Abstract
Childhood maltreatment is a stressor that can lead to the development of behavior problems and affect brain structure and function. This review summarizes the current evidence for the effects of childhood maltreatment on behavior, cognition and the brain in adults and children. Neuropsychological studies suggest an association between child abuse and deficits in IQ, memory, working memory, attention, response inhibition and emotion discrimination. Structural neuroimaging studies provide evidence for deficits in brain volume, gray and white matter of several regions, most prominently the dorsolateral and ventromedial prefrontal cortex but also hippocampus, amygdala, and corpus callosum (CC). Diffusion tensor imaging (DTI) studies show evidence for deficits in structural interregional connectivity between these areas, suggesting neural network abnormalities. Functional imaging studies support this evidence by reporting atypical activation in the same brain regions during response inhibition, working memory, and emotion processing. There are, however, several limitations of the abuse research literature which are discussed, most prominently the lack of control for co-morbid psychiatric disorders, which make it difficult to disentangle which of the above effects are due to maltreatment, the associated psychiatric conditions or a combination or interaction between both. Overall, the better controlled studies that show a direct correlation between childhood abuse and brain measures suggest that the most prominent deficits associated with early childhood abuse are in the function and structure of lateral and ventromedial fronto-limbic brain areas and networks that mediate behavioral and affect control. Future, large scale multimodal neuroimaging studies in medication-naïve subjects, however, are needed that control for psychiatric co-morbidities in order to elucidate the structural and functional brain sequelae that are associated with early environmental adversity, independently of secondary co-morbid conditions.
Keywords: child abuse, maltreatment, fMRI, DTI, PTSD, executive functions, prefrontal cortex, limbic system
Introduction
Child maltreatment, or abuse, is the physical, sexual, emotional mistreatment, or neglect of children. Child maltreatment is defined as any act or series of acts by a parent or caregiver that results in harm, potential for harm, or threat of harm to a child (Leeb et al., 2008). There are four major categories of child abuse: neglect, physical abuse, emotional abuse, and sexual abuse (Giovannoni and Becerra, 1979). Neglect is the failure to provide for the shelter, safety, supervision and nutritional needs of the child and may be physical, e.g., lack of health care, abandonment, inadequate supervision; educational, e.g., allowance of chronic truancy, failure to enrol a child in school, or emotional, e.g., inattention to the child's needs for affection, refusal of or failure to provide needed psychological care, and permission of drug or alcohol use by the child (English et al., 2005). In physical abuse an injury is inflicted on the child by a caregiver via various non-accidental means, including hitting with a hand, stick, strap, or other object; punching; kicking; shaking; throwing; burning; stabbing; or choking (Sedlak and Broadhurst, 1996). Emotional abusers reject, isolate, terrorize, ignore, and corrupt their victims (Garbarino and Garbarino, 1994). Examples of emotional abuse include verbal abuse, penalizing a child for positive/normal behavior and witnessing domestic violence. Child sexual abuse is any sexual act with a child performed by an adult or older child including intercourse, attempted intercourse, oral-genital contact, fondling of genitals directly or through clothing, exhibitionism, exposing children to adult sexual activity or pornography, and the use of the child for prostitution or pornography (Putnam, 2003a).
Early child abuse has many behavioral consequences including most prominently internalizing behavioral problems such as limited stress tolerance, anxiety, affective instability, depression, suicidality, Post-Traumatic Stress Disorder (PTSD), dissociative disturbances and hallucinatory phenomena, but also externalizing behavioral symptoms including poor impulse control, episodic aggression, substance abuse, Attention Deficit Hyperactivity Disorder (ADHD) and conduct disorder (CD) (Rohsenow et al., 1988; van der Kolk et al., 1991; Kendall-Tackett et al., 1993; Kessler et al., 1997; Brodsky et al., 1997, 2008; Kingree et al., 1999a,b; Osofsky, 1999; Thompson et al., 1999a,b; Heffernan and Cloitre, 2000; Heffernan et al., 2000; Kendler et al., 2000; De Bellis, 2002; Clark et al., 2003; Putnam, 2003). Early adversities in the life of a child thus have been shown to have detrimental effects on the mental health of the child. Child abuse and early life stress has furthermore been associated with a series of cognitive problems such as low academic performance and IQ, as well as with specific deficits in language, memory, inhibition, and attention deficits (For a review see Pechtel and Pizzagalli, 2010).
Childhood maltreatment is a severe stressor that, in addition to behavioral and cognitive problems, produces a cascade of physiological, neurochemical, and hormonal changes, which can lead to enduring alterations in brain structure and brain function (Teicher et al., 2003, 2006). The human brain is still developing during childhood through processes of synaptic remodeling, activity dependent myelination and programmed cell death, affecting both gray and white matter organization (de Graaf-Peters and Hadders-Algra, 2006). Longitudinal structural imaging studies show a linear increase with age in white matter that is most pronounced between early childhood and adolescence, but undergoes progressive increase until peaking at around age 45 (Sowell et al., 2003). Gray matter (GM) undergoes substantial non-linear changes, with an increase up to age 10, thought to be due to glial cell proliferation, dendritic and axonal branching and a decrease after age 10 due to synaptic pruning and myelination (Sowell et al., 2003). Hence, trauma or stress during this early life period has the potential to disrupt these neurodevelopmental processes. It is thought that this is partly modulated via three major neurobiological stress response systems: the serotonin system, the sympathetic nervous system (SNS)/catecholamine system and the hypothalamic-pituitary-adrenal (HPA) axis (Watts-English et al., 2006). These systems significantly influence stress reactions, arousal, emotional regulation, brain development and cognitive development and can contribute to long-term negative consequences (for a review see McCrory et al., 2010; Twardosz and Lutzker, 2010).
This article will review the existing evidence for associations between child maltreatment and cognitive and neuroimaging abnormalities. It will outline current findings of neuropsychological studies of the main cognitive deficits implicated in survivors of childhood maltreatment, including IQ, memory, executive functions (EFs), working memory, attention and emotion processing. It will then discuss findings of structural and functional deficits associated with childhood maltreatment as shown by modern imaging techniques, including structural and functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and positron emission tomography (PET). The review will critically discuss the many limitations of previous studies and shape out directions for future work.
Cognitive deficits associated with childhood maltreatment
Academic performance and IQ
Impaired academic performance has been observed in children who experienced neglect (Kendall-Tackett and Eckenrode, 1996) or early institutionalization (Loman et al., 2009) and in adults with a history of childhood sexual abuse (Navalta et al., 2006) or mixed maltreatment histories (Majer et al., 2010). Only one study, however, included subjects that were free of any current and lifetime psychiatric diagnoses (Majer et al., 2010). The other studies either did not screen for co-morbidities (Kendall-Tackett and Eckenrode, 1996; Loman et al., 2009) or did not control for comorbidities (Navalta et al., 2006).
Lower IQ in maltreated children relative to healthy controls is a consistent finding in the literature and has been reported in physically (Carrey et al., 1995; Prasad et al., 2005; Nolin and Ethier, 2007) and sexually abused children (Carrey et al., 1995); neglected children (De Bellis et al., 2009) and post-institutionalized children (Pollak et al., 2010). In many of these studies IQ was found to be related to the severity of maltreatment (e.g., Carrey et al., 1995; De Bellis et al., 2009). However, most of these studies did not control for PTSD or other psychiatric diagnoses (Carrey et al., 1995; Prasad et al., 2005; Nolin and Ethier, 2007; Pollak et al., 2010). Two studies attempted to tease apart the effects of abuse and PTSD on IQ with, however, conflicting results. One reported that lower IQ was related to abuse, regardless of PTSD status (De Bellis et al., 2009), whilst the other suggests that PTSD, and not a history of trauma exposure in the absence of PTSD, is associated with lower verbal IQ (Saigh et al., 2006). The relationship between IQ, maltreatment and PTSD is hence unclear. Studies of adults who were maltreated as children do not generally report IQ differences relative to controls (Bremner et al., 1995; Twamley et al., 2004), suggesting that if IQ is associated with maltreatment it may normalize with age.
Memory
Significant impairments in short and long-term memory functions have been reported in children who have been subjected to unspecified maltreatment (Beers and De Bellis, 2002), traumatic events including physical and sexual abuse, motor accidents and being shot (Yasik et al., 2007), witnessing intimate partner violence (Samuelson et al., 2010), and institutionalization (Bos et al., 2009). This has also been reported in adults with childhood sexual abuse (Bremner et al., 2004; Navalta et al., 2006) and mixed maltreatment including neglect, sexual, physical, and emotional abuse (Bremner et al., 1995; Majer et al., 2010). One of these studies tested non-comorbid patients (Majer et al., 2010). Most of these studies, however, have mostly tested subjects with maltreatment-related PTSD and other psychiatric comorbidities including Major Depressive Disorder (MDD), dysthymic disorder, Oppositional Defiant Disorder (ODD), Dissociative Identity Disorder (DID), Anxiety Disorders, ADHD, Alcohol/drug abuse, (Bremner et al., 1995, 2004; Beers and De Bellis, 2002; Yasik et al., 2007; Samuelson et al., 2010). Some studies have reported a correlation between these memory impairments and the severity or duration of abuse (Bremner et al., 1995; Navalta et al., 2006). However, there have also been negative findings of no memory impairment in adults (Pederson et al., 2004) or children subjected to childhood maltreatment (Nolin and Ethier, 2007). Only three studies have directly compared maltreated subjects with and without PTSD (Bremner et al., 2004; Yasik et al., 2007; Samuelson et al., 2010) and found that only maltreated subjects with PTSD had verbal memory deficits, suggesting that verbal memory deficits are specific to PTSD and not due to maltreatment per se.
Working memory
Impairments of working memory have been reported in adults with a history of emotional abuse and neglect (Majer et al., 2010) or physical abuse (Raine et al., 2001). Negative findings, however, have also been reported (Pederson et al., 2004; Twamley et al., 2004). Working memory was found to be impaired in children with mixed maltreatment histories, including sexual and physical abuse and witnessing domestic violence (DePrince et al., 2009) and post-institutionalized children (Bos et al., 2009; Pollak et al., 2010). With the exception of Majer et al. (2010) the above mentioned studies either did not report or reported but did not control for co-morbidities (Raine et al., 2001; Twamley et al., 2004; Navalta et al., 2006; Bos et al., 2009; DePrince et al., 2009; Pollak et al., 2010) making it impossible to distinguish whether the observed deficits were due to maltreatment or to these co-morbidities.
Attention
Auditory and visual attention deficits have been observed in children who have been subjected to neglect and physical abuse (Nolin and Ethier, 2007), sexual and physical abuse (DePrince et al., 2009), unspecified maltreatment (Beers and De Bellis, 2002) and institutionalization (Pollak et al., 2010). Again, these studies did not report (Nolin and Ethier, 2007; Pollak et al., 2010) or control for co-morbidities in the maltreated groups (Beers and De Bellis, 2002; DePrince et al., 2009).
Inhibitory control
Deficits of inhibitory control have been consistently observed in adults with a history of maltreatment (Navalta et al., 2006), adolescents with early life stress (Mueller et al., 2010) and maltreated and post-institutionalized children (Mezzacappa et al., 2001; DePrince et al., 2009; Pollak et al., 2010). These studies also did not report or control for co-morbidities (Pollak et al., 2010) or reported but did not control for co-morbidities (Mezzacappa et al., 2001; Navalta et al., 2006; DePrince et al., 2009; Mueller et al., 2010). An additional confound of Mezzacappa et al. (2001) study is that many of the subjects were taking medication, including psychostimulants, antidepressants, mood stabilizers and alpha-2 adrenergic agonists, which may have influenced the results. A small sampled study from our group found that Romanian orphans with inattentive and overactive symptoms who had suffered from severe early institutionalized neglect had more inhibition and IQ deficits than standard ADHD clinical cases and healthy controls with reasonable effects sizes (Sonuga-Barke and Rubia, 2008).
Emotion processing
Young maltreated children have been shown to exhibit difficulties correctly identifying and discriminating facial expressions of emotions (During and McMahon, 1991; Pollak et al., 2000; Pollak and Sinha, 2002; Pollak and Tolley-Schell, 2003; Wismer-Fries and Pollak, 2004; Pears and Fisher, 2005; Pine et al., 2005; Vorria et al., 2006). None of these studies, however, reported or screened for co-morbidities. There have been no studies of emotion perception in adults with a history of childhood maltreatment but studies of older children have not shown these same impairments in the ability to discriminate between emotional expressions (Pine et al., 2005; Maheu et al., 2010). This suggests that emotion discrimination difficulties in maltreated children may normalize with age.
It is also likely that the type of maltreatment has an effect on emotion discrimination ability. Neglected and post-institutionalized children may be more likely to have difficulty discriminating emotions (Pollak et al., 2000; Wismer-Fries and Pollak, 2004; Vorria et al., 2006). Physically abused children, however, may be more likely to identify and react to particular negative emotions, such as anger, fear, and pain as they are more likely to witness anger and subsequent adversity and are more likely to experience fear and pain (Pollak and Sinha, 2002; Pollak and Tolley-Schell, 2003; Pine et al., 2005). This is supported by the study of Pollak et al. (2000) who reported that neglected children had more difficulty discriminating between emotional expressions, whereas physically abused children displayed a response bias for angry facial expressions, perceiving more distinction between anger and other negative emotional expressions.
Other studies have shown that physically abused children respond quicker to angry faces and accurately identified facial displays of anger on the basis of less sensory input than did controls (Pollak and Sinha, 2002; Pollak and Tolley-Schell, 2003). Pine et al. (2005) reported a correlation between threat bias and severity of physical maltreatment, suggesting that more severely physically abused children tended to direct attention away from angry/threatening faces. One study has also suggested that post-institutionalized children may respond differently to anger than to other emotions, reporting that these children had difficulty both in identifying and matching all emotions except for anger but performed as well as controls when identifying angry facial expressions (Wismer-Fries and Pollak, 2004). Children exposed to severe early life stress also experience emotion regulation difficulties, which are thought to confer risks for later psychopathology (Lyons-Ruth, 2008; Tottenham et al., 2010).
Summary
There is consistent evidence of decreased IQ in abused children but not in adults with a history of abuse. However, the majority of studies have not controlled for PTSD or other co-morbid conditions and those that have controlled for these produced inconsistent results. Future large sampled studies are needed to disentangle the effects of maltreatment and co-morbid conditions such as PTSD on IQ.
There is evidence for verbal and visual memory deficits in maltreated children and adults and evidence suggests that, while verbal memory impairments may be associated with PTSD, pattern recognition memory may possibly be associated with maltreatment itself. Memory deficits are likely to be due to disruption to the normal development of the underlying neural circuitries including hippocampus, amygdala, dorsolateral prefrontal cortex (DLPFC) and striatum (McGaugh, 2000).
Deficits of working memory, attention and motor inhibition have consistently been observed in maltreated children and adults. These functions are known to develop late in adolescence and to improve between childhood and adulthood (Williams et al., 1999) due to progressively increasing activation in late developing underlying lateral fronto-striatal and fronto-parietal circuitries (Rubia et al., 2006, 2007, 2010; Christakou et al., 2009; Geier and Luna, 2009). It is possible that the development of these circuits may be affected by early stress experiences even in high-functioning abuse survivors.
Younger maltreated children have consistently been shown to exhibit emotion discrimination deficits, but older children with a history of maltreatment do not present with the same deficits suggesting normalization with age. Neglected children tend to have difficulties in discriminating between emotions, whereas physically abused children exhibit a response bias toward negatively valenced emotions, especially anger. However, none of the studies of emotion processing controlled for co-morbid conditions and it is not clear whether abnormal emotion processing is due to abuse or to co-morbid conditions. Emotion discrimination and regulation deficits are likely to be related to disruption to the development of fronto-limbic neural circuits involved in emotion, including the amygdala, ventromedial, and orbital prefrontal cortex, anterior cingulate cortex (ACC), ventral striatum, insula, and cerebellum (Ochsner and Gross, 2005).
Structural brain changes associated with childhood maltreatment
Global volume or GM abnormalities
Abuse or maltreatment in early life has been shown to correlate with structural brain differences. Compared to controls, children with maltreatment-related PTSD have been shown to have smaller cerebral volumes and total lateral ventricles and larger cortical and prefrontal cortical cerebrospinal fluid (CSF) volumes (De Bellis et al., 1999, 2002a). Both of these studies had good sample sizes, but a large proportion of PTSD subjects (86–89%) had co-morbid psychiatric conditions (see Table 1 for details) which could have confounded the findings. Nevertheless, both studies found that brain volume correlated positively with age of onset of trauma and negatively with duration of abuse, suggesting a direct link between abuse and brain structure.
Table 1.
A summary table of the characteristics of structural neuroimaging studies of childhood maltreatment, reporting study type, sample size (N), gender proportion of the maltreated group (female/male), whether the study is on an adult or pediatric population (A/P), maltreatment type(s) included in the maltreated group, percentage of the maltreated group with PTSD and other co-morbidities, whether the maltreated group were taking any psychoactive medication, whether the study was ROI or whole brain (WB) and whether any correlations were reported between the results and maltreatment onset or duration (M), PTSD symptoms (P) or functional impairment (F).
| Article | Type | N (M/C) | Gender (f/m) | Adult/pediatric | Maltreatment type | % PTSD | Other co-morbidities | Medication | ROI/WBA | Correlations (M/P/F) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al., 2008 | sMRI | 26/17 | 26/0 | A | SA | 33 | Lifetime and current: MDD (58%), DD/OCD (8% each), ADHD/BD/SAD/BN (4% each) | N/R | ROI | Yes—M |
| Bremner et al., 1997 | sMRI | 17/17 | 5/12 | A | PA, SA | 100 | MDD (29% current, 86% lifetime), BD (14%), social phobia (21%) | N/R | ROI | / |
| Bremner et al., 2003 | sMRI and PET | 22/11 | 22/0 | A | SA | 45 | Inc MDD (20% current, 80% lifetime), alcohol/substance abuse/dependence (40%). | No—stopped 4 weeks prior | ROI | Yes—P |
| Carrion et al., 2001 | sMRI | 24/24 | 10/14 | P | WDV, PA, SA, N, EA | 50 (& 50 subthreshold) | 65.2% MDD, DD (NOS), social phobia, ADHD, SAD, GAD, simple phobia | No | ROI | / |
| Carrion et al., 2007 | sMRI | 15/0 | 9/6 | P | WDV, PA, SA, N, EA | 100 | N/R | No | ROI | Yes—P |
| Carrion et al., 2009 | sMRI and VBM | 24/24 | 10/14 | P | WDV, PA, SA, N, EA | 50 (& 50 subthreshold) | 65.2% MDD, DD (NOS), social phobia, ADHD, SAD, GAD, simple phobia | No | Both | / |
| Cohen et al., 2006 | sMRI | 265 Healthy-ACEs | Approx 50:50 | A | EA, N, WDV, PA, SA | 0 | 0 | No | ROI | Yes—M |
| De Bellis et al., 1999 | sMRI | 44/61 | 19/25 | P | SA, PA, WDV, N | 100 | 86% MDD, dysthymia, ODD, ADHD | No—stopped 2 weeks prior | ROI | Yes—M |
| De Bellis et al., 2001 | sMRI | 9/9 | 4/5 | P | SA | 100 | 88.9% MDD, ODD, ADHD, SAD | No | ROI | / |
| De Bellis et al., 2002a | sMRI | 28/66 | 14/14 | P | SA, PA, WDV, N | 100 | 89% MDD, dysthymia, ODD, ADHD, suicidal ideation | No | ROI | Yes—M |
| De Bellis et al., 2002b | sMRI | 43/61 | 18/25 | P | PA, SA, EA, WDV | 100 | Not reported but AN, autism, schizophrenia excluded | No | ROI | / |
| De Bellis and Keshavan, 2003 | sMRI | 61/122 | 30/31 | P | SA, PA, WDV, EA | 100 | 86.8% dysthymia, MDD, ODD, ADHD, SAD | No | ROI | / |
| De Bellis and Kuchibhatla, 2006 | sMRI | 58/98 (& 13 GAD) | 28/30 | P | SA, WDV | 100 | Dysthymia (60%), MDD (52%), ODD (43%), ADHD (34%), SAD (5%) | No | ROI | Yes—M |
| Driessen et al., 2000 | sMRI | 21/21 | 21/0 | A | PA, EA, SA, N | 71 | 100% BPD (excluded schizophrenia, anorexia, MDD, SA) | 43% stopped 1 week prior | ROI | Yes—M |
| Jackowski et al., 2008 | sMRI and DTI | 17/15 | 10/7 | P | SA, PA, N, EA, WDV | 100 | MDD (41%), Other depressive diagnoses (30%), ODD (12%), ADHD (6%) | No | ROI | / |
| Kitayama et al., 2006 | sMRI | 8/13 | 7/1 | A | N/R | 100 | Lifetime MDD (62.5%), panic disorder (25%), lifetime alcohol abuse (12.5%) | No | ROI | / |
| Mehta et al., 2009 | sMRI | 14/11 | 8/6 | P | PI | N/R | N/R | N/R | ROI | Yes—M |
| Richert et al., 2006 | sMRI | 23/24 | 10/13 | P | PA, SA, WDV, N, EA | 52 (48 subthreshold) | MDD (13%), DD-NOS (4.4%), social phobia (13%), ADHD (13%), SAD (8.7%), simple phobia (8.7%) | No | ROI | Yes—F |
| Stein et al., 1997 | sMRI | 21/21 | 21/0 | A | SA | 71 | 71% dissociative disorder, MDD, social phobia, OCD | 14% | ROI | / |
| Teicher et al., 2004 | sMRI | 28/115 (& 23 PC) | 15/13 | P | SA, PA, N | 50 | Mood disorders and suicidal ideation or self-destructive behavior (M = 71%; PC = 52%), disruptive disorders (M = 14%; PC = 26%) | No | ROI | Yes—M |
| Tupler and De Bellis, 2006 | sMRI | 61/122 | 30/31 | P | SA, PA, WDV, EA | 100 | 86.8% dysthymia, MDD, ODD, ADHD, SAD | No | ROI | Yes—M,F |
| Vythilingam et al., 2002 | sMRI | 21/14 (& 11 MDD + M) | 21/0 | A | PA, SA | 66 | 100% MDD, panic disorder, phobias, anxiety, OCD, eating disorders, SA | No | ROI | / |
| Weniger et al., 2008 | sMRI | 23/25 | 23/0 | A | PA, SA, N | 43 | 100% BPD, 39% DA, 17% DID, 87% MDD, 28% alcohol abuse, 35% eating disorders | Approx 40% | ROI | / |
| Hanson et al., 2010 | TBM | 31/41 | N/R | P | PA | 0 | CD (6%), eating disorder (6%), MDD (3%) | No | WB | Yes—F |
| Tomoda et al., 2009 | VBM | 23/22 | 8/15 | A | HCP | 0 | 4% lifetime ADHD | No | WB | / |
| Treadway et al., 2009 | VBM | 19 MDD/19 C | 10/9 | A | EA, PA, SA, N | N/R | 100% MDD | No | WB | Yes—M |
| Tomoda et al., 2010 | VBM | 21/19 | 12/9 | A | PVA | N/R | 24% past anxiety; 48% past mood disorders | No | WB | Yes—M |
| Choi et al., 2009 | DTI | 16/16 | 12/4 | A | PVA | N/R | 44% depression, 19% anxiety, 6% ADHD and phobias. | No | ROI | Yes—M |
| Eluvathingal et al., 2006 | DTI | 7/7 | 5/2 | P | PI | N/R | N/R” | No | ROI | / |
Abbreviations: ACEs, adverse childhood events; ADHD, attention deficit hyperactivity disorder; AN, anorexia nervosa; BD, bipolar disorder; BN, bulimia nervosa; BPD, borderline personality disorder; C, control group; CD, conduct disorder; DD, depersonalization disorder; DD (NOS), depressive disorder mot otherwise specified; DTI, diffusion tensor imaging; EA, emotional abuse; EN, emotional neglect; GAD, general anxiety disorder; HCP, harsh corporal punishment; M, maltreated group; MDD, major depressive disorder; N, neglect; N/R, not reported; OCD, obsessive compulsive disorder; ODD, oppositional defiant disorder; PA, physical abuse; PC, psychiatric control group; PET, positron emission tomography; PI, post-institutionalized; PVA, parental verbal abuse; SA, sexual abuse; SAD, separation anxiety disorder; sMRI, structural magnetic resonance imaging; VBM, voxel-based morphometry; WDV, witnessing domestic violence.
Prefrontal cortex
The prefrontal cortex (PFC) has a major role in all functions that characterize mature adult behavior, including higher level motor control, inhibitory control, attention, working memory, personality expression, emotion and motivation regulation, and moderating learned social behavior (Miller and Cohen, 2001; Ochsner and Gross, 2005; Leh et al., 2010). The PFC matures relatively late in life, with progressive gray and white matter changes well into the 1940s and 1950s (Sowell et al., 2003). Due to its protracted development, the PFC is the brain region that is most susceptible to damage in childhood and adolescents and is hence considered an important target for abnormal development in children and adults who have been exposed to severe environmental stressors such as maltreatment.
There are mixed findings from region-of-interest (ROI) studies comparing PFC volume of children with maltreatment-related PTSD and co-morbid psychiatric conditions and non-maltreated children. Some reported smaller PFC volume or GM or increased prefrontal CSF volumes in maltreated subjects, which may indicate abnormal maturation of the prefrontal cortex (De Bellis et al., 1999, 2002a; De Bellis and Keshavan, 2003). Some reported dissociated frontal findings with GM increases in middle-inferior and ventral regions of the PFC, but reductions in dorsal PFC (Richert et al., 2006; Carrion et al., 2009).
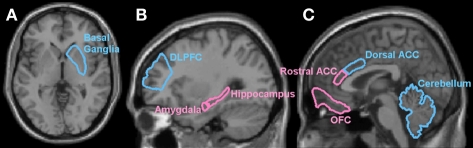
All the above studies have tested for PFC deficits in children with mixed maltreatment histories and PTSD (see Table 1). Hanson et al. (2010), however, using tensor based morphometry (TBM), reported smaller volumes of DLPFC, medial PFC (mPFC) and orbitofrontal cortex (OFC) in physically abused children without PTSD and with only a small proportion of other psychiatric conditions (see Table 1), with the largest differences in the right OFC (see Figure 1 for location of DLPFC and OFC).
Figure 1.
Anatomical brain regions implicated in maltreated subjects based on structural and functional imaging studies, overlaid on a high resolution structural MRI image. Regions highlighted in pink correspond to fronto-limbic areas involved in emotion and motivation processing and regions highlighted in blue to fronto-striatal brain regions involved in executive functions, working memory, inhibition and attention. Panel (A) shows an axial view containing the basal ganglia and panels (B,C) are sagittal views highlighting the hippocampus, amygdala and DLPFC and ACC, OFC, and cerebellum, respectively.
Studies of adults who were maltreated as children also demonstrate decreases in PFC GM. Reductions in GM volume of the frontal cortex as a whole and left DLPFC and right mPFC have been reported in a ROI MRI study of women with childhood sexual abuse (Andersen et al., 2008) and a voxel-based morphometry (VBM) study of young adults exposed to harsh corporal punishment in childhood (Tomoda et al., 2009). Andersen et al. (2008) reported that frontal cortex GM volume was maximally affected by sexual abuse at ages 14–16 years. Many of the maltreated subjects in Andersen et al. study had a current and/or past psychiatric diagnoses, whereas only one subject in Tomoda and colleagues' study met criteria for a lifetime history of ADHD, but no other psychiatric diagnoses were reported (Table 1).
Hippocampus
The hippocampus is part of the limbic system and plays an important role in learning and memory (see Figure 1 for location of hippocampus; Andersen et al., 2007). The relatively few studies examining children with maltreatment-related PTSD or post-institutionalized adolescents have found little evidence of hippocampal volume deficits compared with healthy controls (De Bellis et al., 1999, 2001, 2002a; Carrion et al., 2001; Mehta et al., 2009) with the exception of one relatively small sampled study (Carrion et al., 2007) who found evidence of hippocampal volume reduction developing in a childhood PTSD sample at 12–18 months after the baseline assessment. This finding combined with the prior mentioned negative findings suggests that the hippocampus may be volumetrically normal at the time of initial abuse but become smaller with time compared with healthy controls, which could be related to abnormal cortisol levels which would predict this hippocampal volume reduction over time (Carrion et al., 2007).
Conversely, one study reported increased bilateral hippocampal volume in a large sample of children with maltreatment-related PTSD, approximately 87% of whom had co-morbid psychiatric disorders (see Table 1), compared with healthy controls (Tupler and De Bellis, 2006). The authors suggest that this unexpected increase in hippocampal volume may be due to the possibility that, soon after trauma, the hippocampus sustains a sudden growth spurt in children or suffers a “scar” from a loss of neuropil secondary to elevated cortisol levels in childhood PTSD, and then might shrink in early adulthood when the hippocampus completes its maturation process.
In adults, decreased hippocampal volume has been observed in adult survivors of physical and sexual abuse with PTSD (Bremner et al., 1997); female survivors of sexual abuse 62.5% or 30% of whom had PTSD (Stein et al., 1997); female survivors of neglect, physical, sexual, and emotional abuse with Borderline Personality Disorder (BPD), 57% of whom had co-morbid PTSD (Driessen et al., 2000) and female survivors of physical and/or sexual abuse with MDD, 71% of whom also had PTSD (Vythilingam et al., 2002). There is evidence that hippocampal volume is particularly sensitive to sexual abuse between the ages of 3–5 years and 11–13 years (Andersen et al., 2008).
However, several studies, including a meta-analysis of hippocampal volumes in adults with trauma-related PTSD (Kitayama et al., 2005) found that smaller hippocampal volumes in maltreated subjects were related to PTSD rather than maltreatment itself (Bremner et al., 2003; Weniger et al., 2008), or at least that a small reduction in volume caused by childhood abuse is greatly amplified in those subjects who go on to develop PTSD.
However, in support of a direct link between child abuse and reduced hippocampal volume, some studies report a significant correlation between hippocampal volume and onset or duration of abuse (Driessen et al., 2000; Tupler and De Bellis, 2006). Others, however, found no correlations (Stein et al., 1997; Vythilingam et al., 2002) or report a correlation between hippocampal volume and PTSD (Bremner et al., 2003).
In addition to co-morbid psychiatric conditions, another confounding factor in these studies is that some subjects were on psychoactive medication at the time of testing (Stein et al., 1997; Driessen et al., 2000; Weniger et al., 2008)—see Table 1 for details. Also, all of the above studies of hippocampal volume in abused subjects used a ROI approach, measuring only the hippocampus (Stein et al., 1997; Bremner et al., 2003; Tupler and De Bellis, 2006; Carrion et al., 2007) or one more additional area, including the amygdala, temporal lobe, and basal ganglia (Bremner et al., 1997; De Bellis et al., 1999, 2001, 2002a; Driessen et al., 2000; Carrion et al., 2001; Vythilingam et al., 2002; Weniger et al., 2008).
Amygdala
The amygdala (see Figure 1) plays a key role in emotional processing, assessment of threatening information, behavioral regulation, fear conditioning, and memory for emotional events (Davis and Whalen, 2001; Shin et al., 2006; Dolan, 2007). As these processes are of extreme importance in threatening situations it may be expected that differences in amygdala structure would be associated with exposure to childhood maltreatment.
There is conflicting evidence for structural abnormalities of the amygdala in maltreated children. Some studies showed no volume differences between children with maltreatment-related PTSD and controls (e.g., De Bellis et al., 1999, 2001), whilst others have found effects of maltreatment and PTSD. Thus, Carrion et al. (2001) found a 5.1% reduction in the volume of the amygdala in children with maltreatment-related PTSD compared with controls, although this did not survive total GM correction. In contrast Mehta et al. (2009) reported an increase in amygdala volume in a small sample of post-institutionalized adolescents with severe early deprivation compared to their peers which was correlated with the time spent in institutions. A meta-analysis of children with maltreatment-related PTSD, however, did not find significant differences in amygdala volume between maltreated and non-maltreated children (Woon and Hedges, 2008). Furthermore, none of these studies controlled for co-morbid conditions (Table 1).
The results of studies which have measured amygdala volume in adults with a history of childhood maltreatment are also inconsistent. One study found reduced amygdala volumes in women with a history of maltreatment, PTSD and psychiatric co-morbidities, but not in healthy controls or maltreated women with dissociative amnesia (DA) or DID and similar co-morbidities, suggesting that the decrease is likely to be related to PTSD (Weniger et al., 2008). A confound, however, was uncontrolled medication. Another study reported minor volumetric reductions of the amygdala in maltreated BPD patients with and without PTSD when compared to healthy controls, suggesting this reduction is due to BPD or maltreatment, but not PTSD (Driessen et al., 2000). However, two other studies reported no differences in amygdala volumes in women with a history of childhood sexual abuse with comorbidities (Andersen et al., 2008) and in male and female survivors of childhood physical and sexual abuse with PTSD and co-morbidities (Bremner et al., 1997). Findings on amygdale deficits in relation to maltreatment are hence inconclusive. A further limitation of the above studies is the use of a ROI analysis (see Table 1).
Corpus callosum
The corpus callosum (CC) is the largest white matter structure in the brain. It connects the left and right cerebral hemispheres and facilitates interhemispheric communication for emotion, and higher cognitive abilities, among other processes (Kitterle, 1995; Giedd et al., 1996).
Decreases in CC volume and white matter integrity (particularly middle and posterior regions) have been reported in ROI approach MRI and DTI studies of maltreated children and adolescents, where all (De Bellis et al., 1999, 2002a; De Bellis and Keshavan, 2003; Jackowski et al., 2008) or 50% (Teicher et al., 2004) had PTSD and some had other psychiatric diagnoses (see Table 1), but there have also been negative findings in a study of post-institutionalized adolescents (Mehta et al., 2009).
Two ROI MRI studies of adult females with histories of childhood maltreatment have also reported smaller volume of the CC as compared to healthy controls; one in which all subjects had PTSD and some had histories of, but not generally current psychiatric diagnoses (see Table 1, Kitayama et al., 2007) and one in which approximately one third of subjects had PTSD and some had other co-morbid psychiatric conditions (Andersen et al., 2008). Furthermore the CC was maximally affected if the childhood abuse occurred between ages 9 and 10 (Andersen et al., 2008).
In conclusion, there is evidence for CC impairment in association with childhood maltreatment, but more research is needed to attempt to disentangle the effects of maltreatment, PTSD and other psychiatric disorders on CC volume.
The Anterior Cingulate Cortex
The Anterior Cingulate Cortex (ACC, see Figure 1) lies at the interface between the limbic system and the neocortex. No ROI MRI studies have tested for ACC deficits in children with maltreatment histories and no reduction was observed in a whole brain imaging study of maltreated children (Hanson et al., 2010).
Reductions in ACC volume have, however, been observed in ROI MRI studies of adults with childhood maltreatment-related PTSD and co-morbid disorders (Kitayama et al., 2006); in adults with a history of adverse childhood events, including divorce, family conflict, separation, domestic violence, bullying, neglect, emotional, physical, and sexual abuse, without PTSD or other psychiatric disorders (Cohen et al., 2006); and in adult MDD patients with a history of mixed childhood maltreatment, 70% of whom had current co-morbid non-specified anxiety disorders (Treadway et al., 2009). One whole brain study in adults with a history of harsh corporal punishment reported a decrease in right ACC volume (Tomoda et al., 2009), whereas another adult whole brain study reported no change in ACC (Treadway et al., 2009). Two studies reported a correlation between childhood trauma and ACC volume (Table 1, Cohen et al., 2006; Treadway et al., 2009).
Cerebellum
Decreased cerebellar volumes in children and adolescents with a history of maltreatment and PTSD and co-morbid conditions (see Table 1) has been a consistent finding in the literature (De Bellis and Kuchibhatla, 2006; Carrion et al., 2009) with cerebellar volumes furthermore correlating positively with age of onset and negatively with the duration of trauma (De Bellis and Kuchibhatla, 2006). Hanson et al. (2010), however, reported that relative to controls left cerebellar white matter was larger in a relatively large sample of physically abused children, very few of whom had PTSD or any other psychiatric diagnoses. The cerebellum forms extensive connections with the frontal lobes and is part of fronto-cerebellar neural network that fine-modulate behavior (Arnsten and Rubia, 2012). See Figure 1 for location of cerebellum.
Parieto-temporal regions
A TBM study found smaller volumes in physically abused children, the majority of whom had no psychiatric diagnoses, in the right temporal and bilateral parietal lobes (Hanson et al., 2010). Furthermore, both temporal and parietal volume reductions showed a negative correlation with the academic section of the Life Stress Interview (Hanson et al., 2010)
However, other studies found larger superior temporal gyrus (STG) volumes using a ROI approach in maltreated children and adolescents with PTSD and co-morbidities including MDD, ODD, ADHD (De Bellis et al., 2002b) and a VBM study of young adults with histories of parental verbal abuse, some of whom had a history of non-specified mood and anxiety disorders (Table 1, Tomoda et al., 2010). STG GM volumes furthermore were strongly correlated with levels of parental verbal aggression and inversely associated with parental education (Tomoda et al., 2010).
White matter tracts
Only two studies have tested for structural connectivity deficits in subjects with a history of childhood maltreatment. Eluvathingal et al. (2006), using DTI and fiber tractography, found decreased white matter tract density in the left uncinate fasciculus in post-institutionalized children who experienced socioemotional deprivation, 71% of whom had at least one subscale (subscales included conduct problems, atypicality, attention problems, and depression) in the clinically significant range, compared to controls. The uncinate fasciculus connects OFC to the anterior temporal lobe, including the amygdala. Another DTI study examined the effects of severe parental verbal abuse (PVA, e.g., ridicule, humiliation, and disdain) on brain connectivity in young adults (Choi et al., 2009). 44% of the PVA subjects had a history of depression, 19% had current anxiety disorders and 6% had ADHD and a history of phobias (see Table 1). They reported three white matter tracts to show reduced density: the arcuate fasciculus in left STG; the cingulum bundle by the posterior tail of the left hippocampus and the left body of the fornix. The arcuate fasciculus connects Wernicke's area in the temporoparietal junction with Broca's area in the inferior frontal gyrus and provides a pathway for the prefrontal cortex to receive and modulate auditory information (Makris et al., 2005). The cingulum bundle connects the limbic lobe with the neocortex, particularly the cingulate gyrus and reduction in density of the cingulum bundle has previously been observed in PTSD patients (Kim et al., 2006), indicating that it is susceptible to stress. The left fornix connects together the septohippocampal system, providing a pathway for serotonin from the midbrain raphe to the hippocampus, and plays a major role in the mediation of anxiety (Degroot and Treit, 2004). The authors hypothesized that abnormalities of these three tracts may underlie some of the language and emotional regulation difficulties seen in victims of childhood maltreatment. The emerging evidence from these two DTI studies show that the structural abnormalities in people with a history of child abuse affect interconnected white matter structures and hence the communication between regions in addition to isolated brain areas. Future DTI studies are needed to elucidate the effect of early maltreatment on structural interconnectivity.
Summary
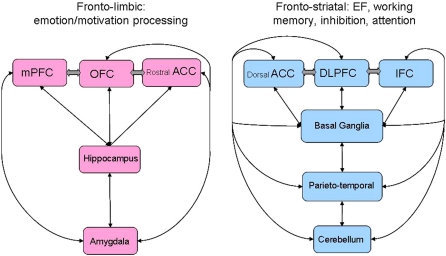
The brain regions most consistently affected by childhood maltreatment are the PFC, ACC, but also hippocampus, amygdala, corpus callosum, and cerebellum, suggesting that fronto-limbic circuitries may be most affected. This finding is restricted to a priori hypothesised areas, though, by the fact that the majority of structural studies in the abuse literature have used ROI approaches that only examined the effect of child abuse on areas of fronto-limbic systems (see Table 1, Bremner et al., 1997, 2003; Stein et al., 1997; De Bellis et al., 1999, 2001, 2002a,b; Driessen et al., 2000; Carrion et al., 2001, 2007; Vythilingam et al., 2002; De Bellis and Keshavan, 2003; Teicher et al., 2004; Cohen et al., 2006; Richert et al., 2006; Tupler and De Bellis, 2006; Kitayama et al., 2006, 2007; Andersen et al., 2008; Jackowski et al., 2008; Weniger et al., 2008; Mehta et al., 2009). Only very few ROI studies have investigated the effect of abuse on the fronto-striatal system. Most found no effect on the basal ganglia (Bremner et al., 1997; De Bellis et al., 1999, 2002a), but one study reported smaller caudate nuclei in adults with a history of traumatic adverse childhood events (Cohen et al., 2006). Only a few studies used whole-brain VBM techniques, none of which reported any basal ganglia changes in maltreated subjects (Carrion et al., 2009; Tomoda et al., 2009, 2010; Treadway et al., 2009; Hanson et al., 2010). Examining previously defined ROIs restricts the search toward a priori hypothesized regions thus providing a biased and inappropriately constrained characterization of anatomy (Friston et al., 2006).
Studies using whole brain analyses have found decreases in GM volume of similar areas, particularly in prefrontal cortex, to those identified by ROI analyses in maltreated subjects, including OFC, DLPFC, mPFC, ACC, but also other areas that have not been studied using ROIs, including the thalamus, parietal, temporal and superior frontal lobes (Tomoda et al., 2009, 2010; Hanson et al., 2010). Whole brain VBM studies have also reported some areas previously implicated by ROI studies to have increased GM in abused subjects including the cerebellum, cingulate cortex, PFC, and STG and, again, some areas that have not been studied using ROI such as the occipital lobe and parahippocampal gyrus (Tomoda et al., 2009, 2010; Hanson et al., 2010). Interestingly, none have reported hippocampal or amygdala differences, which have been the most commonly studied ROIs in subjects with a history of childhood abuse.
A major problem in interpreting the results of structural MRI studies in subjects with a history of childhood maltreatment is that most studies have imaged subjects with associated psychiatric conditions, most prominently PTSD but also MDD, ADHD, ODD, BPD, OCD, Generalized anxiety disorder (GAD), phobias, eating disorders, and substance abuse (see Table 1 for details), making it impossible to determine whether the volumetric changes observed are due to the abuse, the psychiatric conditions or an interaction between the two. The vast majority of studies have tested groups of subjects, in which 100% had PTSD and a large percentage also had other co-morbid conditions (Bremner et al., 1997; De Bellis et al., 1999, 2001, 2002a,b; De Bellis and Keshavan, 2003; Carrion et al., 2001, 2007, 2009; De Bellis and Kuchibhatla, 2006; Kitayama et al., 2006; Richert et al., 2006; Tupler and De Bellis, 2006; Jackowski et al., 2008), whilst in other studies a smaller percentage of participants had PTSD but many participants still had other psychiatric disorders such as MDD, DID, OCD, and BPD (Stein et al., 1997; Driessen et al., 2000; Vythilingam et al., 2002; Bremner et al., 2003; Teicher et al., 2004; Andersen et al., 2008; Weniger et al., 2008). A few studies imaged subjects with unspecified anxiety disorders, which is extremely likely to include PTSD, and co-morbidities such as MDD and unspecified mood disorders (Treadway et al., 2009; Tomoda et al., 2010) and one study did not screen for PTSD or other conditions (Mehta et al., 2009). Another limitation of a few of these studies is that subjects were taking psychoactive medications, such as antidepressants, i.e., selective serotonin reuptake inhibitors (SSRIs), antipsychotics, benzodiazepine, or psychostimulants, all of which are known to affect brain structure and function (Caliguri et al., 2003; Scherck and Falkai, 2006; Rubia et al., 2009, 2011a,b; Murphy, 2010; Nakao et al., 2011) and, therefore, make it difficult to determine clearly the brain alterations associated with abuse (Stein et al., 1997; Driessen et al., 2000; Weniger et al., 2008). In structural MRI in general when comparing two groups of subjects a sample size of ≥20 in each group is desirable for reliability (Brambilla et al., 2003). Some of these studies did not satisfy these criteria (Table 1, Bremner et al., 1997; De Bellis et al., 2001; Kitayama et al., 2006; Carrion et al., 2007; Jackowski et al., 2008; Mehta et al., 2009; Treadway et al., 2009) and so findings of these studies must be considered as weaker statistically than those with larger sample sizes.
PTSD is usually the main outcome of abuse with incidence rates of PTSD resulting from child abuse ranging from 36.3% to 63% (Famularo et al., 1993; McLeer et al., 1998; De Bellis et al., 2001a). It is, therefore, extremely difficult to separate PTSD and child abuse and it may not be the best approach as consequences may be too linked to be separable. It is possible that it is a continuum and that maltreatment causes brain changes that, if the changes are marked enough, can lead to the development of PTSD and other psychiatric conditions, as suggested by studies that report changes in brain structure in survivors of childhood maltreatment without PTSD and more marked changes in abuse survivors with PTSD (e.g., Bremner et al., 2003). However, one study has imaged subjects without PTSD and without co-morbid psychiatric conditions (Cohen et al., 2006) and two have studied subjects without PTSD but included a very small percentage of subjects with psychiatric conditions (Tomoda et al., 2009; Hanson et al., 2010). When including only these three studies, which all have sample sizes in excess of 20, that have controlled for PTSD and co-morbidities and in which the subjects were also medication-free, two of which were whole brain VBM studies (Tomoda et al., 2009; Hanson et al., 2010) and the other studied ROIs including ACC, hippocampus, amygdala, and caudate (Cohen et al., 2006), brain areas identified as having volumetric deficits in maltreated subjects include mPFC, DLPFC, OFC, ACC, caudate, thalamus, parietal lobe and superior frontal lobe. Volume increases were also reported in the cerebellum, cingulate and PFC. Again, none of these studies reported hippocampus or amygdala deficits.
Several studies have found direct correlations between brain abnormalities and age of onset or duration of abuse, including cerebral cortex volume (see Table 1, De Bellis et al., 1999, 2002a), hippocampus (Driessen et al., 2000; Tupler and De Bellis, 2006), amygdala (Mehta et al., 2009), ACC (Cohen et al., 2006; Treadway et al., 2009) and caudate (Cohen et al., 2006), although there have also been findings of no correlation between abuse and hippocampus volumes (Stein et al., 1997; Vythilingam et al., 2002).
Taking into account studies with relatively large sample sizes that have controlled for psychiatric conditions and medication and all studies that have reported correlations of their findings with childhood maltreatment, the main brain regions consistently reported to be affected in maltreated subjects are the DLPFC, mPFC OFC, ACC, hippocampus, amygdala, and cerebellum (Figure 1). DLPFC is involved in aspects of higher level cognitive processing, such as working memory, planning, temporal foresight, interference inhibition, and attention control while the mPFC is central to aspects of social cognition such as self-knowledge and person perception (Amodio and Frith, 2006) and is part of the paralimbic system that regulates motivation and affect, forming interconnections in particular with the amygdala (Compton, 2003). The deficits in DLPFC associated with childhood maltreatment may thus be underlying the observed deficits in motor inhibition, working memory and attention, whilst deficits of the mPFC may manifest as problems with emotion regulation. The OFC and ACC are important for reward processing, and emotion and motivation control (Ochsner and Gross, 2005; Schoenbaum et al., 2006). Structural deficits in regions of the ACC and ventromedial OFC, together with deficits in interconnected limbic areas such as hippocampus and amygdala, therefore, may be associated with problems in emotion and motivation control and with deficits in emotion processing. Alterations to the hippocampus and amygdala associated with childhood maltreatment are likely to be neural correlates of the impairments in memory and emotion processing discussed previously. There is growing evidence that the cerebellum plays a crucial role in executive functioning, most prominently in attention and timing functions, (Rubia and Smith, 2004; Schmahmann et al., 2007; Arnsten and Rubia, 2012) and in emotion processing and fear conditioning (Schutter and van Honk, 2005). Alterations in the cerebellum due to childhood maltreatment, therefore, may be involved in the deficits of emotion discrimination and executive functioning described previously.
DTI has shown decreased white matter density of four white matter tracts that form pathways between structures that have been implicated in structural MRI studies in abused subjects, suggesting that structural abnormalities affect the communication between regions as well as isolated brain areas (Eluvathingal et al., 2006; Choi et al., 2009). The uncinate fasciculus and cingulum bundle connect the anterior temporal lobe (including the amygdala) to the OFC and cingulate and play a role in emotion control; the arcuate fasciculus connects frontal and temporal lobe language areas and the fornix is involved in anxiety as it connects the midbrain with the hippocampus.
Functional brain differences associated with childhood maltreatment
In contrast to the number of studies examining structural brain differences, only a few have investigated possible functional correlates associated with maltreatment using imaging techniques such as fMRI or PET and a very small proportion of these have been conducted in children (see Table 2 for details).
Table 2.
A summary table of the characteristics of functional neuroimaging studies of childhood maltreatment, reporting study type and task, sample size (N), gender proportion of the maltreated group (female/male), whether the study is of an adult or pediatric population (A/P), maltreatment type(s) included in the maltreated group, percentage of the maltreated group with PTSD and other co-morbidities, whether the maltreated group were taking any psychoactive medication, whether the study was ROI or whole brain (WB) and whether any correlations were reported between the results and maltreatment onset or duration (M), PTSD symptoms (P) or functional impairment (F).
| Article | Type | N (M/C) | Gender (f/m) | Adult/pediatric | Maltreatment type | % PTSD | Other Co-morbidities | Medication | ROI/WBA | Correlations (M/P/F) |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al., 2002 | fMRI—Resting | 8/16 | 7/1 | A | SA | 12.5 | Yes 37.5% axis I diagnosis- details not reported | No | ROI | / |
| Carrion et al., 2008 | fMRI—Go/NoGo | 16/14 | 9/7 | P | PA, SA, WDV | 25 (& 75 subthreshold) | MDD (19%); panic disorder (6%), OCD (6%), ADHD (6%), enuresis (6%) | No | WB | Yes—P |
| Croy et al., 2010 | fMRI—Olfactory | 12/10 | 12/0 | A | PA, SA | 50 | M and C matched for diagnoses (inc Substance abuse, depression, anxiety disorders, OCD, DD, somatoform disorders and eating disorders) | N/R | WB | / |
| Dillon et al., 2009 | fMRI—Reward | 13/31 | 9/4 | A | EA, PA, SA | 7 | MDD (7%), agoraphobia (7%), GAD (14%) | 14% | ROI | / |
| Lanius et al., 2001 | fMRI—Traumatic scripts | 18/0 | N/R | A | SA, motor accident | 50 (& 50 subthreshold) | MDD (11%), dysthymia (16.6%), panic disorder (11%), lifetime alcohol abuse/dependence (22%), nicotine abuse (22%) | No—stopped 2 weeks prior | WB | / |
| Lanius et al., 2003 | fMRI—Traumatic scripts | 20/0 | N/R | A | SA, motor accident | 50 (& 50 subthreshold) | MDD (10%), Dysthymia (20%), panic disorder (15%), lifetime drug abuse/dependence (10%), lifetime alcohol abuse/dependence (20%), nicotine abuse (20%) | No—stopped 2 weeks prior | WB | / |
| Maheu et al., 2010 | fMRI—Emotion | 11/19 | 8/3 | P | PI, EN | 0 | Specific phobia (9%), SAD (9%) | No | ROI | / |
| Mueller et al., 2010 | fMRI—STOP | 12/21 | 9/3 | P | EN, N, non-specified M | 0 | GAD (8%), ODD (8%), enuresis (8%), specific phobia (16%) | 8% but stopped 30 h prior | WB | / |
| Noll-Hussong et al., 2010 | fMRI—Multisomatoform pain | 8/8 | 7/1 | A | SA | 0 | 100% multisomatoform pain disorder. No others | N/R | WB | / |
| Raine et al., 2001 | fMRI—Working memory | 10/9 (& 5 violent) | 0/10 | A | PA | N/R | N/R | N/R | ROI | / |
| Bremner et al., 1999 | PET—Traumatic scripts | 22/0 | 22/0 | A | SA | 45 | Lifetime and current: MDD (50%), alcohol/ substance depen- dences (41%), panic disorder with agora- phobia (22.7%), dysthymia/OCD/GAD/anorexia (4.5% each) | No—stopped 4 weeks prior | WB | / |
| Bremner et al., 2003a | PET—retrieval emotional word pairs | 10/11 | 10/0 | A | SA | 100 | Lifetime and current: MDD (70%), panic disorder without agoraphobia (10%), alcohol and substance dependences (past, 60%) | No—stopped 4 weeks prior | WB | / |
| Bremner et al., 2004a | PET—Emotional stroop | 21/0 | 21/0 | A | SA | 57 | Lifetime and current: MDD (71%), alcohol and substance dependence (past, 71%), social phobia (19%), GAD (14%), dysthymia/panic disorder without agoraphobia/simple phobia/Bulimia (5% each) | No—stopped 4 weeks prior | WB | / |
| Bremner et al., 2005 | PET—Fear conditioning | 8/11 | 8/0 | A | SA | 100 | Lifetime and current MDD (75%), dysthymia (25%) panic disorder with agoraphobia (13%), GAD (25%), social phobia (13%), simple phobia (13%), alcohol and substance dependences (past, 62.5%) | No—stopped 4 weeks prior | WB | / |
| Chugani et al., 2001 | PET—Resting state | 10/7 (& 17 adult C) | 4/6 | P | PI | N/R | N/R | No | Both | |
| Schmahl et al., 2004 | PET—Traumatic scripts | 20/0 | 20/0 | A | SA, PA | 45 | BPD (50%), 75% other diagnoses including MDD, dysthymia, BD, panic disorder with agoraphobia, BN, OCD, alcohol and substance abuse/dependence | 50% | WB | / |
| Shin et al., 1999 | PET—Traumatic scripts | 16/0 | 16/0 | A | SA | 50 | Current MDD (31%), dysthymia (6%), cyclothymia (6%), panic with agoraphobia (6%), panic without agoraphobia (6%), GAD (6%), somatoform disorder (6%), bulimia (6%), simple phobia (6%) past alcohol abuse/dependence (19%) | No—stopped 2 weeks prior (2 months for SSRIs) | WB | / |
Abbreviations as for Table 1.
Resting state
Chugani and colleagues used PET to investigate the awake resting state brain activation in pre-adolescent children adopted from Romanian orphanages, where the children had experienced adverse early rearing environments (Chugani et al., 2001). The adopted children relative to controls showed decreased metabolism in a network of areas implicated in stress regulation, including the OFC, ventromedial PFC, amygdala, head of hippocampus, lateral temporal cortex and the brain stem.
Working memory
One fMRI study tested for neural correlates of working memory in four subgroups of adult males with relatively small sample sizes: a group with a history of childhood physical abuse, a group of violent offenders, a group of physically abused violent offenders and a control group (Raine et al., 2001, see Table 2 for study characteristics). Activation was measured in three slices that allowed assessment of partial areas of frontal, temporal and occipital lobes but psychiatric conditions were not reported or controlled for. Abused subjects, irrespective of their violence status, showed reduced activation during the working memory task, in PFC including DLPFC, anterior PFC, and inferior prefrontal gyrus (BA 10/44/45/46/47), lateral temporal (BA 21/22) and occipital cortex (BA 18/19). Abused non-violent subjects had decreased activation of the left STG, increased activation of right STG and a strong deficit in task performance. This performance deficit supports the neuropsychological studies discussed earlier which report working memory deficits in abused subjects.
Inhibitory control
Two fMRI studies investigated response inhibition in maltreated adolescents using whole brain analyses. One was in adolescents with mixed maltreatment histories whilst they carried out a Go/No-Go task (Carrion et al., 2008); the other in adolescents with early life stress who were adopted because of early caregiver deprivation whilst carrying out a stop task (Mueller et al., 2010). In Carrion et al. study 25% of the maltreated group had PTSD and the remaining 75% demonstrated significant PTSD symptoms but were subthreshold for diagnosis and some had co-morbid psychiatric diagnoses (see Table 2 for details); whereas in Mueller et al. study none of the subjects had PTSD and only a very few had other psychiatric conditions (Table 2). In both studies, increased activation was observed in maltreated subjects in ACC (BA 24/32) and both report activation differences in PFC for maltreated subjects. Carrion et al. (2007); reported decreased activation of DLPFC (BA 9/46) and increased activation in mPFC (BA 8/9), whereas Mueller and colleagues reported increased activation of inferior PFC (BA 44/45/46) as well as in pre- and post-central gyri, the striatum, and the posterior insula. Both studies had relatively good sample sizes, but Mueller et al. (2010) study was better controlled with respect to psychiatric conditions.
Emotion processing
An fMRI study of emotion processing in neglected 8–18 year olds with no PTSD and a low proportion of psychiatric comorbidities used a ROI approach to measure activation in the hippocampus and amygdala whilst subjects viewed emotional faces (Maheu et al., 2010, Table 2). Reaction times were faster for neglected youths than controls when rating angry faces and relative to controls, neglected youths showed significantly greater left amygdala and left anterior hippocampus activation when viewing angry or fearful faces. These findings support the previous neuropsychological findings of a different response to angry faces in maltreated children discussed earlier (e.g., Pollak et al., 2000; Pine et al., 2005) and are also inline with the event-related potential (ERP) literature. ERP studies have shown that, when compared to non-maltreated controls, those with a history of physical abuse or neglect generated altered ERP response amplitudes when presented with angry or fearful faces, or voices, compared to happy or neutral targets (Pollak et al., 2001; Cicchetti and Curtis, 2005; Parker and Nelson, 2005; Shackman et al., 2007).
Traumatic scripts
A number of PET studies of adults have compared women survivors of childhood sexual abuse with and without PTSD (Bremner et al., 1999, 2003a, 2004a, 2005; Shin et al., 1999) or BPD (Schmahl et al., 2004). Unfortunately, the absence of a control group of non-abused women without PTSD/BPD in four of these studies (Bremner et al., 1999, 2004a; Shin et al., 1999; Schmahl et al., 2004) presents a crucial confound. Furthermore, in all of these studies subjects in the abused groups with and without PTSD or BPD had co-morbid conditions (see Table 2) and in Schmahl et al. study of BPD patients 50% were taking medications that affect brain function, including SSRIs, typical and atypical antipsychotics and selective noradrenaline reuptake inhibitors (SNRIs) thus making it even more difficult to determine effects specifically related to abuse. Three of the above studies used traumatic script-driven imagery (Bremner et al., 1999; Shin et al., 1999; Schmahl et al., 2004) and another used the emotional Stroop paradigm to investigate the influence of emotion on attention (Bremner et al., 2004a). These studies have reported group differences in blood flow in PTSD subjects, compared to subjects with abuse but without PTSD, in regions including DLPFC, mPFC, OFC, ACC, posterior cingulate, hippocampus, insula, visual association cortex, cuneus, and inferior parietal lobule, whilst Schmahl et al. (2004) reported altered activation of DLPFC, mPFC, and ACC in abused women with BPD, compared to abused women without BPD.
Two studies have used PET to compare women with a history of childhood sexual abuse, PTSD and similar co-morbid conditions to the above studies with a control group of women with no history of abuse, PTSD or other psychiatric disorder and reported group differences in the blood flow of areas previously implicated in childhood maltreatment (Bremner et al., 2003a, 2005). Bremner et al. (2003a) used PET during the retrieval of neutral and emotionally valenced word pairs and reported alterations in mPFC, OFC, MFG, ACC, posterior cingulate, inferior temporal lobe, and hippocampus. Using a fear-conditioning paradigm increased amygdala activation was reported during fear acquisition and reduced ACC activation during extinction in the abused group with PTSD (Bremner et al., 2005). However, PTSD or comorbid psychiatric conditions were not controlled for.
Two fMRI studies compared the brain activation of traumatized adults with PTSD as a result of childhood sexual abuse or a motor vehicle accident with traumatized adults with PTSD symptoms but who did not meet the full DSM-IV criteria for PTSD, when listening to traumatic scripts (Lanius et al., 2001, 2003). They reported decreased activation in the thalamus, ACC and MFG for subjects with full PTSD criteria, when compared to those with PTSD symptoms but not full PTSD. These effects are, therefore, likely to be due to PTSD.
Reward processing
Dillon and colleagues used ROI fMRI to investigate the effect of reward processing on the activation of the basal ganglia in adults with a history of mixed childhood maltreatment and psychiatric disorders (Table 2, Dillon et al., 2009), some of whom (14%) were taking psychotropic medications (citalopram, an SSRI, and hydrocodone, an opiate analgesic), in the weeks prior to scanning which is likely to have had an effect on the brain function of these individuals (Murphy, 2010). Using a monetary incentive delay task they found that these adults, relative to non-abused controls, rated reward-predicting cues as less positive, and displayed a weaker response to reward cues in the left globus pallidus.
Sensory processing
Processing of olfactory stimuli was investigated in women with a history of childhood physical and/or sexual abuse and non-maltreated controls using whole brain fMRI (Croy et al., 2010). The groups did not differ significantly according to the diagnosis of mental disorders (see Table 2 for details), so the authors could be relatively confident that any difference in activation between the groups was due to their maltreatment history. Both groups showed normal activation in olfactory projection areas. However, the abused group had enhanced activation in PFC (BA 44/45/47), posterior parietal lobe, occipital lobe, and the posterior cingulate cortex, and decreased activation in OFC, hippocampus, ACC, and cerebellum, indicating that childhood maltreatment may be associated with an altered processing of olfactory stimuli. Another whole brain fMRI study investigated the neural substrates of empathy-induced pain in a small sample of multisomatoform pain patients with and without a history of childhood sexual abuse, none of whom had PTSD or other psychiatric co-morbidities (Noll-Hussong et al., 2010). Abused patients showed increased activation in PFC (BA 44/45/46/47) while non-abused participants showed increased activation of the left hippocampus. The findings of these two fMRI studies complement other studies of maltreatment in so far as enhanced activation in associative and emotional regions includes the fronto-limbic pathways previously implicated in childhood maltreatment.
Cerebellum
Anderson and colleagues (Anderson et al., 2002) suggested that as the cerebellar vermis has a protracted ontogeny and a high density of glucocorticoid receptors, it is highly susceptible to early stress. They, therefore, used steady-state fMRI (T2 relaxometry) to compare resting blood flow in the cerebellar vermis of a small group of young adults with a history of childhood sexual abuse, 62.5% of whom had no diagnosable Axis I psychiatric disorder (Table 2), and non-abused controls. Abused subjects had higher T2 relaxation time (T2-RT) than controls in the vermis but not in cerebral or cerebellar hemispheres. These findings support their hypothesis that the functional activity of the cerebellar vermis may be affected by exposure to repetitive sexual abuse.
Summary
All PET and most fMRI studies carried out in abused subjects have used whole brain analysis with only four fMRI studies focusing on ROIs (Table 2, Raine et al., 2001; Anderson et al., 2002; Dillon et al., 2009; Maheu et al., 2010). Whole brain fMRI and PET studies of abused subjects have consistently reported altered activation of prefrontal regions. fMRI studies of inhibition and working memory have consistently reported altered activation of DLPFC and inferior PFC (Raine et al., 2001; Carrion et al., 2008; Mueller et al., 2010), whilst fMRI and PET studies of non-executive function tasks have demonstrated altered activation of mPFC, OFC, and DLPFC (Bremner et al., 1999, 2003a, 2004a; Shin et al., 1999; Schmahl et al., 2004; Croy et al., 2010; Noll-Hussong et al., 2010). The ACC, considered part of the medial frontal cortex, also consistently showed altered activation in abused subjects in motor inhibition (Carrion et al., 2008; Mueller et al., 2010) and non-executive function tasks (Bremner et al., 1999, 2003a, 2004a, 2005; Shin et al., 1999; Schmahl et al., 2004; Croy et al., 2010). The findings of abnormal activation patterns of PFC and ACC are in line with structural MRI findings which reported volume deficits of PFC and ACC and support the theory that top-down fronto-limbic and fronto-cortical systems are most affected by childhood maltreatment. Abnormal activation in OFC and vmPFC, including ACC, in association with abuse, supports the structural evidence for deficits in fronto-limbic systems mediating affect control. Altered activation of DLPFC is consistent with deficits in working memory, inhibition and attention.
In support of the theory that that the fronto-limbic system is affected in people with a history of childhood maltreatment, altered hippocampal activation has been observed in four whole brain functional neuroimaging studies of traumatic scripts, retrieval of emotional word pairs, olfaction, and pain (Bremner et al., 1999, 2003a; Croy et al., 2010; Noll-Hussong et al., 2010) and a PET study of fear conditioning reported altered amygdala activation in abused subjects (Bremner et al., 2005). An ROI fMRI study of emotion processing focusing on the amygdala and hippocampus also reported an increase in hippocampus and amygdala activation in response to angry and fearful faces in maltreated adolescents when compared to controls (Maheu et al., 2010).
Relatively few functional neuroimaging studies have tested for altered function of fronto-striatal pathways in maltreated subjects as only a few studies have utilized tasks that load on the basal ganglia. However, a study of response inhibition reported increased activation in subjects with early life stress compared to controls in the inferior frontal cortex (IFC) and striatum (Mueller et al., 2010). These regions form fronto-striatal neural networks for inhibitory control (Rubia et al., 2003, 2007; Aron et al., 2004) which could explain inhibitory deficits. An fMRI study of reward processing that focused on a ROI encompassing the basal ganglia reported weaker response to reward cues in the left globus pallidus (Dillon et al., 2009) indicating that abuse may be associated with dysfunction in left basal ganglia regions implicated in reward-related learning and motivation.
Other areas in which altered activation has been observed in maltreated subjects include the insula and cerebellum. Increased insula activation has been observed in maltreated adolescents (none of whom had PTSD) using fMRI during the stop task (Mueller et al., 2010) and insula activation was shown to differ between groups of women with a history of maltreatment listening to traumatic scripts, depending on whether they had developed PTSD or not (Bremner et al., 1999; Shin et al., 1999). The insula plays a role in diverse functions including perception, motor control, self-awareness, cognitive functioning, and is associated with emotion processing and the limbic system (Augustine, 1996; Phan et al., 2002). A dysfunction of the insula in abused subjects may, therefore, be linked to the functional deficits in emotion processing discussed earlier. Decreased activation of the cerebellum was observed in women with a history of child abuse compared to controls in an fMRI study of olfaction (Croy et al., 2010) and a ROI fMRI study focusing on the cerebellum reported higher T2 relaxation time for sexually abused subjects than controls in the cerebellar vermis (Anderson et al., 2002). Functional cerebellar dysfunction in abused subjects is in line with structural MRI studies, discussed earlier, which report alterations in cerebellar volume in maltreated subjects and may be related to the deficits of emotion discrimination and attention previously described.
As with structural MRI and neuropsychology, functional neuroimaging studies of childhood maltreatment are also confounded by co-morbid psychiatric conditions. PTSD is, again, the most common psychiatric confound and most functional neuroimaging studies have studied subjects with PTSD and other co-morbidities (see Table 2, Bremner et al., 1999, 2003a, 2004a, 2005; Shin et al., 1999; Lanius et al., 2001, 2003; Anderson et al., 2002; Schmahl et al., 2004; Carrion et al., 2008; Croy et al., 2010) or did not screen for psychiatric conditions (Raine et al., 2001). Only very few studies have tested groups of abused subjects with no PTSD and very few subjects with other psychiatric conditions (Maheu et al., 2010; Mueller et al., 2010) or in which only a very few have PTSD and other co-morbidities (7%, Dillon et al., 2009). Similar brain regions have been shown to have altered fMRI activation in studies using abused subjects with and without PTSD. For example, two fMRI studies of response inhibition reported altered activation in ACC and PFC, one in which subjects had PTSD (Carrion et al., 2008) and one in which they did not (Mueller et al., 2010). However, a few PET studies compared women with a history of childhood sexual abuse with and without PTSD and reported group differences in blood flow in PTSD subjects, compared to subjects with abuse but without PTSD, in regions implicated in abuse including DLPFC, mPFC, OFC, ACC, hippocampus and insula, suggesting that these differences were due to PTSD and not abuse itself (Bremner et al., 1999, 2004; Shin et al., 1999). This again highlights the difficulties involved in attempting to separate the effects of abuse and of PTSD.
Most functional neuroimaging studies have used subjects that were free of psychotropic medications for at least 2–4 weeks prior to scanning (Bremner et al., 1999, 2003a, 2004a, 2005; Shin et al., 1999; Maheu et al., 2010) or were completely medication-naïve (Carrion et al., 2008). Some, however, have not reported the medication history/state of their subjects (Lanius et al., 2001, 2003; Raine et al., 2001; Anderson et al., 2002; Croy et al., 2010; Noll-Hussong et al., 2010) and others have used subjects who were taking medications such as SSRIs, opioid analgesics, typical and atypical antipsychotics and SNRIs at the time of scanning or in the weeks immediately prior to scanning (Schmahl et al., 2004; Dillon et al., 2009). See Table 2 for details of medication. This is a potential confound as these medications, especially SSRIs, are known to affect brain function and have been shown to alter activation measured using fMRI (Caliguri et al., 2003; Scherck and Falkai, 2006; Peran et al., 2008; Murphy, 2010).
For result reliability, it is generally thought that sample sizes should be ≥12 for fMRI (Desmond and Glover, 2002) and between 10 and 20 for PET (Andreasen et al., 1996), but some studies discussed here do not satisfy these criteria (Table 2, e.g., Raine et al., 2001; Anderson et al., 2002; Bremner et al., 2005; Noll-Hussong et al., 2010) so their results should be treated as preliminary. Another limitation of the functional neuroimaging studies is that there have been very few studies of abused children as most fMRI and all PET studies have tested adults with a history of childhood maltreatment (Table 2). It is important that both adults and children with histories of maltreatment are studied as brain changes can develop or normalise over time and the potential effects are likely to be very different in adults compared to adolescents, as has been observed for structural changes, for example, the fact that reduction of hippocampal volume is consistently found to be reduced in abused adults, but not in children (see Section “Hippocampus”).
Overall conclusions
In conclusion, there is consistent evidence that childhood maltreatment is associated with neuropsychological impairments in academic achievement, IQ, memory, emotion processing, working memory, attention, and response inhibition. There is also evidence for changes in brain structure and function, most consistently in ventromedial and orbitofrontal-limbic regions and networks of affect control, but with emerging evidence for some deficits also in lateral fronto-striatal and parieto-temporal regions that mediate EFs, such as response inhibition, attention, and working memory (see Figure 2 for a schematic representation of these networks). However, the majority of the studies investigating childhood maltreatment are characterized by several limitations, which are discussed here and summarized in Tables 1 and 2. Firstly, the presence of psychiatric disorders is very high in individuals who have experienced childhood maltreatment (72%, De Bellis et al., 2001a) and, therefore, most studies of maltreatment have been conducted in abused children or adults who also had associated psychiatric conditions, most prominently PTSD but also depression, anxiety and antisocial behaviors. This makes it difficult to determine effects that are unique to maltreatment as it is not clear whether the structural and functional differences can be attributed to the child abuse or the associated psychiatric condition or a combination of both. Therefore, an important direction for future research would be to attempt to tease apart the effect of child abuse from the effect of psychiatric co-morbidities on cognitive functioning, brain structure, and brain function. However, this may not be the best approach as the psychiatric conditions discussed are not independent from the maltreatment. This is particularly true for PTSD where, by definition, to be diagnosed with PTSD patients must have undergone or observed a highly traumatic event, or events, such as child abuse. Therefore, it is possible that PTSD, and other psychiatric conditions, can occur as a direct result of maltreatment and it may be the case that brain changes in abuse survivors may only be detectable if they are severe enough to produce psychiatric symptoms. If this is true, however, it does not rule out the possibility that the brain changes were present before symptom onset and may have ultimately led to the symptoms of PTSD, depression, anxiety etc., observed. Some studies have shown direct correlations between brain deficits and the onset or duration of abuse (De Bellis et al., 1999, 2002a; Driessen et al., 2000; Cohen et al., 2006; De Bellis and Kuchibhatla, 2006; Tupler and De Bellis, 2006; Andersen et al., 2008; Choi et al., 2009; Mehta et al., 2009; Treadway et al., 2009; Tomoda et al., 2010), whilst others have shown correlations between brain deficits and PTSD symptoms—see Table 1 (Bremner et al., 2003; Carrion et al., 2007, 2008). This also highlights the inseparable nature of maltreatment and PTSD and suggests that they may both contribute to the brain changes observed. What is of interest and is important to attempt to elucidate is what makes some people resilient to maltreatment whilst others suffer the negative consequences of psychiatric conditions.
Figure 2.
Schematic representation of the brain regions and networks that have been implicated childhood maltreatment in functional and structural imaging studies. Deficits of fronto-limbic regions and networks have most consistently been associated with childhood abuse. However, there is some evidence from more recent studies, including whole brain imaging analyses, for deficits in fronto-striatal and fronto-cerebellar networks.
Second, and as a result of these psychiatric conditions, some subjects were being treated at the time of the study with psychotropic medication (see Tables 1 and 2), most commonly anti-depressants (SSRIs), but also antipsychotics, mood stabilizers, alpha-2 adrenergic agonists, opioid analgesics, benzodiazepine, SNRIs, or psychostimulants (Stein et al., 1997; Driessen et al., 2000; Mezzacappa et al., 2001; Schmahl et al., 2004; Weniger et al., 2008; Dillon et al., 2009) which are known to affect brain structure and function (Murphy, 2010; Nakao et al., 2011), thus making it impossible to determine whether observed effects were due to maltreatment, psychiatric co-morbid conditions or psychotropic medication.
Thirdly, many studies have studied subject samples that are characterized by very different maltreatment histories, using the blanket term “maltreatment” to include subjects with a history of sexual abuse, physical abuse, emotional abuse, neglect, and witnessing domestic violence in one sample (Tables 1 and 2). Different forms of abuse present differently clinically, for example, self-harm and eating disorders are more common in survivors of sexual abuse (Wonderlich et al., 1997; Weierich and Nock, 2008) and it is, therefore, likely that different types of abuse also have different effects on behavior and neurobiology. Therefore, ideally the effects of all forms of abuse should be considered separately which some studies (e.g., Hanson et al., 2010) have already started to address.
Another limitation is that many of the studies discussed in this review have used relatively small sample sizes limiting their statistical power (see Tables 1 and 2 for details of sample sizes, e.g., De Bellis et al., 2001; Kitayama et al., 2006; Noll-Hussong et al., 2010). Also, many studies of child abuse have used mixed gender groups of subjects (Tables 1 and 2). There is evidence for gender differences on brain structure and function in both normal brain development (Giedd et al., 1999, 2006; Lenroot et al., 2007; Christakou et al., 2009; Rubia et al., 2010) and psychiatric patients, e.g., ADHD patients (Gur et al., 2002; Valera et al., 2010). It is, therefore, possible that childhood abuse may manifest differently in different genders and so male and female subjects should be imaged separately.
The majority of structural MRI, but also some fMRI studies, have not tested for whole brain effects but have only examined a priori ROIs (Tables 1 and 2). This is a limited approach as we do not yet have extensive knowledge of all brain areas affected by childhood maltreatment and it is possible that ROI studies are not focusing on the most affected brain regions. This is even more problematic since most ROI studies were based on prior studies of relatively small sample sizes that furthermore have included high rates of comorbidities. This has in particular lead to a bias of ROIs to focus on limbic areas of amygdala and hippocampus, while whole brain studies suggest that other brain regions in PFC and ACC are more consistently affected.
Electroencephalography (EEG) and ERP studies have also reported differences in brain activation in subjects who have been exposed to childhood maltreatment, compared to controls, in the resting state (Ito et al., 1998; Miskovic et al., 2010), during emotion processing (Pollak et al., 2001; Pollak and Tolley-Schell, 2003; Cicchetti and Curtis, 2005) and whilst recalling traumatic memories (Schiffer et al., 1995). However, whilst the temporal resolution of EEG and ERP is very good, the spatial resolution is limited and currents from deep brain structures contribute much less than those generated in superficial layers of the cortex (Fabiani et al., 2000). In this review the primary interest was to elucidate the effect of child abuse on specific brain regions and networks and we have, therefore, focused our review primarily on the functional imaging techniques of fMRI and PET.
MRI and PET techniques, however, also have their limitations. Whilst fMRI has relatively high spatial resolution and the capacity to demonstrate the entire network of brain areas engaged when subjects undertake particular tasks, there are limitations relating to the interpretation of the fMRI signal. The signal cannot easily differentiate between function-specific processing and neuromodulation, between top-down and bottom-up signals, and it may potentially confuse excitation and inhibition. Also, the magnitude of the fMRI signal cannot be quantified to reflect accurately differences between brain regions, or between tasks within the same region (Logothetis, 2008). PET has similar limitations to fMRI, but additionally has lower temporal resolution due to signal averaging and the need to give intravenous injections to the subjects (Peyron et al., 2000).
DTI has shown decreased density of four white matter tracts in abused subjects, suggesting the communication between brain regions is affected (Eluvathingal et al., 2006; Choi et al., 2009). However, this the current evidence for WM tract deficits is only based on two studies and more DTI studies are needed to further investigate the effect of childhood maltreatment on white matter tracts. The limitations of DTI must also be taken into consideration. As well as the uncertainty produced by background noise, patient movement, and distortion from imaging artefacts, accuracy of the constructed fiber tract is limited by the information contained in the diffusion tensor and in the different methods of constructing the tracts. Other limitations are that the anatomy of the white matter tracts must already be known, DTI tractography cannot distinguish anterograde from retrograde information flow and the analysis is based on the assumption that diffusion has Gaussian characteristics, which is not correct when diffusion is restricted. Furthermore, tractography is a mathematical estimation of white matter tracts rather than an anatomic depiction so axonal fibers can appear disproportionately large because of higher FA (for a review see Mori and van Zijl, 2002).
While there is some evidence for abnormal structural connectivity, so far there are no studies that have tested for functional connectivity deficits. Early environmental adversities are likely to compromise the development of complex neural networks rather than isolated brain regions and hence more focus should be on structural and functional regional interconnectivity measures. Further, most studies conducted in patients with childhood abuse have used one imaging modality only. Future studies should combine several imaging modalities to elucidate to what extent abnormal brain structure in fronto-limbic and fronto-cortical regions is associated with abnormal function in these areas and how this relates to the abnormal behavioral and cognitive endophenotype of the disorder. For example, a combination of MRI, DTI, fMRI, and ERP would allow investigation of brain structure, connectivity and function with both spatial and temporal resolution within the same study.
Unfortunately, so far no PET studies have investigated the underlying neurotransmitter abnormalities in patients with childhood abuse. There is consistent evidence for the implication of monoamines both in normal development as well as in early stress-induced deviation of normal brain development (Watts-English et al., 2006; McCrory et al., 2010; Twardosz and Lutzker, 2010). There is evidence for monoamine neurotransmitter receptor and transporter abnormalities in child psychiatric conditions that are typically associated with childhood abuse such as depression (Drevets et al., 2007), ADHD (Nakao et al., 2011), and anxiety (Tauscher et al., 2001). Therefore, it is likely that childhood abuse is associated with abnormal development of monoaminergic pathways, which could be measured using PET.
In summary, despite all the limitations of the existing literature, there is emerging evidence that specific brain areas are affected by childhood maltreatment. When taking into account only high resolution structural and functional neuroimaging studies that have controlled for co-morbid psychiatric conditions and medication, the main brain regions consistently reported to have abnormalities in subjects with a history of childhood maltreatment are DLPFC, mPFC, OFC, ACC, hippocampus, amygdala, and the cerebellum (see Figures 1 and 2). If ROI studies are omitted, the hippocampus, and amygdala deficits are not reported as consistently as the other brain regions. Furthermore, several structural MRI studies reported that abnormalities of cerebral cortex, PFC, hippocampus, amygdala, ACC, and caudate correlated with the age of onset or duration of abuse, further strengthening the direct link between childhood abuse and abnormalities in these brain regions (see Table 1, De Bellis et al., 1999, 2002a; Driessen et al., 2000; Cohen et al., 2006; Tupler and De Bellis, 2006; Andersen et al., 2008; Mehta et al., 2009; Treadway et al., 2009).
In conclusion, the best available evidence suggests that the pathways affected in subjects with a history of childhood maltreatment are predominantly in fronto-limbic networks, including mPFC, OFC, ACC, hippocampus, and amygdala. These pathways are involved in emotion and motivation processing (Adolphs, 2002; Ochsner and Gross, 2005; Amodio and Frith, 2006; Andersen et al., 2007) as well as the control of aggression (Davidson et al., 2000). Disruption to these pathways in abused subjects may, therefore, underlie the observed deficits in emotion and reward processing as well as excessive aggression or violent behavior (for a review see Heide and Solomon, 2006). Deficits in these fronto-limbic networks may be due to the vulnerability of the frontal lobe to stress effects due to the fact that it has a high density of glucocorticoid receptors and dopaminergic projections that are stress-susceptible (Brake et al., 2000).
There is emerging evidence for deficits in other prefrontal regions such as DLPFC and IFC that, together with their striatal, cerebellar and parieto-temporal connections, are important for the normal development of EFs, working memory, inhibition, and attention (Rubia et al., 2006, 2007, 2010; Christakou et al., 2009; Geier and Luna, 2009; Arnsten and Rubia, 2012), and which could be the underlying substrates for poor performance on tasks that tap into these functions (see Figure 2).
While childhood abuse research lends itself optimally to the study of adverse environmental effects on brain plasticity, there is recent evidence to suggest gene-environment interaction effects. Studies of gene-environment interaction have shown that specific genotypes moderate the association between childhood maltreatment and psychopathology. Several studies, including a meta-analysis, have shown that carriers of genotypes conferring low MAO-A activity are more susceptible to antisocial behaviors and mental illness following early environmental adversity such as maltreatment (Caspi et al., 2002; Kim-Cohen et al., 2006; Taylor and Kim-Cohen, 2007; Weder et al., 2009). Furthermore, polymorphisms for the promoter region of the serotonin transporter (5-HTTLPR) gene have also been found to moderate the association, so that childhood maltreatment increased the risk of depression in early adulthood for persons with the common “short” allele compared to persons with the long allele (Caspi et al., 2003; Cicchetti et al., 2007), although some studies found the association only in girls (Aslund et al., 2009). Imaging genetics studies, furthermore, have found an association between these two genetic polymorphisms (i.e., low activity MAO-A VNTR genotype and S allele of 5-HTTLPR) and the structure and function of emotion processing brain regions such as the OFC, anterior cingulate and orbitofrontal-amygdala networks (for review see Scharinger et al., 2010). Future neuroimaging studies of childhood abuse should, therefore, include testing for specific candidate genotypes that may moderate the association between childhood abuse, psychiatric comorbidities and brain structure and function deficits in order to elucidate the interaction between genes, environment, the emergence of psychopathology and underlying brain deficits.
In conclusion, future studies of the neuroimaging substrates of childhood abuse need to address the limitations of previous imaging studies by applying multimodal imaging that combine structure and function measures, by focusing more on structural and functional neural networks in addition to isolated regions by differentiating between different types of abuse, and by addressing and controlling confounds of psychiatric co-morbidities, medication and gender effects. Testing for candidate genes that have been found to be associated with adverse mental health outcome after child abuse could furthermore elucidate gene-environment-imaging interactions.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Heledd Hart was supported by a grant from the Kids Company.
References
- Adolphs R. (2002). Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 12, 169–177 10.1016/S0959-4388(02)00301-X [DOI] [PubMed] [Google Scholar]
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andersen P., Morris R., Amaral D., Bliss T., O'Keefe J. (2007). The Hippocampus Book. Oxford Neurosciences Series. New York, NY: Oxford University Press [Google Scholar]
- Andersen S. L., Tomada A., Vincow E. S., Valente E., Polcari A., Teicher M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 20, 292–301 10.1176/appi.neuropsych.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. M., Teicher M. H., Polcari A., Renshaw P. I. (2002). Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology 27, 231–244 10.1016/S0306-4530(01)00047-6 [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., Arndt S., Cizadlo T., O'Leary D. S., Watkins G. L., Boles Ponto L. L., Hichwa R. D. (1996). Sample size and statistical power in [15O]H2O studies of human cognition. J. Cereb. Blood Flow Metab. 16, 804–816 10.1097/00004647-199609000-00005 [DOI] [PubMed] [Google Scholar]
- Arnsten A., Rubia K. (2012). Neurobiological circuits regulating attention, movement, and emotion: disruptions in Pediatric disorders. J. Am. Acad. Child Adolesc. Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- Aron A. R., Robbins T. W., Poldrack R. A. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Aslund C., Leppert J., Comasco E., Nordquist N., Oreland L., Nilsson K. W. (2009). Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav. Genet. 39, 524–531 10.1007/s10519-009-9285-9 [DOI] [PubMed] [Google Scholar]
- Augustine J. R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Brain Res. Rev. 22, 229–244 10.1016/S0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Beers S. R., De Bellis M. D. (2002). Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. Am. J. Psychiatry 159, 483–486 10.1176/appi.ajp.159.3.483 [DOI] [PubMed] [Google Scholar]
- Bos K. J., Fox N., Zeanah C. H., Nelson C. A. (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Front. Behav. Neurosci. 3:16 10.3389/neuro.08.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake W. G., Sullivan R. M., Gratton A. (2000). Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J. Neurosci. 20, 5538–5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Hardan A., di Nemic S. U., Perez J., Soares J. C., Barale F. (2003). Brain anatomy and development in autism: review of structural MRI studies. Brain Res. Bull. 61, 557–569 10.1016/j.brainresbull.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Narayan M., Staib L. H., Southwick S. M., McGlashan T., Charney D. S. (1999). Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 156, 1787–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J., Randall P., Capelli S., Scott T., McCarthy G., Charney D. (1995). Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 59, 97–105 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Randall P., Vermetten E., Staib L., Bronen R. A., Mazure C., Capelli S., McCarthy G., Innis R. B., Charney D. S. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biol. Psychiatry 41, 23–32 10.1016/S0006-3223(96)00162-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. D., Vermetten E., Afzal N., Vythilingam M. (2004). Deficits in verbal declarative memory function in women with childhood sexual abuse related posttraumatic stress disorder. J. Nerv. Ment. Dis. 192, 643–664 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Vermetten E., Schmahl C., Vaccarino V., Vythilingam M., Afzal N., Grillon C., Charney D. S. (2005). Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol. Med. 35, 791–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. D., Vermetten E., Vythilingam M., Afzal N., Schmahl C., Elzinga B., Charney D. S. (2004a). Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry 55, 612–620 10.1016/j.biopsych.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Vythilingam M., Vermetten E., Southwick S. M., McGlashan T., Nazeer A., Khan S., Vaccarino L. V., Soufer R., Garg P. K., Ng C. K., Staib L. H., Duncan J. S., Charney D. S. (2003). MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 160, 924–932 10.1176/appi.ajp.160.5.924 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Vythilingam M., Vermetten E., Southwick S. M., Mc-Glashan T., Staib L. H., Soufer R., Charney D. S. (2003a). Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol. Psychiatry 53, 879–889 10.1016/S0006-3223(02)01891-7 [DOI] [PubMed] [Google Scholar]
- Brodsky B. S., Malone K. M., Ellis S. P., Dulit R. A., Mann J. J. (1997). Characteristics of borderline personality disorder associated with suicidal behavior. Am. J. Psychiatry 154, 1715–1719 [DOI] [PubMed] [Google Scholar]
- Brodsky B. S., Mann J. J., Stanley B., Tin A., Oguendo M., Birmaher B., Greenhill L., Kolko D., Zelazny J., Burke A. K., Nelhem N. M., Brent D. (2008). Familial transmission of suicidal behavior: factors mediating the relationship between childhood abuse and offspring suicide attempts. J. Clin. Psychiatry 69, 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguri M. P., Brown G. G., Meloy M. J., Eberson S. C., Kindermann S. S., Frank L. R., Zorrilla L. E., Lohr J. B. (2003). An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 123, 171–182 10.1016/S0925-4927(03)00075-1 [DOI] [PubMed] [Google Scholar]
- Carrey N. J., Butter H. J., Persinger M. A., Bialik R. J. (1995). Physiological and cognitive correlates of child abuse. J. Am. Acad. Child Adolesc. Psychiatry 34, 1067–1075 10.1097/00004583-199508000-00017 [DOI] [PubMed] [Google Scholar]
- Carrion V. G., Garrett A., Menon V., Weems C. F., Reiss A. L. (2008). Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress. Anxiety 25, 514–526 10.1002/da.20346 [DOI] [PubMed] [Google Scholar]
- Carrion V. G., Weems C. F., Eliez S., Patwardhan A., Brown W., Ray R. D., Reiss A. L. (2001). Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol. Psychiatry 50, 943–951 [DOI] [PubMed] [Google Scholar]
- Carrion V. G., Weems C. F., Reiss A. L. (2007). Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119, 509–516 10.1542/peds.2006-2028 [DOI] [PubMed] [Google Scholar]
- Carrion V. G., Weems C. F., Watson C., Eliez S., Menon V., Reiss A. L. (2009). Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 172, 226–234 10.1016/j.pscychresns.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., McClay J., Moffitt T. E., Mill J., Martin J., Craig I. W., Taylor A., Poulton R. (2002). Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854 10.1126/science.1072290 [DOI] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T. E., Taylor A., Craig I. W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- Choi J., Jeong B., Rohan M. L., Polcari A. M., Teicher M. H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry 65, 227–234 10.1016/j.biopsych.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Brammer M., Giampietro V., Rubia K. (2009). Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J. Neurosci. 29, 11020–11028 10.1523/JNEUROSCI.1279-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H. T., Behen M. E., Muzik O., Juha C., Ferenc N., Chugani D. C. (2001). Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14, 1290–1301 10.1006/nimg.2001.0917 [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Curtis W. J. (2005). An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Dev. Psychopathol. 17, 641–677 10.1017/S0954579405050315 [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F. A., Sturge-Apple M. L. (2007). Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptornatology among adolescents from low socioeconomic status backgrounds. Dev. Psychopathol. 19, 1161–1180 10.1017/S0954579407000600 [DOI] [PubMed] [Google Scholar]
- Clark D. B., De Bellis M. D., Lynch K. G., Cornelius J. R., Martin C. S. (2003). Physical and sexual abuse, depression and alcohol use disorders in adolescents: onsets and outcomes. Drug Alcohol Depend. 69, 51–60 10.1016/S0376-8716(02)00254-5 [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Grieve S., Hoth K. F., Paul R. H., Sweet L., Tate D., Gunstad J., Stroud L., McCaffery J., Hitsman B., Niaura R., Clark C. R., MacFarlane A., Bryant R., Gordon E., Williams L. M. (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 59, 975–982 10.1016/j.biopsych.2005.12.016 [DOI] [PubMed] [Google Scholar]
- Compton R. J. (2003). The interface between emotion and attention: a review of evidence from psychology and neuroscience. Behav. Cogn. Neurosci. Rev. 2, 115–129 10.1177/1534582303255278 [DOI] [PubMed] [Google Scholar]
- Croy I., Schellong J., Gerber J., Joraschky P., Iannilli E., Hummel T. (2010). Women with a history of childhood maltreatment exhibit more activation in association areas following non-traumatic olfactory stimuli: a fMRI study. PLoS One 5:e9362 10.1371/journal.pone.0009362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. J., Putnam K. M., Larson C. L. (2000). Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science 289, 591–594 [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P. J. (2001). The amygdala: vigilance and emotion. Mol. Psychiatry 6, 3–34 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D. (2002). Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 27, 155–170 10.1016/S0306-4530(01)00042-7 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Baum A. S., Birmaher B., Keshavan M. S., Eccard C. H., Boring A. M., Jenkins F. J., Ryan N. D. (1999). Developmental traumatology part I: biological stress systems. Biol. Psychiatry 45, 1259–1270 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Broussard E. R., Herring D. J., Wexlerd S., Moritze G., Benitez J. G. (2001a). Psychiatric co-morbidity in caregivers and children involved in maltreatment: a pilot research study with policy implications. Child Abuse Negl. 25, 923–944 10.1016/S0145-2134(01)00247-2 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Hall J., Boring A. M., Frustaci K., Moritz G. (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry 50, 305–309 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Hooper S. R., Spratt E. G., Woolley D. P. (2009). Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J. Int. Neuropsychol. Soc. 15, 868–878 10.1017/S1355617709990464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M. D., Keshavan M. S. (2003). Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci. Biobehav. Rev. 27, 103–117 10.1016/S0376-8716(02)00254-5 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Keshavan M. S., Frustaci K., Shifflett H., Iyengar S., Beers S. R., Hall J. (2002b). Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol. Psychiatry 51, 544–552 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Keshavan M. S., Shifflett H., Iyengar S., Beers S. R., Hall J., Moritz G. (2002a). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry 52, 1066–1078 [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Kuchibhatla M. (2006). Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry 60, 697–703 10.1016/j.biopsych.2006.04.035 [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters V. B., Hadders-Algra M. (2006). Ontogeny of the human central nervous system: what is happening when? Early Hum. Dev. 82, 257–266 10.1016/j.earlhumdev.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Degroot A., Treit D. (2004). Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 1001, 60–71 10.1016/j.brainres.2003.10.065 [DOI] [PubMed] [Google Scholar]
- DePrince A. P., Weinzierl K. M., Combs M. D. (2009). Executive function performance and trauma exposure in a community sample of children. Child Abuse Negl. 33, 353–361 10.1016/j.chiabu.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Desmond J. E., Glover G. H. (2002). Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods 118, 115–128 10.1016/S0165-0270(02)00121-8 [DOI] [PubMed] [Google Scholar]
- Dillon D. G., Holmes A. J., Birk J. L., Brooks N., Lyons-Ruth K., Pizzagalli D. A. (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry 66, 206–213 10.1016/j.biopsych.2009.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan R. J. (2007). The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 787–799 10.1098/rstb.2007.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W. C., Thase M. E., Moses-Kolko E. L., Price J., Frank E., Kupfer D. J., Mathis C. (2007). Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 34, 865–877 10.1016/j.nucmedbio.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M., Herrmann J., Stahl K., Zwaan M., Meier S., Hill A., Osterheider M., Petersen D. (2000). Magnetic resonance imaging volumes of the hippocampus and amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiatry 57, 1115–1122 [DOI] [PubMed] [Google Scholar]
- During S., McMahon R. (1991). Recognition of emotional facial expressions by abusive mothers and their children. J. Clin. Child Psychol. 20, 132–139 [Google Scholar]
- Eluvathingal T. J., Chugani H. T., Behen M. E., Juhasz C., Muzik O., Maqbool M., Chugani D. C., Makki M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- English D. J., Thompson R., Graham J. C., Briggs E. C. (2005). Toward a definition of neglect in young children. Child Maltreat. 10, 190–206 10.1177/1077559505275178 [DOI] [PubMed] [Google Scholar]
- Fabiani M., Gratton G., Colesm M. G. H. (2000). “Event-related brain potentials: methods, theory and appications,” in Handbook of Psychophysiology, 2nd edn, eds Cacioppo J. T., Tassinary L. G., Berntson G. G. (Cambridge, UK: Cambridge University Press; ), 53–84 [Google Scholar]
- Famularo R., Fenton T., Kinscherff R. (1993). Child maltreatment and the development of post traumatic stress disorder. Am. J. Dis. Child. 147, 755–760 [DOI] [PubMed] [Google Scholar]
- Friston K. J., Rotshtein P., Geng J. J., Sterzer P., Henson R. N. (2006). A critique of functional localisers. Neuroimage 30, 1077–1087 10.1016/j.neuroimage.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Garbarino J., Garbarino A. (1994). Emotional Maltreatment of Children, 2nd edn. Chicago, IL: National Committee to Prevent Child Abuse [Google Scholar]
- Geier C., Luna B. (2009). The maturation of incentive processing and cognitive control. Pharmacol. Biochem. Behav. 93, 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Blumenthal J., Jeffries N. O., Castellanos F. X., Liu H., Zijdenbos A., Paus T., Evans A. C., Rapoport J. L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Giedd J. N., Clasen L. S., Lenroot R., Greenstein D., Wallace G. L., Ordaz S., Molloy E. A., Blumenthal J. D., Tossell J. W., Stayer C., Samango-Sprouse C. A., Shen D., Davatzikos C., Merke D., Chrousos G. P. (2006). Puberty-related influences on brain development. Mol. Cell. Endocrinol. 254–255, 154–162 10.1016/j.mce.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Giedd J. N., Rumsey J. M., Castellanos F. X., Rajapakse J. C., Kaysen D., Vaituzis A. C., Vauss C., Hamburger S. D., Rapoport J. L. (1996). A quantitative MRI study of the corpus callosum in children and adolescents. Dev. Brain Res. 91, 274–280 10.1016/0165-3806(95)00193-X [DOI] [PubMed] [Google Scholar]
- Giovannoni J. M., Becerra R. M. (1979). Defining Child Abuse. New York, NY: The Free Press [Google Scholar]
- Gur R., Gunning-Dixon F., Bilker W. B., Gur R. E. (2002). Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb. Cortex 12, 998–1003 10.1093/cercor/12.9.998 [DOI] [PubMed] [Google Scholar]
- Hanson J. L., Chung M. K., Avants B. B., Shirtcliff E. A., Gee J. C., Davidson R. J., Pollak S. D. (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci. 30, 7466–7472 10.1523/JNEUROSCI.0859-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan K., Cloitre M. (2000). A comparison of posttraumatic stress disorder with and without borderline personality disorder among women with a history of childhood sexual abuse: etiological and clinical characteristics. J. Nerv. Ment. Dis. 188, 589–595 [DOI] [PubMed] [Google Scholar]
- Heffernan K., Cloitre M., Tardiff K., Marzuk P. M., Portera L., Leon A. C. (2000). Childhood trauma as a correlate of lifetime opiate use in psychiatric patients. Addict. Behav. 25, 797–803 10.1016/S0306-4603(00)00066-6 [DOI] [PubMed] [Google Scholar]
- Heide K. M., Solomon E. P. (2006). Biology, childhood trauma, and murder: rethinking justice. Int. J. Law Psychiatry 29, 220–233 10.1016/j.ijlp.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Ito Y., Teicher M., Glod C., Ackerman E. (1998). Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. J. Neuropsychiatry Clin. Neurosci. 10, 298–307 [DOI] [PubMed] [Google Scholar]
- Jackowski A. P., Douglas-Palumberi H., Jackowski M., Win L., Schultz R. T., Staib L. W., Krystal J. H., Kaufman J. (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 162, 256–261 10.1016/j.pscychresns.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K. A., Eckenrode J. (1996). The effects of neglect on academic achievement and disciplinary problems: a developmental perspective. Child Abuse Negl. 20, 161–169 10.1016/S0145-2134(95)00139-5 [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K. A., Williams L. M., Finkelhor D. (1993). Impact of sexual abuse on children: a review and synthesis of recent empirical studies. Psychol. Bull. 113, 164–180 [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Bulik C. M., Silberg J., Hettema J. M., Myers J., Prescott C. A. (2000). Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch. Gen. Psychiatry 57, 953–959 [DOI] [PubMed] [Google Scholar]
- Kessler R., Berglund P., Foster C., Saunders W., Stang P., Walters E. (1997). Social consequences of psychiatric disorders, II: teenage parenthood. Am. J. Psychiatry 154, 1405–1411 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Jeong D. U., Sim M. E., Bae S. C., Chung A., Kim M. J., Chang K. H., Ryu J., Renshaw P. F., Lyoo I. K. (2006). Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology 54, 120–125 10.1159/000098262 [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J., Caspi A., Taylor A., Williams B., Newcombe R., Craig I. W., Moffitt T. E. (2006). MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol. Psychiatry 11, 903–913 10.1038/sj.mp.4001851 [DOI] [PubMed] [Google Scholar]
- Kingree J. B., Sullivan B. F., Thompson M. P. (1999a). Attributions for the development of substance addiction among participants in a 12-step oriented treatment program. J. Psychoactive Drugs 31, 129–135 [DOI] [PubMed] [Google Scholar]
- Kingree J. B., Thompson M. P., Kaslow N. J. (1999b). Risk factors for suicide attempts among low-income women with a history of alcohol problems. Addict. Behav. 24, 583–587 10.1016/S0306-4603(98)00109-9 [DOI] [PubMed] [Google Scholar]
- Kitayama N., Brummer M., Hertz L., Quinn S., Kim Y., Bremner J. D. (2007). Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: a preliminary study. J. Nerv. Ment. Dis. 195, 1027–1029 10.1097/NMD.0b013e31815c044f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N., Quinn S., Bremner J. D. (2006). Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J. Affect. Disord. 90, 171–174 10.1016/j.jad.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N., Vaccarino V., Kutner M., Weiss P., Bremner J. D. (2005). Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 88, 79–86 10.1016/j.jad.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Kitterle F. (1995). Hemispheric Communication: Mechanisms and Models. Hillsdale, NJ: Laurence Erlbaum Associates [Google Scholar]
- Lanius R. A., Williamson P. C., Densmore M., Boksman K., Gupta M. A., Neufeld R. W., Gati J. S., Menon R. S. (2001). Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry 158, 1920–1922 10.1176/appi.ajp.158.11.1920 [DOI] [PubMed] [Google Scholar]
- Lanius R. A., Williamson P. C., Hopper J., Densmore M., Boksman K., Gupta M. A., Neufeld R. W., Gati J. S., Menon R. S. (2003). Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 53, 204–210 10.1016/S0006-3223(02)01466-X [DOI] [PubMed] [Google Scholar]
- Leeb R. T., Paulozzzi L., Melanson C., Simon T., Arias I. (2008). Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements. Atlanta: Centers for Disease Control and Prevention [Google Scholar]
- Leh S., Petrides M., Strafella A. (2010). The neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacology 35, 70–85 10.1038/npp.2009.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R. K., Gogtay N., Greenstein D. K., Wells E. M., Wallace G. L., Clasen L. S., Blumenthal J. D., Lerch J., Zijdenbos A. P., Evans A. C., Thompson P. M., Giedd J. N. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. K. (2008). What we can do and what we cannot do with fMRI. Nature 453, 869–878 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Loman M. M., Wiik K. L., Frenn K. A., Pollak S. D., Gunnar M. R. (2009). Postinstitutionalized children's development: growth, cognitive, and language outcomes. J. Dev. Behav. Pediatr. 30, 426–434 10.1097/DBP.0b013e3181b1fd08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K. (2008). Contributions of the mother-infant relationship to dissociative, borderline, and conduct symptoms in young adulthood. Infant Ment. Health J. 29, 203–218 10.1002/imhj.20173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu F. S., Dozier M., Guyer A. E., Mandell D., Peloso E., Poeth K., Jenness J., Lau J. Y. F., Ackerman J. P., Pine D. S., Ernst M. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 10, 34–49 10.3758/CABN.10.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M., Nater U., Lin J. M., Capuron L., Reeves W. (2010). Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 10, 61–71 10.1186/1471-2377-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Kennedy D. N., McInerney S., Sorensen A. G., Wang R., Caviness V. S. Jr., Pandya D. N. (2005). Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex 15, 854–869 10.1093/cercor/bhh186 [DOI] [PubMed] [Google Scholar]
- McCrory E., De Brito S. A., Viding E. (2010). Research review: the neurobiology and genetics of maltreatment and adversity. J. Child Psychol. Psychiatry 51, 1079–1095 10.1111/j.1469-7610.2010.02271.x [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. (2000). Memory-a century of consolidation. Science 287, 248–251 [DOI] [PubMed] [Google Scholar]
- McLeer S. V., Dixon J. F., Henry D., Ruggiero K., Escovitz K., Niedda T., Scholle R. (1998). Psychopathology in non-clinically referred sexually abused children. J. Am. Acad. Child Adolesc. Psychiatry 37, 1326–1333 10.1097/00004583-199812000-00017 [DOI] [PubMed] [Google Scholar]
- Mehta M. A., Golembo N. I., Nosarti C., Colvert E., Mota A., Williams S. C. R., Rutter M., Sonuga-Barke E. J. S. (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J. Child Psychol. Psychiatry 50, 943–951 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- Mezzacappa E., Kindlon D., Earls F. (2001). Child abuse and performance task assessments of executive functions in boys. J. Child Psychol. Psychiatry 42, 1041–1048 [DOI] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L. A., Georgiades K., Boyle M., Macmillan H. (2010). Adolescent females exposed to child maltreatment exhibit atypical EEG coherence and psychiatric impairment: linking early adversity, the brain, and psychopathology. Dev. Psychopathol. 22, 419–432 10.1017/S0954579410000155 [DOI] [PubMed] [Google Scholar]
- Mori S., van Zijl P. C. (2002). Fiber tracking: principles and strategies a technical review. NMR Biomed. 15, 468–480 10.1002/nbm.781 [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Maheu F. S., Dozier M., Peloso E., Mandell D., Leibenluft E., Pinea D. S., Ernsta M. (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia 48, 3037–3044 10.1016/j.neuropsychologia.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. E. (2010). Using functional neuroimaging to investigate the mechanisms of action of selective serotonin reuptake inhibitors (SSRIs). Curr. Pharm. Des. 16, 1990–1997 [DOI] [PubMed] [Google Scholar]
- Nakao T. C., Radua Q., Rubia K., Mataix-Cols D. (2011). Gray matter volume abnormalities in ADHD and the effects of stimulant medication: voxel-based meta-analysis. Am. J. Psychiatry 168, 1154–1163 10.1176/appi.ajp.2011.11020281 [DOI] [PubMed] [Google Scholar]
- Navalta C. P., Polcari A., Webster D. M., Boghossian A., Teicher M. H. (2006). Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J. Neuropsychiatry Clin. Neurosci. 18, 45–53 10.1176/appi.neuropsych.18.1.45 [DOI] [PubMed] [Google Scholar]
- Nolin P., Ethier L. (2007). Using neuropsychological profiles to classify neglected children with or without physical abuse. Child Abuse Negl. 31, 631–643 10.1016/j.chiabu.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Noll-Hussong M., Ottia A., Laeerb L., Wohlschlaegerb A., Zimmerb C., Lahmanna C., Henningsena P., Toellec T., Guendeld H. (2010). Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J. Psychosom. Res. 68, 483–487 10.1016/j.jpsychores.2010.01.020 [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Gross J. J. (2005). The cognitive control of emotion. Trends Cogn. Sci. 9, 242–249 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Osofsky J. D. (1999). The impact of violence on children. Future Child. 9, 33–49 [PubMed] [Google Scholar]
- Parker S. W., Nelson C. A. (2005). Bucharest early intervention project core group: an event-related potential study of the impact of institutional rearing on face recognition. Dev. Psychopathol. 17, 621–639 10.1017/S0954579405050303 [DOI] [PubMed] [Google Scholar]
- Pears K. C., Fisher P. A. (2005). Emotion understanding and theory of mind among maltreated children in foster care: evidence of deficits. Dev. Psychopathol. 17, 47–65 [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D. A. (2010). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl.) 214, 55–70 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson C. L., Maurer S. H., Kaminski P. L., Zander K. A., Peters C. M., Stokes-Crowe L. A., Osborn R. E. (2004). Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J. Trauma. Stress 17, 37–40 10.1023/B:JOTS.0000014674.84517.46 [DOI] [PubMed] [Google Scholar]
- Peran P., Demonet J. F., Cardebat D. (2008). Paroxetine-induced modulation of cortical activity supporting language representations of action. Psychopharmacology (Berl.) 195, 487–496 10.1007/s00213-007-0939-0 [DOI] [PubMed] [Google Scholar]
- Peyron R., Laurent B., García-Larrea L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol. Clin. 30, 263–288 [DOI] [PubMed] [Google Scholar]
- Phan K. L., Wager T., Taylor S. F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Pine D. S., Mogg K., Bradley B. P., Montgomery L. A., Monk C. S., McClure E., Guyer A. E., Ernst M., Charney D. S., Kaufman J. (2005). Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am. J. Psychiatry 162, 291–296 10.1176/appi.ajp.162.2.291 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Cicchetti D., Hornuny K., Reed A. (2000). Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 36, 679–688 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Klorman R., Thatcher J. E., Cicchetti D. (2001). P3b reflects maltreated children's reactions to facial displays of emotion. Psychophysiology 38, 267–274 10.1111/1469-8986.3820267 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Nelson C. A., Schlaak M. F., Roeber B. J., Wewerka S. S., Wiik K. L., Frenn K. A., Loman M. M., Gunnar M. R. (2010). Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. 81, 224–236 10.1111/j.1467-8624.2009.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S. D., Sinha P. (2002). Effects of early experience on children's recognition of facial displays of emotion. Dev. Psychol. 38, 784–791 [DOI] [PubMed] [Google Scholar]
- Pollak S. D., Tolley-Schell S. A. (2003). Selective attention to facial emotion in physically abused children. J. Abnorm. Psychol. 112, 323–338 [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Kramer L. A., Ewing-Cobbs L. (2005). Cognitive and neuroimaging findings in physically abused preschoolers. Arch. Dis. Child 90, 82–85 10.1136/adc.2003.045583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W. (2003). Cherish your exceptions. J. Child Sex. Abus. 12, 133–135 10.1300/J070v12n02_10 [DOI] [PubMed] [Google Scholar]
- Putnam F. W. (2003a). Ten-year research update review: child sexual abuse. J. Am. Acad. Child Adolesc. Psychiatry 42, 269–278 10.1097/00004583-200303000-00006 [DOI] [PubMed] [Google Scholar]
- Raine A., Park S., Lencz T., Bihrle S., LaCasse L., Widom C. S., Al-Dayeh L., Singh M. (2001). Reduced right hemisphere activation in severely abused violent offenders during a working memory task: an fMRI study. Aggress. Behav. 27, 111–129 [Google Scholar]
- Richert K. A., Carrion V. G., Karchemskiy A., Reiss A. L. (2006). Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress. Anxiety 23, 17–25 10.1002/da.20131 [DOI] [PubMed] [Google Scholar]
- Rohsenow D. J., Corbett R., Devine D. (1988). Molested as children: a hidden contribution to substance abuse? J. Subst. Abuse Treat. 5, 13–18 [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A-M., Brammer M., Taylor E. (2009). Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 57, 640–652 10.1016/j.neuropharm.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A-M., Brammer M., Taylor E. (2011a). Methylphenidate normalises fronto-striatal underactivation during interference inhibition in medication-naive boys. Neuropsychopharmacology 36, 1575–1586 10.1038/npp.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A-M., Brammer M., Taylor E. (2011b). Methylphenidate normalises fronto-cingulate underactivation during error processing in children with attention-deficit hyperactivity disorder. Biol. Psychiatry 70, 255–262 10.1016/j.biopsych.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Hyde Z., Halari R., Giampietro V., Smith A. (2010). Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage 51, 817–827 10.1016/j.neuroimage.2010.02.058 [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A. (2004). The neural correlates of cognitive time management: a review. Acta Neurobiol. Exp. (Wars.) 64, 329–340 [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A. B., Brammer M. J., Taylor E. (2003). Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20, 351–358 10.1016/S1053-8119(03)00275-1 [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A. B., Taylor E., Brammer M. (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum. Brain Mapp. 28, 1163–1177 10.1002/hbm.20347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A. B., Wooley J., Nosarti C., Heyman I., Taylor E., Brammer M. (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum. Brain Mapp. 27, 973–993 10.1002/hbm.20237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K. D., Hammen C. (1999). Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 70, 660–677 10.1111/1467-8624.00048 [DOI] [PubMed] [Google Scholar]
- Saigh P., Yasik A., Oberfield R., Halamandaris P., Bremner J. (2006). The intellectual performance of traumatized children and adolescents with or without posttraumatic stress disorder. J. Abnorm. Psychol. 115, 332–340 10.1037/0021-843X.115.2.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson K. W., Krueger C. E., Burnett C., Wilson C. K. (2010). Neuropsychological functioning in children with posttraumatic stress disorder. Child Neuropsychol. 16, 119–133 10.1080/09297040903190782 [DOI] [PubMed] [Google Scholar]
- Scharinger C., Rabl U., Sitte H. H., Pezawas L. (2010). Imaging genetics of mood disorders. Neuroimage 53, 810–821 10.1016/j.neuroimage.2010.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherck H., Falkai P. (2006). Effects of antipsychotics on brain structure. Curr. Opin. Psychiatry 19, 145–150 10.1097/01.yco.0000214339.06507.d8 [DOI] [PubMed] [Google Scholar]
- Schiffer F., Teicher M., Papanicolaou A. (1995). Evoked potential evidence for right brain activity during the recall of traumatic memories. J. Neuropsychiatry Clin. Neurosci. 7, 169–175 [DOI] [PubMed] [Google Scholar]
- Schmahl C. G., Vermetten E., Elzinga B. M., Bremner J. D. (2004). A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol. Psychiatry 55, 759–765 10.1016/j.biopsych.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Weilburg J. B., Sherman J. C. (2007). The neuropsychiatry of the cerebellum: insights from the clinic. Cerebellum 6, 254–267 10.1080/14734220701490995 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M. R., Stalnaker T. A. (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 29, 116–124 10.1016/j.tins.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D. J. L. G., van Honk J. (2005). The cerebellum on the rise in human emotion. Cerebellum 4, 290–294 10.1080/14734220500348584 [DOI] [PubMed] [Google Scholar]
- Sedlak A. J., Broadhurst D. D. (1996). Third national Incidence Study of Child Abuse and Neglect (NIS-3 Final Report). U.S. Dept. of Health and Human Service Contract No. 1 (05–94–1840). 10.1016/j.chiabu.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Shackman J. E., Shackman A. J., Pollak S. D. (2007). Physical abuse amplifies attention to threat and increases anxiety in children. Emotion 7, 838–852 10.1037/1528-3542.7.4.838 [DOI] [PubMed] [Google Scholar]
- Shin L. M., McNally R. J., Kosslyn S. M., Thompson W. L., Rauch S. L., Alpert N. M., Metzger L. J., Lasko N. B., Orr S. P., Pitman R. K. (1999). Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584 [DOI] [PubMed] [Google Scholar]
- Shin L. M., Rauch S. L., Pitman R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N.Y. Acad. Sci. 1071, 67–79 10.1196/annals.1364.007 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J. S., Rubia K. (2008). Inattentive/overactive children with histories of profound institutional deprivation compared with standard ADHD cases: a brief report. Child Care Health Dev. 34, 596–602 10.1111/j.1365-2214.2008.00863.x [DOI] [PubMed] [Google Scholar]
- Sowell E. R., Peterson B. S., Thompson P. M., Welcome S. E., Henkenius A. L., Toga A. W. (2003). Mapping cortical change across the human life span. Nat. Neurosci. 6, 309–315 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- Stein M. B., Koverola C., Hanna C., Torchia M. G., McClarty B. (1997). Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 27, 951–959 [DOI] [PubMed] [Google Scholar]
- Tauscher J., Bagby R. M., Javanmard M., Christensen B. K., Kasper S., Kapur S. (2001). Inverse relationship between serotonin 5-HT1A receptor binding and anxiety: a [11C]WAY-100635 PET investigation in healthy volunteers. Am. J. Psychiatry 158, 1326–1328 [DOI] [PubMed] [Google Scholar]
- Taylor A., Kim-Cohen J. (2007). Meta-analysis of gene-environment interactions in developmental psychopathology. Dev. Psychopathol. 19, 1029–1037 10.1017/S095457940700051X [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Andersen S. L., Polcari A., Anderson C. M., Navalta C. P., Kim D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 27, 33–44 10.1016/S0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Dumont N. L., Ito Y., Vaituzis C., Giedd J. N., Andersen S. L. (2004). Childhood neglect is associated with reduced corpus callosum area. Biol. Psychiatry 56, 80–85 10.1016/j.biopsych.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Samson J. A., Polcari A., McGreenery C. E. (2006). Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am. J. Psychiatry 163, 993–1000 10.1176/appi.ajp.163.6.993 [DOI] [PubMed] [Google Scholar]
- Thompson M. P., Kaslow N. J., Kingree J. B., Puett R., Thompson N. J., Meadows L. (1999a). Partner abuse and posttraumatic stress disorder as risk factors for suicide attempts in a sample of low-income, inner-city women. J. Trauma. Stress 12, 59–72 10.1023/A:1024742215337 [DOI] [PubMed] [Google Scholar]
- Thompson M. P., Kaslow N. J., Kingree J. B., Puett R., Thompson N. J., Meadows L. (1999b). Partner abuse and posttraumatic stress disorder as risk factors for suicide attempts in a sample of low-income, inner city women. J. Trauma. Stress 12, 59–72 10.1023/A:1024742215337 [DOI] [PubMed] [Google Scholar]
- Tomoda A., Sheu Y. S., Rabi K., Suzuki H., Navalta C. P., Polcari A., Teicher M. H. (2010). Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 54(Suppl. 1), S280–S286 10.1016/j.neuroimage.2010.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Suzuki H., Rabi K., Sheu Y-S., Polcari A., Teicher M. H. (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 47(Suppl. 2), T66–T71 10.1016/j.neuroimage.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T. A., Quinn B. T., McCarry T. W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M. C., Eigsti I. M., Thomas K. M., Freed P. J., Booma E. S., Gunnar M. R., Altemus M., Aronson J., Casey B. J. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M. T., Grant M. M., Ding Z., Hollon S. D., Gore J. C., Shelton R. C. (2009). Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One 4:e4887 10.1371/journal.pone.0004887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler L. A., De Bellis M. D. (2006). Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry 59, 523–529 10.1016/j.biopsych.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Twamley E. W., Hami S., Stein M. B. (2004). Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatr. Res. 126, 265–274 10.1016/j.psychres.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Twardosz S., Lutzker J. R. (2010). Child maltreatment and the developing brain: a review of neuroscience perspectives. Aggress. Violent Behav. 15, 59–68 [Google Scholar]
- Valera E., Brown A., Biederman J., Faraone S., Makris N., Monuteaux M., Whitfield-Gabrieli S., Vitulano M., Schiller M., Seidman L. (2010). Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry 167, 86 10.1176/appi.ajp.2009.09020249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk B. A., Perry J. C., Herman J. L. (1991). Childhood origins of self-destructivebeha- vior. Am. J. Psychiatry 148, 1665–1672 [DOI] [PubMed] [Google Scholar]
- Vorria P., Papaligoura Z., Sarafidou J., Kopakaki M., Dunn J., Van Ijzendoorn M. H., Kontopoulou A. (2006). The development of adopted children after institutional care: a follow-up study. J. Child Psychol. Psychiatry 47, 1246–1253 10.1111/j.1469-7610.2006.01666.x [DOI] [PubMed] [Google Scholar]
- Vythilingam M., Heim C., Newport J., Miller A. H., Anderson E., Bronen R., Brummer M., Staib L., Vermetten E., Charney D. S., Nemeroff C. B., Bremner J. D. (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry 159, 2072–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts-English T., Fortson B. L., Gibler N., Hooper S. R., DeBellis M. D. (2006). The psychobiology of maltreatment in childhood. J. Soc. Issues 62, 717–736 [Google Scholar]
- Weder N., Yang B. Z., Douglas-Palumberi H., Massey J., Krystal J. H., Gelernter J., Kaufman J. (2009). MAOA Genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol. Psychiatry 65, 417–424 10.1016/j.biopsych.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierich M. R., Nock M. K. (2008). Posttraumatic stress symptoms mediate the relation between childhood sexual abuse and nonsuicidal self-injury. J. Consult. Clin. Psychol. 76, 39–44 10.1037/0022-006X.76.1.39 [DOI] [PubMed] [Google Scholar]
- Weniger G., Lange C., Sachsse U., Irle E. (2008). Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr. Scand. 118, 281–290 10.1111/j.1600-0447.2008.01246.x [DOI] [PubMed] [Google Scholar]
- Williams B. R., Ponesse J. S., Schachar R. J., Logan G. D., Tannock R. (1999). Development of inhibitory control across the life span. Dev. Psychol. 35, 205–213 [DOI] [PubMed] [Google Scholar]
- Wismer-Fries A. B., Pollak S. D. (2004). Emotion understanding in postinstitutionalized Eastern European children. Dev. Psychopathol. 16, 355–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich S. A., Brewerton T. D., Jocic Z., Dansky B. S., Abbott D. W. (1997). Relationship of childhood sexual abuse and eating disorders. J. Am. Acad. Child. Adolesc. Psychiatry 36, 1107–1115 10.1097/00004583-199708000-00018 [DOI] [PubMed] [Google Scholar]
- Woon F. L., Hedges D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 18, 729–736 10.1002/hipo.20437 [DOI] [PubMed] [Google Scholar]
- Yasik A. E., Saigh P. A., Oberfield R. A., Halamandaris P. V. (2007). Posttraumatic stress disorder: memory and learning performance in children and adolescents. Biol. Psychiatry 61, 382–388 10.1016/j.biopsych.2006.06.005 [DOI] [PubMed] [Google Scholar]