Abstract
Objectives
This study describes the antileishmanial efficacy of the novel drug formulation of amphotericin B (AmB) attached to functionalized carbon nanotubes (f-CNTs) and compares it with AmB.
Methods
f-CNTs were prepared in a two-step chemical carboxylation and amidation process. The AmB was then attached to make f-CNT–AmB and its construction was confirmed by Fourier transform infrared (FTIR) spectroscopy and transmission electron microscopy (TEM). The cytotoxicity of the constructed compound, f-CNT–AmB, was assessed in vitro using the J774A.1 macrophage cell line and in vivo using healthy BALB/c mice. Antileishmanial activity of AmB and f-CNT–AmB was assessed in vitro using a macrophage (J774A.1 cell line) model of Leishmania donovani infection. Antileishmanial activity was assessed in vivo by comparing the parasite load of hamsters treated with a 5 day course of AmB, f-CNTs or f-CNT–AmB initiated at 30 days after infection with L. donovani parasites.
Results
The FTIR spectroscopy and TEM data demonstrate the successful attachment of AmB to f-CNTs. The in vitro cytotoxicity of AmB, f-CNTs and f-CNT–AmB was measured by the cytotoxic concentration required to kill 50% of the cells: 0.48 ± 0.06 μg/mL; 7.31 ± 1.16 μg/mL; 0.66 ± 0.17 μg/mL, respectively, in the J774A.1 cell line. The in vivo toxicity assessment of the compounds in BALB/c mice revealed no hepatic or renal toxicity. Against intracellular amastigotes the in vitro antileishmanial efficacy of f-CNT–AmB was significantly higher than that of AmB (IC50 0.00234 ± 0.00075 μg/mL versus 0.03263 ± 0.00123 μg/mL; P ≤ 0.0001). The percentage inhibition of amastigote replication in hamsters treated with f-CNT–AmB was significantly more than that with AmB (89.85% ± 2.93% versus 68.97% ± 1.84%; P = 0.0004).
Conclusions
The results of these experiments clearly demonstrate that f-CNT–AmB has significantly greater antileishmanial efficacy than AmB and had no significant cytotoxic effects.
Keywords: L. donovani, nanomedicine, VL
Introduction
Visceral leishmaniasis (VL) is a fatal protozoan disease in which the haemoflagellate intracellular parasite Leishmania donovani infects the macrophages of the reticuloendothelial system. The current treatment options for VL are improving but remain limited by the issues of toxicity, resistance, high cost, requirement for a cold chain and the practicalities of parenteral administration.1 In the hyperendemic state of Bihar, India, where there is widespread clinical failure with pentavalent antimonials, amphotericin B (AmB) deoxycholate, paromomycin and miltefosine are being used as alternative first-line drugs. The liposomal formulation of AmB has minimal toxicity and good efficacy, even with a single-dose regimen,2 but it is not widely available due to expense, despite the preferential pricing (US$18/50 mg) negotiated by the WHO.
The development of new drug delivery systems is attractive as it allows optimization of the pharmacological profile and the therapeutic properties of existing drugs.3 Within the family of nanomaterials, carbon nanotubes (CNTs) have emerged as a new and efficient tool for transporting therapeutic molecules,4 due to their unique physical and chemical properties. Pristine CNTs (non-functionalized) are difficult to dissolve in most organic or inorganic solvents because of their long structured features or aggregation. This problem can be overcome by functionalized CNTs (f-CNTs), which enable chemical covalent or non-covalent bonding between the CNTs and the material of interest.5–7 Formation of amino-terminated CNTs is a critical step in covalently linking nanotubes and biomolecules because amino groups act as chemical bridges. Furthermore, the f-CNTs, irrespective of functional group and chemistry, offer significant improvements over the toxicity profile of CNTs, in vitro and in vivo.6,8,9
The ability of f-CNTs to cross cell membranes and to deliver peptides, proteins and nucleic acids into cells makes them useful as vehicles for drug delivery against intracellular targets. Studies on the impact of f-CNTs on cells of the immune system have demonstrated in vitro that f-CNTs are taken up by lymphocytes and macrophages, without affecting cell viability.10 The present paper deals with an important formulation of f-CNTs and their ability to facilitate the action of the established antileishmanial drug AmB when employed to kill L. donovani.
Materials and methods
Attachment of AmB to f-CNTs
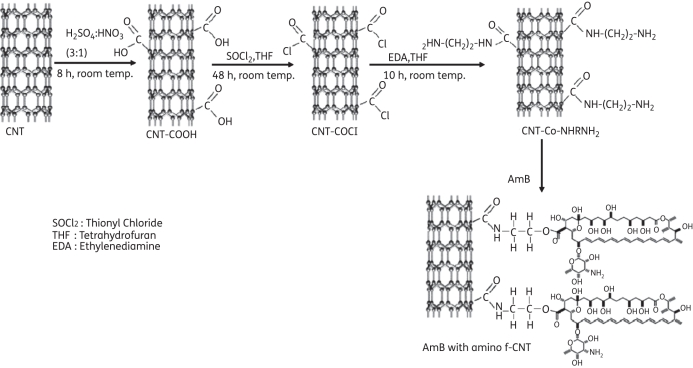
Multiwalled CNTs were synthesized by spray pyrolysis of benzene and ferrocene at 850°C under an argon atmosphere.11 These CNTs were treated with a concentrated H2SO4/HNO3 mixture to form a stable aqueous suspension containing individual oxidized CNTs with carboxyl groups (Figure 1). Then carboxylated CNTs were treated with ethylenediamine [NH2(CH2)2NH2], forming an active amine group on the nanotube surface.12 The amino f-CNTs (∼25 mg) were dispersed in deionized water (∼10 mL) and 25 mg of AmB powder was added and sonicated for 2 h. The AmB solution was mixed with f-CNT solution and sonicated in an ultrasonication bath at room temperature for 24 h. During this process the amine group of CNTs reacts with the carboxylic group of AmB, forming an ether bond between the f-CNTs and AmB. This solution was centrifuged and washed with deionized water four times to remove unbound AmB. Functionalization and attachment were confirmed by Fourier transform infrared (FTIR) spectroscopy.13,14 The FTIR spectra of the prepared samples were recorded in transmittance mode using Nicolet 5700 (Thermonicolet, USA) and Perkin-Elmer (Spectrum 100, USA) spectrometers. Transmission electron microscopy (TEM) observations of the sample were performed using a 200 kV Tecnai 20 G2 electron microscope.
Figure 1.
Schematic representation of the formation of amino f-CNTs and attachment of AmB to f-CNTs. The amino f-CNTs were prepared by reaction of ethylenediamine with carboxylic groups of f-CNTs. These amino f-CNTs were reacted with the carboxylic group of AmB.
AmB loading efficiency on f-CNTs
AmB dissolved in DMSO is known to show maximum absorbance at a wavelength of 408 nm.15 A preparation of f-CNT–AmB in DMSO was centrifuged at 6000 rpm for 10 min. The optical density of unattached, dissolved AmB remaining in the supernatant was measured using a UV–visible spectrophotometer (SpectraMax-190; Molecular Device) and compared with the estimated optical density of a known concentration of AmB (1 mg/mL) to calculate loading efficiency. Four separate preparations of f-CNT–AmB were assessed for loading efficiency to ensure reproducibility.
Cell lines and culture conditions
The WHO reference strain of L. donovani MON2 (MHOM/IN/80/DD8) was used for both in vitro and in vivo work. These parasites and the macrophage cell line J774A.1 were grown in supplemented RPMI 1640 medium as described previously.16
Animals
Commercially acquired female BALB/c mice (25–35 g) were used as an in vivo model for assessing cytotoxicity.17 Syrian golden male hamsters, Mesocricetus auratus (45–50 g), were used to study the antileishmanial effects of the compounds because the hamster is an optimal model of L. donovani infection as it most closely resembles the clinical and immunopathological response in humans.18 The animals were maintained in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals, Delhi, India. All animal studies were carried out in accordance with protocols approved by the Central Animal Ethics Committee (CAEC) at the Institute of Medical Sciences, Banaras Hindu University (CAEC number Dean/10-11/185).
In vitro assessment of cytotoxicity
The cytotoxicity of AmB, f-CNTs and f-CNT–AmB was assessed as described previously.16 Briefly, 5 × 104 J774A.1 macrophages were aliquotted into 96-well plates and incubated in triplicate with AmB (0.005–0.64 μg/mL), f-CNTs (0.0625–8.0 μg/mL) or f-CNT–AmB (0.005–0.64 μg/mL) for 72 h and analysed using a 3-(4,5-dimethylthiazole-2-yl)-2-5-diphenyl tetrazolium bromide (MTT) assay. The experiment was performed twice for reproducibility and the concentration required to kill 50% of the cells (CC50) was calculated.
In vivo assessment of toxicity
Forty BALB/c mice were used to assess adverse effects in vivo of a 5 day course of daily intraperitoneal injections of AmB, f-CNTs or f-CNT–AmB, using 5 mg/kg, 10 mg/kg and 20 mg/kg dose regimens. Each drug was tested in 12 mice with 4 mice for each concentration. The control group consisted of four mice injected daily with PBS. On day 5 the mice were euthanized and serum was collected for assessment of hepatic (alkaline phophatase and sodium glucose phosphatase) and renal (urea and creatinine) function using commercially available kits (Merck, India).
In vitro assessment of antileishmanial activity
An in vitro macrophage model was used to assess the antileishmanial activity of AmB and f-CNT–AmB against intracellular amastigotes. Drug dilutions of AmB (0.005–0.02 μg/mL) and f-CNT–AmB (0.00125–0.005 μg/mL) were added in duplicate wells with two control wells to macrophages pre-infected with L. donovani promastigotes. Following 72 h of incubation, the numbers of amastigotes per 100 macrophages were counted and the IC50 of each compound was calculated.16
In vivo assessment of antileishmanial activity
AmB powder (Bharat Serum and Vaccines Ltd, Mumbai, India) dissolved in DMSO at 2.5 mg/mL and the prepared f-CNT–AmB at 2.5 mg/mL were used as stock solutions. AmB, f-CNT and f-CNT–AmB were reconstituted for in vivo administration in 1× PBS at 1.5 mg/mL (injection volume ranged from 200 to 400 μL according to animal weight). Twenty hamsters were infected by intracardiac injection of 1 × 108 L. donovani promastigotes. At 30 days, the infection was confirmed in four randomly selected hamsters by splenic biopsy and performance of splenic dab. AmB, f-CNTs or f-CNT–AmB were injected intraperitoneally at 5 mg/kg body weight per day for 5 days, and in controls PBS was used. The splenic dab, performed on all animals, was analysed by calculating Leishman Donovan units (LDUs) and the percentage inhibition of parasites.16 This experiment was repeated for reproducibility with a further 20 hamsters and the results were collated.
Statistical analysis
GraphPad Prism5 version was used to calculate the CC50 and IC50 values. An unpaired t-test (two-tailed) was applied to determine the significance of the differences between the cytotoxicity and antileishmanial activity of the two drugs AmB and f-CNT–AmB.
Results
Characterization of the construction of f-CNT–AmB by FTIR spectroscopy and TEM
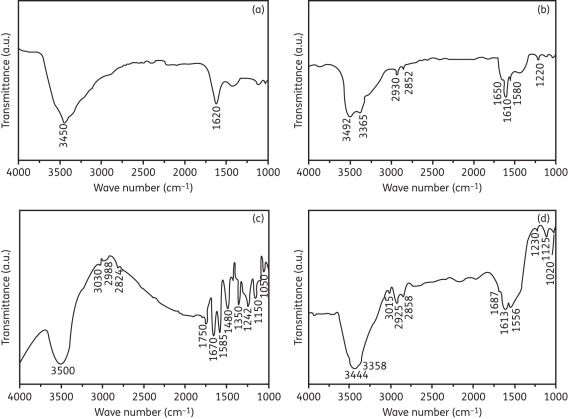
The attachment of functional groups onto the surface of CNTs and linkage of f-CNTs to AmB were examined by FTIR spectroscopy. Figure 2(a) represents the FTIR spectrum of the grown CNTs. The peak at 1620 cm–1 corresponds to the vibration of the carbon skeleton of the CNTs. Another peak at 3450 cm–1 corresponds to the presence of OH groups on the surface of CNTs. Following treatment with concentrated H2SO4/HNO3 to attach carboxylic (COOH) groups, the FTIR spectrum of amino group f-CNTs (Figure 2b) showed a peak at 1650 cm–1, which is associated with the amide carbonyl (C O) stretch. In addition, the presence of new peaks at 1560 and 1220 cm–1 corresponds to N–H in plane and C–N bond stretching, respectively. Two peaks at around 2930 and 2852 cm–1 were due to the C–H stretching mode of CH2 in the ethylenediamine molecule. The small peak at 3365 cm–1 can be assigned to N–H stretching of amide or amine groups. The peak at 3492 cm–1 was due to the N–H stretching of the amine (NH2) group. All these observations confirm that the surfaces of the CNTs have been functionalized with amine groups via amide formation.
O) stretch. In addition, the presence of new peaks at 1560 and 1220 cm–1 corresponds to N–H in plane and C–N bond stretching, respectively. Two peaks at around 2930 and 2852 cm–1 were due to the C–H stretching mode of CH2 in the ethylenediamine molecule. The small peak at 3365 cm–1 can be assigned to N–H stretching of amide or amine groups. The peak at 3492 cm–1 was due to the N–H stretching of the amine (NH2) group. All these observations confirm that the surfaces of the CNTs have been functionalized with amine groups via amide formation.
Figure 2.
FTIR spectra of (a) CNTs, (b) amino f-CNTs, (c) AmB and (d) AmB with amino f-CNTs. In (a) the peak at 1620 cm–1 shows the carbon skeleton of non-functionalized CNTs. In (b) the peaks at 2930 and 3492 cm–1 show the C–H bond of CH2 and the N–H bond of NH2, confirming the functionalization of CNTs. In (c) the peaks at 1750 and 1150 cm–1 represent C O and C–O bonds, respectively, of the ester group and the peak at 1050 cm–1 confirms the C–OH bond present in AmB. In (d) the peak at 1125 cm–1 indicates the existence of the ether group in f-CNT–AmB.
O and C–O bonds, respectively, of the ester group and the peak at 1050 cm–1 confirms the C–OH bond present in AmB. In (d) the peak at 1125 cm–1 indicates the existence of the ether group in f-CNT–AmB.
The FTIR spectrum of AmB confirmed the presence of carboxylic, amine and ester groups (Figure 2c). The peak at 3500 cm–1 was due to the N–H stretching of the amine groups. The peaks at 3030, 2988 and 2824 cm–1 represent the C–H stretching vibrations of the alkene and alkane groups, and C–N stretching vibration appears at 1350 cm–1. The peak at 1585 cm–1 can be assigned to the N–H stretching vibration of amine groups and C C of alkene groups. The peaks at 1670, 1480 and 1242 cm–1 represent C
C of alkene groups. The peaks at 1670, 1480 and 1242 cm–1 represent C O, OH and C–O stretching vibrations of carboxylic groups. The peaks at 1750 and 1150 cm–1 represent C
O, OH and C–O stretching vibrations of carboxylic groups. The peaks at 1750 and 1150 cm–1 represent C O and C–O bonds, respectively, indicating the existence of an ester group. The peak at 1050 cm–1 was associated with the C–OH bond present in AmB.
O and C–O bonds, respectively, indicating the existence of an ester group. The peak at 1050 cm–1 was associated with the C–OH bond present in AmB.
During attachment the carboxylic group of AmB reacted with the amine group of f-CNTs, turning the O–H bond of the carboxylic group into a new ether bond, which was represented by the peak at 1125 cm–1 (Figure 2d). The peak at 3492 cm–1 of the NH2 group of f-CNTs was absent in the f-CNT–AmB spectrum; this provides evidence of AmB attachment to the f-CNTs. The peaks at 1687, 1556 and 1230 cm–1 represent C O, N–H and C–N stretching, respectively, indicating the presence of an amide group. The assignments of specific radicals/chemical species to the observed wave numbers have been done based on previously described results.19,20
O, N–H and C–N stretching, respectively, indicating the presence of an amide group. The assignments of specific radicals/chemical species to the observed wave numbers have been done based on previously described results.19,20
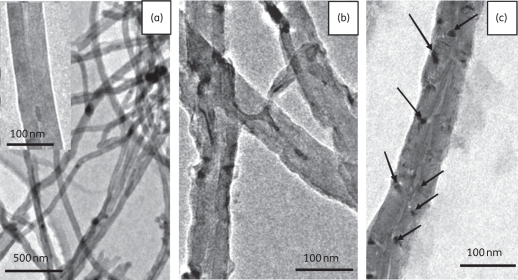
The morphology of the multiwalled CNTs (non-functionalized) [Figure 3(a)], f-CNTs and f-CNT–AmB was observed by TEM. The non-functionalized CNTs were almost free of amorphous carbon and catalyst particles and their diameter and length were ∼40–70 nm and ∼2–8 μm, respectively. The f-CNTs shown in Figure 3(b) have rough outer surfaces in comparison with nanotubes without functionalization. The treatment of CNTs with strong oxidizing agents caused some etching of the graphitic surfaces of the material, leading to nanotubes with disordered sites. These sites were helpful for providing points for the attachment of AmB. In Figure 3(c), which shows the f-CNT–AmB molecules, a dark mark can be observed on the surface of each f-CNT, indicating the attachment of AmB. The synthesis of f-CNT–AmB was performed on a large scale and it was stored at room temperature for at least 6 months without any loss of efficacy.
Figure 3.
TEM images of (a) synthesized CNTs (the inset shows the magnified image of a CNT), (b) amino f-CNTs and (c) AmB with amino f-CNT (the arrows show the attachment of AmB to an amino f-CNT). (a) Smooth surfaces of non-functionalized CNTs. (b) After functionalization, surfaces become rough, providing attachment sites for AmB.
Drug loading efficiency on f-CNTs
The UV–visible spectrophotometer showed absorbances of 3.547 and 1.007 at 408 nm for 1 mg/mL AmB and unattached AmB from the supernatant of f-CNT–AmB, respectively. Therefore the loading efficiency of AmB onto f-CNTs was calculated to be 72.4%. This percentage loading efficiency was reproducible.
In vitro cytotoxicity
The rank order of cytotoxicity of the three compounds against the J774A.1 cell line was AmB (0.48 ± 0.06 μg/mL) > f-CNT–AmB (0.66 ± 0.17 μg/mL) > f-CNTs (7.31 ± 1.16 μg/mL) (P < 0.05). These data represent the mean ± SD of two experiments performed in triplicate.
In vivo toxicity
No renal or hepatic toxicity in BALB/c mice was noted at any drug concentration with AmB, f-CNTs or f-CNT–AmB. There were no significant differences observed in the assessment of urea (range = 42.4–75.3 mg/dL), creatinine (range = 0.3–0.4 mg/dL) or alkaline phosphatase (range = 118.8–123.5 U/L) between the control and tested compound groups at all doses. In the measurement of sodium glucose phosphatase (range = 57.3–80.0 U/L) a slight elevation was observed between the control group (72.5 ± 4.04 U/L) and the group that received 20 mg/kg f-CNT–AmB (85.25 ± 6.71 U/L). The body weight of animals did not decrease in any groups during the experiment but a mild inflammatory reaction was noted at the injection site in the f-CNT and f-CNT–AmB groups at all concentrations. This reaction was well tolerated by the mice.
In vitro antileishmanial activity
The antileishmanial activity of the three compounds was AmB (IC50 0.03263 ± 0.00123 μg/mL), f-CNT (0.00544 ± 0.00143 μg/mL) and f-CNT–AmB (IC50 0.00234 ± 0.00075 μg/mL). The IC50 of f-CNT–AmB shows that it is 13.94 times more potent than AmB against intramacrophage amastigotes (P = 0.0001).
In vivo drug assay in hamsters
The results from both in vivo studies involving 20 hamsters were merged and mean values were taken for analysis. Four hamsters died during the course of the experiments due to reasons unrelated to the experiment. The rank order of antileishmanial efficacy of the three compounds was f-CNT–AmB > AmB > f-CNTs. The percentage suppression of parasites in the spleen was significantly higher with f-CNT–AmB (89.8%) than with AmB (68.9%) (P = 0.0004; Table 1).
Table 1.
In vivo tests in Syrian golden hamsters
| Group | Before treatment, n = 4 | f-CNT–AmB (group A), n = 10 | AmB (group B), n = 8 | f-CNT (group C), n = 5 | Control (group D), n = 9 | P valuea |
|---|---|---|---|---|---|---|
| Spleen weight (g), mean ± SD | 0.9 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.1 | 0.0208 |
| Amastigotes/500 nuclei | 2627 ± 141.2 | 266.7 ± 76.9 | 815.2 ± 48.3 | 2822.7 ± 196.2 | 5234.9 ± 982.6 | 0.0004 |
| Parasite burden (LDUb) (post-treatment) (×104) | 2.6 ± 0.4 | 0.2 ± 0.1 | 0.6 ± 0.1 | 3.2 ± 0.7 | 7.9 ± 1.7 | 0.0004 |
| Percentage inhibition of splenic parasite load | 89.8 ± 2.9 | 68.9 ± 1.8 | 44.7 ± 2.5 | 0.0004 | ||
| Percentage suppression of parasite replication | 94.9 ± 1.5 | 84.4 ± 0.9 | 0.0004 |
aP value between groups A (f-CNT–AmB) and B (AmB).
bNumber of amastigotes per 500 nuclei × tissue weight (mg).
Discussion
These experiments show a novel approach to antileishmanial therapy. By employing the drug delivery system of f-CNTs to the known antileishmanial drug AmB, its antileishmanial efficiency is significantly increased in both in vivo and in vitro settings. This, together with low cytotoxicity of f-CNT–AmB, means that it is a viable compound for further drug development.
The f-CNTs have several advantages when used as a nanovector for therapeutic molecules. The functional groups of CNTs when modified with therapeutic molecules are stably attached to the nanotube backbone and therefore avoid the risk of macromolecule desorption.21 The chemical synthesis of f-CNT–AmB involves covalent coupling rather than biological molecules, which makes it cheaper to make than existing liposomal AmB.
The intracellular nature of L. donovani makes it suitable for targeted drug delivery by f-CNTs. Both in vitro and in vivo experiments demonstrated a marked increase in the efficacy of f-CNT–AmB compared with AmB. Furthermore, the addition of f-CNTs to AmB reduced the cytotoxicity of AmB in vitro as demonstrated by the CC50. The therapeutic window between the IC50 (0.00234 μg/mL) and CC50 (0.66 μg/mL) of f-CNT–AmB is wider than that of AmB, thus improving its safety. This potential was confirmed by minimal observed cytotoxicity in our in vivo studies.
To the best of our knowledge, this is the first description of the use of f-CNT as therapeutic nanovector for the most effective antileishmanial drug, AmB, in the treatment of this neglected tropical disease, VL. Our data demonstrate the superiority of f-CNT–AmB over AmB. This provides a possibility of eventually using this f-CNT–AmB formulation in the clinical setting with greater antileishmanial efficacy and lower toxicity. Furthermore its production profile is favourable in comparison with liposomal AmB with low-cost mass production and stable storage at room temperature. Further studies are ongoing to develop it as an antileishmanial drug, to compare its efficacy with that of liposomal AmB and to explore its potential for oral administration.
Funding
CSIR and DST (UNANST: BHU) provided financial assistance. The work was partly supported by NIAID, NIH grant no. 1P50AI074321. ICMR (New Delhi, India) provided financial assistance to V. K. P.
Transparency declarations
None to declare.
Acknowledgements
We are extremely grateful to Professor C. N. R. Rao (FRS) and Professor D. P. Singh (Vice Chancellor, BHU) for their encouragement and support. Helpful discussion with Professor A. S. K. Sinha is gratefully acknowledged.
References
- 1.Sundar S, More DK, Singh MK, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–7. doi: 10.1086/318121. doi:10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Chakravarty J, Agarwal D, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–12. doi: 10.1056/NEJMoa0903627. doi:10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. doi:10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 4.Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomedicine. 2008;4:183–200. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Song C, Pehrsson PE. Water-soluble and optically pH-sensitive single-walled carbon nanotubes from surface modification. J Am Chem Soc. 2002;124:12418–9. doi: 10.1021/ja027861n. doi:10.1021/ja027861n. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Sethuraman A, Jin C, et al. Biological properties of carbon nanotubes. J Nanosci Nanotechnol. 2007;7:1284–97. doi: 10.1166/jnn.2007.655. doi:10.1166/jnn.2007.655. [DOI] [PubMed] [Google Scholar]
- 7.Georgakilas V, Tagmatarchis N, Pantarotto D, et al. Amino acid functionalisation of water soluble carbon nanotubes. Chem Commun (Camb) 2002:3050–1. doi: 10.1039/b209843a. [DOI] [PubMed] [Google Scholar]
- 8.Lacerda L, Bianco A, Prato M, et al. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460–70. doi: 10.1016/j.addr.2006.09.015. doi:10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Smart SK, Cassady AI, Lu GQ, et al. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–47. doi:10.1016/j.carbon.2005.10.011. [Google Scholar]
- 10.Dumortier H, Lacotte S, Pastorin G, et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–8. doi: 10.1021/nl061160x. doi:10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava A, Srivastava ON, Talapatra S, et al. Carbon nanotube filters. Nat Mater. 2004;3:610–4. doi: 10.1038/nmat1192. doi:10.1038/nmat1192. [DOI] [PubMed] [Google Scholar]
- 12.Awasthi K, Singh DP, Singh SK, et al. Attachment of biomolecules (protein and DNA) to amino-functionalized carbon nanotubes. New Carbon Materials. 2009;24:301–6. doi:10.1016/S1872-5805(08)60053-0. [Google Scholar]
- 13.Wu W, Wieckowski S, Pastorin G, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed Engl. 2005;44:6358–62. doi: 10.1002/anie.200501613. doi:10.1002/anie.200501613. [DOI] [PubMed] [Google Scholar]
- 14.Vossoughi M, Gojgini S, Kazemi A, et al. Conjugation of amphotericin B to carbon nanotubes via amide-functionalization for drug delivery applications. Engineering Letters. 2009;17 EL_17_4_2. [Google Scholar]
- 15.Mbongo N, Loiseau PM, Billion MA, et al. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 1998;42:352–7. doi: 10.1128/aac.42.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manandhar KD, Yadav TP, Prajapati VK, et al. Antileishmanial activity of nano-amphotericin B deoxycholate. J Antimicrob Chemother. 2008;62:376–80. doi: 10.1093/jac/dkn189. doi:10.1093/jac/dkn189. [DOI] [PubMed] [Google Scholar]
- 17.Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, et al. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2010;4:1481–92. doi: 10.1021/nn901573w. doi:10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- 18.Kaur J, Sundar S, Singh N. Molecular docking, structure-activity relationship and biological evaluation of the anticancer drug monastrol as a pteridine reductase inhibitor in a clinical isolate of Leishmania donovani. J Antimicrob Chemother. 2010;65:1742–8. doi: 10.1093/jac/dkq189. doi:10.1093/jac/dkq189. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Huang W, Wu L, et al. Study on amino-functionalized multiwalled carbon nanotubes. Materials Science and Engineering: A. 2007;464:151–6. doi:10.1016/j.msea.2007.02.091. [Google Scholar]
- 20.Rao CNR. Chemical Applications of Infrared Spectroscopy. New York: Academic Press; 1963. pp. 611–7. [Google Scholar]
- 21.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4:627–33. doi: 10.1038/nnano.2009.241. doi:10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]