), but a contrasting increase in the incidence of other non-affective psychoses (F21–29) (IRR 1.04; 95% CI 1.01–1.07). The incidence of manic or depressive psychoses remained stable over time, after adjustment for age and sex (Table 2 and Figure 2). We did not observe any non-linear change in incidence rates over time nor any age–period interaction effects for any psychotic outcome (data available from authors).
Figure 2.
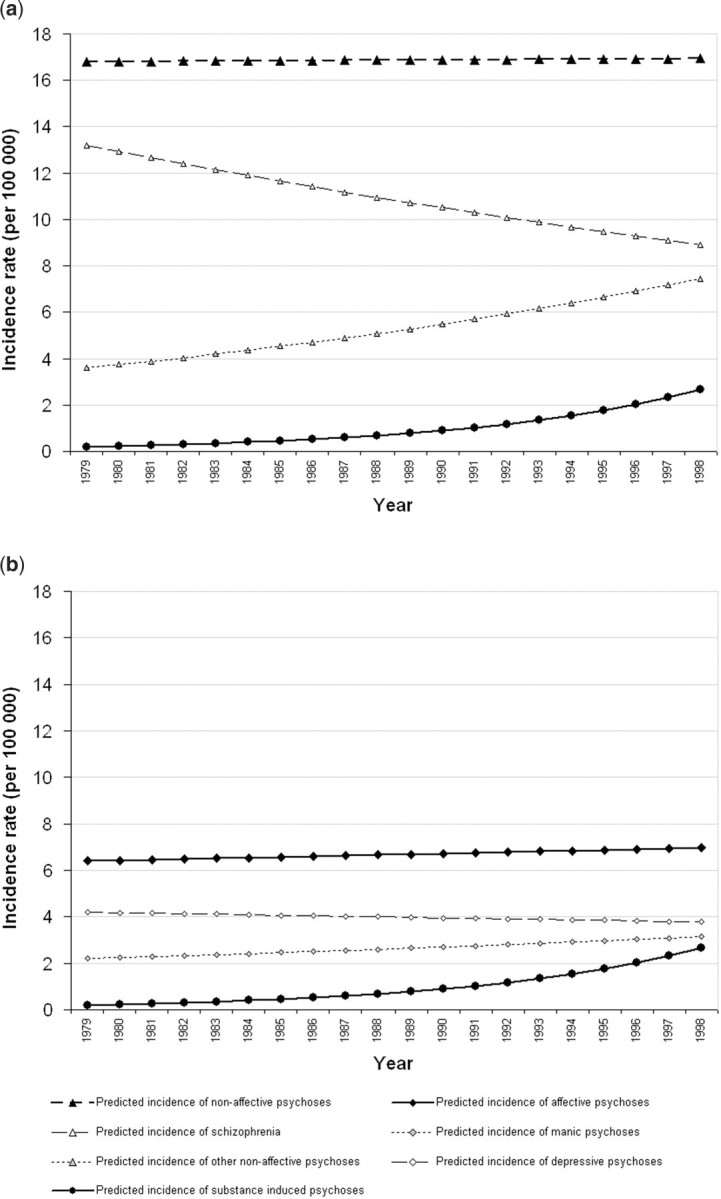
Interpolated incidence of specific psychotic outcomes, 1979–98. (For graphical comparison, the scale is kept the same on both graphs. Substance-induced psychoses are also included on both graphs.) (a) Non-affective and substance-induced psychoses, (b) affective and substance-induced psychoses
When we examined changes in incidence over time for men and women separately, we found that the direction and magnitude of the trends reported above were generally upheld, despite some loss of precision in our estimates (data available from authors).
We went onto consider the possibility that changes in the ethnic structure of the population at-risk could have confounded changes in the incidence of psychotic disorders over time. Because we only had data available on ethnicity at TP2 and TP3 we excluded TP1 from these analyses. Census data confirmed that the BME population in Nottingham had increased from 8.3% to 11.9% between 1991 and 2001 (χ2 P < 0.01; Table 1). However, the additional effect of ethnicity did not alter any of the patterns in incidence rates between TP2 and TP3 observed in the same model adjusted for age and sex only (data available from authors). Further, when we restricted the analysis to the white group there was no evidence that the age and sex standardized incidence rate of non-affective or affective psychoses differed between TP2 and TP3. A trend remained for increased incidence of substance-induced psychosis between time periods (IRR 2.0; 95% CI 0.9–4.4; P = 0.09).
Discussion
Principal findings
We observed heterogeneity between outcomes in terms of changes in incidence over time. There was a notable increase in the incidence of substance-induced psychoses in Nottingham over the last two decades of the 20th Century, but a decline in the incidence of schizophrenia. The decline in schizophrenia appeared to be offset, however, by a corresponding increase in the incidence of other non-affective psychoses over the same time period. Given the overall stability in the rates of non-affective psychoses, these findings may reflect genuine changes in the syndromal presentation of psychotic disorders.9 The incidence of both manic and depressive psychoses were stable over time. We did not observe a change in the overall incidence of psychotic disorders over time, despite weak evidence of a slight increase in point estimates. Changes in the demographic composition of the sample by age, sex and ethnicity did not appear to confound our findings.
Strengths and limitations of the study
This study utilized data from three methodologically robust and highly similar first onset studies over a 20-year period in a well-defined catchment area, overseen by an internationally recognized centre of excellence in psychosis epidemiology. We controlled for age and sex, and where possible, ethnicity, making it unlikely that these factors concealed or explained changes in incidence. There was considerable continuity in clinical personnel, with largely the same panel of senior clinicians responsible for diagnoses, using the same methods, materials and operational criteria (ICD-10). There was also some commonality in analytical support/data management. Further, participants at TP1 (ICD-9) were re-diagnosed to ICD-10 diagnoses, using the original data. It is therefore unlikely that changes in diagnostic fashion could account for the reversed patterns observed for schizophrenia and other non-affective psychoses over the study period (Figure 1). Rather, these patterns support secular changes in the clinical presentation of non-affective psychoses over time.9
A limitation of our study is the definition of substance-induced psychosis, whether this definition changed over time, and subsequent interpretation of the meaning of the observed increase over time. It is possible that the increase in substance-induced psychoses is entirely attributable to period effects associated with changing diagnostic fashion over time, as both clinical and popular awareness of the detrimental effects of substance misuse on the development of psychotic symptoms increased. The Present State Examination used at TP1 did not include the comprehensive drug section included in the SCAN at later time periods, and we therefore acknowledge the possibility that substance-induced psychosis may have been under-diagnosed at TP1. Nevertheless, this could not explain the increased incidence of substance-induced psychosis between our latter two time periods where identical standardized diagnostic criteria were used, making it unlikely that greater clinical awareness of substance misuse entirely explains this increase. We did however, use a self-report measure of substance misuse in our study rather than an objective measure, and acknowledge that increased popular awareness of substance misuse over time—a form of recall bias—may have led to an increase in diagnosis of substance-induced psychoses.
An earlier analysis of TP1 and TP29 was able to address changes, but not trends in incidence, having only considered two time periods. Unlike the previous study, which used the same denominator at each time period, we estimated the population at-risk using the census closest to each study period.
Unlike some other studies,1,5,23 our study was not truly longitudinal in design, but estimated incidence at three different 2-year time periods. We do not believe this will have affected our results, though it makes it difficult to disentangle possible period from cohort effects. Nevertheless, we did not observe any age–period interaction effects, which may have indicated the presence of cohort effects. One study in Finland has examined the contribution of period and cohort effects on the incidence of psychoses,24 finding stronger evidence for the latter, suggesting changes in risk factors around the time of birth may have been more important than period effects. However, like our study, they also reported a change in the syndromal presentation of non-affective psychoses, and suggested a period effect—increased clinical reluctance to diagnose schizophrenia at first presentation—may have explained these findings. We cannot refute this explanation for our findings, but given the reasons stated above, we believe a genuine change in the syndromal presentation of disorders provides a more parsimonious reading of our results.
Previous research in the UK has often been contradictory regarding changes in the incidence of psychotic disorders,3,11,25 with several studies,1–3 but not all,25 reporting a decline in the incidence of schizophrenia. Several of these studies were based on first admissions rather than first contact with services,2,3 and may have underreported the true incidence of psychotic disorders with the movement towards community-based services over this time. We used an over-inclusive screen to identify all potential participants, and a leakage study was conducted at each time period to maximize ascertainment. Boydell et al.11 have shown that the incidence of non-affective psychoses, including schizophrenia, has increased in Southeast London over the last three decades, but this finding may be explained by the substantial increase in BME groups in this area over the same period; something we were partially able to control for in this study.
Meaning of the findings
Overall, there was little evidence of change in the total incidence of psychoses over a 20-year period. This finding is discrepant with a trend observed in a recent systematic review,26 which suggested higher rates tended to be reported in older studies. We have also shown that the incidence of non-affective and affective psychoses, when broadly defined, was relatively constant over time. Our findings are useful for health service planning as we believe they demonstrate a change in the syndromal presentation of non-affective psychoses over time. The increasing incidence of substance-induced psychoses, whose incidence became comparable with both the manic and depressive psychoses by TP3, is a noteworthy finding. We have shown that these changes are unlikely to be due to chance. Further, we can almost certainly discount possible confounding by important sociodemographic factors. We have attempted to minimize bias within the study by using similar methodologies at each time period, including making consensus diagnoses, but we cannot completely exclude increased clinical and/or popular awareness of substance misuse as an explanation of the changing syndromal presentation of disorders.
Having acknowledged the potential for chance, bias and confounding, it is reasonable to speculate about other possible explanations for the increasing incidence of substance-induced psychosis. Arguably, such an increase could be driven by changes in the substance(s) used, or changes in the population at-risk in terms of either vulnerability to, or prevalence of substance use.
An increase in vulnerability to psychosis following substance misuse may arise if the age at which people begin using drugs is lower. There is evidence that earlier age of cannabis use is associated with a greater risk of schizophrenia symptoms in adulthood.27 Although there are several potential explanations for this,28 including a straightforward dose–response relationship or reverse causality, it is possible that use at a younger age impinges negatively on critical periods of brain development. If an increased vulnerability to psychoses due to substance use explains the increasing incidence of substance-induced psychosis, then one would expect to observe an increase in the prevalence of substance use among people with psychotic disorder. However, the prevalence of any reported substance misuse amongst people with psychosis was constant between TP2 and TP3 (19.0% vs 19.5%, respectively).29
The prevalence of substance use within the population at-risk is reported to have increased from 28% to 33% between 1994 and 1998 for men aged 19–29 years in England and Wales;30 a group at high risk of developing a substance-induced psychosis.17 It is, however, unlikely that this change could account for the increasing incidence of substance-induced psychosis we have observed, since one would expect the prevalence of substance misuse amongst people with psychosis to have also risen. The stable prevalence of substance misuse, together with a rising incidence of substance-induced psychosis, is consistent with an increase in the strength or dose of illicit substances over time. This could either be due to a change in the type of substances used over time (towards substances with greater toxicity), though the available evidence does not support this,31 or through changes in the strength of the same substance. There is some evidence for this; the average content of the principal psychoactive chemical in cannabis, tetrahydrocannabinol (THC), has risen from an estimated 1–5% in the 1960s to 9.4% in 1997.32 In our sample, however, the use of stimulants, such as amphetamine, and poly-substance abuse, were attributable to a considerably greater proportion of substance-induced psychoses than were cannaboids (data available from authors), though a large proportion of these cases were reported to also use cannabis (G.A.D.). An important caveat of our study is that the increase in substance-induced psychoses observed here does not provide evidence—either for or against—an increase due to cannabis use per se, but we cannot exclude it as a possible explanation of our findings. Anecdotally, it has been suggested that substance-induced psychoses patterns may have reflected the geographically and temporally transient nature of illicit substance availability, particularly amphetamine (P.B.J.).
Further challenges
Recent research has provide valuable information about the incidence of psychotic disorders according to different sociodemographic characteristics, including age,17 sex,17 ethnicity13 and place.17,33 In one of these places (Nottingham), we found the overall incidence of psychotic disorder was predominantly stable over a 20-year period, though this masked changes in the syndromal presentation of specific disorders, including a notable increase in the incidence of substance-induced psychoses. The risk of psychosis due to substance misuse is yet to be fully understood, and delineating the syndromal overlap between substance-induced psychoses and non-affective psychoses remains an important caveat in this context. A recent meta-analysis suggested prior cannabis use increased the risk of psychotic disorder by ∼40%,28 though risk increased in a dose–response fashion according to the frequency of consumption. Our study could not provide specific evidence about cannabis used, but if cannabis is a causal risk factor for psychoses, perhaps interacting with genetic susceptibility,34 then model projections suggest we should begin to see an increase in the incidence of psychotic disorders by 2010.35 It will be vital to have accurate and detailed figures for both the incidence of psychotic disorders and substance misuse in clinical as well as epidemiological samples to address these issues.
Funding
Medical Research Council; Stanley Medical Research Institute; WHO; National Institute of Mental Health of the United States of America (grant MH 29969); defunct UK Department of Health and Social Security; former Trent Regional Health Authority (now Trent Strategic Health Authority).
Acknowledgement
We are grateful to Dr Stuart Leask for his help in preparation of this manuscript. J.B.K. was supported by a Sir Henry Wellcome Research Fellowship from the Wellcome Trust (grant code: WT085540).
Conflict of interest: None declared.
KEY MESSAGES.
There was no evidence that the overall incidence of psychotic disorders was in decline in a single study location over a 20-year period.
However, there is evidence of changes of a syndromal shift in presentation, with a notable decrease in the incidence of schizophrenia in place of an increase in the incidence of other non-affective and substance-induced psychoses.
Our findings could not be explained by demographic changes over time, but are consistent with a genuine change in the syndromal presentation of first episode psychoses.
References
- 1.Der G, Gupta S, Murray RM. Is schizophrenia disappearing? Lancet. 1990;335:513–16. doi: 10.1016/0140-6736(90)90745-q. [DOI] [PubMed] [Google Scholar]
- 2.Eagles JM, Hunter D, McCance C. Decline in the Diagnosis of Schizophrenia among 1st Contacts with Psychiatric-Services in Northeast Scotland, 1969–1984. Br J Psychiatry. 1988;152:793–98. doi: 10.1192/bjp.152.6.793. [DOI] [PubMed] [Google Scholar]
- 3.Geddes JR, Black RJ, Whalley LJ, Eagles JM. Persistence of the decline in the diagnosis of schizophrenia among first admissions to Scottish hospitals from 1969 to 1988. Br J Psychiatry. 1993;163:620–26. doi: 10.1192/bjp.163.5.620. [DOI] [PubMed] [Google Scholar]
- 4.Munk-Jorgensen P, Mortensen PB. Is schizophrenia really on the decrease? Eur Arch Psychiatry Clin Neurosci. 1993;242:244–47. doi: 10.1007/BF02189970. [DOI] [PubMed] [Google Scholar]
- 5.Osby U, Hammar N, Brandt L, et al. Time trends in first admissions for schizophrenia and paranoid psychosis in Stockholm County, Sweden. Schizophr Res. 2001;47:247–54. doi: 10.1016/s0920-9964(00)00124-9. [DOI] [PubMed] [Google Scholar]
- 6.Kendell RE, Malcolm DE, Adams W. The problem of detecting changes in the incidence of schizophrenia. Br J Psychiatry. 1993;162:212–18. doi: 10.1192/bjp.162.2.212. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan M, Boydell J, Murray R, Susser E. Temporal variation in the incidence, course and outcome of schizophrenia. In: Murray R, Jones PB, Susser E, van Os J, Cannon M, editors. The Epidemiology of Schizophrenia. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 8.Harrison G, Cooper JE, Gancarczyk R. Changes in the administrative incidence of schizophrenia. Br J Psychiatry. 1991;159:811–16. doi: 10.1192/bjp.159.6.811. [DOI] [PubMed] [Google Scholar]
- 9.Brewin J, Cantwell R, Dalkin T, et al. Incidence of schizophrenia in Nottingham—A comparison of two cohorts, 1978–80 and 1992–94. Br J Psychiatry. 1997;171:140–44. doi: 10.1192/bjp.171.2.140. [DOI] [PubMed] [Google Scholar]
- 10.Castle D, Wessely S, Der G, Murray RM. The incidence of operationally defined schizophrenia in Camberwell, 1965–84. Br J Psychiatry. 1991;159:790–94. doi: 10.1192/bjp.159.6.790. [DOI] [PubMed] [Google Scholar]
- 11.Boydell J, Van Os J, Lambri M, et al. Incidence of schizophrenia in south-east London between 1965 and 1997. Br J Psychiatry. 2003;182:45–49. doi: 10.1192/bjp.182.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Boydell J, van Os J, McKenzie K, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323:1336–38. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon P, Kirkbride JB, Morgan C, et al. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol Med. 2006;36:1541–50. doi: 10.1017/S0033291706008774. [DOI] [PubMed] [Google Scholar]
- 14.van Os J, Takei N, Castle DJ, et al. The incidence of mania: time trends in relation to gender and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 1996;31:129–36. doi: 10.1007/BF00785759. [DOI] [PubMed] [Google Scholar]
- 15.Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monograph Supplement. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JE, Goodhead D, Craig T, Harris M, Howat J, Korer J. The incidence of schizophrenia in Nottingham. Br J Psychiatry. 1987;151:619–26. doi: 10.1192/bjp.151.5.619. [DOI] [PubMed] [Google Scholar]
- 17.Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-Center ÆSOP Study. Arch Gen Psychiatry. 2006;63:250–58. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- 18.Wing JK, Cooper JE, Sartorius N. Cambridge & London: Cambridge University Press; 1974. The Measurement and Classification of Psychiatric Symptoms. [Google Scholar]
- 19.WHO. Geneva: WHO; 1992. Schedule for Clinical Assessment in Neuropsychiatry. [Google Scholar]
- 20.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch of Gen Psychiatry. 1982;39:784–88. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 21.WHO. World Health Organisation. 10th edn. Geneva: World Health Organisation; 1994. International Classification of Diseases. [Google Scholar]
- 22.OPCS/GRO(S) Undercoverage in Great Britain: 1991 Census User Guide 58. London: 1994. [Google Scholar]
- 23.Munk-Jorgensen P, Mortensen PB. Incidence and other aspects of the epidemiology of schizophrenia in Denmark, 1971-87. Br J Psychiatry. 1992;161:489–95. doi: 10.1192/bjp.161.4.489. [DOI] [PubMed] [Google Scholar]
- 24.Suvisaari JM, Haukka JK, Tanskanen AJ, Lonnqvist JK. Decline in the incidence of schizophrenia in Finnish cohorts born from 1954 to 1965. Archf Gen Psychiatry. 1999;56:733–40. doi: 10.1001/archpsyc.56.8.733. [DOI] [PubMed] [Google Scholar]
- 25.Allardyce J, Morrison G, Van Os J, Kelly J, Murray RM, McCreadie RG. Schizophrenia is not disappearing in south-west Scotland. Br J Psychiatry. 2000;177:38–41. doi: 10.1192/bjp.177.1.38. [DOI] [PubMed] [Google Scholar]
- 26.McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2 doi: 10.1186/1741-7015-2-13. Available at http://www.ncbi.nlm.nih.gov/pubmed/15115547?dopt=AbstractPlus&holding=f1000,f1000m,isrctn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–13. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. The Lancet. 2007;370:319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 29.Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150–53. doi: 10.1192/bjp.174.2.150. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay M, Partridge S. London: Home Office; 1999. Drug misuse declared in 1998: results from the British Crime Survey. [Google Scholar]
- 31.Murphy R, Roe S. London: Home Office; 2007. Drug misuse declared: findings from the 2006/7 British Crime Survey. [Google Scholar]
- 32.Bone C, Waldron SJ. New trends in illiait cannabis cultivation in the United Kingdom of Breat Britain and Northern Ireland. Bull Narcotics. 1998;50:117–28. [Google Scholar]
- 33.Kirkbride JB, Morgan C, Fearon P, Dazzan P, Murray RM, Jones PB. Neighbourhood-level effects on psychoses: re-examining the role of context. Psychol Med. 2007;37:1413–25. doi: 10.1017/S0033291707000499. [DOI] [PubMed] [Google Scholar]
- 34.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–27. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Hickman M, Vickerman P, Macleod J, Kirkbride JB, Jones PB. Cannabis and Schizophrenia: model projections of the impact of the rise in cannabis use on historical and future trends in schizophrenia in England and Wales. Addiction. 2007;102:597–606. doi: 10.1111/j.1360-0443.2006.01710.x. [DOI] [PubMed] [Google Scholar]