Abstract
Objectivers
To evaluate pain severity and distribution in relation to sleep difficulty in older adults.
Design
Population-based cross-sectional study
Setting
Community within a 5-mile radius of the study center at the Institute for Aging Research, Hebrew SeniorLife (HSL) in Boston
Participants
765 participants of the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly (MOBILIZE) Boston Study, aged 64 and older.
Measurements
Pain severity was measured using the Brief Pain Inventory (BPI), Pain Severity Subscale. Musculoskeletal pain distribution was grouped according to no pain, single site, ≥2 sites, and widespread pain (upper and lower extremities and back pain). We measured 3 aspects of sleep difficulty using items from the CESD-R (trouble getting to sleep, sleep more than usual, and restless sleep).
Results
Prevalence of trouble getting to sleep according to BPI severity was 17.8%, 19.7%, 32.0%, and 37.0% for the lowest to highest pain severity quartiles, respectively. Similar relationships between pain and sleep were observed across sleep measures according to pain severity and distribution. Adjusted for sociodemographic characteristics, chronic conditions and health behaviors, chronic pain was strongly associated with trouble sleeping (≥1d/week), (single site pain, OR=1.77, 95%CI, 1.10–2.87; multisite pain OR=2.38, 95% CI, 1.48–3.83; and widespread pain, OR=2.55, 95% CI, 1.43–4.54, each compared with no pain). Similar associations were observed for restless sleep and sleeping more than usual. With specific pain sites alone or in combination with other sites of pain, only modest associations were observed with sleep problems.
Conclusion
Widespread or other multisite pain and moderate to severe pain are strongly associated with sleep difficulty in older adults. Further research is needed to better understand the burden and consequences of pain-related sleep problems in the older population.
INTRODUCTION
Sleep problems are common in the older population; about 40% of older adults report having sleep difficulty.1 Sleep disturbances contribute to a number of health consequences in the older population including decreased cognitive and physical function, interference with family and social relationships, increased pain, poor self-rated health, and risk for falls.2,3
Sleep problems of older people are often related to other chronic health conditions. Multiple causes such as age-related changes, pain, anxiety, depression, and chronic diseases are associated with sleep disturbances in older adults.4,5 A 2003 report from the National Sleep Foundation survey revealed that sleep problems seem more often related to comorbidities rather than normal aging.6 In addition, growing evidence points to poor general health, cognitive impairment and mortality among the serious consequences of sleep difficulties in older adults.7
Sleep problems in persons with arthritis are thought to be related to pain symptoms.8 In a review of sleep problems related to osteoarthritis, researchers found that knee pain, less education, poorer self-rated health, and poorer physical functioning were associated with sleep disturbances in the older population. 9 Chronic pain from arthritis is common in this population: national estimates show that 59% of the population aged 65 and older report chronic pain from arthritis.10 In a study examining sleep disturbances and daily functioning in patients with chronic pain, greater sleep disturbance was associated with more disability and physical symptoms.11 An experimental study demonstrated that sleep deprivation had a hyperalgesic effect and recovery of slow wave sleep had an analgesic effect in health adults. 12 In addition, Dzierzewski and colleagues revealed day-to-day associations between sleep and pain in older adults with insomnia.13
Although studies have been performed examining the association between chronic pain and sleep problems in the general adult population, research is lacking on the relationship between chronic pain and sleep problems in older adults. Studies in older adults have primarily addressed selected pain sites such as knee pain or knee arthritis in relation to sleep difficulty.9 Sleep disturbances in relation to both pain severity and the problem of multisite pain in the older population have not been studied. The purpose of this population-based investigation from the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly (MOBILIZE) Boston Study is to better understand the relationship between sleep difficulty and domains of chronic pain in the community-living older population.
METHODS
From September 2005 to January 2008, study participants were recruited door-to-door based on a simple random sample from town and city lists identifying persons age 70 and older, living within a 5-mile radius of the study clinic at the Institute for Aging Research, Hebrew SeniorLife (HSL) in Boston. The recruitment area included most of Boston and parts of 5 nearby suburbs. Demographics of the town lists were comparable to the U.S. Census 2000 according to the age and sex distributions. The geographic area was chosen to minimize the transportation burden and costs, and to include a racially and socioeconomically diverse population. Details of the study recruitment and methods were published previously.14,15
Study eligibility criteria included age 70 years or older, ability to understand and communicate in English, plans to stay in the area for 2 years, and ability to walk 20 feet without personal assistance (walking aids permitted). Persons with advanced cancer and other terminal conditions were not enrolled in the study. In the initial recruitment, 5655 households were sampled, and 4319 people aged 70 years and older were confirmed to be living in the selected households. Of 3822 people who completed the initial eligibility screen at the door, 2382 were eligible. Reasons for ineligibility included non-English speaking (44%), living in a nursing home (18%), too ill to participate (15%), unable to walk twenty feet (8%), impaired cognition (8%), planning to leave the area (3%), and impaired hearing (3%). Of those who were eligible, 1616 (68% of eligible screenees) agreed to participate and were referred to the HSL study center. Finally, 765 persons, including 16 spouses aged 64 and older who joined the study, completed the two-part baseline enrollment which included a home interview and clinic visit. Signed informed consent was obtained during the home interview. Persons who scored less than 18 on the Mini-Mental State Exam, used as a screen for moderate to severe cognitive impairment, were excluded from participation.16,17 All procedures and methods were approved by the institutional review boards of the HSL and collaborating institutions.
Chronic Pain Assessment
The Brief Pain Inventory (BPI), which measures pain in general without reference to pain distribution, was originally developed for assessing pain in cancer patients and was later validated for use in patients with non-cancer pain.18,19,20 The 4-item BPI Pain Severity Subscale was used to measure overall pain: at its worst, at its least, on average in the past week and at present, using a 0–10 numeric rating scale, where 0 indicated no pain and 10 corresponded to “severe or excruciating pain as bad as you can imagine”. Pain distribution was measured by a 13-item joint pain questionnaire, adapted from Women’s Health and Aging Study (WHAS). Chronic musculoskeletal pain in the hands and wrists, shoulders, back, hips, knees, and feet was defined as pain lasting 3 or more months in the previous year and present in the past month.21 We classified chronic joint pain into four categories as follows: (1) widespread pain (pain in upper and lower extremities and back); (2) pain in two or more sites (not meeting criteria for widespread pain); (3) pain in a single site; (4) no pain.22 To evaluate site-specific pain in the 6 musculoskeletal sites, an additional set of 6 pain distribution measures was designated as: (1) pain in the selected joint and 1 or more other joints (2) pain only in the selected joint; (3) pain other than in the selected joint; (4) no pain.
Sleep Measurement
Three aspects of sleep difficulty were assessed using 3 items from the Center for Epidemiologic Studies Depression Scale (CESD): trouble getting to sleep, sleeping more than usual, and restless sleep.23 For example, the CESD question was phrased as follows: “Was your sleep restless?” Response options for each aspect of sleep were the following: not at all, less than 1 day over the past week, 1–2 days over the past week, 3–4 days over the past week, 5–7 days over the past week, or nearly every day for 2 weeks. For each of the 3 aspects of sleep, sleep difficulty was classified according to report of difficulty present one or more days in the past week. Although brief, the sleep items from the CESD have been used previously in population-based studies of sleep problems.24–26 We used an empirical approach in classifying a sleep problem as report of difficulty 1 or more days in the past week, based on the distribution of the responses in the population, allowing for adequate group sizes for the analyses.
Sociodemographic and Health Characteristics
Sociodemographic characteristics including age, sex, race, and education were obtained during the baseline home visit. Participants self reported physician diagnosed medical conditions including heart disease, lung diseases, and spinal stenosis/disc disease. Heart disease included reports of heart attack, angina, congestive heart failure, cardiac arrhythmia or having a pacemaker. Peripheral neuropathy was assessed using the Semmes-Weinstein monofilament testing, applying a modified method from the Health, Aging, and Body Composition Study.27,28 Peripheral arterial disease was defined according to a disease algorithm, based on ankle-arm index (AAI) < 0.90 and the Rose Intermittent Claudication Questionnaire.29,30 Diabetes was defined according to a disease algorithm based on self-reported diabetes, antidiabetic medication use, and laboratory measures from the baseline clinic visit including random glucose (>200mg/dl) and hemoglobin A1c (>7%). American College of Rheumatology (ACR) clinical criteria for osteoarthritis(OA) of the hand and knee were assessed in the clinic examination by experienced clinical nurses trained and certified by the study rheumatologist (R.H.S.).31,32 Body Mass Index (BMI), which is weight in kilograms divided by height in meters squared, was calculated from measured height and weight. Cognitive status was measured using the Mini-Mental State Examination.16 Anxiety was measured by the anxiety subscale of the well validated Hospital Anxiety and Depression Scale (HADS).33 Depression was measured by using a modification of the 20-item CESD scale.34 Based on the CESD score excluding the 3 sleep items, we classified participants as having minor or major depression using a diagnostic algorithm applying DSM-IV criteria.23, 27 The Physical Activity Scale for the Elderly (PASE) is a summary measure of activities performed in the previous 7 days.35 Chronic disease management self-efficacy was measured using the validated Lorig self-efficacy scale.36
Medication
During the home visit, the interviewer examined all containers of prescription and over-the-counter medications used in the previous 2 weeks, and recorded the names and amount of use. Analgesic medications included opioid and nonopioid analgesics. Psychotherapeutic medications included sedatives, hypnotic, anxiolytic, antidepressant, and antipsychotic agents. Use of analgesic and psychotherapeutic medications was dichotomized as daily use versus no daily use.
Analytical Procedures
All data used in the study were analyzed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). The prevalence of baseline characteristics according to BPI pain severity quartiles is presented using descriptive statistics and χ2 tests for trend (1df) for categorical variables and analysis of variance for continuous variables. To determine the relation between chronic pain and the three sleep problems, we performed a series of multivariate logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI) for occurrence of sleep difficulties related to the two domains of chronic pain (severity and distribution). In the first model, we adjusted for demographic characteristics (age, sex, race, and education). In the second model, we added chronic medical conditions including heart disease, lung disease, diabetes, peripheral artery disease, neuropathy, cognitive function, and self-efficacy for chronic disease management. BMI, physical activity, anxiety, and daily analgesic and psychotherapeutic medication use plus all variables from the second model were included in the third model. In our final model, we added depression based on the CESD without the sleep items. We used the same approach to examine the association between chronic pain in selected joints and the occurrence of sleep difficulties.
RESULTS
Older adults with more severe pain were more likely to be female, obese, less physically active, and have depression, anxiety, less education, lower income, poorer cognitive function, and less confidence in managing chronic pain (Table 1). Pain severity was not associated with age. Medical conditions associated with pain severity included hand and knee osteoarthritis, spinal stenosis/disc disease, diabetes, lung disease, peripheral artery disease, and neuropathy.
Table 1.
Baseline characteristics according to the Brief Pain Inventory Severity Scale(Quartiles)*
| Characteristic | BPI 1st Quart (n=185) | BPI 2nd Quart (n=188) | BPI 3rd Quart (n=200) | BPI 4th Quart (n=189) | p-value (trend)† | |
|---|---|---|---|---|---|---|
| Percent | ||||||
| Age | 70–74 | 28.7 | 27.7 | 30.0 | 28.0 | |
| 75–79 | 24.9 | 37.8 | 33.0 | 31.8 | ||
| 80–84 | 29.2 | 20.7 | 20.5 | 23.8 | .57 | |
| >85 | 16.2 | 10.6 | 14.0 | 14.8 | ||
| Women | 52.4 | 58.0 | 67.0 | 77.8 | <.001 | |
| Race | White | 78.9 | 86.2 | 80.5 | 64.4 | |
| Black | 15.7 | 6.9 | 14.0 | 28.2 | ||
| Other | 5.4 | 6.9 | 5.5 | 7.5 | .004 | |
| Education | < High school | 9.2 | 3.7 | 9.5 | 21.8 | |
| High school graduate | 21.1 | 15.4 | 23.5 | 33.5 | ||
| College graduate | 69.7 | 80.9 | 67.0 | 44.7 | <.001 | |
| Body Mass Index | < 25 | 36.5 | 30.3 | 29.2 | 23.0 | |
| 25–29.99 | 40.9 | 48.7 | 40.5 | 41.5 | ||
| ≥ 30 | 22.7 | 21.1 | 30.3 | 35.5 | <.001 | |
| Income | < 15,000 | 16.8 | 13.3 | 20.5 | 38.1 | |
| ≥15,000 | 83.2 | 86.7 | 79.5 | 61.9 | <.001 | |
| Depression (Minor &Major) | 1.1 | 0.5 | 2.0 | 8.5 | <.001 | |
| Anxiety (HADS)¶ | 2.7 | 10.7 | 11.2 | 16.8 | <.001 | |
| Physical activity | Least active tertile | 33.5 | 23.0 | 37.8 | 39.0 | |
| (PASE) | Middle active tertile | 29.2 | 35.3 | 32.1 | 36.9 | |
| Most active tertile | 37.3 | 41.7 | 30.1 | 24.1 | .002 | |
| MMSE score | <24 | 9.7 | 7.5 | 12.5 | 18.5 | .003 |
| Daily analgesic use | 8.7 | 11.2 | 29.0 | 49.2 | <.001 | |
| Daily psychoactive medications use | 10.8 | 11.2 | 18.5 | 20.1 | .003 | |
| Chronic disease management self-efficacy (mean (± SD)) | 9.2 (1.3) | 9.2 (1.0) | 8.9 (1.2) | 8.8 (1.3) | <.001 | |
| Osteoarthritis: | None | 86.5 | 67.6 | 55.0 | 44.7 | |
| Knee OA only | 6.0 | 21.3 | 18.5 | 24.5 | ||
| Hand OA only | 6.0 | 8.0 | 17.0 | 14.9 | ||
| Hand and knee OA | 1.6 | 3.2 | 9.5 | 16.0 | <.001 | |
| Spinal stenosis/disc disease | 12.4 | 15.4 | 17.0 | 28.6 | <.001 | |
| Heart disease | 41.6 | 34.0 | 48.5 | 42.3 | .29 | |
| Diabetes | 16.8 | 16.0 | 19.0 | 28.6 | .003 | |
| Lung Disease | 14.1 | 8.5 | 16.0 | 25.4 | .002 | |
| Peripheral artery disease | 3.2 | 3.7 | 12.0 | 19.1 | <.001 | |
| Neuropathy | 9.8 | 9.6 | 12.8 | 17.0 | .023 | |
Three persons of the original 765 were missing Brief Pain Inventory (BPI) Severity Subscalepain information. Percentages may not sum to 100 due to rounding.
Mantel-Haenszel χ 2 test for trend (1d.f.) for categorical variables and analysis of variance for continuous variables.
Abbreviations: HADS= Hospital Anxiety Depression Scale, PASE = Physical Activity Scale for the Elderly, BMI = body mass index, MMSE = Mini-Mental State Examination, S.D. = standard deviation, OA= osteoarthritis
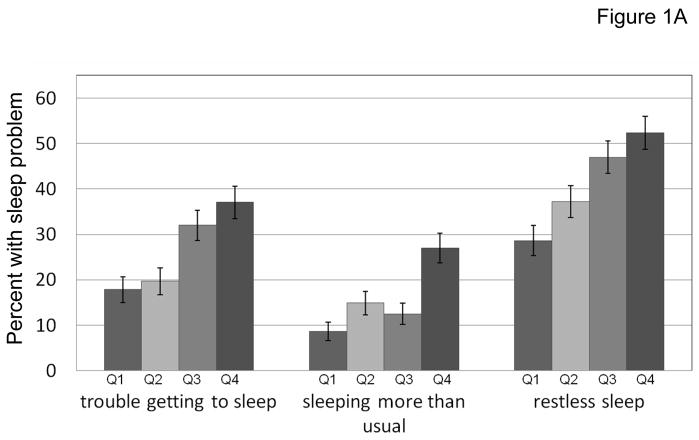
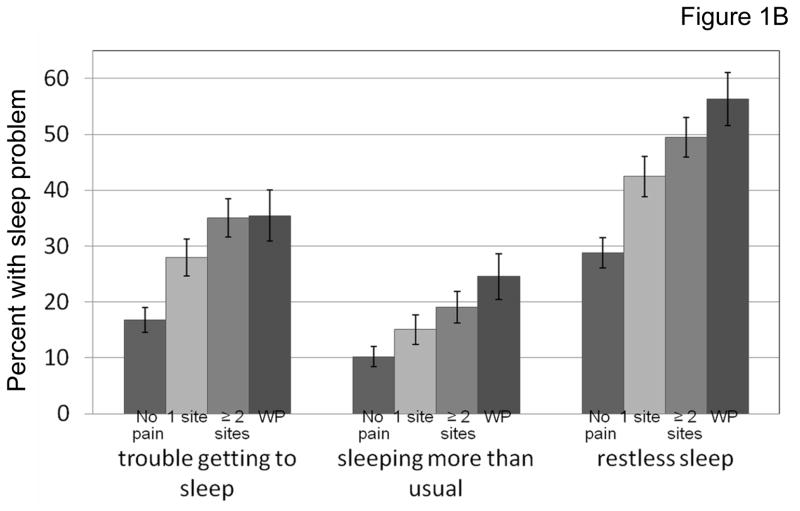
Approximately one-quarter of participants reported having trouble getting to sleep one or more days in the previous week. Reports of restless sleep were common (41%) and sleeping more than usual was somewhat less common (16%) in this older population. The prevalence of trouble getting to sleep increased with more severe pain (17.8%, 18.7%, 32% and 37%, respectively, according to BPI severity scale quartiles). Similar significant trends were observed for sleeping more than usual and restless sleeping (Figure 1A). For pain categorized according to pain distribution (no pain, single site pain, multisite pain, and widespread pain), there were strong trends for higher prevalence of sleep difficulties with more disseminated pain for each of the sleep measures (Figure 1B).
Figure 1.
Figure 1A. Pain severity was grouped into quartiles, indicated by Q1 to Q4, from least pain to most severe pain. The trend was statistically significant for associations between pain severity and each sleep problem using Mantel-Haenszel χ21 test, p <0.0001.
The error bars indicate the standard error of the proportion.
Figure 1B. Pain distribution was grouped as follows: no pain, pain in 1 site, pain in 2 or more sites, and widespread pain (WP). The trend was statistically significant for the association between pain measures and each sleep problem using Mantel-Haenszel χ21 test, p <0.0001.
The error bars indicate the standard error of the proportion.
After adjusting for sociodemographic characteristics, chronic conditions, health behaviors, body mass index, and anxiety, persons with the most severe pain had more than a two-fold likelihood of having trouble getting to sleep one or more days per week compared to those with the lowest pain severity scores (OR=2.33, 95% C.I. 1.35, 4.01). Similar strong associations were observed according to pain distribution and with other measures of sleep difficulty, restless sleep and sleeping more than usual. Additional adjustment for use of daily analgesic and psychotherapeutic medications, and depression score modestly attenuated the risk estimates, but all associations remained significant except the relation between pain severity and trouble getting to sleep (Table 2).
Table 2.
Odds Ratios for sleep difficulties according to baseline pain measures, MOBILIZE Boston Study, 2005–2008.
| Pain categories | N | Model 1* Adjusted for sociodemographic characteristics |
Model 2† (+chronic conditions, disease management self-efficacy & cognitive status) |
Model 3‡ (+BMI, physical activity, anxiety, daily analgesic/psychoactive drug use,) |
Model 4§ (+ depression without sleep item) |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | ||
|
Trouble getting to sleep (≥ 1 day a week)
| |||||||||
| Chronic Musculoskeletal Pain | |||||||||
| None | 273 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Single site | 186 | 1.94 | 1.23, 3.06 | 1.84 | 1.16, 2.93 | 1.77 | 1.10, 2.87 | 1.70 | 1.04, 2.77 |
| Multisite | 194 | 2.54 | 1.64, 3.94 | 2.32 | 1.47, 3.67 | 2.38 | 1.48, 3.83 | 2.06 | 1.26, 3.36 |
| Widespread Pain | 110 | 2.53 | 1.51, 4.23 | 2.14 | 1.23, 3.73 | 2.55 | 1.43, 4.54 | 1.91 | 1.05, 3.49 |
|
| |||||||||
| BPI Pain Severity | |||||||||
| 1st quartile | 185 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd quartile | 188 | 1.09 | 0.64, 1.85 | 1.04 | 0.61, 1.77 | 1.01 | 0.58,1.75 | 1.02 | 0.58,1.78 |
| 3rd quartile | 200 | 2.04 | 1.26, 3.32 | 1.76 | 1.06, 2.90 | 1.92 | 1.14, 3.22 | 1.66 | 0.98, 2.82 |
| 4th quartile(greatest severity) | 189 | 2.48 | 1.51, 4.08 | 2.21 | 1.32, 3.73 | 2.33 | 1.35, 4.01 | 1.61 | 0.90, 2.88 |
|
| |||||||||
|
Sleep much more than usual (≥ 1 day a week)
| |||||||||
| Chronic Musculoskeletal Pain | |||||||||
| None | 273 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Single site | 186 | 1.56 | 0.88, 2.77 | 1.56 | 0.86, 2.83 | 1.58 | 0.86, 2.90 | 1.54 | 0.83, 2.84 |
| Multisite | 194 | 2.22 | 1.29, 3.83 | 2.20 | 1.22, 3.95 | 2.17 | 1.19, 3.94 | 1.96 | 1.07, 3.62 |
| Widespread Pain | 110 | 2.86 | 1.56, 5.25 | 2.81 | 1.45, 5.46 | 2.72 | 1.37, 5.40 | 2.16 | 1.06, 4.39 |
|
| |||||||||
| BPI Pain Severity | |||||||||
| 1st quartile | 185 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd quartile | 188 | 1.92 | 0.99, 3.73 | 1.73 | 0.88, 3.40 | 1.74 | 0.86, 3.49 | 1.75 | 0.87, 3.52 |
| 3rd quartile | 200 | 1.60 | 0.82, 3.13 | 1.21 | 0.59, 2.45 | 1.33 | 0.64, 2.75 | 1.21 | 0.58, 2.53 |
| 4th quartile(greatest severity) | 189 | 3.94 | 2.10, 7.44 | 3.67 | 1.88, 7.16 | 4.01 | 2.00, 8.07 | 3.22 | 1.54, 6.74 |
|
| |||||||||
|
Sleep restless (≥ 1 day a week)
| |||||||||
| Chronic Musculoskeletal Pain | |||||||||
| None | 271 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Single site | 185 | 1.88 | 1.26,2.79 | 1.75 | 1.17, 2.61 | 1.76 | 1.16, 2.66 | 1.71 | 1.12, 2.60 |
| Multisite | 193 | 2.45 | 1.66, 3.62 | 2.05 | 1.36, 3.10 | 1.99 | 1.31, 3.04 | 1.85 | 1.20, 2.85 |
| Widespread Pain | 110 | 3.26 | 2.04, 5.22 | 2.43 | 1.46, 4.04 | 2.58 | 1.51, 4.39 | 2.27 | 1.31, 3.93 |
|
| |||||||||
| BPI Pain Severity | |||||||||
| 1st quartile | 185 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 2nd quartile | 188 | 1.53 | 0.98, 2.38 | 1.48 | 0.95, 2.33 | 1.45 | 0.92, 2.30 | 1.46 | 0.92, 2.31 |
| 3rd quartile | 200 | 2.29 | 1.49, 3.52 | 1.97 | 1.26, 3.07 | 2.06 | 1.30, 3.25 | 1.91 | 1.20, 3.04 |
| 4th quartile (greatest severity) | 189 | 2.84 | 1.81, 4.45 | 2.18 | 1.36, 3.49 | 2.15 | 1.32, 3.51 | 1.79 | 1.06, 3.01 |
Odds ratios (OR) and 95% confidence intervals (CI) from logistic regression models predicting sleep problems; Model 1 covariates included age, sex, race, education.
Model 2 included all variables from model 1 and heart disease, lung disease, diabetes, peripheral arterial disease (PAD), neuropathy, Mini-Mental State Examination score (MMSE), chronic disease management self-efficacy
Model3 included all variables from model 2 and body mass index (BMI), Physical Activity Scale for the Elderly (PASE), Anxiety subscale of the Hospital Anxiety and Depression Scale (HADS), and daily use of analgesic medications, daily use of psychotherapeutic medications.
Model4 included all variables from model 3 and depression.
When sleep difficulty measures were analyzed according to pain in individual musculoskeletal sites alone or in combination with other pain sites, we found modest and somewhat inconsistent associations (Table 3). Pain in the upper body sites, the hands and shoulders, were the only single sites independently associated with some sleep difficulties. However, similar to the findings from Table 2, pain in 2 or more sites was independently associated with a 16% to 41% increase in likelihood for having sleep difficulties.
Table 3.
Odds ratios for the prevalence of sleep difficulties according to selected pain sites*.
| Pain categories | Trouble sleeping† | Sleeping more‡ | Restless sleep§ | ||||
|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Back and other joint pain | |||||||
| None | 272 | 1.00 | 1.00 | 1.00 | |||
| Pain other than back | 290 | 1.17 | 1.03,1.33 | 1.18 | 1.03,1.35 | 1.21 | 1.08,1.36 |
| Back only | 23 | 1.12 | 0.81,1.53 | 0.94 | 0.65,1.36 | 1.13 | 0.85,1.51 |
| Back and other pain | 177 | 1.13 | 0.98,1.32 | 1.21 | 1.03,1.41 | 1.29 | 1.13,1.48 |
|
| |||||||
| No. in model N=762
| |||||||
| Hip and other joint pain | |||||||
| None | 273 | 1.00 | 1.00 | 1.00 | |||
| Pain other than hip | 332 | 1.18 | 1.04,1.33 | 1.18 | 1.03,1.34 | 1.22 | 1.09,1.37 |
| Hip only | 21 | 0.65 | 0.40,1.07 | 1.28 | 0.88,1.85 | 0.85 | 0.57,1.25 |
| Hip and other pain | 137 | 1.17 | 0.99,1.38 | 1.16 | 0.97,1.39 | 1.33 | 1.14,1.54 |
|
| |||||||
| No. in model N=763
| |||||||
| Knee and other joint pain | |||||||
| None | 273 | 1.00 | 1.00 | 1.00 | |||
| Pain other than knee | 259 | 1.22 | 1.07,1.39 | 1.20 | 1.05,1.38 | 1.26 | 1.12,1.42 |
| Knee only | 53 | 0.98 | 0.78,1.24 | 1.00 | 0.78,1.27 | 1.00 | 0.81,1.23 |
| Knee and other pain | 176 | 1.11 | 0.95,1.29 | 1.21 | 1.03,1.41 | 1.24 | 1.08,1.42 |
|
| |||||||
| No. in model N=761
| |||||||
| Hands and other joint pain | |||||||
| None | 272 | 1.00 | 1.00 | 1.00 | |||
| Pain other than hands | 299 | 1.11 | 0.97,1.26 | 1.15 | 1.00,1.31 | 1.22 | 1.09,1.37 |
| Hands only | 32 | 1.51 | 1.19,1.94 | 1.12 | 0.83,1.51 | 1.29 | 1.02,1.64 |
| Hands and other pain | 159 | 1.18 | 1.01,1.37 | 1.27 | 1.08,1.48 | 1.23 | 1.07,1.41 |
|
| |||||||
| No. in model N=762
| |||||||
| Shoulder and other joint pain | |||||||
| None | 273 | 1.00 | 1.00 | 1.00 | |||
| Pain other than shoulder | 332 | 1.11 | 0.98,1.25 | 1.16 | 1.02,1.32 | 1.16 | 1.03,1.29 |
| Shoulder only | 21 | 1.11 | 0.80,1.56 | 1.52 | 1.13,2.06 | 1.48 | 1.13,1.95 |
| Shoulder and other pain | 137 | 1.31 | 1.12,1.54 | 1.16 | 0.97,1.37 | 1.41 | 1.22,1.62 |
|
| |||||||
| No. in model N=763
| |||||||
| Feet and other joint pain | |||||||
| None | 274 | 1.00 | 1.00 | 1.00 | |||
| Pain other than feet | 302 | 1.13 | 1.00,1.28 | 1.14 | 1.00,1.30 | 1.22 | 1.09,1.37 |
| Feet only | 32 | 1.26 | 0.98,1.64 | 1.16 | 0.87,1.55 | 1.19 | 0.94,1.51 |
| Feet and other pain | 154 | 1.19 | 1.02,1.39 | 1.28 | 1.08,1.50 | 1.25 | 1.09,1.44 |
|
| |||||||
| No. in model N=762 | |||||||
Odds ratio (OR) and 95% confidence interval (CI) from logistic regression models predicting sleep problems; model covariates include age, sex, race, education, heart disease, lung disease, peripheral artery disease (PAD), diabetes, neuropathy, Mini-Mental State Examination score (MMSE), chronic disease management self-efficacy, body mass index (BMI), physical activity (PASE), anxiety, daily use of analgesic medications and daily use of psychotherapeutic medications, and depression without sleep items.
Trouble getting to sleep.
Sleeping much more than usual.
Sleep restless.
DISCUSSION
Similar to previous reports, we found that chronic pain and sleep difficulties were common in the older population living in the community. We observed strong and consistent associations between more severe and disseminated chronic pain and heterogeneous sleep complaints. Adjusting for potential underlying pathology and comorbidity as well as health practices did not materially alter the association. Our findings also demonstrated that joint pain was associated with sleep difficulties primarily when multiple pain sites were involved.
A number of mechanisms have been identified as potential causes of sleep disturbances in elderly people. Smith and Haythornthwaite discussed the inter-relationship between chronic pain and sleep disturbance and reported that certain parts of brain (specifically, the thalamus and mesencephalic periaqueductal gray matter) were associated with both pain and sleep/arousal.37 Dysfunction of the HPA axis has been found to be associated with an increased risk of developing chronic widespread pain.38 Wolkove and colleagues reviewed several studies and found that aging was associated with sleep pattern changes such as reduced sleep time, delayed onset of sleep, earlier morning awakening, more fragmented sleep, and increased daytime napping.4 Although changes in sleep patterns are not necessarily considered as sleep disorders, older adults have a higher prevalence of sleep disturbances. Alternatively, others have concluded that sleep difficulties were not related to aging itself but instead were related to medical and psychiatric disorders and related health burdens.6,39 We did not find that the prevalence of sleep disturbances varied according to age in our population of adults aged 70 and older (data not shown). Others have found that sleep disturbances were associated with some medical disorders related to pain symptoms such as arthritis, low back pain, headache, and fibromyalgia.8,40,41 In our study, severity and the distribution of pain were both strongly associated with sleep disturbances. However, we did not find consistent associations between individual sites of pain and sleep difficulties, and only shoulder and hand pain independently are associated with some sleep disturbances. It is possible that upper limb pain may be more noticeable than lower body pain during sleep particularly if people sleep on their sides.
Our results point to the complexity of pain problems and their consequences in older adults and the importance of assessing characteristics of pain, specifically pain severity and distribution. We found that diffuse distribution of pain is a key factor in the association between pain and sleep. These findings are consistent with other reports of functional consequences of widespread or multisite pain in older adults, including links between chronic musculoskeletal pain and the occurrence of falls in the MOBILIZE Boston population42 and between widespread musculoskeletal pain and the progression of disability in older disabled women.43 The traditional view of pain as a site-specific condition or one related to a single pathology may not be the most informative approach in assessing pain that could contribute to sleep problems or other functional consequences in older persons. Current pain assessment guidelines 44 emphasize pain intensity and pain interference with function, important domains; however, the common problem of disseminated or widespread pain is not addressed. In fact, assessment of pain distribution is only minimally addressed in the current guidelines.
In a national study of Canadian adults, Power and colleagues demonstrated that arthritis-related sleep disturbances were largely due to pain severity.8 In that study, arthritis was assessed by self report and was included in the same analytic models with pain. In contrast, we assessed arthritis using the ACR clinical criteria which include pain in the definition. Therefore, we did not adjust for presence of arthritis in our multivariate regression analysis as it would represent an overadjustment for pain. Pain severity was associated with presence of knee and/or hand arthritis in our study, but nearly half of participants who had the most severe pain did not have clinically assessed hand or knee arthritis. This suggests that there are other pain conditions that contribute to the high prevalence of sleep problems in older adults.
The relationship between sleep disturbances and pain may be more complex when associated with depression and anxiety. Nicassio and Wallston evaluated the relationship between pain, sleep problems and depression in patients with rheumatoid arthritis over a 2-year interval.26 Their longitudinal analysis revealed that pain predicted subsequent sleep problems but the reverse was not true, sleep problems did not predict subsequent pain. In addition, sleep problems contributed to depression but only among persons who reported high levels of pain. In our study, when we adjusted for anxiety and depression, it did not substantially alter the relationship between pain and sleep difficulty.
Our study showed that after adjustment for use of psychotherapeutic medications and daily analgesics, the association between pain and sleep difficulties decreased modestly. Use of these medications may be an indicator of how burdensome sleep difficulties are for older people who have pain. Because use of psychotherapeutic and analgesic medications may be on the causal pathway from pain to sleep problems including these measures in our analyses may represent an over-adjustment for pain severity. Nonetheless, we found that pain severity and pain distribution continued to be strongly and independently associated with sleep difficulty even after adjusting for psychotherapeutic and analgesic medications.
Smith and Haythornthwaite indicated that the analgesics and sedative hypnotic medications prescribed for chronic pain and promoting sleep have both analgesic and sedating effects, so it is difficult to ascertain whether reducing sleep disturbances contributed to the reduction of pain interference on sleep or the soporific effects of the medications.37 A recent pilot study suggested that better management of sleep can reduce pain in older adults with arthritis.45 In contrast, a study on the use of a synthetic cannabinoid improved sleep in fibromyalgia patients, but had no effect on pain.46 Research is needed to determine whether better management of more severe or disseminated pain conditions in older adults also leads to improved sleep.
One of the limitations of our study is that it is a cross-sectional analysis, thus portraying the concurrent relationship between pain and sleep disturbance. Longitudinal studies are needed to examine the persistence of these concurrent problems and to further elucidate the temporal aspects of these two chronic conditions. The advantages of our study over previous reports is that we examined domains of both pain severity and distribution, and we controlled for multiple potential confounders of the pain and sleep relationship. An important strength of our study is that our findings are generalizable to the urban and suburban population of older adults living in the community.
In summary, sleep difficulties are common among older individuals and are significantly associated with more severe and disseminated pain. Both sleep and pain are treatable with currently available pharmacologic and non-pharmacologic approaches although use of the former may be limited by side-effects. Our findings suggest that chronic pain characteristics such as presence of multisite or widespread pain may be an important factor contributing to sleep difficulty and may need to be added to pain assessments in older patients. More research is needed to develop effective interventions for these prevalent and co-occurring disabling conditions including a better understanding of the barriers to implementation of effective pain and sleep disorder management in older patients.
Acknowledgments
The authors acknowledge and thank the MOBILIZE Boston research team and study participants for the contribution of their time, effort, and dedication. This work was funded by the National Institute on Aging, Research Nursing Home Program Project Grant, # P01AG004390. Ms. Chen was supported by a graduate assistantship from the College of Nursing and Health Sciences, University of Massachusetts Boston.
Footnotes
None of the authors have financial or any other kind of conflicts with this paper.
References
- 1.Vitiello MV. Sleep disorders and aging: Understanding the causes. J Gerontol A Biol Sci Med Sci. 1997;52(4):M189–M191. doi: 10.1093/gerona/52a.4.m189. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 3.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2002;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolkove N, Elkholy O, Baltzan M, et al. Sleep and aging: Sleep disorders commonly found in older people. CMAJ. 2007;176(9):1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(2):S366–S372. [PubMed] [Google Scholar]
- 6.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10:S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53(6):911–919. doi: 10.1002/art.21584. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox S, Brenes GA, Levine D, et al. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Prevalence of self-reported arthritis or chronic joint symptoms among adults—United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(42):948–950. [PubMed] [Google Scholar]
- 11.McCracken LM. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 12.Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective interruption and sleep recovery of pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 13.Dzierzewski JM, Williams JM, Roditi D, et al. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: Evidence of covariation over time. Journal of the American Geriatrics Society. 2010;58:925–930. doi: 10.1111/j.1532-5415.2010.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;18(8):16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samelson EJ, Kelsey JL, Kiel DP, et al. Issues in conducting epidemiologic research among elders: lessons from the MOBILIZE Boston Study. Am J Epidemiol. 2008;168(12):1444–1451. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Escobar JI, Burnam A, Karno M, et al. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity. Cultural and linguistic artifacts. J Nerv Ment Dis. 1986;174:607–14. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in Pain Research and Therapy, Volume 12: Issues in Pain Measurement. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 19.Keller S, Bann CM, Dodd SL, et al. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tan G, Jensen MP, Thornby JI, et al. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Maryland: National Institute on Aging; 1995. [Google Scholar]
- 22.Eggermont LH, Bean JF, Guralnik JM, et al. Comparing pain severity versus pain location in the MOBILIZE Boston Study: chronic pain and lower extremity function. J Gerontol A Biol Sci Med Sci. 2009;64(7):763–770. doi: 10.1093/gerona/glp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. App Psych Meas. 1977;1:385–401. [Google Scholar]
- 24.Hartz AJ, Daly JM, Kohatsu ND, et al. Risk factors for insomnia in a rural population. Ann Epidemiol. 2007;17:940–947. doi: 10.1016/j.annepidem.2007.07.097. [DOI] [PubMed] [Google Scholar]
- 25.Kutner NG, Bliwise DL, Zhang R. Linking race and well-being within a biopsychosocial framework: variation in subjective sleep quality in two racially diverse older adult samples. J Health Soc Behav. 2004;45:99–113. doi: 10.1177/002214650404500107. [DOI] [PubMed] [Google Scholar]
- 26.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101(3):514–520. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 27.Perkins BA, Olaleye D, Zinman B, et al. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes care. 2001;24(2):250–256. doi: 10.2337/diacare.24.2.250. [DOI] [PubMed] [Google Scholar]
- 28.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2006;21(11):1803–1810. doi: 10.1359/jbmr.060725. [DOI] [PubMed] [Google Scholar]
- 29.Rose G. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull WHO. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 30.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54(3):294–300. doi: 10.1016/s0895-4356(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 31.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 32.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Eaton WW, Muntaner C, Smith C, et al. Center for Epidemiologic Studies Depression Scales: Review and Revision (CESD and CESD-R) In: Maruish ME, Mahwah NJ, editors. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3. Vol. 3. New Jersey: Lawrence Erlbaum Assoc Inc; 2004. pp. 363–377. [Google Scholar]
- 35.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 36.Lorig K, Chastain RL, Ung E, et al. Development and evaluation of a scale to measure perceived self- efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 37.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 38.McBeth J, Silman AJ, Gupta A, et al. Moderation of psychosocial risk factors through dysfunction of the hypothalamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56 (1):360–371. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]
- 39.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53(1):555–559. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 40.Menefee LA, Cohen MJ, Anderson WR, et al. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1(2):156–172. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 41.Lamberg L. Chronic pain linked with poor sleep; exploration of causes and treatment. JAMA. 1999;281(8):691–692. [PubMed] [Google Scholar]
- 42.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135(12):1038–46. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 44.American Geriatrics Society (AGS) Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(6):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 45.Vitiello MV, Rybarczyk B, Von Korff M, et al. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 46.Ware MA, Fitzcharles MA, Joseph L, et al. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):604–610. doi: 10.1213/ANE.0b013e3181c76f70. [DOI] [PubMed] [Google Scholar]