Abstract
Objective
Compare survival of patients with poorly compressible arteries (PCA) to those with a normal ankle-brachial index (ABI) and those with peripheral arterial disease (PAD).
Background
Limited data are available regarding survival in patients with PCA identified in the clinical setting by non-invasive lower extremity arterial evaluation.
Methods
We conducted a historical cohort study of consecutive patients who underwent outpatient, non-invasive lower extremity arterial evaluation at Mayo Clinic, Rochester, Minnesota, from January 1998 through December 2007, and were followed for a mean duration of 5.8±3.1 years. An ABI 1.00-1.30 was considered normal, PAD was defined as a resting or post-exercise ABI ≤0.90, and PCA defined as an ABI ≥1.4 and/or an ankle systolic blood pressure >255 mm Hg. Patients were followed for all-cause mortality through 09/30/2009.
Results
Of 16,493 individuals (mean age ± standard deviation = 67.8±13.0 years, 59% male); 29% had normal ABI, 54% had PAD, and 17% had PCA. During follow-up (mean duration = 5.8±3.1 years), 4365 patients (26%) died. The percent alive at the end of the study period was 88%, 70%, and 60% for normal ABI, PAD, and PCA respectively. After adjustment for age, sex, cardiovascular risk factors, comorbid conditions, and medication use, the hazard ratios (confidence interval) of death associated with PCA were 2.0 (1.8-2.2) and 1.3 (1.2-1.4) compared to normal ABI and PAD groups, respectively.
Conclusions
Patients identified by non-invasive vascular testing to have poorly compressible leg arteries have poor survival, worse than those with a normal ABI or those with PAD.
Keywords: peripheral arterial disease, arterial calcification, mortality, ankle-brachial index
INTRODUCTION
Patients with known or suspected lower extremity peripheral artery disease (PAD) are typically evaluated in the outpatient setting by measuring the ankle brachial index (ABI), the ratio of lower of the ankle systolic blood pressures (BP) at each ankle to the higher arm systolic BP (1). An ABI between 1.00-1.30 is considered normal and an ABI ≤0.90 indicates presence of PAD (2). An ABI ≥1.40 is abnormal and a consequence of medial arterial calcification (3) in the lower extremities leading to poor compressibility or “stiffness” of arteries. As a result, higher cuff pressure is needed to cause compression or occlusion of arteries, resulting either in an inability to obtain an ABI (ankle BP >255 mmHg) or a falsely elevated ABI (≥1.40) (4). The presence of poorly compressible arteries (PCA) in the lower extremities has been found to be highly specific for calcification of the medial layer in these arteries (4-6). The phenomenon has also been referred to in the literature as “calcified,” “stiff,” “non compressible,” or “poorly compressible” vessels/arteries. In this report, we use the term “poorly compressible arteries.”
Based on data from National Health and Nutritional Examination Survey, 1.6 million adults aged ≥40 years in the United States have an ABI >1.40 (7). These individuals have a higher prevalence of diabetes, chronic kidney disease and cardiovascular disease, and lower quality of life than those with normal ABI (8-10). Three previous studies (11-13) of random samples of community-dwelling volunteers have shown that participants with a high ABI had survival rates similar to those with PAD (ABI ≤0.90). Other than small studies (n = 8 and 403, respectively) (14,15), limited data are available regarding survival of patients identified in the clinical setting as having PCA.
The goal of the present study was to characterize patients with PCA identified in the non-invasive vascular laboratory relative to patients with a normal ABI as well patients with PAD, in terms of prevalence of cardiovascular risk factors, comorbid conditions, and survival. We estimated survival free of death and identified predictors of survival in patients undergoing non-invasive lower extremity arterial evaluation in the outpatient setting. Knowledge of life expectancy may be useful in the care of patients with PCA by informing clinical decision-making.
METHODS
Study design and population
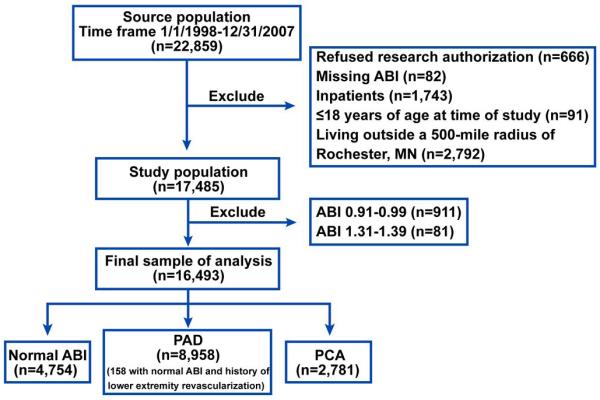
This study used a historical cohort design. The sample consisted of consecutive adult patients who underwent outpatient lower extremity arterial evaluation for suspected or known PAD in the non-invasive vascular laboratory of Mayo Clinic, Rochester, MN, between January 1, 1998, and December 31, 2007. Since January of 1998, the results of all non-invasive lower extremity arterial evaluations have been entered into a database that becomes part of the Mayo electronic medical record (EMR). We excluded the following patients who: 1) refused research authorization, 2) had missing results of arterial evaluation, 3) resided outside a 500-mile radius of Rochester, MN, 4) were hospitalized at the time of arterial evaluation, and 5) were aged ≤18 years at the time of arterial evaluation (Figure 1). Additionally, to ease interpretation of our analyses, we excluded patients with borderline high ABI (1.31-1.39) and borderline low ABI (0.91-0.99) (Figure 1).
Figure 1. Study patients.
Flow diagram of patients in our study.
Non-invasive lower extremity arterial evaluation
Non-invasive lower extremity arterial evaluation was performed using standardized protocols in the non-invasive vascular laboratory, which is certified by the Intersocietal Commission for the Accreditation of Vascular Laboratories (http://www.icavl.org/). Systolic BP was measured in each arm and in the dorsalis pedis and posterior tibial arteries bilaterally using a hand-held 8.3-MHz Doppler probe. The higher of the two arm pressures and the lower of the two ankle pressures was used to calculate the ABI in each leg. The ABI based on the lower ankle pressure identifies a greater number of patients at risk for adverse cardiovascular events than an ABI based on the higher ankle pressure (16). However, since the latter has been used for calculating the ABI in several prior studies, we also performed analyses based on the higher ankle pressure (results provided in the Supplement) to facilitate comparison to these studies. A standard treadmill test with a speed of 1 to 2 mph and a fixed grade of 10° with continuous electrocardiographic monitoring is performed (except in patients with severe PAD, i.e., ABI <0.5, and in patients with PCA) to obtain post-exercise ABI. Doppler wave forms were recorded from each major lower extremity artery. The data were interpreted and reported by a vascular medicine specialist.
Definition of normal ABI, PAD, and PCA
Since January of 1998, the results of all non-invasive lower extremity arterial evaluations have been entered into a database that becomes part of the Mayo electronic medical record (EMR). We defined normal ABI as 1.0-1.3, borderline low ABI as 0.91-0.99, and borderline high ABI as 1.31-1.39. We defined PAD as an ABI ≤0.9 at rest or 1 min after exercise. Since patients with PAD can have a normal ABI after revascularization, we used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for surgical or percutaneous revascularization supplemented by manual review of medical records, to identify such individuals. Patients were classified as having PCA if any ABI was ≥1.40 or the ankle systolic BP was >255 mmHg (upper limit of commonly used BP apparatuses) or the term “non-compressible vessels” was mentioned in the lower extremity arterial evaluation. In patients with PCA, we additionally ascertained whether abnormal Doppler waveforms (absent, monophasic, or reduced biphasic) in a lower extremity artery had been noted during the laboratory evaluation.
Cardiovascular risk factors and comorbid conditions
Demographic characteristics including area of residence, ethnicity, height and weight were obtained from the Mayo EMR which contains both structured and free text elements. Cardiovascular risk factors were ascertained from the EMR using previously validated algorithms that have good sensitivity and specificity compared to manual chart abstraction (17). Smoking status and medication use were ascertained using Natural Language Processing (NLP) as previously described (18,19). NLP was used to discover drug ‘named-entity’ mentions from clinical notes since medications are often recorded in clinical notes as free-text. Laboratory data were obtained from the EMR using a window of 1 year centered around the time of arterial evaluation. Hypertension was considered present if there were two BP readings of ≥140/90 mm Hg within three months of the date of arterial evaluation, or a prior diagnosis of hypertension and current treatment with antihypertensive medication. Diabetes was ascertained based on fasting plasma glucose ≥126 mg/dL, or random glucose >200 mg/dl, or hemoglobin A1c of >6.5%, or a prior diagnosis and use of oral hypoglycemic agent(s) or insulin. Comorbid conditions such as coronary heart disease (CHD), cerebrovascular disease, heart failure, chronic kidney disease, malignancy, and chronic obstructive pulmonary disease were ascertained based on the presence of relevant ICD-9-CM diagnosis and procedure codes for up to 6 months following the date of arterial evaluation (17). The presence of critical limb ischemia in the study groups was ascertained by the presence of the ICD-9-CM diagnosis codes 440.22-24.
Ascertainment of survival
The follow-up period for each patient was the time between first arterial evaluation and the earliest of date of death or final censoring date (September 30, 2009). Death was ascertained initially from the Mayo EMR. For persons who were not noted as being deceased, we used the Accurint® database (Seisint, Inc., West Palm Beach, FL) to ascertain vital status. Based on the EMR and Accurint® database, the number of decedents was 4,365 (26% of the study cohort).
Statistical analysis
Demographic and physical characteristics, laboratory values, cardiovascular risk factors, and comorbid conditions were summarized as mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. To assess the effect of age and sex on the prevalence of PCA, we performed logistic regression analyses. Differences in demographic and clinical characteristics between the three study groups were tested with an analysis of covariance for continuous variables and by logistic regression for binary categorical variables, adjusting for age and sex. A P-value of <0.05 was considered statistically significant. We computed the Kaplan-Meier survival curves in the three groups of patients and compared survival using the one-sample log rank test.
We constructed a series of multivariable Cox proportional hazards models to estimate hazard ratios for death in the PCA patients compared to the normal ABI group and PAD groups, starting with an unadjusted model and then adjusting for age and sex, and then additionally for body mass index (BMI), CHD, cerebrovascular disease, heart failure, diabetes, hypertension, chronic kidney disease, malignancy, chronic obstructive pulmonary disease, smoking, and medication (statin and aspirin) use, and finally, for the presence of critical limb ischemia. We assessed whether interactions existed between PCA and age and sex in predicting hazard of dying compared to patients with a normal ABI and PAD. The multivariable Cox proportional hazards models were repeated in strata defined by any significant interactions. We furthermore identified predictors of survival and estimated the associated hazard ratios, using these same Cox proportional hazards models. To address the possibility of a referral bias, we included Olmsted County residency as a predictor variable, and also repeated the multivariable Cox proportional hazards models stratified by Olmsted County residency. Finally, we assessed a) whether the presence of abnormal arterial Doppler signals in PCA patients resulted in an additional increase in risk of mortality and b) whether defining PAD as ABI <1.0 and PCA as ABI ≥1.3 altered inferences. Statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 22,859 patients underwent non-invasive lower extremity arterial evaluation between January 1, 1998 and December 31, 2008. After applying the initial exclusion criteria, 17,485 remained (Figure 1). Additionally, we removed patients with borderline low ABI (0.91-0.99) and borderline high ABI (1.31-1.39) to arrive at the final study sample of 16,493 patients (59% men, 98% non-Hispanic white, mean age 67.8±13.0 years). Of these, 4754 (29%) patients had a normal ABI (1.00-1.30) and 8,958 patients (54%) had PAD. The latter included 158 patients with a normal ABI who had previously undergone lower extremity revascularization for symptomatic PAD. A significant proportion (17%, n=2781) of patients had PCA (Table 1). The prevalence of critical limb ischemia was significantly higher in patients with PCA than in patients with PAD (Table 1). Abnormal Doppler arterial waveforms were present in 1976 (71%) of PCA patients. The proportion of patients with normal ABI, PAD, and PCA among men and women of various age ranges is shown in Figure 2. The prevalence of PCA was higher in men (P<0.001) than in women and increased with age (P<0.001) in both men and women. Patients with PAD or PCA were older than the reference group. Other patient characteristics were compared after adjustment for age and sex (Table 1).
Table 1.
Patient characteristics Normal ABI
| Normal ABI (n=4754) |
PAD (n=8958) |
PCA (n=2781) |
|
|---|---|---|---|
| Age, years | 63.0±14.3 | 69.3±11.7 | 71.3±12.1 |
| Men, n (%) | 2541 (53.5) | 5270 (58.8) | 1869 (67.2) |
| Olmsted County residents, n (%) | 618 (13.0) | 1027 (11.5) | 302 (10.9) |
| BMI, kg/m2 | 29.7±6.7 | 28.4±5.7 | 29.9±6.7 |
| Systolic BP, mmHg | 133±19 | 142±23 | 143±26 |
| Diastolic BP, mmHg | 77±10 | 74±11 | 73±11 |
| Pulse pressure, mmHg | 56±15 | 68±19 | 69±20 |
| Plasma glucose, mg/dL | 109.5±35.0 | 119.9±45.2 | 136.0±61.9 |
| Serum creatinine, mg/dL | 1.2±0.5 | 1.3±0.7 | 1.8±1.6 |
| Ever smoked | 3457 (72.7) | 7773 (86.8) | 2011 (72.3) |
| Diabetes | 862 (18.1) | 2542 (28.4) | 1628 (58.6) |
| Hypertension | 2491 (52.4) | 6578 (73.4) | 2171 (78.1) |
| Coronary heart disease | 1559 (32.8) | 4918 (54.9) | 1637(58.9) |
| Heart failure | 343 (7.2) | 1233 (13.7) | 738 (26.6) |
| Chronic kidney disease | 198 (4.2) | 635 (7.1) | 531 (19.1) |
| Cerebrovascular disease | 673 (14.2) | 2958 (33.0) | 780 (28.1) |
| COPD | 401 (8.4) | 1634 (18.3) | 331 (11.9) |
| Malignancy | 899 (18.9) | 1728 (19.3) | 611 (22.0) |
| Critical limb ischemia | - | 1657 (18.5) | 1029 (37) |
| Statin use | 1282 (27.0) | 3382 (37.8) | 1024 (36.8) |
| Aspirin use | 1677 (35.28) | 4167 (46.5) | 1270 (45.7) |
ABI=ankle brachial index, BMI=body mass index, BP=blood pressure, COPD=chronic obstructive pulmonary disease, PAD=peripheral arterial disease, PCA=poorly compressible arteries. Shown are mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables. Differences in age and sex between study groups were significant at P<0.001. Age- and sex-adjusted differences in the remaining patient characteristics were also significant at P<0.001, except for Olmsted County residency (P=0.007).
Figure 2. Age and sex distribution in the three study groups.
The proportion of patients with normal ABI, PAD, and PCA among men and women of various age ranges. The prevalence of PCA was higher in men (P<0.001) than in women and increased with age (P<0.001) in both men and women.
Pulse pressure was higher in the PCA and PAD groups than the normal ABI group. Fasting serum glucose and serum creatinine were highest in the PCA group. Among cardiovascular risk factors, diabetes and hypertension were more prevalent in patients with PCA, while history of smoking was more common in patients with PAD (Table 1). In the PCA group, a higher proportion of patients had coexisting CHD, heart failure, and chronic kidney disease than in the normal ABI and PAD groups (Table 1). On the other hand, the proportion of patients with cerebrovascular disease and chronic obstructive pulmonary disease was highest in the PAD group (Table 1). The proportion of patients on a statin medication or aspirin was higher in the PCA and PAD groups than the normal ABI group, but was not significantly different between PCA and PAD groups (Table 1).
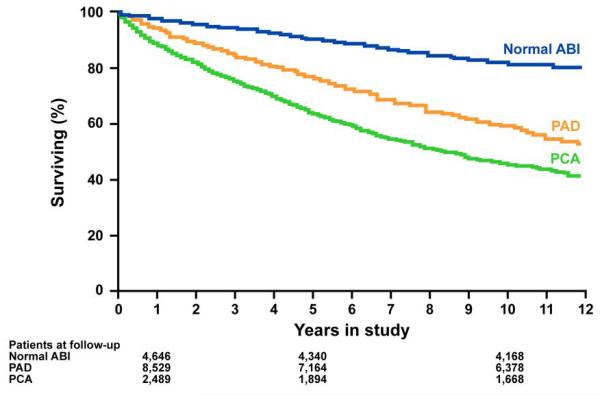
The mean follow-up for the entire group was 5.8±3.1 years (median: 5.6 years, inter-quartile range: 3.2-8.3 years). As of 09/30/2009, 4,365 (26%) patients had died; a greater proportion of patients with PCA died (n=1123, 40%), followed by PAD patients (n=2649, 30%), and patients with a normal ABI (n=593, 12%). The estimated percent survival at 5 years was lowest in the PCA group (74%), followed by the PAD (79%) and normal ABI (86%) groups. Kaplan Meier survival curves over 12 years of follow-up are shown in Figure 3. The curves start to separate early and survival in PCA group was worse than the PAD and normal ABI groups.
Figure 3. Survival curves in the three study groups.
Kaplan-Meier survival curves. The number of patients in each study group at 1, 5, and 10 years of follow-up are listed.
We estimated the relative hazard of death in patients with PAD and PCA using multivariable Cox proportional hazards regression. In the unadjusted model, compared to patients with normal ABI, the hazard of death was 2.5-fold higher in patients with PAD and 4.0-fold higher in patients with PCA (Table 2). In the model that adjusted for age, sex and comorbid conditions, the hazard of death was 1.6-fold higher in patients with PAD, and 2.0-fold higher in patients with PCA than those with normal ABI (Table 2). When we compared patients with PCA to patients with PAD, the former had 60% higher hazard of death (HR=1.6, CI=1.5-1.7) in the unadjusted model and 30% higher hazard of death (HR=1.3, CI=1.2-1.4) after adjustment for age sex and comorbid conditions (Table 2). The presence of PCA was associated with increased hazard of death even after additional adjustment for critical limb ischemia (Table 2). We found significant interactions between presence of PCA and sex in influencing the hazard of death compared to patients with a normal ABI (P<0.001) and PAD (P<0.007). We therefore performed analyses stratified by sex. Compared to patients with normal ABI, the hazard of death associated with PCA was greater in women (2.6) than in men (1.7) (Table 2). Compared to patients with PAD, the hazard of death associated with PCA was also greater in women (1.5) than in men (1.3) (Table 2). Olmsted County residency was associated with a greater hazard of death, consistent with a modest referral bias. However, in analyses stratified by Olmsted County residency, the hazard ratios associated with PCA and other predictor variables were similar in magnitude (Table 3).
Table 2.
Hazard of death associated with the presence of PCA, compared to the normal ABI and PAD groups
| Hazard ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Group |
||||
| Normal ABI | PAD | PCA | PCA vs. PAD* | |
| Model A | ||||
| All patients, n=16,493 | 1.0 | 2.5 (2.3-2.7) | 4.0 3.6-4.4) | 1.6 (1.5-1.7) |
| Men, n=9,680 | 1.0 | 2.0 (1.8-2.3) | 3.0 (2.7-3.4) | 1.5 (1.4-1.6) |
| Women, n=6,813 | 1.0 | 3.3 (2.8-3.8) | 6.0 (5.1-7.1) | 1.9 (1.7-2.1) |
| Olmsted County patients, n=1,947 | 1.0 | 2.3 (1.9-2.9) | 4.6 (3.5-5.9) | 2.0 (1.6-2.3) |
| Non-Olmsted County patients, n=14,546 | 1.0 | 2.5 (2.3-2.8) | 3.9 (3.5-4.4) | 1.6 (1.5-1.7) |
| Model B | ||||
| All patients | 1.0 | 1.9 (1.8-2.1) | 2.9 (2.6-3.3) | 1.5 (1.4-1.6) |
| Men | 1.0 | 1.7 (1.5-1.9) | 2.4 (2.2-2.8) | 1.4 (1.3-1.5) |
| Women | 1.0 | 2.3 (2.0-2.7) | 4.2 (3.5-5.0) | 1.7 (1.5-2.0) |
| Olmsted County patients | 1.0 | 1.6 (1.3-2.0) | 2.8 (2.1-3.6) | 1.8 (1.5-2.1) |
| Non-Olmsted County patients | 1.0 | 2.0 (1.8-2.2) | 3.0 (2.7-3.4) | 1.5 (1.4-1.6) |
| Model C | ||||
| All patients | 1.0 | 1.6 (1.5-1.8) | 2.0 (1.8-2.3) | 1.3 (1.2-1.4) |
| Men | 1.0 | 1.3 (1.5-1.6) | 1.7 (1.5-2.0) | 1.3 (1.2-1.4) |
| Women | 1.0 | 1.9 (1.6-2.2) | 2.7 (2.2-3.4) | 1.4 (1.2-1.6) |
| Olmsted County patients | 1.0 | 1.3 (1.0-1.6) | 1.8 (1.4-2.5) | 1.4 (1.1-1.7) |
| Non-Olmsted County patients | 1.0 | 1.7 (1.5-1.9) | 2.1 (1.9-2.4) | 1.3 (1.2-1.4) |
| Model D | ||||
| All patients | 1.0 | 1.5 (1.4-1.6) | 1.8 (1.6-2.0) | 1.2 (1.1-1.3) |
| Men | 1.0 | 1.4 (1.2-1.5) | 1.5 (1.3-1.7) | 1.2 (1.1-1.3) |
| Women | 1.0 | 1.7 (1.5-2.1) | 2.5 (2.0-3.1) | 1.3 (1.2-1.5) |
| Olmsted County patients | 1.0 | 1.2 (0.9-1.5) | 1.6 (1.1-2.2) | 1.3 (1.0-1.5) |
| Non-Olmsted County patients | 1.0 | 1.6 (1.4-1.7) | 1.9 (1.6-2.1) | 1.2 (1.1-1.3) |
PAD=peripheral arterial disease, PCA=poorly compressible arteries.
Model A: Unadjusted; Model B: Adjusted for age and sex; Model C: Adjusted for age, sex, body mass index, systolic blood pressure, coronary heart disease, cerebrovascular disease, congestive heart failure, diabetes, hypertension, chronic kidney disease, malignancy, chronic obstructive pulmonary disease, smoking, statin use, and aspirin use. Model D: adjusted for Model C variables plus presence of critical limb ischemia. The sex stratified models were not adjusted for sex.
Table 3.
Variables associated with relative hazard of dying
| Hazard ratio (confidence interval) | |||
|---|---|---|---|
| All patients | Olmsted County patients |
Non-Olmsted County patients |
|
| Age (per decade increase) | 1.50 (1.45-1.55) | 1.64 (1.51-1.77) | 1.48 (1.43-1.53) |
| Male sex | 1.11 (1.04-1.18) | 1.24 (1.05-1.46) | 1.08 (1.01-1.16) |
| BMI (5 kg/m2 increase) | 0.91 (0.89-0.94) | 0.92 (0.85-0.99) | 0.91 (0.89-0.94) |
| Ever smoked | 1.13 (1.04-1.23) | 0.99 (0.82-1.21) | 1.17 (1.07-1.28) |
| Diabetes | 1.53 (1.43-1.63) | 1.64 (1.39-1.94) | 1.51 (1.40-1.62) |
| Hypertension | 1.14 (1.06-1.24) | 1.23 (0.97-1.56) | 1.14 (1.05-1.24) |
| Coronary heart disease | 1.32 (1.23-1.41) | 1.11 (0.91-1.35) | 1.35 (1.25-1.45) |
| Heart failure | 2.13 (1.98-2.30) | 2.25 (1.87-2.71) | 2.14 (1.97-2.31) |
| Chronic kidney disease | 1.83 (1.68-1.99) | 1.74 (1.41-2.14) | 1.84 (1.68-2.03) |
| Cerebrovascular disease | 1.18 (1.10-1.26) | 1.19 (1.00-1.40) | 1.17 (1.09-1.25) |
| COPD | 1.52 (1.42-1.64) | 1.42 (1.18-1.71) | 1.55 (1.44-1.68) |
| Malignancy | 1.50 (1.40-1.60) | 1.34 (1.14-1.58) | 1.53 (1.43-1.65) |
| Statin use | 0.70 (0.65-0.75) | 0.71 (0.59-0.85) | 0.70 (0.65-0.76) |
| Aspirin use | 0.92 (0.86-0.98) | 1.14 (0.96-1.35) | 0.88 (0.83-0.95) |
| Olmsted County residency | 1.21 (1.11-1.32) | - | - |
BMI=body mass index, COPD=chronic obstructive pulmonary disease.
In the fully adjusted model, factors in addition to PCA that were associated with an increased hazard of dying were hypertension, diabetes, history of smoking, CHD, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease, chronic kidney disease, and malignancy (Table 3). A higher BMI, and statin and aspirin use were associated with a reduced hazard of death (Table 3). In patients with PCA, the presence of abnormal Doppler arterial waveforms was associated with an incremental hazard of death independent of age, sex, cardiovascular risk factors, comorbid conditions and medication use – HR 1.55 (1.31-1.83; P<0.0001). When we used criteria for PAD as ABI <1.0 and for PCA as ABI >1.3, the fully adjusted hazards of death associated with PAD and PCA, compared to an ABI of 1.0-1.3 were 1.58 (1.44-1.74) and 1.98 (1.76-2.20), respectively, similar to when using the initial definitions for PAD and PCA.
When analyses were repeated using the higher of the two ankle pressures to calculate the ABI, the inferences were unchanged. The results of these analyses are summarized in the Supplement.
DISCUSSION
In this historical cohort study of patients referred for a non-invasive arterial evaluation, patients who met criteria for PCA had significantly lower survival than patients with PAD and those with normal ABI. Among patients with PCA, 40% died by the end of the study period, a significantly higher proportion than in patients with PAD (30%) and normal ABI (12%). Having PCA was associated with 4.0- and 1.6-fold unadjusted higher hazard of death than the normal ABI and PAD groups, respectively. The higher risk of death appeared to be due in part to greater age and higher prevalence of comorbid conditions and cardiovascular risk factors. After adjustment for age, sex, cardiovascular risk factors, comorbid conditions, and medication use, patients with PCA were at a 2.0- and 1.3-fold higher risk of death than the normal ABI and PAD groups, respectively. The hazard of death due to PCA was higher in women than in men.
Three previous cohort studies showed a U-shaped association between ABI and survival (11-13). These studies used different criteria to label patients as having normal ABI, PAD, and PCA. The Strong Heart Study (11) included 4393 American Indians, grouped into low (≤0.90), normal (0.90-1.40), and high ABI (>1.40) categories. Participants with borderline-low and borderline-high ABI were included in the “reference category.” During a mean follow-up of 8.3±2 years, 1,022 participants died. The adjusted HR of death was 1.7 in the PAD group and 1.8 in the high ABI group (11). In the Cardiovascular Health Study (12), 5748 Medicare-eligible adults were followed for a mean of 11.1 years. The adjusted HR of death increased at ABI values above and below the normal range (1.00-1.30). Compared to the normal ABI group, the HR of death in high ABI (>1.40) group was 1.60. However, this study excluded patients in whom ABI could not be calculated, and therefore may have included only a “milder” form of PCA (12). In the Multiethnic Study of Atherosclerosis (MESA) (13), 6647 non-Hispanic white, African-American, Hispanic, and Chinese men and women age 45-84 years without history of myocardial infarction or stroke, underwent measurement of ABI. After adjustment for both traditional and newer cardiovascular risk factors, hazard ratios for a low (<1.0) and high (>1.40) ABI were 1.77 and 1.85 respectively.
By contrast to these three studies (11-13) in which survival of patients with high ABI was similar to those with PAD, we found that adjusted HR for death was higher in patients with PCA than those with PAD. A cross-sectional study of 7155 patients age >50 years from 350 primary care sites found those with ABI ≥1.40 at higher odds for foot ulcer, neuropathy, heart failure and stroke after adjustment for traditional risk factors, compared to those with normal ABI (20). Our results confirm that both PAD and PCA are associated with reduced survival compared to patients with a normal ABI, and in addition, demonstrate that having PCA carries 30% (adjusted) higher risk of death than having PAD. This is an important finding given that the presence of PAD is associated with a relatively poor prognosis (21-24). Olmsted County residency was associated with a modestly greater hazard of death in our study cohort, suggesting a ‘healthy person’ referral bias. In other words, patients from outside of the Olmsted County were ‘healthier’ and able to travel to Rochester, MN, for further evaluation of leg symptoms. However, in analyses stratified by Olmsted County residency, the relative hazards associated with the predictor variables, including the presence of PCA, were similar.
In a study (15) of 403 hospitalized diabetic patients, the presence of PCA and abnormal Doppler, but not PCA alone, was associated with a higher risk of cardiovascular events. By contrast, the presence of PCA with normal Doppler waveforms in our study was associated with increased risk of mortality compared to those with normal ABI (fully adjusted HR 1.4, 1.2-1.6) but not when compared to patients with PAD (fully adjusted HR 0.9, 0.8-1.1). The presence of abnormal Doppler in patients with PCA was associated with a significant increment in the risk of death, suggesting that concomitant atherosclerosis adds to the risk due to medial arterial calcification. Whether the presence of PCA is merely a marker of increased risk or directly contributes to increased mortality is unknown.
One potential mechanism of increased risk is greater arterial stiffness in patients with PCA. We found systolic BP and pulse pressure to be higher in the PCA and PAD groups than in normal ABI group. In MESA (6), participants with PCA had greater left ventricular mass than patients with normal or low ABI. The authors postulated that medial artery calcification leads to increased arterial stiffness and increased left ventricular afterload, thereby increasing left ventricular mass (6). Consistent with this hypothesis, we previously showed that serum N-terminal pro-B-type natriuretic peptide levels are significantly higher in patients with PCA than in patients with normal ABI and those with PAD (25). An additional mechanism could be the high prevalence of critical limb ischemia in patients with PCA. However, even after additional adjustment for the presence of critical limb ischemia, patients with PCA had a higher hazard of dying than patients with or without PAD.
Our study has several important clinical implications. First, a substantial proportion of patients (17% in the present study) referred for non-invasive lower extremity arterial evaluation had PCA. The prevalence of PCA is likely to increase as a result of aging and increased prevalence of diabetes, highlighting the need for increased awareness of this entity (26). Second, the presence of PCA in older adults indicates increase risk of mortality and motivates further investigation to identify factors whose modification would improve survival. Finally, among the three groups in our study, patients with PCA had the highest prevalence of diabetes, heart failure, and chronic kidney disease. Together with prior studies that have reported an increased risk of amputation and poor quality of life in patients (20,27-29). these data indicate high level of comorbidity and health care burden associated with PCA. Identifying risk factors for medial arterial calcification and treating such factors is important given that treatment options for vascular calcification are limited (30).
Strengths and limitations
A strength of the present study is the large sample of patients who underwent standardized evaluation in an accredited non-invasive vascular laboratory. To the best of our knowledge, the 2781 patients with PCA comprise the largest reported cohort of such patients to date. Although we attempted to limit referral bias by excluding patients who lived outside of a 500-mile radius of our medical center, and also performed sensitivity analyses using Olmsted County residency as a stratification variable, our survival estimates need to be validated in additional studies. The study cohort was almost entirely non-Hispanic white and whether our findings can be generalized to other racial and ethnic groups needs further study. We did not have imaging studies available to confirm medial arterial calcification in patients with PCA, nor did we have information on all adverse cardiovascular events or the cause of death.
Conclusion and future directions
A significant proportion of patients referred for non-invasive lower extremity arterial evaluation had PCA and survival in such patients was lower than in those with a normal ABI, and even lower than in patients with PAD. After adjusting for age, sex, cardiovascular risk factors, comorbid conditions, and medications, survival in the PCA group was 2.0-fold lower than with normal ABI and 1.3-fold lower than those with ABI ≤0.90. The hazard of dying associated with PCA was higher in women than in men. There was an increased prevalence of comorbid conditions in patients with PCA, especially diabetes, heart failure, and chronic kidney disease. However, other as yet unidentified factors may contribute to reduced survival in patients with PCA. Further investigation is needed to identify mechanisms that lead to development of PCA (30-36), identify mediators of poor survival, and explore treatment options to improve survival in these high risk patients.
Supplementary Material
Acknowledgments
Financial Support: Dr. Arain was supported by the NIH Vascular Medicine Training Program K12 grant (HL083797). Dr. Kullo was supported by NIH grants HL75794 and HL81331.
Role of the funding sources Dr. Arain was supported by the NIH Vascular Medicine Training Program K12 grant (HL083797). Dr. Kullo was supported by NIH grants HL75794 and HL81331.
Acknowledgments
We acknowledge Vicki Schmidt for help with manuscript preparation and the technicians of the non-invasive Vascular Laboratory at Mayo Clinic, Rochester, MN.
ABBREVIATIONS
- ABI
Ankle-brachial index
- BMI
Body mass index
- BP
Blood pressure
- CHD
Coronary heart disease
- EMR
Electronic medical record
- HR
Hazard ratio
- ICD-9 CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NLP
Natural language processing
- PAD
Peripheral arterial disease
- PCA
Poorly compressible arteries
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have nothing to disclose and have no relationships to industry.
Conflicts of interest There are no potential conflicts of interest, financial or otherwise, from any of the authors. All the authors had access to the data and a role in writing the manuscript.
References
- 1.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. 1969;207:1869–74. [PubMed] [Google Scholar]
- 2.Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation. 1985;71:516–22. doi: 10.1161/01.cir.71.3.516. [DOI] [PubMed] [Google Scholar]
- 3.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 4.Orchard TJ, Strandness DE., Jr Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the American Diabetes Association and the American Heart Association, September 18-20, 1992 New Orleans, Louisiana. Circulation. 1993;88:819–28. doi: 10.1161/01.cir.88.2.819. [DOI] [PubMed] [Google Scholar]
- 5.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Ix JH, Katz R, Peralta CA, et al. A high ankle brachial index is associated with greater left ventricular mass MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:342–9. doi: 10.1016/j.jacc.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resnick HE, Foster GL. Prevalence of elevated ankle-brachial index in the United States 1999 to 2002. Am J Med. 2005;118:676–9. doi: 10.1016/j.amjmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 9.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 10.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167:962–9. doi: 10.1093/aje/kwm402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 12.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–93. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 13.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–42. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aboyans V, Lacroix P, Tran MH, et al. The prognosis of diabetic patients with high ankle-brachial index depends on the coexistence of occlusive peripheral artery disease. J Vasc Surg. 2011;53:984–91. doi: 10.1016/j.jvs.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118:961–7. doi: 10.1161/CIRCULATIONAHA.107.763227. [DOI] [PubMed] [Google Scholar]
- 17.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010;17:568–574. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savova GK, Ogren PV, Duffy PH, Buntrock JD, Chute CG. Mayo Clinic NLP system for patient smoking status identification. J Am Med Inform Assoc. 2008;15:25–8. doi: 10.1197/jamia.M2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner DJ, Coyle JF, Rocha BH, Haug P, Huff SM. Medical data abstractionism: fitting an EMR to radically evolving medical information systems. Stud Health Technol Inform. 2004;107:550–4. [PubMed] [Google Scholar]
- 20.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292–8. doi: 10.1016/j.jacc.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 21.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New Engl J Med. 1992;326:381. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 22.Kollerits B, Heinrich J, Pichler M, et al. Intermittent claudication in the Erfurt Male Cohort (ERFORT) Study: its determinants and the impact on mortality. A population-based prospective cohort study with 30 years of follow-up. Atherosclerosis. 2008;198:214–22. doi: 10.1016/j.atherosclerosis.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. Jama. 2006;295:180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 24.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 25.Jouni H, Rodeheffer RJ, Kullo IJ. Increased NT-pro-BNP levels in patients with medial artery calcification and poorly compressible vessels. Arterioscler Thromb Vasc Biol. 2011;31:197–202. doi: 10.1161/ATVBAHA.110.216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestro A, Diehm N, Savolainen H, et al. Falsely high ankle-brachial index predicts major amputation in critical limb ischemia. Vasc Med. 2006;11:69–74. doi: 10.1191/1358863x06vm678oa. [DOI] [PubMed] [Google Scholar]
- 28.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–74. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification: A neglected harbinger of cardiovascular complications in non–insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 30.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–24. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 31.Demer LL, Sage AP, Tintut Y. Nanoscale architecture in atherosclerotic calcification. Arterioscler Thromb Vasc Biol. 2008;28:1882–4. doi: 10.1161/ATVBAHA.108.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–34. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 33.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–7. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 34.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 36.Ix JH, De Boer IH, Peralta CA, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–15. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.