Abstract
Purpose
To provide cross-sectional normative data for [-2]pro-prostate-specific antigen ([-2]proPSA) from the Olmsted County Study of Urinary Symptoms and Health Status among Men (OCS) and the Flint Men’s Health Study (FMHS) and to describe the associations with clinical urologic measures and risk of prostate cancer diagnosis.
Materials and Methods
Measurements of [-2]proPSA were obtained from n=420 white men from Olmsted County, Minnesota and n=328 black men from Genesee County, Michigan. Cross-sectional associations between [-2]proPSA and prostate enlargement / elevated PSA level were assessed. Cox proportional hazard models were used to assess associations between [-2]proPSA and incident diagnosis of prostate cancer.
Results
Baseline [-2]proPSA level was slightly higher among black men (median (25th, 75th percentiles) =6.3 (4.1, 8.9) pg/mL) than among white men (median=5.6 (3.9, 7.7) pg/mL), p=0.01. Baseline [-2]proPSA level was highly predictive of biopsy-confirmed prostate cancer (CaP) in the OCS cohort. Relative to men in the lower quartile of [-2]proPSA, men in the upper quartile had almost an 8-fold increase in the risk of CaP (hazard ratio (HR): 7.8, 95% confidence interval (CI): 2.2, 27.8) after adjustment for age and baseline PSA.
Conclusions
Levels of [-2]proPSA in these cohorts of community-dwelling black and white men are much lower than seen in previous studies. These data suggest that levels of [-2]proPSA may help identify prostate cancer in men with serum PSA levels in an indeterminate range, although the reference ranges for white and black men may differ slightly.
Keywords: prostate enlargement, prostate cancer, biomarkers, proPSA
INTRODUCTION
Prostate cancer is the most common non-cutaneous cancer in U.S. men and it is estimated that there will be 217,730 new cases and 32,050 deaths from prostate cancer in the United States in 2010.1 Prostate-specific antigen (PSA) is widely used as a serum marker for prostate cancer screening. However, the diagnostic value of PSA testing is limited by its lack of specificity,2, 3 particularly in the range of 2–10 ng/mL where elevated PSA levels are often due to benign disease.
Precursor forms of PSA, such as [-2]pro-prostate-specific antigen ([-2]proPSA) have been identified as promising new biomarkers for distinguishing men with prostate cancer.4, 5 Preliminary studies suggest that [-2]proPSA may enhance the sensitivity and specificity for distinguishing benign disease from prostate cancer.6 This may, in turn, result in fewer biopsies, particularly in men who have PSA levels in the 4–10 ng/mL range, where specificity may be lower. Moreover, this biomarker was reported to distinguish men with less aggressive tumors from men with more aggressive tumors7–10 as well as to predict prostate cancers requiring treatment11. In the 2–10 ng/mL range of PSA level, [-2]proPSA had the highest specificity to detect prostate cancer6, particularly when the ratio of [-2]proPSA to free PSA level (%[-2]proPSA) was considered5, 9, 12. In a prospective screening study, Le and colleagues reported that %[-2]proPSA provided better discrimination to detect biopsy-confirmed prostate cancer on biopsy than PSA level or % free PSA.12 On immunostaining, [-2]proPSA has shown the most cancer specificity.7
These initial studies of [-2]proPSA have, however, been limited to select patient groups including clinical series of patients, men selected for biopsy or radical prostatectomy, or prostate cancer screening studies, which do not reflect the full range of [-2]proPSA levels.4, 7–9, 12 The [-2]proPSA levels in these select samples may not reflect the levels in the general community.13, 14 In order to gain a broader understanding of the utility of [-2]proPSA, we utilized data from the Olmsted County Study of Urinary Symptoms and Health Status among Men (OCS) and the Flint Men’s Health Study (FMHS) to describe baseline levels of [-2]proPSA and cross-sectional associations between [-2]proPSA and urologic measures in two population-based samples of black and white men. In addition, we examined associations between baseline values of [-2]proPSA and risk of biopsy-confirmed prostate cancer (CaP).
METHODS
Subjects
Many of the details regarding the construction of both the OCS 15 and FMHS 16 studies have been described previously.
Briefly, the OCS is a cohort of white men 40 to 79 years old who were randomly selected from an enumeration of the 1990 Olmsted County, Minnesota population. Men who had a history of prostate cancer, prostate surgery or other conditions known to interfere with voiding were excluded. After excluding men with these pre-existing conditions, 3,874 men were asked to join the study, and 2,115 (55%) agreed to participate and completed a previously validated questionnaire. A 25% random sub-sample 476/537 (89%) participated in a full urologic exam which included a transrectal ultrasound to measure prostate volume and a blood draw to measure serum PSA level.15, 17 The cohort has been followed biennially since 1990. Men who died or were lost to follow-up were replaced during rounds 2 and 3. The OCS study received approval from the Mayo Clinic and Olmsted Medical Center Institutional Review Boards (IRB).
The FMHS cohort consists of a probability sample of black men from households located in Genesee County, Michigan in 1996. 16, 18 Subjects were ineligible if they reported a history of prostate cancer or a prior operation on the prostate gland. A trained interviewer contacted each sample household, identified 730 eligible subjects, and performed a detailed in-home interview which included completion of the American Urological Association Symptom Index (AUASI). All participants were invited to participate in a comprehensive urologic examination, similar to that received by men participating in the OCS study. Of the 730 men, 379 (52%) completed the exam phase of the study. The FMHS study received approval from the University of Michigan Medical School IRB.
The FMHS samples and baseline information were collected in 1996. To make the two data sources comparable, the corresponding 1996 OCS measurements and blood samples were used for the initial/baseline values. Levels of [-2]proPSA were available for 420 OCS men and 328 FMHS men.
Prostate volume
During the in-clinic exam, total prostate volume was measured via transrectal ultrasound for the OCS19 and FMHS16 participants. Anteroposterior and transverse diameters were measured at the maximal dimensions, and the superior-inferior diameter was measured at the maximal length from the base to the apex of the midline sagittal plane and combined to calculate the total volume of the prostate assuming a prolate ellipsoid shape.20
Prostate cancer
Community medical records were reviewed for all men in the OCS cohort and dates of biopsy-confirmed prostate cancer were recorded. As community medical records were not available for the FMHS men, analyses examining associations between [-2]proPSA and future prostate cancer diagnosis were limited to the OCS cohort.
Assays
Stored blood samples were used to measure [-2]proPSA levels using serum samples drawn in 1996 for both the OCS (n=420) and FMHS (n=328) cohorts. Serum samples were obtained prior to any prostatic manipulations and were frozen at −70°C in separate aliquots. A central lab performed the [-2]proPSA assay on previously unthawed samples for both study sites. The Tandem-E PSA assay (Hybritech, Incorporated, San Diego, CA) and Abbott AxSYM polyclonal-monoclonal immunoassay (Abbott Diagnostics, Abbott Park, IL) were used to measure serum PSA levels for men in the OCS and FMHS cohorts, respectively. In earlier reports, we showed that PSA determinations were consistent across assays and laboratories, with slightly, but non-significantly higher results for the Tandem-E PSA assay at the levels measured in these cohorts.21, 22 In this study, [-2]proPSA levels were assessed in the same laboratory using an automated, sequential, two-step immunoenzymatic (“sandwich”) assay developed for use on the Beckman Coulter (Brea, CA) Access® instrument using investigational-use-only two-site immunoenzymatic reagents provided by Beckman Coulter, Inc. In this laboratory, the intra-assay variation in [-2]proPSA levels ranged from 2.0% to 3.2% while the inter-assay variation ranged from 3.0% to 9.9%.
Statistical analysis
Descriptive statistics were used to describe the distributions of [-2]proPSA and urologic measures. The cumulative distribution function by age decade was plotted for each race. Cross-sectional associations of [-2]proPSA with prostatic enlargement (prostate volume >30 cc) and elevated PSA (PSA level >2.5 ng/mL) were assessed using logistic regression models with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). Multivariable models were used to adjust for age and PSA. Longitudinal follow-up data, available only in the OCS cohort, was used to assess the association between baseline [-2]proPSA level and risk of a subsequent diagnosis of biopsy-confirmed CaP using Cox proportional hazards regression. Hazard ratios (HRs) and 95% CIs were used to describe the associations between these measures.
RESULTS
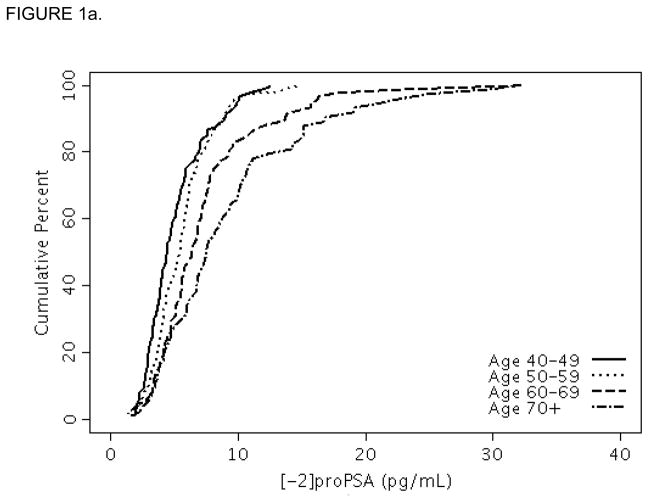
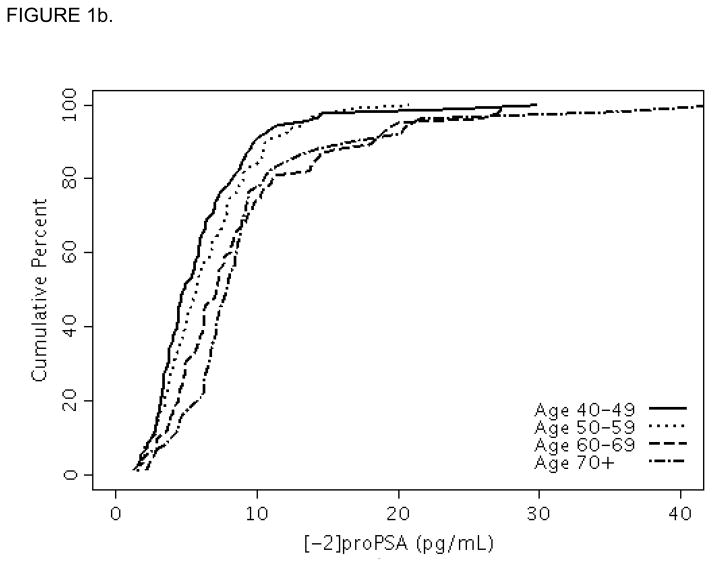
Baseline characteristics of the OCS and FMHS cohorts are shown in Table 1. Men in the FMHS had slightly smaller prostates and lower median PSA levels than OCS men. The median [-2]proPSA level was higher in the FMHS cohort [median (25th, 75th percentiles) =6.3 (4.1, 8.9) pg/mL] than in the OCS cohort [median=5.6 (3.9, 7.7) pg/mL], p=0.01. For reference, selected percentiles (e.g., minimum, 5, 10, 25, 50, 75, 90, 95, and maximum) of [-2]proPSA are presented in Table 2 for the individual and combined samples. The 95th percentiles of the [-2]proPSA levels were <14.7 pg/mL and <18.4 pg/mL for men in the OCS and FMHS cohorts, respectively. The percentile levels of [-2]proPSA across the entire distribution increased consistently with age decade (Figures 1a and 1b) as seen by the shift to the right in the distributions of [-2]proPSA level with increasing age decade.
TABLE 1.
Baseline characteristics of Olmsted County Study of Urinary Symptoms and Health Status among Men and Flint Men’s Health Study.
| Variable | OCS | FMHS | P value* |
|---|---|---|---|
| N | 420 | 328 | |
| Age (mean ± SD) | 58.7 ± 10.4 | 57.4 ± 10.4 | 0.24 |
| Family history of prostate cancer (N (%)) | 65 (15.5) | 55 (16.8) | 0.63 |
| Median (Q1, Q3) | Median (Q1, Q3) | ||
| Prostate volume (cc) | 26.7 (21.7, 35.0) | 26.2 (20.1, 33.4) | 0.06 |
| PSA (ng/mL) | 1.2 (0.8, 1.8) | 1.0 (0.5, 1.7) | 0.001 |
| [-2]proPSA (pg/mL) | 5.6 (3.9, 7.7) | 6.3 (4.1, 8.9) | 0.01 |
SD: Standard Deviation, Q1: 25th percentile, Q3: 75th percentile
Rank-sum P value reported for continuous variables, chi-square P value reported for dichotomous variables.
TABLE 2.
Selected empirical quantiles of [-2]proPSA (pg/mL) in the OCS, FMHS and combined FMHS and OCS cohorts.
| Quantile | OCS | FMHS | Combined |
|---|---|---|---|
| 0 (minimum) | 1.36 | 1.28 | 1.28 |
| 2.5 | 2.06 | 1.87 | 1.99 |
| 5 | 2.34 | 2.23 | 2.31 |
| 10 | 2.98 | 2.90 | 2.95 |
| 25 | 3.94 | 4.09 | 3.99 |
| 50 (median) | 5.58 | 6.26 | 5.84 |
| 75 | 7.67 | 8.91 | 8.28 |
| 90 | 10.70 | 13.46 | 11.44 |
| 95 | 14.71 | 18.36 | 15.25 |
| 97.5 | 16.92 | 21.53 | 20.21 |
| 100 (maximum) | 32.32 | 42.91 | 42.91 |
FIGURE 1.
FIGURE 1a. Cumulative distribution function plot of [-2]proPSA by age decade in the Olmsted County Study.
FIGURE 1b. Cumulative distribution function plot of [-2]proPSA by age decade in the Flint Men’s Health Study.
In multivariable models, after adjusting for age, [-2]proPSA levels were correlated with prostate volume (age-adjusted Spearman correlation(rS): OCS rS=0.46, FMHS rS=0.47) and PSA level (OCS rS=0.63, FMHS rS=0.71), all P<0.0001. For both blacks and whites, men in the upper quartile of [-2]proPSA were more likely to have an enlarged prostate and an elevated PSA level than those in the lowest quartiles, even after adjusting for age (supplementary data available on journal website ). These associations increased with increasing quartile of [-2]proPSA (supplementary data available on journal website, test for trend, P<0.0001). The wide confidence intervals for elevated PSA (i.e., PSA > 2.5 ng/ml) is primarily due to the low number of men in either cohort with elevated PSA.
There were 41 (9.8%) incident cases of prostate cancer in the OCS cohort after the baseline [-2]proPSA measurements. Men in the upper quartile of the [-2]proPSA distribution were more likely to be diagnosed with biopsy-confirmed prostate cancer (age-adjusted HR: 10.8, 95% CI: 3.1, 36.9), compared to men in the lowest quartile (Table 3). The magnitude of the association with prostate cancer increased with increasing [-2]proPSA quartile (tests for trend, P<0.0001). The association was slightly attenuated after further adjusting for baseline PSA, but remained nearly 8-fold higher for men in the upper quartile of [-2]proPSA relative to men in the lowest quartile (HR: 7.8, 95% CI: 2.2, 27.8). Similar results were seen when adjusting for baseline prostate volume, and for both baseline PSA and prostate volume (data not shown).
TABLE 3.
Crude and age- and PSA-adjusted association of baseline [-2]proPSA levels and future diagnosis of prostate cancer in the Olmsted County Study.
| [-2]proPSA quartile (range, pg/mL) | Biopsy-Confirmed Prostate Cancer
|
||
|---|---|---|---|
| Crude HR (95% CI) | Age-adj. HR (95% CI) | Age-, PSA-adj. HR (95% CI) | |
| Quartile 1 (min–3.99) | reference | reference | reference |
| Quartile 2 (4.00–5.84) | 1.2 (0.3, 5.5) | 1.2 (0.3, 5.5) | 1.2 (0.3, 5.2) |
| Quartile 3 (5.85–8.28) | 4.0 (1.1, 14.5) | 3.9 (1.1, 14.1) | 3.5 (0.96, 12.7) |
| Quartile 4 (8.29–max) | 11.4 (3.4, 38.1) | 10.8 (3.1, 36.9) | 7.8 (2.2, 27.8) |
| P trend | <0.0001 | <0.0001 | <0.0001 |
HR: hazard ratio, CI: confidence interval
DISCUSSION
These results provide normative values from population-based cohorts of black and white men. Although the levels of [-2]proPSA were slightly higher in black men than in white men in this study, previous studies based on clinical and screening cohorts of [-2]proPSA have reported values that on average were much higher (i.e., >90th percentile) than that observed in this study. Median levels in these previous studies6,23 ranged from 18 to 65 pg/mL. These levels are at the 95th percentile or greater of the two cohorts included in this study. As men in these screening studies had higher PSA levels than the general community, these differences are likely due to differences between these groups and men in the general community. Alternatively, differences in laboratory measurements or calibration could also lead to differences in the results.
Several other papers have reported improved prostate cancer detection using [-2]proPSA or %[-2]proPSA, particularly in the PSA range of 2–10 ng/mL,6,9, 12 and better differentiation of aggressive disease.7, 9, 10 A large screening study12 also reported improved prostate cancer detection of %[-2]proPSA in lower PSA ranges (median 1.05 ng/ml). In our study, higher baseline levels of [-2]proPSA were associated with future diagnosis of biopsy-confirmed CaP after adjustment for age and baseline PSA level. This result provides additional support to the potential use of this marker in distinguishing men with or without prostate cancer.
Many of these studies report sensitivities and specificities for various thresholds and functions of these biomarkers; however the study samples from these previous reports may not accurately reflect the assay performance in distinguishing benign prostate disease from prostate cancer. Compared to the general population, the previous studies are based on men with elevated serum PSA and [-2]proPSA levels. Because of this, the differences between men with and without cancer in the overall PSA range and therefore the utility of identifying men with prostate cancer may be greater than these previous reports have suggested.14
This study included only black and white men and the generalizability of the results to other races or ethnic groups may be limited; however there were few differences between black and white men in this study. Previous studies investigating the utility of [-2]proPSA in distinguishing men with CaP have reported results specifically in the range of PSA level from 4–10 ng/mL. The prevalence of men with PSA levels in this range was <10% in each of our cohorts. Consequently, it was not possible to analyze the ability of this biomarker in predicting risk of cancer in this subgroup. Previous studies have also assessed the ratio of [-2]proPSA level to free PSA however free PSA level was not measured at baseline in these cohorts. In addition, the stability of the analytes in samples frozen for several years is currently unknown, however [-2]proPSA levels were stable when frozen up to 12 months at −70°C.24 Additionally, the confidence intervals around the estimated HR were wide due to the increased variability that may be attributed to the low number of overall events observed in the study.
Conclusions
These data suggest that levels of [-2]proPSA may help identify prostate cancer in men with serum PSA levels in an indeterminate range, although the reference ranges for white and black men may differ slightly.
Supplementary Material
Acknowledgments
The authors thank the men who participate in the Olmsted County Study and the Flint Men’s Health Study, the study personnel, and Ms. Kristie Shorter for her assistance in preparation of this manuscript. This project was supported by research grants from the Public Health Service, National Institutes of Health (Grants DK58859, AR30582 and 1UL1 RR024150-01), National Cancer Institute (P50CA69568), and Merck Research Laboratories. Beckman Coulter (Brea, CA) provided the test kits for [-2]proPSA free of charge and with no obligation.
ABBREVIATIONS
- [-2]proPSA
[-2]pro-prostate-specific antigen
- CaP
prostate cancer
- CI
confidence intervals
- HR
hazard ratios
- OR
odds ratios
- PSA
prostate-specific antigen
Footnotes
Disclosures
Dr. Klee has received research grants and royalties for unrelated technologies from Beckman Coulter, Inc. Dr. Jacobsen has received research grants from Beckman Coulter, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges.[see comment] JAMA. 1993;270:860. [PubMed] [Google Scholar]
- 3.Catalona WJ, Southwick PC, Slawin KM, et al. Comparison of percent free PSA, PSA density, and age-specific PSA cutoffs for prostate cancer detection and staging. Urology. 2000;56:255. doi: 10.1016/s0090-4295(00)00637-3. [DOI] [PubMed] [Google Scholar]
- 4.Mikolajczyk SD, Millar LS, Wang TJ, et al. A precursor form of prostate-specific antigen is more highly elevated in prostate cancer compared with benign transition zone prostate tissue. Cancer Res. 2000;60:756. [PubMed] [Google Scholar]
- 5.Mikolajczyk SD, Catalona WJ, Evans CL, et al. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem. 2004;50:1017. doi: 10.1373/clinchem.2003.026823. [DOI] [PubMed] [Google Scholar]
- 6.Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J Urol. 2003;170:2181. doi: 10.1097/01.ju.0000095460.12999.43. [DOI] [PubMed] [Google Scholar]
- 7.Chan TY, Mikolajczyk SD, Lecksell K, et al. Immunohistochemical staining of prostate cancer with monoclonal antibodies to the precursor of prostate-specific antigen. Urology. 2003;62:177. doi: 10.1016/s0090-4295(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 8.Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro-prostate specific antigen preferentially detects aggressive prostate cancers in men with 2 to 4 ng/ml prostate specific antigen. J Urol. 2004;171:2239. doi: 10.1097/01.ju.0000127737.94221.3e. [DOI] [PubMed] [Google Scholar]
- 9.Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010;19:1193. doi: 10.1158/1055-9965.EPI-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephan C, Kahrs AM, Cammann H, et al. A [-2]proPSA-based artificial neural network significantly improves differentiation between prostate cancer and benign prostatic diseases. Prostate. 2009;69:198. doi: 10.1002/pros.20872. [DOI] [PubMed] [Google Scholar]
- 11.Makarov DV, Isharwal S, Sokoll LJ, et al. Pro-prostate-specific antigen measurements in serum and tissue are associated with treatment necessity among men enrolled in expectant management for prostate cancer. Clin Cancer Res. 2009;15:7316. doi: 10.1158/1078-0432.CCR-09-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le BV, Griffin CR, Loeb S, et al. [-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J Urol. 2010;183:1355. doi: 10.1016/j.juro.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guess HA, Jacobsen SJ, Girman CJ, et al. The role of community-based longitudinal studies in evaluating treatment effects. Example: benign prostatic hyperplasia. Med Care. 1995;33:AS26. [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Bergstralh EJ, Guess HA, et al. Predictive properties of serum-prostate-specific antigen testing in a community-based setting. Arch Intern Med. 1996;156:2462. [PubMed] [Google Scholar]
- 15.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 16.Wei JT, Schottenfeld D, Cooper K, et al. The natural history of lower urinary tract symptoms in black American men: relationships with aging, prostate size, flow rate and bothersomeness. J Urol. 2001;165:1521. [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Guess HA, Panser L, et al. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch Fam Med. 1993;2:729. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 18.Sarma AV, Wei JT, Jacobson DJ, et al. Comparison of lower urinary tract symptom severity and associated bother between community-dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men’s Health Study. Urology. 2003;61:1086. doi: 10.1016/s0090-4295(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes T, Girman CJ, Jacobsen SJ, et al. Longitudinal prostate growth rates during 5 years in randomly selected community men 40 to 79 years old. J Urol. 1999;161:1174. [PubMed] [Google Scholar]
- 20.Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991;145:984. doi: 10.1016/s0022-5347(17)38508-7. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen SJ, Klee GG, Lilja H, et al. Stability of serum prostate-specific antigen determination across laboratory, assay, and storage time. Urology. 1995;45:447. doi: 10.1016/S0090-4295(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 22.Oesterling JE, Moyad MA, Wright GL, Jr, et al. An analytical comparison of the three most commonly used prostate-specific antigen assays: Tandem-R, Tandem-E, and IMx. Urology. 1995;46:524. doi: 10.1016/S0090-4295(99)80266-0. [DOI] [PubMed] [Google Scholar]
- 23.de Vries SH, Raaijmakers R, Blijenberg BG, et al. Additional use of [-2] precursor prostate-specific antigen and “benign” PSA at diagnosis in screen-detected prostate cancer. Urology. 2005;65:926. doi: 10.1016/j.urology.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Semjonow A, Kopke T, Eltze E, et al. Pre-analytical in-vitro stability of [-2]proPSA in blood and serum. Clin Biochem. 2010;43:926. doi: 10.1016/j.clinbiochem.2010.04.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.