Abstract
Exposure to abused drugs and stressful experience, two factors that promote the development of addiction, also modify synaptic function in the mesolimbic dopamine system. Here, we show that exposure to a novel environment produces functional synaptic adaptations in the nucleus accumbens (NAc) that mirror the effect of conventional forms of stress. We find an enhancement of excitatory synaptic strength in the NAc shell one day after exposure to a novel environment for 60 minutes – an effect not observed in NAc core. This effect disappeared following repeated exposure to the same environment, but then reappeared if mice are returned to the same environment 10-14 days later. There were no interactions between the effects of environmental novelty and a single exposure to cocaine (15 mg/kg), with no effect of the latter on synaptic strength in NAc shell. These results have important implications for designing studies of NAc synapses in the context of behavioral analysis, and expand our understanding of how different forms of stress modify NAc synaptic function.
Keywords: Novelty, Stress, Cocaine, Nucleus Accumbens, Synaptic Plasticity, Glutamate
1. Introduction
The addictive potential of abused drugs may involve their ability to commandeer synaptic plasticity in the mesolimbic dopamine system (Kauer and Malenka, 2007). Synaptic adaptations in this neural circuit – which includes the nucleus accumbens (NAc) and ventral tegmental area (VTA) - develop over the course of chronic drug exposure and have been associated with drug craving and persistent vulnerability to relapse (Kalivas, 2009; Robinson and Berridge, 2003; Vezina, 2004). This association between synaptic plasticity and addiction has been forged through the union of neurophysiology and increasingly sophisticated animal models of addiction-related behavior. Behavioral responses to drug exposure can be evaluated in vivo using paradigms such as psychomotor activation, conditioned place preference, and operant self-administration, and then subsequently correlated with ex vivo measures of synaptic function.
Measures of addiction-related behavior are commonly collected in a testing apparatus distinct from the daily living environment, and the importance of habituating animals to novel testing environments is widely recognized but perhaps not fully appreciated. This is particularly critical in light of evidence that exposure to novel environments can increase circulating levels of corticosterone (Badiani et al., 1998; Piazza et al., 1991) and cause dopamine release in the NAc (Rebec et al., 1997) – effects also associated with more conventional forms of stress (Kalivas and Duffy, 1995). Indeed, exposure to novel environments is often utilized as an experimental manipulation to produce psychological stress (e.g., Antelman et al., 1992; de Groote and Linthorst, 2007; Gesing et al., 2001; Pfister, 1979; Rivat et al., 2007). Stressful experience has been shown to alter synaptic function in both the VTA and NAc (Campioni et al., 2009; Saal et al., 2003), highlighting the need to minimize stress-like responses to environmental novelty when evaluating the behavioral and neurobiological impact of drug exposure.
Here, we report that exposure to a novel environment causes an increase in NAc synaptic strength that resembles the effect of more conventional forms of stress (Campioni et al., 2009). This stress-like effect is no longer detected following daily habituation to the same environment, but is again observed if animals are returned to the same environment 10-14 days later. This latter result has important implications for designing studies in which behavioral responses are examined following prolonged drug-free periods, suggesting rehabituation to the testing environment is important for avoiding the confounding effects of novelty. Furthermore, these results expand our understanding of how different forms of stress affect synaptic function in NAc.
2. Materials and Methods
2.1. In vivo treatments
Male C57Bl/6J mice (Jackson Lab) were given at least one week to acclimate to housing conditions and were at least 4 weeks old at the beginning of each experiment. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Minnesota Institutional Animal Care and Use Committee. Mice were exposed to environmental novelty in a behavioral testing apparatus we have used previously to record locomotor activity following drug exposure (Kourrich et al., 2007; Kourrich and Thomas, 2009; Rothwell et al., 2010). This apparatus was located in a room distinct from the mouse colony and consisted of a clear plastic cage (40 × 20 × 20 cm) with ground corncob bedding on the floor. The cage was equipped with an array of infrared photobeams (Applied Concepts; Ann Arbor, MI) that monitored the number of “crossovers” (i.e., successive interruption of beams on opposite ends of the cage) during each test session. Mice were placed in these cages for 60 minutes and then returned to their home cage in the animal colony. Injections of sterile 0.9% saline (5 mL/kg, i.p.) or cocaine (15 mg/kg) were given 20 minutes after placement in the apparatus, and then mice were returned to the novel environment following injection for an additional 40 minutes.
2.2. Synaptic Physiology
Twenty-four hours after the last exposure to the behavioral testing apparatus, we prepared parasagittal brain slices (240 μm) containing the NAc as described (Thomas et al., 2001). Slices recovered in a holding chamber at least 1 h before being superfused with aCSF (22-23°C) saturated with 95% O2/5% CO2 and containing (in mM) 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, 26.2 NaHCO3 and 11 glucose. Picrotoxin (100 μM) was added to block GABAA receptor-mediated IPSCs. Cells were visualized using IR-DIC optics and medium spiny neurons were identified by their morphology and hyperpolarized resting membrane potential (−75 to −85 mV). As previously described (Kourrich and Thomas, 2009; Thomas et al., 2001), recordings in NAc shell and core were performed in slices that lacked or contained dorsal striatal tissue, respectively.
To assess excitatory synaptic transmission, neurons were voltage-clamped at −80 mV using a Multiclamp 700A amplifier (Molecular Devices). Electrodes (3–5 MΩ) contained (in mM) 117 cesium gluconate, 2.8 NaCl, 20 HEPES, 0.4 EGTA, 5 TEA-Cl, 2 MgATP, and 0.3 MgGTP, pH 7.2–7.4 (~270 mOsm). Series resistance (10–40 MΩ) and input resistance were monitored on-line with a 4 mV depolarizing step (100 ms) given with each stimulus. Excitatory post-synaptic currents (EPSCs) were evoked at 0.1 Hz by glass monopolar microelectrodes placed at the border between prelimbic cortex and NAc. Data were filtered at 2 kHz, digitized at 5 kHz and collected and analyzed using custom software (Igor Pro; Wavemetrics).
2.3. Data Analysis
Locomotor responses were assessed in 5-minute bins during exposure to environmental novelty, and total crossovers were also calculated across the entire 60-minute session. AMPAR/NMDAR ratios were computed by recording EPSCs at +40 mV and then applying 50 μM D-AP5 to specifically block NMDARs. The residual AMPAR current was digitally subtracted from the initial signal at +40mV to derive the NMDAR current, and peak amplitudes were used to calculate the AMPAR/NMDAR ratio (Kourrich and Thomas, 2009; Thomas et al., 2001). The current-voltage relationship of AMPAR EPSCs was recorded in the presence of 50 μM D-AP5 and holding potentials were corrected for the liquid junction potential (~8 mV). The rectification index of AMPAR EPSCs was calculated by dividing the peak current at +40 mV by the absolute value of the peak current at -80 mV. Traces in figures represent the average of 20-30 consecutive responses and stimulus artifacts have been removed.
Statistical analysis was performed with ANOVA followed by Student-Newman-Keuls (SNK) post-hoc tests, as well as Pearson’s correlation coefficient. All analysis was conducted using SPSS (version 13.0) with a Type I error rate of α = .05 (two-tailed). All data are presented as mean +/- SEM; sample sizes and results of statistical tests for each experiment are provided in figure legends.
3. Results
3.1. One exposure to a novel environment persistently modifies NAc synaptic function
We initially identified the effect of environmental novelty while investigating whether a single exposure to cocaine affects NAc excitatory synaptic transmission. Whole-cell voltage-clamp recordings from medium spiny neurons in the NAc shell were performed in acute brain slices prepared 24 hours after one injection of cocaine (15 mg/kg) or saline (Figure 1A). As previously reported (Kourrich et al., 2007; Thomas et al., 2001), the AMPAR/NMDAR ratio did not differ between animals receiving one injection of cocaine or saline (Figure 1C). However, both the saline and cocaine groups exhibited a significant increase in this parameter relative to a naïve control group that did not undergo any experimental manipulation (Figure 1C).
Figure 1.
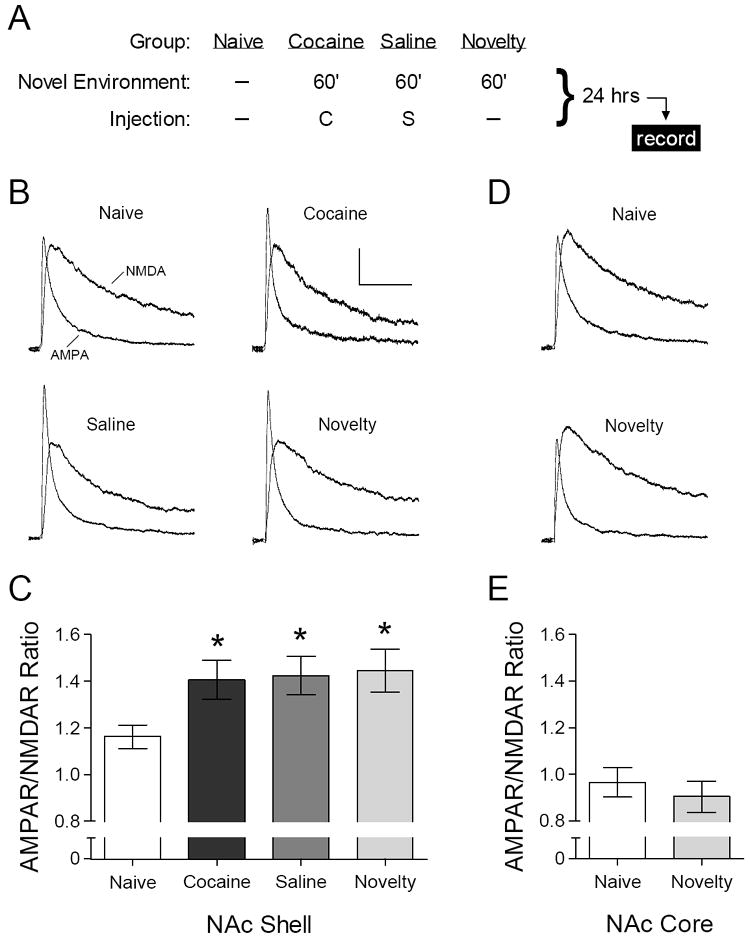
Acute exposure to environmental novelty increases the AMPAR/NMDAR ratio in NAc shell. (A) Summary of experimental design – acute injections of saline (“S”) and cocaine (“C”, 15 mg/kg) were administered in a novel environmental context. (B) Representative AMPAR and NMDAR ESPCs recorded in NAc shell. Calibration: 100 ms, 20 pA. (C) Mean AMPAR/NMDAR ratio in NAc shell from naïve mice (n = 32 cells) and 24 hours after acute exposure to cocaine (n = 18 cells), saline (n = 24 cells), or novelty alone (n = 16 cells). [ANOVA: F3,86 = 3.93, p = .011; *p < .05 compared to naïve group, SNK post-hoc test]. (D) Representative AMPAR and NMDAR EPSCs recorded in NAc core; same calibration as in (B). (E) Mean AMPAR/NMDAR ratio in NAc core from naïve mice (n = 9 cells) and 24 hours after exposure to novelty alone (n = 7 cells). [ANOVA: F1,14 < 1].
The increased AMPAR/NMDAR ratio in the cocaine group was unlikely to be caused by the pharmacological effects of cocaine itself, because the saline group exhibited a comparable increase in this parameter. We thus considered what other aspects of our experimental procedure could account for this change. In our lab and many others, rodents injected with cocaine or saline are routinely placed in an apparatus that records locomotor activity, because changes in psychomotor activation following repeated drug exposure (i.e., “psychomotor sensitization”) represent a form of drug-induced neurobehavioral plasticity with potential relevance to human addiction (Robinson and Berridge, 2003). When exposure to this apparatus occurs for the first time in combination with injection of saline or cocaine, as in the present experiment, the apparatus constitutes a novel environment. We thus considered whether mere placement in the behavioral testing apparatus was sufficient to cause the change observed in the saline and cocaine groups. Indeed, the AMPAR/NMDAR ratio in NAc shell was increased to a similar degree 24 hours after exposure to this novel environment for 60 minutes, in the absence of any injection (Figure 1C). This effect of environmental novelty was not observed when we recorded from medium spiny neurons in NAc core (Figure 1E). We also did not detect changes in the current-voltage relationship (data not shown) or rectification index of AMPAR EPSCs in any treatment group (Naïve: 0.45 +/- 0.01, n = 19 cells; Cocaine: 0.45 +/- 0.02, n = 17 cells; Saline: 0.45 +/- 0.02, n = 14 cells; Novelty: 0.45 +/- 0.01, n = 13 cells) [ANOVA: F3,59 < 1].
We considered whether changes in the AMPAR/NMDAR ratio in NAc shell might correlate with the magnitude of the locomotor response to novelty, which can predict the individual propensity of rodents to acquire psychosimulant self-administration (Piazza et al., 1989). As expected, mice that were injected with cocaine 20 minutes after being placed in the testing apparatus showed a robust increase in locomotor activity, while the response of mice injected with saline resembled that of the “novelty” control group that did not receive any injection (Figure 2A). For each individual mouse, we compared the total amount of locomotor activity with the average AMPAR/NMDAR ratio of all cells recorded in the NAc shell (Figure 2B). The correlation between these variables was not statistically significant in any treatment group (Novelty: r2 = .25, p = .20; Saline: r2 = .24, p = .078; Cocaine: r2 = .05, p = .55). To maximize the statistical power of this comparison, we compared individual differences in the AMPAR/NMDAR ratio of all mice with locomotor activity during the first 20 minutes of the session (Figure 2C), as all three treatment groups had similar average activity levels during this time period. Again, this correlation failed to achieve statistical significant (r2 = .04, p = .25), indicating the locomotor response to novelty is not a robust predictor of the AMPAR/NMDAR ratio measured 24 hours later in NAc shell.
Figure 2.
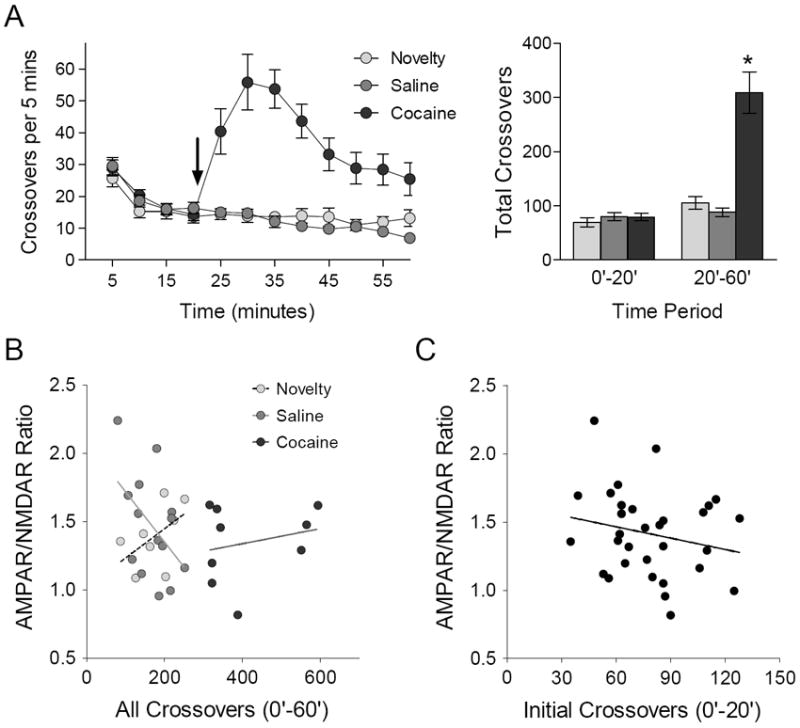
The locomotor response to novelty does not predict synaptic adaptations in NAc shell. (A) Left: time course of locomotor activity during exposure to environmental novelty; arrow indicates injection of saline or cocaine (15 mg/kg). Right: total number of crossovers before (0’-20’) and after (20’-60’) exposure to saline (n = 14 mice), cocaine (n = 10 mice), or novelty alone (n = 8 mice). [ANOVA, 20’-60’: F2,29 = 30.34, p < .001; *p < .05 compared to novelty and saline, SNK post-hoc test]. (B) Relationship between average AMPAR/NMDAR ratio in NAc shell and total locomotor activity for individual mice in each group; see text for statistics. (C) Relationship between average AMPAR/NMDAR ratio in NAc shell and initial locomotor activity for individual mice in all groups.
3.2. The effect of novelty habituates with repeated exposure to the same environment
Our previous study did not reveal a difference between naïve mice and those injected with saline in the behavioral testing apparatus (Kourrich et al., 2007). A potential explanation for this difference is that, in our previous study (Kourrich et al., 2007), mice exposed to the same apparatus once each day over the course of five days. Repeated exposure to the same environment is quite likely to diminish the novelty of that environment, as well as any neurobiological effect specifically associated with novelty. To directly assess this possibility, we placed mice in the same apparatus and injected them with saline once each day over five days (Figure 3A). In acute brain slices prepared 24 hours after the fifth exposure, there was no difference in the AMPAR/NMDAR ratio in NAc shell compared with interleaved recordings from naïve control mice (Figure 3B). This results confirms that the impact of environmental novelty habituates with repeated exposure, consistent with our previous results (Kourrich et al., 2007).
Figure 3.
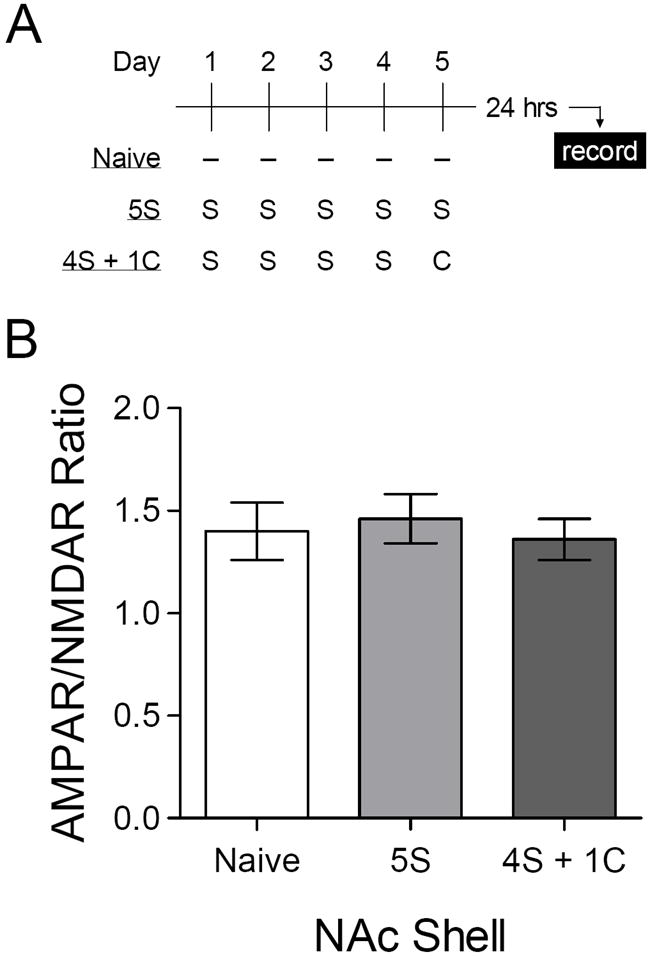
Habituation to the impact of environmental novelty in NAc shell. (A) Experimental time line – repeated injections of saline (“S”) or cocaine (“C”, 15 mg/kg) were administered in the same environmental context. (B) Mean AMPAR/NMDAR ratio in naïve mice (n = 5 cells) or 24 hours after the last of five daily saline injections (“5S”, n = 8 cells) or four daily saline injections followed by a single cocaine injection (“4S + 1C”, n = 9 cells). [ANOVA: F2,19 < 1].
We also sought to determine whether habituation to the impact of environmental novelty might reveal a synaptic effect of a single cocaine injection. In the same experiment just described, an additional group of mice were placed in the apparatus over five days, but received an injection cocaine (15 mg/kg) rather than saline on the fifth and final day (Figure 3A). The AMPAR/NMDAR ratio in the NAc shell of these mice was similar to that of the other two groups (Figure 3B), confirming that even under these conditions, we do not detect any lasting effect of a single cocaine exposure on synaptic strength in the NAc shell (Kourrich et al., 2007; Thomas et al., 2001).
3.3. Renewed impact of the same environment 10-14 days after repeated exposure
Several prominent tests of addiction-related behavior involve removing animals from the testing apparatus for periods of days or weeks, and then returning them to the same apparatus to evaluate a behavioral response of interest (Fuchs et al., 2006; Grimm et al., 2001; Paulson et al., 1991). It is therefore important to determine whether habituation to environmental novelty persists when animals are returned to the same context following a prolonged delay. To evaluate this possibility, mice were again injected with saline in the same apparatus once each day over five days, as in the preceding experiment. After the last exposure, mice remained in their home cages for 10-14 days. At this point, we prepared acute brain slices from some mice with no additional experimental manipulation; recordings from this control group provided a baseline for comparison with separate groups that were returned to the same environment and injected with saline or cocaine (15 mg/kg; Figure 4A).
Figure 4.
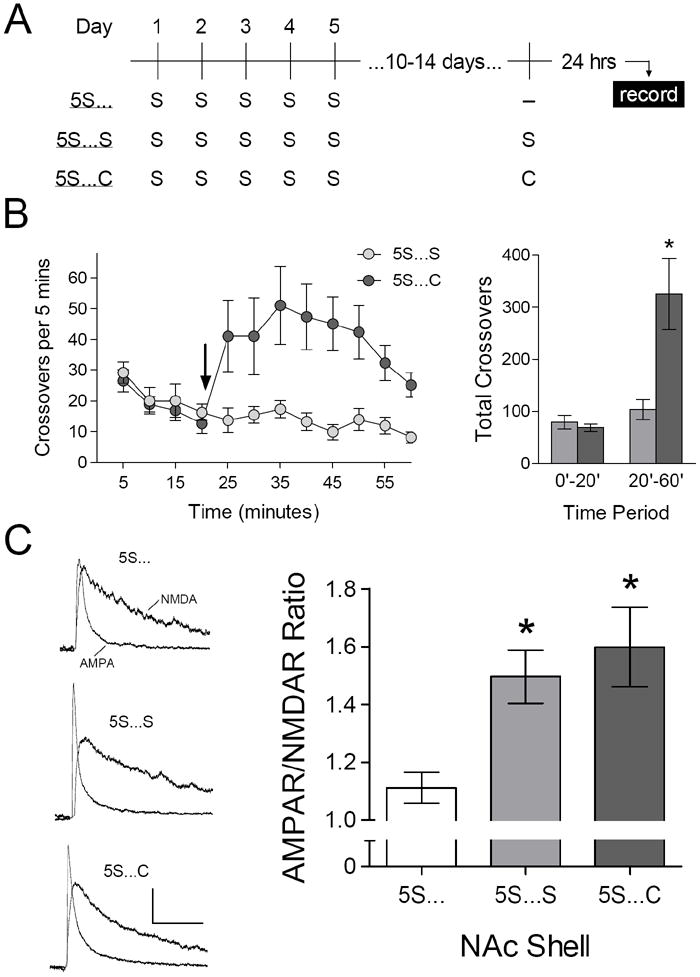
Renewed impact of the same environment 10-14 days after repeated exposure. (A) Experimental time line – all groups received five daily injections of saline (“S”) in the same environmental context, 10-14 days before brain slice preparation. (B) Left: time course of locomotor activity during re-exposure to the same environment; arrow indicates injection of saline or cocaine (15 mg/kg). Right: total number of crossovers before (0’-20’) and after (20-60’) injection of saline (“5S…S”, n = 8 mice) or cocaine (“5S…C”, n = 7 mice). [ANOVA, 20’-60’: F1,13 = 11.20, p = .005]. (C) Mean AMPAR/NMDAR ratio in control mice (“5S…”, n = 12 cells) or mice that were injected with saline (“5S…S”, n = 13 cells) or cocaine (“5S…C”, n = 13 cells) in the same environment, 24 hours before brain slice preparation. [ANOVA: F2,35 = 6.13, p = .005; *p < .05 compared to control, SNK post-hoc test].
As expected (cf. Figure 2), mice re-exposed to the same environment and injected with cocaine exhibited a significant increase in locomotor activity, compared to mice re-exposed to the same environment and injected with saline (Figure 4B). Recording from these two groups were conducted 24 hours later, and revealed a significant increase in the AMPAR/NMDAR ratio in the NAc shell (Figure 4C). The magnitude of this effect was similar following injection with either saline or cocaine, suggesting it was caused by re-exposure to the apparatus rather than any pharmacological effect of cocaine.
4. Discussion
Exposure to novel environments is an intrinsic facet of measuring behavioral responses to drug exposure and correlating addiction-related behavior with measures of synaptic function. However, it is important to recognize that environmental novelty represents a form of psychological stress (Pfister, 1979), and stressful experience can alter synaptic function in the mesolimbic dopamine system (Campioni et al., 2009; Saal et al., 2003). We have now shown that a single exposure to a novel environment causes an increase in the AMPAR/NMDAR ratio in NAc shell, a change that persists at least 24 hours. The effect of environmental novelty was not observed in NAc core and disappeared in the shell following five daily exposures to the same apparatus. However, spontaneous recovery of the effect was observed in NAc shell if mice were returned to the same apparatus 10-14 days later – an important finding given that tests of addiction-related behavior are often conducted following prolonged drug-free periods.
4.1. Novelty as stress: potential mechanisms
The specific effect of environmental novelty on synapses in NAc shell, but not NAc core, mirrors previous results regarding the synaptic impact of more conventional forms of stress. It was recently reported that two days of forced swim stress also increase the AMPAR/NMDAR ratio selectively in NAc shell (Campioni et al., 2009) – a pattern remarkably similar to our present findings. The change in AMPAR/NMDAR ratio caused by forced swim stress involves increased AMPAR currents in the NAc (Campioni et al., 2009) as well as the VTA (Dong et al., 2004), a mechanism also likely to contribute to our present results.
Campioni et al. (2009) also reported that forced swim stress caused a change in the current-voltage relationship of AMPAR currents, which exhibited inward rectification in control mice but not following forced swim stress. Other studies have not observed significant inward rectification of AMPAR currents in the mouse NAc shell under control conditions (Kourrich et al., 2007; Mameli et al., 2009; Vialou et al., 2010). We did not detect any changes in rectification following exposure to environmental novelty in the present study, indicating that changes in AMPAR subunit composition do not mediate the increase in AMPAR/NMDAR ratio caused by environmental novelty. It should be noted that the internal pipette solution used in the present experiments did not include spermine or other polyamines, so we cannot rule out the possibility that washout of endogenous polyamines prevented the detection of increased rectification of AMPAR currents following exposure to environmental novelty (cf. Vialou et al., 2010). However, other groups have observed clear rectification of AMPAR currents in the absence of exogenous polyamines (Clem and Barth, 2006; Jia et al., 1996), making this scenario unlikely.
The effect of forced swim stress on NAc synapses can be blocked by administration of a glucocorticoid receptor antagonist, and was also recapitulated by peripheral injection of corticosterone (Campioni et al., 2009). It is important to note that exposure to a novel environment also increases circulating levels of corticosterone in rodents (Badiani et al., 1998; Piazza et al., 1991), suggesting this stress hormone may also contribute to synaptic changes caused by environmental novelty. Corticosterone could act directly on MSNs (Ambroggi et al., 2009) to regulate the trafficking of AMPARs to synapses, as has been demonstrated in other cell types (Krugers et al., 2010). The variable expression of corticosterone receptor subtypes in NAc shell and core (Ahima et al., 1991; Ahima and Harlan, 1990) may contribute to the differential effect of environmental novelty across these subregions.
An alternative mechanism that may explain the synaptic impact of environmental novelty is the release of dopamine, which is enhanced in NAc shell but not core during exploration of a novel environment (Rebec et al., 1997). A similar pattern has been reported following electric footshock, a more conventional form of stress (Kalivas and Duffy, 1995). Dopamine receptor stimulation has been shown to facilitate synaptic incorporation of AMPAR in the NAc (Sun et al., 2008) as well as other brain regions (Smith et al., 2005; Sun et al., 2005), which could contribute to the increase in AMPAR/NMDAR ratio observed here. The enhanced release of dopamine may involve interactions between corticosterone and dopaminergic neurons in the VTA (Piazza and Le Moal, 1996), as potentiation of synaptic transmission in the VTA caused by forced swim stress has been shown to depend on glucocorticoid signaling (Dong et al., 2004; Saal et al., 2003). It will be important for future studies to compare the time course of synaptic modification in the VTA and NAc following stressful events, to determine whether synaptic changes in the VTA precede subsequent adaptations in NAc, as has been demonstrated following cocaine exposure (Mameli et al., 2009).
4.2. Novelty, reward, and individual differences
Despite the numerous parallels between conventional stressors and exposure to novel environments, it is important to note that novelty can also be rewarding in rodents. Rats prefer to spend time in novel environments (Bardo et al., 1989) and contexts associated with novel objects (Bevins et al., 2002), and mice will learn to perform operant responses to obtain novel environmental stimuli (Olsen and Winder, 2009). As with other types of reward, the rewarding impact of novelty appears to depend on activation of the mesolimbic dopamine system (Bardo et al., 1996; Olsen and Winder, 2009). Thus, it is difficult to uniquely associate NAc dopamine release with either stress or reward. The line between these psychological constructs is further blurred by the fact that rats will learn to perform an operant response to receive intravenous injections of corticosterone (Piazza et al., 1993), suggesting exposure to this stress hormone can be reinforcing.
The dichotomous nature of novelty as both stress and reward may be linked to individual differences in sensation-seeking, in that large behavioral and neurochemical responses to novelty may indicate the mesolimbic dopamine system is particularly vulnerable to activation by stress. Individuals that respond strongly to novelty are also more sensitive to the rewarding and reinforcing effects of psychostimulants, and may thus be prone to develop drug addiction. In rodents, sensation-seeking is frequently assessed by measuring the locomotor response to a novel environment, which has been associated with susceptibiliy to develop psychostimulant self-administration (Piazza et al., 1989). However, we did not find any relationship between the locomotor response to novelty and subsequent synaptic adaptations in NAc (Figure 2). This is consistent with recent reports that the locomotor response to novelty does not predict all facets of sensation-seeking (Olsen and Winder, 2009) or vulnerability to addiction (Belin et al., 2010).
4.3. Habituation to environmental novelty and spontaneous recovery: interactions with cocaine
The intrinsic novelty of a given environment would be expected to diminish with increasing exposure to that environment. Consistent with this notion, we found that five daily exposures to the same environment no longer affected the AMPAR/NMDAR ratio in NAc shell (Figure 3). This explains why the effect of environmental novelty was not previously detected when naïve mice were compared to those that received five daily injections of saline in the same environmental context (Kourrich et al., 2007). This pattern is consistent with a broader literature indicating that physiological reponses to mild stress, such as environmental novelty and physical restraint, are attenuated by previous exposure to the same form of stress (Grissom and Bhatnagar, 2009). It also highlights the importance of habituating rodents to a behavioral testing apparatus prior to drug exposure (Pierce and Kalivas, 2007), particularly when examining how drug exposure alters NAc glutamate transmission. In contrast, physiological responses to more intense forms of stress, such as forced swim (Kreibich et al., 2009) and social defeat (Covington and Miczek, 2005), do not necessarily habituate during chronic exposure (Grissom and Bhatnagar, 2009). This may explain why more persistent synaptic adaptations are observed in the mesolimbic dopamine system following repeated exposure to these latter types of stress (Campioni et al., 2009; Christoffel et al., 2011; Saal et al., 2003; Vialou et al., 2010).
In our previous studies, we reported that a single injection of cocaine did not alter excitatory synaptic transmission in the NAc shell (Kourrich et al., 2007; Thomas et al., 2001). However, cocaine was administered in a novel environment in some of these experiments, raising the possibility that the impact of environmental novelty obscured a pharmacological effect of one cocaine exposure. In the present report, after diminishing environmental novelty through repeated exposure to the testing apparatus, we still did not detect any effect of a single cocaine injection (Figure 3). In contrast, recent biochemical analyses suggest that a single cocaine injection alters expression of AMPARs on the cell surface of NAc neurons, causing an increase in naïve rats (Ferrario et al., 2011a) but a decrease in rats that previously received repeated saline injections (Ferrario et al., 2010). The source of this discrepancy is not clear, as physiological and biochemical studies of cocaine’s effects on NAc AMPARs have yielded remarkably consistent results in several domains, including the potentiation of AMPAR number and function during withdrawal from repeated cocaine exposure (Boudreau and Wolf, 2005; Kourrich et al., 2007), as well as depotentiation following re-exposure to cocaine (Boudreau et al., 2007; Kourrich et al., 2007). The increased incorporation of GluA2-lacking AMPARs during incubation of cocaine craving has also been demonstrated through both biochemical and physiological approaches (Conrad et al., 2008).
As previously suggested (Ferrario et al., 2011a), one exposure to cocaine may have divergent effects on AMPAR trafficking in the NAc shell and core. This would explain why biochemical analysis of tissue samples containing both shell and core (Ferrario et al., 2010; Ferrario et al., 2011a) would lead to different conclusions that our physiological studies, where recordings were conducted specifically in NAc shell (present results and Kourrich et al., 2007). Another possibility is that a single cocaine injection modulates extrasynaptic rather than synaptic AMPARs (Ferrario et al., 2011a). AMPAR trafficking in these distinct dendritic domains can be differentially regulated (Wolf, 2010), and extrasynaptic receptors would be detected in biochemical but not electrophysiological assays. New biochemical approaches that can distinguish synaptic and extrasynaptic membranes (Ferrario et al., 2011b) provide an opportunity to advance our understanding of potential roles and regulation of extrasynaptic AMPARs.
A key finding of the present experiments is that habituation to environmental novelty does not persist over a period of 10-14 days (Figure 4). A number of prominent tests of addiction-related behavior, including psychomotor sensitization (Paulson et al., 1991), incubation of cocaine craving (Grimm et al., 2001), and cocaine-seeking after abstinence (Fuchs et al., 2006), involve re-exposing animals to a previously familiar testing apparatus following an extended drug-free period. Our results may provide the first demonstration that established familiarity with a testing apparatus can dissipate over prolonged periods away from that apparatus, indicating that “rehabituation” to the testing apparatus is critical for avoiding the confounding effects of environmental novelty (Kourrich et al., 2007; Rothwell et al., 2010). This finding may also be relevant to the basic biology of stress responses, as spontaneous recovery following habituation to a single form of stress has not clearly been demonstrated (Grissom and Bhatnagar, 2009).
This renewed impact of a previously familiar environment may explain apparent differences between previous studies. We have reported that daily cocaine injections lead to an increased AMPAR/NMDAR ratio in NAc shell, which is reversed to control levels following re-exposure to cocaine (Kourrich et al., 2007) – a finding independently confirmed using biochemical approaches (Boudreau et al., 2007). In contrast, an earlier study reported that mice re-exposed to cocaine have an AMPAR/NMDAR ratio that is reduced below control levels (Thomas et al., 2001). However, in the latter study, the control group (which received daily saline injections) was also exposed to cocaine in the previously familiar testing apparatus, so the AMPAR/NMDAR ratio may have been boosted by renewed environmental novelty. Cocaine challenge thus decreased the AMPAR/NMDAR ratio in NAc shell below a control level elevated by novelty (Thomas et al., 2001), whereas in the former study (Kourrich et al., 2007), cocaine challenge only decreased the AMPAR/NMDAR ratio to naïve control levels when mice had been rehabituated to the testing apparatus. Thus, re-exposure to cocaine not only depotentiates NAc synapses (Boudreau et al., 2007; Kourrich et al., 2007), but intriguingly, this depotentiation appears to override the boosting effect that environmental novelty would otherwise provide (present results and Thomas et al., 2001). These issues further highlight the importance of controlling for the effects of environmental novelty and other potentially stressful aspects of experimental procedure, such as injection stress (Argilli et al., 2008; Barrot et al., 1999), when evaluating changes in synaptic function caused by drug exposure.
4.4. Conclusions
As discussed above, the synaptic impact of environmental novelty in NAc shell raises important considerations for designing studies of synaptic plasticity caused by exposure to addictive drugs. Furthermore, the stress-like impact of exposure to a novel environment provides an important confirmation of previous studies employing different forms of stress (Campioni et al., 2009). It is well established that different types of stress can exert divergent physiological effects (e.g., de Groote and Linthorst, 2007; Gesing et al., 2001), so the similarity between one exposure to a mild form of psychological stress (environmental novelty) and repeated exposure to a more intense and physical form of stress (forced swim) indicates a robust and consistent effect of stressful experience on excitatory synapses in NAc shell. The influence of stressful events on synaptic plasticity in the mesolimbic dopamine system may help explain why stressful life experience is a key predisposing factor to the development of drug addiction as well as other forms of psychiatric disease (de Kloet et al., 2005; Heim et al., 2004; Sinha, 2001).
Exposure to a novel environment alters synaptic function in nucleus accumbens shell
Conventional forms of stressful experience cause similar synaptic changes
This effect is no longer observed after repeated exposure to the same context
The synaptic impact of the same context is renewed 10-14 days after repeated exposure
Acknowledgments
We thank Jason Klug and Bonnie LaCroix for technical assistance, Dr. William Engeland for helpful comments on the manuscript, and members of the Thomas lab for stimulating discussions. This work was supported by funding from the University of Minnesota Graduate School (to PER) and grants from National Institute on Drug Abuse (DA007234 and DA023750 to PER, DA019666 to MJT) and the Whitehall Foundation (to MJT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Kocan D, Knopf S, Edwards DJ, Caggiula AR. One brief exposure to a psychological stressor induces long-lasting, time-dependent sensitization of both the cataleptic and neurochemical responses to haloperidol. Life Sci. 1992;51:261–266. doi: 10.1016/0024-3205(92)90084-3. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Pierce RC. Novelty-induced place preference behavior in rats: effects of opiate and dopaminergic drugs. Pharmacol Biochem Behav. 1989;32:683–689. doi: 10.1016/0091-3057(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11:1155–1166. doi: 10.1046/j.1460-9568.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-Novelty-Preference Rats are Predisposed to Compulsive Cocaine Self-administration. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129:41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. I{kappa}B Kinase Regulates Social Defeat Stress-Induced Synaptic and Behavioral Plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- de Groote L, Linthorst AC. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience. 2007;148:794–805. doi: 10.1016/j.neuroscience.2007.06.030. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(-/-) mice. Proc Natl Acad Sci U S A. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wolf ME. Effects of acute cocaine or dopamine receptor agonists on AMPA receptor distribution in the rat nucleus accumbens. Synapse. 2011a;65:54–63. doi: 10.1002/syn.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci Lett. 2011b doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst AC, Holsboer F, Reul JM. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J Neurosci. 2001;21:4822–4829. doi: 10.1523/JNEUROSCI.21-13-04822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology. 2009;34:2609–2617. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister HP. The glucocorticosterone response to novelty as a psychological stressor. Physiol Behav. 1979;23:649–652. doi: 10.1016/0031-9384(79)90154-9. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Locomotor behavior. Curr Protoc Neurosci. 2007;Chapter 8(Unit 8):1. doi: 10.1002/0471142301.ns0801s40. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebe P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–2228. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Gewirtz JC, Thomas MJ. Episodic Withdrawal Promotes Psychomotor Sensitization to Morphine. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotox Res. 2010;18:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


