Abstract
Bilateral loss of vestibular sensation causes difficulty maintaining stable vision, posture and gait. An implantable prosthesis that partly restores vestibular sensation could significantly improve quality of life for individuals disabled by this disorder. We have developed a head-mounted multichannel vestibular prosthesis (MVP) that restores sufficient semicircular canal function to recreate a 3D angular vestibulo-ocular reflex (aVOR). In this study, we evaluated effects of chronic MVP stimulation on locomotion in chinchillas. Two of three animals examined exhibited significant improvements in both locomotion and aVOR.
I. Introduction
The 3-dimensional angular vestibulo-ocular reflex (3D aVOR) helps stabilize gaze during head rotation by moving the eyes opposite the head to keep visual images stable on the retinae. Head rotations are sensed by the three semicircular canals (SCCs) of each inner ear, which modulate firing rates on ampullary branches of each vestibular nerve, driving central pathways that cause a reflex eye movement. While individuals with unilateral loss of vestibular sensation usually compensate well, bilateral loss can lead to chronic inability to maintain stable vision, posture and gait [1].
An implantable prosthesis that senses head rotation and electrically stimulates the corresponding ampullary nerves could help individuals with bilateral vestibular loss by restoring vestibular sensation in the implanted ear. Previous studies [2-4] have shown that prototypes of these devices can partially restore the 3D aVOR in rodents and primates with bilateral vestibular deficiency. Adaptive changes in aVOR with chronic prosthetic stimulation have also been studied. Dai et al. [5] showed that during 7 days of continuous stimulation in chinchillas, aVOR gain remained stable while 3D misalignment between head and eye rotation axes improved. Merfeld et al. found that 1D aVOR gain remained stable in squirrel monkeys during months of continuous prosthetic modulation [6] and that cycling a prosthesis on and off in guinea pigs led to gradual reduction in the magnitude and time constant of nystagmus evoked by each transition [7]. These studies demonstrate that the aVOR may undergo adaptive changes over time to augment (or make better use of) the effects of prosthetic stimulation.
Ideally, a vestibular implant would successfully restore not only stable vision but also stable posture and gait. Few studies have examined the impact of prosthetic vestibular stimulation on these behaviors [8]. Although the newest version of our multichannel vestibular prosthesis (MVP) includes triaxis linear accelerometers [9], no head-mounted prosthesis yet described can stimulate the utricle and saccule (which normally encode tilt and translational movements) with sufficiently high selectivity to restore their normal function. It is unclear whether restoring SCC sensation of head angular velocity alone is sufficient to restore stable posture and gait. We hypothesized that over time, animals with bilateral vestibular hypofunction can recover more normal locomotion when provided with sensation of 3D head angular velocity via chronic SCC stimulation by a head-mounted MVP. To investigate this, we measured aVOR performance and locomotion before, during and after a period of chronic prosthetic stimulation.
II. METHODS
A. Experimental subjects and design
Adult wild-type 300-650g female chinchillas (Chinchilla lanigera) were used for all experiments, which were performed in accordance with a protocol approved by the Johns Hopkins Animal Care and Use Committee. During surgical implantation of electrodes into one labyrinth, animals were rendered bilaterally vestibular deficient by canal plugging of the contralateral labyrinth followed by bilateral intratympanic membrane gentamicin injection. Loss of aVOR was confirmed by 3-dimensional video-oculography testing during sinusoidal head rotations about each SCC axis on a turntable in the dark [10, 11].
Three chinchillas were subjected to chronic stimulation using a head-mounted second-generation Johns Hopkins MVP2 Multichannel Vestibular Prosthesis with each gyroscope axis aligned parallel to its corresponding canal axis [9]. After locomotion was recorded with the prosthesis powered off, each chinchilla was placed on the turntable to measure aVOR with and without prosthetic stimulation before undergoing chronic adaptation. During adaptation, the MVP2 was continuously powered by a secure yet non-restrictive battery vest containing three AAA-size 3.7 volt batteries in parallel. The batteries were changed every 24 to 48 hours to maintain stimulation. The total stimulation period was 9 days for 1 chinchilla (ch105) and 10 days for 2 chinchillas (ch207 and ch108), during which animals were kept in their usual housing facilities under diurnal lighting conditions. During each stimulation period, the MVP2 was periodically cycled on and off 3 to 4 times. On the final day of chronic stimulation, each chinchilla's locomotion and aVOR performance were measured with and without prosthetic stimulation encoding head rotation. Locomotion was recorded immediately after the prosthesis was powered off and again after at least 4 days later. Each chinchilla underwent the experimental paradigm twice: once with MVP2 outputs modulated by head motion and a second time with only constant-rate stimulation.
B. Electrode implantation and bilateral vestibular ablation
Surgical implantation of electrodes and contralateral canal plugging were performed as described previously [4, 12]. Stimulating and reference electrodes were made from Teflon-insulated 75-μm and 125-μm Pt-Ir wire, respectively (Cooner Wire, Chatsworth, CA). All electrodes were implanted in the left labyrinth except in 1 chinchilla (ch105), who was implanted on the right. To restrain animals during whole body rotation and to mount the prosthesis, a head cap was fashioned using dental acrylic under isoflurane anesthesia. After recovery from surgery, gentamicin was instilled bilaterally into the middle ears, as described previously [4, 12, 13]. Each chinchilla then underwent 3-dimensional video-oculography (3D VOG) testing in darkness to verify that no residual aVOR remained.
C. Prosthetic stimulation paradigm
For each chinchilla, stimulation-reference electrode pairs and stimulation current were chosen to maximize the amplitude of eye movements while minimizing the effects of current spread to nontarget SCCs. Care was taken to use a stimulation current low enough to avoid facial nerve stimulation. For all chinchillas, symmetric, biphasic pulse trains with an interphase duration of 200μs were modulated between 0 and 350 pulses per second (pps) using a sigmoidal mapping function that transformed the angular velocity component sensed by each gyroscope into pulse rate, as described previously [5]. The baseline pulse rate was set at 60pps for two chinchillas (ch105 and ch108) and 100pps for one chinchilla (ch207). To mimic physiologic aVOR gain [10], a precompensation matrix was programmed, as described previously [12], such that a gain of at least 0.5 was achieved with good eye-head alignment for every direction of head rotation. During chronic stimulation of the animal, prosthesis output was periodically probed to confirm appropriate function.
D. Eye movement recording and analysis
Angular VOR for each chinchilla was characterized using a 180 frame/s binocular 3D VOG system adapted from one described previously [11]. Briefly, each chinchilla was mounted in a gimbal bolted to a servo-controlled motor. The motor was positioned so that the center of the animal's skull aligned with the motor's Earth-vertical axis. The gimbal was reoriented as needed to sequentially align the motor's axis with each of three mean semicircular canal pair axes: horizontal, left anterior / right posterior with left ear down nose up (LARP-LEDNU), and right anterior / left posterior with right ear down nose up (RALP-REDNU). The apparatus was rotated sinusoidally at 2 Hz and 20-100°/s peak velocity for 20 cycles. Animals were anesthetized with isoflurane during mounting but fully alert during testing (>20 minutes after last isoflurane).
Eye movement data were analyzed in 3D with respect to a head-referenced coordinate system (yaw, LARP and RALP) and quantified as rotation vectors using software described previously [11, 14]. Each of the three components was separately averaged over ≥5 saccade-free cycles. From this, 3D aVOR gain, eye-head misalignment and disconjugacy were calculated. Gain was defined as the ratio of angular eye speed and head speed. Eye-head misalignment and disconjugacy were defined as the 3D angle between eye and head axes or between the two eyes’ rotation axes, respectively. Data from the eye ipsilateral to the implanted electrodes were used to compute gain and misalignment.
E. Locomotion recording and analysis
Locomotion recordings were performed in darkness while the animal was allowed to roam freely in a cylindrical enclosure ~1.5 m in diameter and 0.5 m tall. Before each session, the battery vest was replaced by a light harness attached to two colored light-emitting diodes (LEDs) aligned with the midsagittal plane. As the chinchilla roamed in the enclosure, LED positions were recorded at 15 – 30 frame/s by an overhead camera. Custom software was used to analyze image data, including the chinchilla's head-tail direction, horizontal angular velocity, linear velocity, and 2D position within enclosure with respect to time. Each recording lasted at least 15 min. Circling frequency was defined as the number of 360° revolutions made by the chinchilla per second. A 2-tailed t-test was used to compare mean circling frequency between experimental conditions. Statistical significance was defined as a p < 0.05. Angular and linear velocity spectra were computed via Fourier analysis.
III. RESULTS
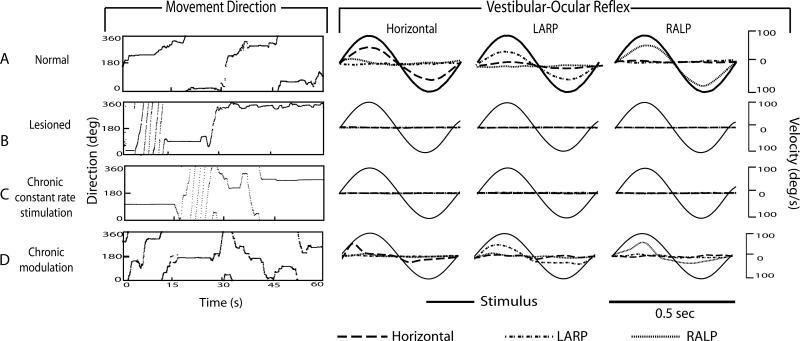
Figure 1 shows representative locomotion and aVOR responses (during 2Hz, 100°/s sinusoidal head rotation in darkness about the horizontal, LARP, and RALP axes) for a normal chinchilla (Fig. 1A) and for a bilaterally vestibular-deficient chinchilla (ch207) prior to device activation (Fig. 1B), after 10 days of continual stimulation at a fixed, constant baseline rate (Fig. 1C) and after 10 days of continual motion-modulated stimulation using a head-mounted MVP (Fig. 1D). Findings for all normal and bilaterally vestibular-deficient (BVD) animals examined are summarized in Figure 2.
Fig. 1.
Movement analysis and aVOR gain for: A) Normal chinchilla, B) chinchilla with bilateral vestibular hypofunction (lesioned) without prosthetic stimulation, C) same chinchilla after chronic constant-rate stimulation, and D) same chinchilla after chronic stimulation with modulation to head motion. Normal chinchilla exhibited normal aVOR (data from [4]) and no high frequency circling. Lesioned chinchilla initially exhibited high frequency circling, which disappeared after chronic modulation but not after chronic constant-rate stimulation. Angular VOR was absent in the lesioned chinchilla with the prosthesis powered off or with constant-rate stimulation, but was partially restored with prosthetic modulation. Asymmetry in gain is due to inherent limitations in encoding inhibitory head motions using a unilaterally-implanted prosthesis. All sinusoidal eye responses were measured at 2Hz, 100°/s in the dark. Although movements were recorded for at least 15 minutes in the dark, only a representative 1 minute sample is shown for purpose of clarity.
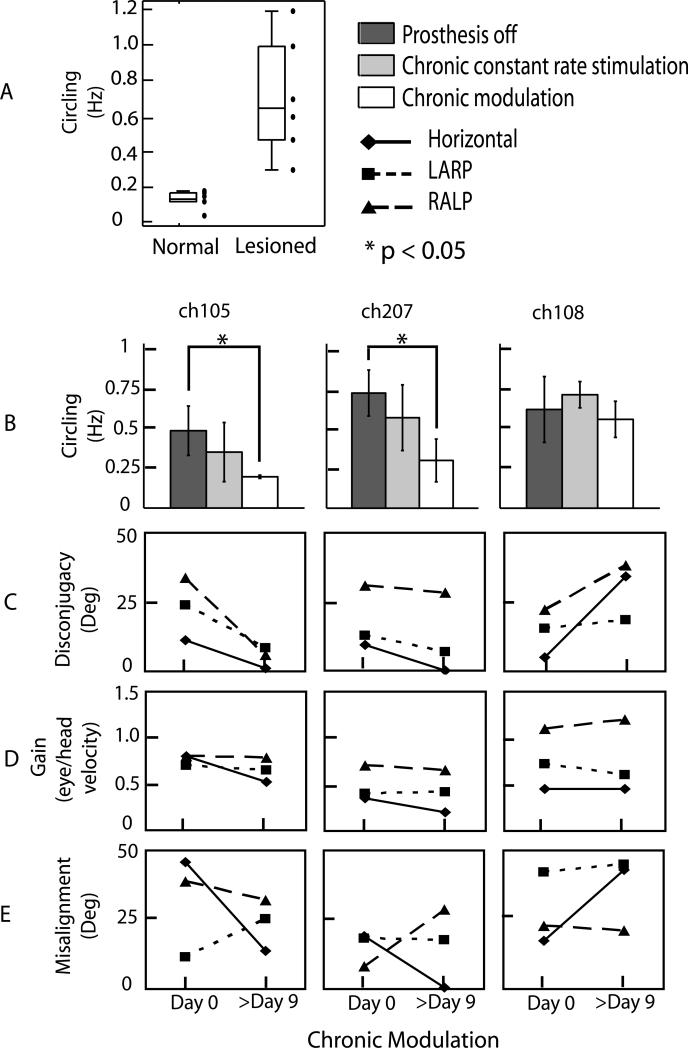
Fig. 2.
Effects of chronic adaptation on circling and aVOR. (A) Chinchillas with bilateral vestibular hypofunction (lesioned, n=6) demonstrated higher circling frequency than normal chinchillas (n=6). Three chinchillas underwent chronic prosthetic stimulation with and without modulation to head movement (B). In two (ch105 and ch207), circling frequency improved with chronic prosthetic modulation but not with chronic constant rate stimulation. Ch108 did not improve with prosthetic modulation. VOR disconjugacy (C), gain (D), and misalignment (E) for each chinchilla were analyzed at the start (day 0) and end (day 9 or 10) of chronic modulation. Disconjugacy improved in both chinchillas that also showed decrease in circling frequency but not for ch108. Gains for all chinchillas remained nearly constant, while misalignment changes were variable.
Normal chinchillas tended to dart to and along the edges of the enclosure, exploring and occasionally trying to climb over its walls (e.g., Fig. 1A and supplemental movie). They rarely or never ran in tight circles (Fig. 2A). Normal chinchillas exhibit a mean aVOR gain of 0.61±0.07 and little to no misalignment of the 3D aVOR with respect to the axis of head rotation [10].
In contrast, BVD chinchillas tested prior to prosthesis activation exhibited periods of rapid circling during which they ran in tight loops at high angular velocity (Fig. 1B). As shown in Fig. 2A, mean (±SD) circling frequency was significantly (p<0.005) larger in the 6 BVD chinchillas tested (0.71±0.3 Hz) than in 6 normal animals (0.13±0.05 Hz). Circling was unidirectional (i.e., any given BVD animal always circled in the same direction) and it most often occurred when the BVD animal was initially placed in the enclosure or in response to tactile stimuli (such as the experimenter's gloved hand gently touching the animal).
Immediately after MVP activation, BVD chinchillas exhibited a roll head tilt bringing the unimplanted ear down, as expected for an acute imbalance of vestibular nerve tone. Animals did not circle during the immediate post-activation period; instead, they tended to remain still.
After 9-10 days of continual stimulation with constant, baseline-rate stimuli that did not modulate with head movement, no significant improvements were observed in either locomotion or aVOR performance (Figs. 1C and 2B). Animals continued to circle as before, and they exhibited no aVOR response to head rotation.
After 9-10 days of stimulation with motion-modulated stimuli, significant locomotion and aVOR performance improvement was observed for two of the three animals tested. As shown in Fig. 2B, mean circling frequency after chronic prosthetic modulation decreased from 0.47±0.15 Hz to 0.19±0.01 Hz for ch105 (p<0.005) and from 0.7±0.14 Hz to 0.28±0.13 Hz for ch207 (p<0.001), but did not change significantly for ch108. All three animals exhibited a significant increase in mean excitatory half-cycle gain, from essentially undetectable without stimulation and with only nonmodulated stimulation to 0.53±0.24, 0.61±0.18 and 0.87±0.21 for the horizontal, LARP, and RALP axes, respectively, on the first day of motion-modulated stimulation.
After 9-10 days of motion-modulated stimulation, the aVOR gain was no different than its value on the first day (Fig. 2D). All three animals exhibited greater aVOR misalignment and asymmetry than normal chinchillas [10], consistent with prior studies[4]. In contrast to previous studies [5], misalignment did not improve significantly during chronic motion-modulated stimulation; however, the small sample size precludes drawing well-powered inferences from the absence of a change.
The same two animals that exhibited improved locomotion also exhibited a reduction in aVOR disconjugacy (i.e., the difference in 3D aVOR axis measured separately for the two eyes), while the animal that had no improvement in locomotion exhibited an increase in disconjugacy. (Fig. 2C)
By four days after cessation of motion-modulated stimulation, circling reverted toward prestimulation levels in the two animals that had improved significantly during stimulation (ch207 and ch105). However, circling frequency did not change immediately. As Fig. 3 shows, circling frequency initially stayed low, despite a sudden withdrawal of both motion-modulated and baseline stimulation.
Fig. 3.
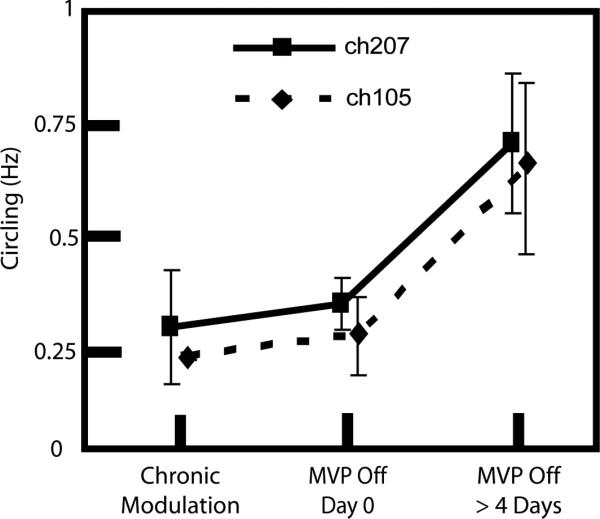
Reversion to circling after cessation of motion-modulated prosthetic stimulation was not immediate. The two chinchillas that showed improvement in circling frequency with chronic modulation, reverted to rapid circling after the prosthesis was turned off. However, a significant increase in circling frequency was not observed until 4 days after the prosthesis was powered off.
IV. DISCUSSION
Although this study's sample size is too small to support drawing strong inferences, our findings suggest that a multichannel vestibular prosthesis that partially replaces semicircular canal function can improve both 3D aVOR performance and locomotion. Rapid circling has been used as a phenotypic marker of BVD in many quadruped animals including mice and chinchillas [15-17], although the exact mechanism by which this behavior arises is unknown. While some have proposed that CNS involvement is necessary for the development of circling behavior [18], observations of this study are consistent with the view that peripheral vestibular lesions alone may engender circling. In this study, restoration of unilateral SCC input improved locomotion in 2 of 3 animals, consistent with the hypothesis [16] that circling arises due to a lack of vestibular feedback to signal the completion of a planned trajectory as an animal changes its heading during locomotion.
Whereas the circling frequency was consistently elevated in all BVD chinchillas compared to normal animals, two of three BVD animals undergoing chronic motion-modulated MVP stimulation achieved significant improvements in circling frequency. While the third BVD animal exhibited no such improvement, the two that did demonstrate that restoring angular sensation alone, without any attempt to provide tilt or translational acceleration information via the prosthesis, can yield improved stability of locomotion.
Interestingly, the chinchilla that did not improve in circling frequency during motion-modulated stimulation (ch108) also exhibited a worsening of aVOR disconjugacy (Fig. 2C). This could be due to inadvertent stimulation of otolith endorgans. Natural stimulation of ampullary nerves typically evokes conjugate eye movements (e.g., during episodes of benign paroxysmal positional vertigo or stimulation via a superior canal dehiscence[19]), whereas natural stimulation of the utricle and saccule can evoke disconjugate eye movements [20, 21]. To the extent that aVOR disconjugacy in the present study implies inadvertent electrical stimulation of the utricular or saccular nerve, ch108's failure to improve in either circling or disconjugacy could indicate that sensory confusion occurred when angular velocity signals from the MVP's gyro sensors were inadvertently encoded as changes in activity on the utricular and saccular nerves.
Circling is not unique to animals with bilateral vestibular deficiency. Like many quadruped species, chinchillas typically circle spontaneously for up to a several hours after acute onset of asymmetry in vestibular tone, as occurs after unilateral labyrinthectomy, vestibular nerve section, and intratympanic injection of gentamicin or lidocaine.[16, 17, 22] All three animals examined during chronic stimulation in this study were studied >12 months after bilateral vestibular injury, so acute post-lesion effects were unlikely.
Since the normal chinchillas we examined did not have head posts to which LEDs could be attached, we measured body direction instead of head direction to maintain consistency across all animals. Differences may emerge when head direction is analyzed quantitatively.
If decreases in circling frequency are due to restoration of some level of labyrinthine sensation, then powering off the prosthesis should cause animals to revert to circling as they did before prosthesis activation. In fact, animals did revert to baseline circling frequencies by 4 days after cessation of prosthetic stimulation (Fig. 3). However, circling frequency did not increase immediately on the day the device was turned off, despite immediate disappearance of aVOR.
Why circling did not instantly worsen along with aVOR performance is unclear. One possibility is that animals simply avoided movement of any kind in the first day after prosthesis deactivation, when they would presumably be experiencing vertigo due to a sudden change in vestibular tone on the implant side.
Alternatively, it is possible that the benefits of chronic stimulation may persist for a few days after discontinuation of stimulation. For this to be true, one would have to posit another source of sensory input regarding spatial orientation and movement. For example, the prosthetic input could have served as a training signal against which proprioceptive sensation is compared to result in enhancement of cervicoocular and related reflexes. A similar line of reasoning has been used in attempts to define a mechanism for persistence of improvements in balance and posture after a period of electrotactile stimulation [23, 24]; however, whether such effects can persist beyond cessation of artificial sensory input is unclear.
Conclusion
This study demonstrates that even without restoration of utricular or saccular function, prosthetic restoration of unilateral SCC input can improve not only aVOR but also locomotion in BVD chinchillas. Future work will be directed toward (1) identifying factors that determine whether a given animal will achieve improved locomotion performance with prosthetic SCC stimulation and (2) examining the effects of prosthetic stimulation encoding head gravitoinertial acceleration via electrodes implanted near the utricular and saccular nerves.
Supplementary Material
Acknowledgments and disclosures
We gratefully acknowledge: Americo Migliaccio (Neuroscience Research Australia) and Hamish MacDougall (University of Sidney), who helped create a precursor to the VOG system used in this work. Disclosures: patents pending on related technology (CCDS, GYF, BC); equity interest in Labyrinth Devices LLC (CCDS).
This work was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD) grants R01DC009255, R01DC002390 and 1F31DC010099.
References
- 1.Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; 1999. [Google Scholar]
- 2.Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000 May;28(5):572–81. doi: 10.1114/1.293. [DOI] [PubMed] [Google Scholar]
- 3.Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng. 2002 Feb;49(2):175–81. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- 4.Della Santina CC, Migliaccio AA, Patel AH. A Multi-channel Semicircular Canal Neural Prosthesis Using Electrical Stimulation to Restore 3D Vestibular Sensation. IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1016–30. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai C, Fridman GY, Chiang B, Davidovics NS, Melvin TA, Cullen KE, Della Santina CC. Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp Brain Res. 2011 Mar 4; doi: 10.1007/s00221-011-2591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1005–15. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- 7.Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006 Nov;53(11):2362–72. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- 8.Lewis R, Haburcakova C, Gong W, Lee D, Merfeld D. Book Responses Evoked by a Vestibular Implant Providing Chronic Stimulation: II. Responses Evoked in Rhesus Monkeys, vol. Abstract, Series Responses Evoked by a Vestibular Implant Providing Chronic Stimulation: II. Responses Evoked in Rhesus Monkeys. Association for Research in Otolaryngology; City: 2011. Responses Evoked by a Vestibular Implant Providing Chronic Stimulation: II. Responses Evoked in Rhesus Monkeys; p. 263. [Google Scholar]
- 9.Chiang B, Fridman G, Dai C, Rahman M, Della Santina C. Design and performance of a multichannel vestibular prosthesis that restores semicircular canal sensation in rhesus monkey. IEEE Trans Neural Systems and Rehab Eng. 2010 doi: 10.1109/TNSRE.2011.2164937. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliaccio AA, Minor LB, Della Santina CC. Adaptation of the vestibulo-ocular reflex for forward-eyed foveate vision. The Journal of Physiology. 2010 October;58815(20):3855–3867. doi: 10.1113/jphysiol.2010.196287. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio AA, Macdougall HG, Minor LB, Della Santina CC. Inexpensive system for real-time 3-dimensional video-oculography using a fluorescent marker array. J Neurosci Methods. 2005;143(2):141–50. doi: 10.1016/j.jneumeth.2004.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman GY, Davidovics NS, Dai C, Migliaccio AA, Della Santina CC. Vestibulo-ocular reflex responses to a multichannel vestibular prosthesis incorporating a 3D coordinate transformation for correction of misalignment. J Assoc Res Otolaryngol. 2010 Sep;11(3):367–81. doi: 10.1007/s10162-010-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93(2):643–55. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 14.Davidovics NS, Fridman GY, Chiang B, Della Santina CC. Effects of Biphasic Current Pulse Frequency, Amplitude, Duration, and Interphase Gap on Eye Movement Responses to Prosthetic Electrical Stimulation of the Vestibular Nerve. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2011;19(1):84–94. doi: 10.1109/TNSRE.2010.2065241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vervoort R, Ceulemans H, Van Aerschot L, D'Hooge R, David G. Genetic modification of the inner ear lateral semicircular canal phenotype of the Bmp4 haplo-insufficient mouse. Biochemical and Biophysical Research Communications. 2010;394:780–785. doi: 10.1016/j.bbrc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Vidal PP, Degallaix L, Josset P, Gasc JP, Cullen KE. Postural and locomotor control in normal and vestibularly deficient mice. J Physiol. 2004;559(Pt 2):625–38. doi: 10.1113/jphysiol.2004.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS. Disruption of the Head Direction Cell Signal after Occlusion of the Semicircular Canals in the Freely Moving Chinchilla. The Journal of Neuroscience. 2009 November;2918(46):14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser A, Fedrowitz M, Ebert U, Zimmermann E, Hedrich HJ, Wedekind D, Loscher W. Auditory and vestibular defects in the circling (ci2) rat mutant. Eur J Neurosci. 2001;14:1129–42. doi: 10.1046/j.0953-816x.2001.01726.x. France. [DOI] [PubMed] [Google Scholar]
- 19.Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000 Dec 26;55(12):1833–41. doi: 10.1212/wnl.55.12.1833. [DOI] [PubMed] [Google Scholar]
- 20.Fluur E, Mellstrom A. Utricular stimulation and oculomotor reactions - Fluur - 2009 - The Laryngoscope - Wiley Online Library. The Laryngoscope. 1970;(80):1701–1712. doi: 10.1288/00005537-197011000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969 Oct;68(4):350–62. doi: 10.3109/00016486909121573. [DOI] [PubMed] [Google Scholar]
- 22.Shima F, Hassler R. Circling behavior produced by unilateral lesions of the central vestibular system. Appl Neurophysiol. 1982;45(3):255–60. doi: 10.1159/000101609. [DOI] [PubMed] [Google Scholar]
- 23.Barros CG, Bittar RS, Danilov Y. Neurosci Lett. Vol. 476. Elsevier Ireland Ltd; Ireland: 2010. Effects of electrotactile vestibular substitution on rehabilitation of patients with bilateral vestibular loss; pp. 123–6. 2010. [DOI] [PubMed] [Google Scholar]
- 24.Danilov YP, Tyler ME, Skinner KL, Hogle RA, Bach-y-Rita P. Efficacy of electrotactile vestibular substitution in patients with peripheral and central vestibular loss. J Vestib Res. 2007;17(2-3):119–30. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.