Abstract
BACKGROUND
Early termination of prolonged seizures with intravenous administration of benzodiazepines improves outcomes. For faster and more reliable administration, paramedics increasingly use an intramuscular route.
METHODS
This double-blind, randomized, noninferiority trial compared the efficacy of intramuscular midazolam with that of intravenous lorazepam for children and adults in status epilepticus treated by paramedics. Subjects whose convulsions had persisted for more than 5 minutes and who were still convulsing after paramedics arrived were given the study medication by either intramuscular autoinjector or intravenous infusion. The primary outcome was absence of seizures at the time of arrival in the emergency department without the need for rescue therapy. Secondary outcomes included endotracheal intubation, recurrent seizures, and timing of treatment relative to the cessation of convulsive seizures. This trial tested the hypothesis that intramuscular midazolam was noninferior to intravenous lorazepam by a margin of 10 percentage points.
RESULTS
At the time of arrival in the emergency department, seizures were absent without rescue therapy in 329 of 448 subjects (73.4%) in the intramuscular-midazolam group and in 282 of 445 (63.4%) in the intravenous-lorazepam group (absolute difference, 10 percentage points; 95% confidence interval, 4.0 to 16.1; P<0.001 for both noninferiority and superiority). The two treatment groups were similar with respect to need for endotracheal intubation (14.1% of subjects with intramuscular midazolam and 14.4% with intravenous lorazepam) and recurrence of seizures (11.4% and 10.6%, respectively). Among subjects whose seizures ceased before arrival in the emergency department, the median times to active treatment were 1.2 minutes in the intramuscular-midazolam group and 4.8 minutes in the intravenous-lorazepam group, with corresponding median times from active treatment to cessation of convulsions of 3.3 minutes and 1.6 minutes. Adverse-event rates were similar in the two groups.
CONCLUSIONS
For subjects in status epilepticus, intramuscular midazolam is at least as safe and effective as intravenous lorazepam for prehospital seizure cessation. (Funded by the National Institute of Neurological Disorders and Stroke and others; ClinicalTrials.gov number, NCT00809146.)
Early termination of prolonged epileptic seizures in response to intravenous administration of benzodiazepines by paramedics in the prehospital setting is associated with better patient outcomes. The randomized, controlled Prehospital Treatment of Status Epilepticus (PHTSE) trial (ClinicalTrials.gov number, NCT00004297) compared diazepam, lorazepam, and placebo given intravenously by paramedics to treat subjects with prolonged convulsive seizures.1 The trial showed that both these benzodiazepines were an effective prehospital treatment for seizures, as compared with placebo. The proportion of subjects whose seizures were terminated at the time of arrival in the emergency department was 59.1% in the group receiving intravenous lorazepam, 42.6% in the group receiving intravenous diazepam, and 21.1% in the group receiving intravenous placebo.
Many emergency medical services (EMS) systems, however, have begun to use intramuscular midazolam rather than an intravenous agent, largely because intramuscular administration is faster and is consistently achievable.2 This practice has become increasingly common despite the lack of clinical-trial data regarding the efficacy and safety of intramuscular midazolam. Although intravenous lorazepam is the preferred treatment for patients with seizures in the emergency department (and was the most effective treatment in the PHTSE trial), it is rarely used by paramedics in the prehospital setting because of the potential difficulty with intravenous administration, as well as the short shelf-life of lorazepam when it is not refrigerated.3 EMS medical directors need a practical alternative that is at least as safe and effective as intravenous lorazepam. We therefore performed a noninferiority study to determine whether intramuscular midazolam is as effective as intravenous lorazepam, with a similar degree of safety, for terminating status epilepticus seizures before arrival at the hospital.
METHODS
STUDY DESIGN
The Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) was a randomized, double-blind, phase 3, noninferiority clinical trial. It was designed and conducted by the Neurological Emergencies Treatment Trials (NETT) network, a multi-disciplinary clinical trials infrastructure funded by the National Institute of Neurological Disorders and Stroke (NINDS). The investigators were responsible for all elements of the trial, including design, data collection, and analysis. The authors wrote the manuscript and vouch for the data and analysis. The trial was performed under an Investigational New Drug application with the Food and Drug Administration (FDA). Autoinjectors with active medication and placebo were purchased by the Department of Defense and provided to the NINDS through a cooperative agreement. The Department of Defense had no role in the design of the study, accrual or analysis of data, or preparation of the manuscript. The study was conducted in accordance with the protocol, which is available with the full text of this article at NEJM.org.
RAMPART involved 4314 paramedics, 33 EMS agencies, and 79 receiving hospitals across the United States. Paramedics received continuing medical education in the management of seizures and other neurologic emergencies, as well as supplemental training in human subjects research and protections and in the study protocol, with refresher protocol training provided throughout the trial.
The trial met the exception from informed-consent requirements for emergency research under the FDA code of regulations 21 CFR 50.24.4 Institutional review boards for all entities engaged in this research reviewed local community consultation activity, according to the regulations regarding the exception from informed consent, and provided approval. Subjects or their legally authorized representatives were notified about enrollment in the trial by the study team as soon as possible, usually while the subject was still in the emergency department, and provided written informed consent to allow continued data collection until follow-up was completed.
STUDY SUBJECTS
The intended study population included children with an estimated body weight of 13 kg or more and adults requiring treatment with benzodiazepines for status epilepticus in the prehospital setting. Subjects were enrolled if they were having convulsive seizures at the time of treatment by paramedics and were reported by reliable witnesses to have been continuously convulsing for longer than 5 minutes or if they were having convulsive seizures at the time of treatment after having intermittent seizures without regaining consciousness for longer than 5 minutes.
Subjects were excluded for the following reasons: the acute precipitant of the seizures was major trauma, hypoglycemia, cardiac arrest, or a heart rate of less than 40 beats per minute (since these conditions require alternative treatments); they had a known allergy to midazolam or lorazepam; they were known to be pregnant or a prisoner; they were being treated as part of another study; or, preemptively, they opted out of this study by wearing a medical-alert tag marked “RAMPART declined.”
STUDY INTERVENTION
When they arrived at the scene, the study paramedics rapidly performed an initial assessment and stabilized subjects who were in status epilepticus, according to their local EMS protocols. For subjects who met the eligibility criteria, the paramedics began the study procedure by opening an instrumented box containing a study drug kit. Each kit contained two color-coded, shrink-wrapped study-drug bundles, one for each dose tier; each bundle consisted of one intramuscular autoinjector (Investigational Midazolam Autoinjector [Meridian Medical Technologies]) and one prefilled intravenous syringe (Carpuject System [Hospira]). All adults and those children with an estimated body weight of more than 40 kg received either 10 mg of intramuscular midazolam followed by intravenous placebo or intramuscular placebo followed by 4 mg of intravenous lorazepam. In children with an estimated weight of 13 to 40 kg, the active treatment was 5 mg of intramuscular midazolam or 2 mg of intravenous lorazepam. Blinding and simple randomization with equal numbers of subjects assigned to the two study groups were achieved with the use of a double-dummy strategy, in which each kit was randomly assigned at the central pharmacy to contain either the active intramuscular drug with intravenous placebo or intramuscular placebo with the active intravenous drug. All subjects were treated with the intramuscular autoinjector, after which venous access was immediately achieved and treatment was administered by means of intravenous syringe. Subjects were considered to be enrolled in the trial when the intramuscular autoinjector was applied, regardless of whether the intramuscular dose was successfully delivered.
A voice recorder was activated by opening the study box. Paramedics were instructed to record oral statements when intramuscular treatment was administered, when intravenous access was obtained, when the intravenous study drug was administered, when any rescue treatments were given, and when convulsions were observed to stop. Each statement was time-stamped by the study box’s internal clock. Paramedics also stated whether the subject was convulsing on arrival at the emergency department.
When it was difficult to obtain intravenous access, paramedics were instructed to continue attempts for at least 10 minutes, but they were permitted to use intraosseous access at any time in lieu of intravenous access. For the purposes of this trial, intraosseous access to the vascular space was considered equivalent to intravenous access. Rescue therapy, as dictated by local EMS protocol, was recommended for use in subjects who were still convulsing 10 minutes after the last study medication was administered. If there was a delay in obtaining intravenous access and the subject stopped having seizures before the intravenous study drug could be given, the intravenous study medication was not used. If convulsions resumed later during EMS transport, rescue therapy (according to the local protocol) was to be given.
STUDY OUTCOMES
The primary outcome was termination of seizures before arrival in the emergency department without the need for the paramedics to provide rescue therapy. Subjects did not reach the primary outcome if they were having seizures on arrival in the emergency department or if they received rescue medication before arrival. Termination of seizures on arrival was determined according to the clinical judgment of the attending emergency physician and was based on examination of the subjects, their clinical course, and results of any routine diagnostic testing (Section 6.1 of the protocol). This outcome measure was previously used in the PHTSE trial.1,5
Key secondary outcome measures included the time from study-box opening to termination of convulsions and the time from initiation of active-drug administration to termination of convulsions (among subjects in whom convulsions ceased before arrival in the emergency department), the frequency and duration of hospitalization and of admissions to the intensive care unit, and the frequencies of acute endotracheal intubation and acute seizure recurrence. Acute endotracheal intubation was defined as intubation performed or attempted by EMS personnel or performed within 30 minutes after arrival in the emergency department. Acute seizure recurrence was defined as any further convulsive or electrographic seizures that required additional antiepileptic medications during the first 12 hours of hospitalization in subjects who did not have seizures on arrival in the emergency department. Serious adverse events were recorded through the end of the study for every subject (see Table A2 in the Supplementary Appendix, available at NEJM.org).
STATISTICAL ANALYSIS
The primary objective of the study was to show that the proportion of subjects whose seizures were terminated before arrival in the emergency department (without the use of rescue medications) in the intramuscular midazolam group was not inferior to that in the intravenous lorazepam group by more than a prespecified amount (the noninferiority margin). The null hypothesis of inferiority was tested with the use of a one-sided z statistic.6 The primary analysis was followed by a one-sided test (conditional on the finding of noninferiority) for superiority at a significance level of 0.025, although this was not prespecified in the protocol. On the basis of published studies of similar patient populations, and accounting for differences in the dose of lorazepam and in the definition of efficacy, we estimated that after an initial dose of intravenous lorazepam had been administered, seizures would be terminated in 70% of subjects before arrival in the emergency department. Sample size was estimated on the basis of the comparison of independent proportions, with two planned interim analyses for futility with respect to the primary outcome; 90% power to show the noninferiority of intramuscular midazolam; a noninferiority margin of 10 percentage points; and a one-sided test with the probability of a type I error of 0.025. The maximum sample size required for randomization was 890 subjects (445 per treatment group). Because some patients have recurring episodes of status epilepticus, the total sample size was inflated by 15% (1024 subjects) to account for inadvertent repeated enrollment of the same subjects. (Repeated enrollments of the same subject were not analyzed.) Secondary outcomes were compared in a superiority framework with the use of a two-sided test with the probability of a type I error of less than 0.05. All analyses were conducted with the intention-to-treat population defined as all subjects randomly assigned to a study medication. A sensitivity analysis was conducted with the per-protocol population, which excluded subjects with any of the following three predefined protocol deviations: eligibility violation, incorrect dose of study medication, or incorrect administration.
RESULTS
SUBJECTS AND ENROLLMENT
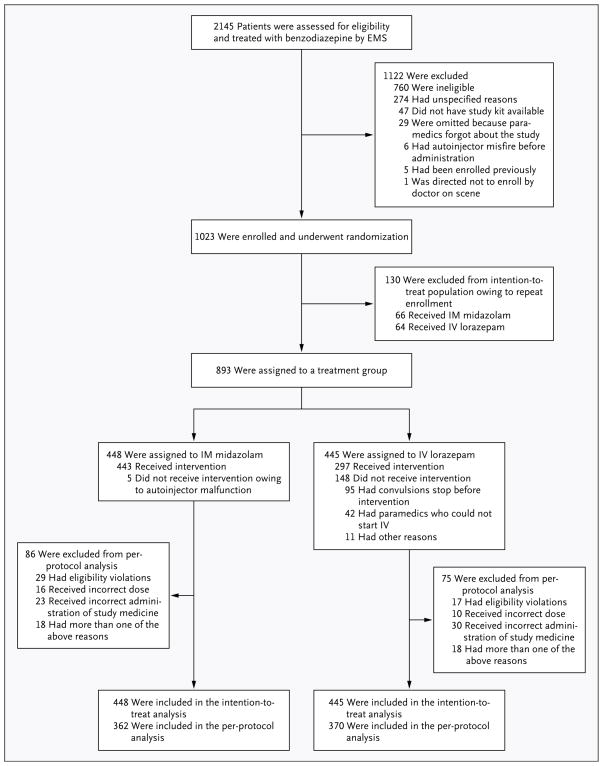
Between June 15, 2009, and January 14, 2011, a total of 893 subjects were enrolled (with a total of 1023 enrollments and a reenrollment rate of 13%) (Fig. 1). The two treatment groups were well balanced with respect to demographic and clinical characteristics, dose tier, presence or absence of a history of epilepsy, accuracy of the diagnosis of status epilepticus (vs. a discharge diagnosis of a non-epileptic spell), and the diagnosis of the underlying cause of status epilepticus (Table 1). The overall number of subjects who were black reflected the proportion of blacks in the subject population from which the sample was drawn.
Figure 1. Screening, Enrollment, Randomization, and Inclusion in Intention-to-Treat and Per-Protocol Analyses.
The number of patients who were assessed and enrolled includes any repeat assessments and enrollments for those who presented to emergency medical services (EMS) with status epilepticus more than once. The number assigned to treatment in the intention-to-treat analysis includes every patient who was enrolled in the study but only the initial enrollment for those enrolled more than once. Randomization was defined as occurring when an autoinjector was applied to the subject. “Misfire” refers to instances when the autoinjector was inadvertently triggered before it could be applied to the subject. “Malfunction” refers to instances when the autoinjector was applied but the drug was not administered because of operator error or mechanical failure. IM denotes intramuscular, and IV intravenous.
Table 1.
Characteristics of the Subjects at Baseline.*
| Characteristic | IM Midazolam (N = 448) | IV Lorazepam (N = 445) |
|---|---|---|
| Age | ||
| Mean (range) — yr | 43±22 (0–102) | 44±22 (1–94) |
| Age group — no. (%) | ||
| 0–5 yr | 32 (7) | 29 (7) |
| 6–10 yr | 15 (3) | 20 (4) |
| 11–20 yr | 28 (6) | 21 (5) |
| 21–40 yr | 114 (25) | 112 (25) |
| 41–60 yr | 169 (38) | 169 (38) |
| ≥61 yr | 90 (20) | 94 (21) |
| Male sex — no. (%) | 250 (56) | 238 (53) |
| Race — no. (%)† | ||
| Black | 229 (51) | 224 (50) |
| White | 165 (37) | 183 (41) |
| Other, mixed, or unknown | 54 (12) | 38 (9) |
| Ethnic group — no. (%)† | ||
| Non-Hispanic | 310 (69) | 290 (65) |
| Hispanic | 49 (11) | 57 (13) |
| Unknown | 89 (20) | 98 (22) |
| Dose tier — no. (%)‡ | ||
| Low | 62 (14) | 59 (13) |
| High | 386 (86) | 386 (87) |
| History of epilepsy — no. (%) | ||
| Yes | 293 (65) | 295 (66) |
| No | 111 (25) | 103 (23) |
| Not documented | 44 (10) | 47 (11) |
| Final diagnosis — no. (%) | ||
| Status epilepticus | 404 (90) | 399 (90) |
| Nonepileptic spell | 31 (7) | 32 (7) |
| Undetermined | 13 (3) | 14 (3) |
| Precipitating cause of status epilepticus — no. (%) | ||
| Noncompliance with or discontinuation of anticonvulsant therapy | 137 (31) | 141 (32) |
| Idiopathic or breakthrough status epilepticus | 121 (27) | 121 (27) |
| Coexisting condition that lowered seizure threshold | 33 (7) | 29 (7) |
Plus–minus values are means ±SD. There were no significant differences between the two groups with respect to baseline characteristics.
Race and ethnic group were reported by the investigators. More detailed data for race are provided in Table A3 in the Supplementary Appendix.
The high-dose tier included children whose estimated body weight was above 40 kg and all adults, and active treatment consisted of either 10 mg of intramuscular (IM) midazolam or 4 mg of intravenous (IV) lorazepam. The low-dose tier included children whose estimated body weight was 13 to 40 kg, and active treatment consisted of either 5 mg of IM midazolam or 2 mg of IV lorazepam.
PRIMARY OUTCOME
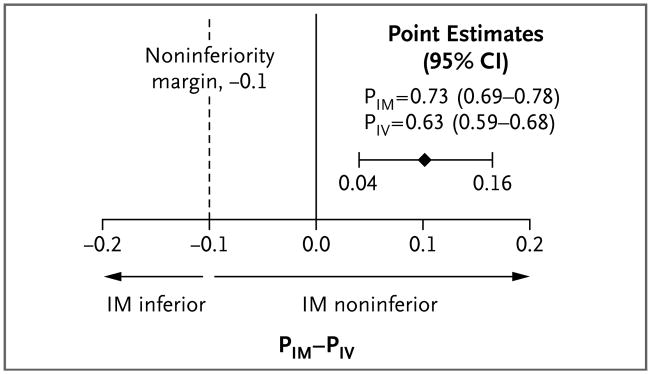
Seizures were absent without rescue therapy on arrival in the emergency department in 329 of 448 subjects assigned to active treatment with intramuscular midazolam (73.4%) and in 282 of 445 assigned to active treatment with intravenous lorazepam (63.4%) (difference, 10 percentage points; 95% confidence interval [CI], 4.0 to 16.1; P<0.001 for noninferiority and P<0.001 for superiority) (Fig. 2). The primary results were similar in the per-protocol analysis. Table 2 shows the number of subjects who were having seizures at the time of arrival in the emergency department and the number who needed rescue medication. Subjects randomly assigned to the intramuscular group were less likely to be having seizures on arrival in the emergency department (regardless of the use or nonuse of rescue therapy) than were those randomly assigned to the intravenous group (proportion of subjects without seizures, 83.9% vs. 76.2%; difference, 7.7 percentage points; 95% CI, 2.5 to 12.9). Inability to start an intravenous infusion was anticipated to be a common reason for failure of intravenous therapy. Among subjects in the intravenous group who did not reach the primary outcome, 31 never received the intravenous study medication because of failure to obtain vascular access, whereas only 5 in the entire intramuscular group did not receive the intramuscular study medication owing to malfunction or misapplication of the autoinjector.
Figure 2. Primary Outcome According to Treatment Group.
PIM-PIV represents the absolute difference in the primary outcome between the proportion of subjects treated with IM midazolam and the proportion treated with IV lorazepam (i.e., the proportion of subjects who did not have seizures on arrival in the emergency department and who did not receive rescue medication). CI denotes confidence interval.
Table 2.
Primary and Secondary Outcomes.*
| Outcome | Intention-to-Treat Analysis† (N = 893) | Per-Protocol Analysis‡ (N = 732) | ||
|---|---|---|---|---|
| IM Midazolam (N = 448) | IV Lorazepam (N = 445) | IM Midazolam (N = 362) | IV Lorazepam (N = 370) | |
|
Primary outcome
| ||||
| Seizures terminated, no rescue therapy given
| ||||
| No. of subjects | 329 | 282 | 271 | 238 |
|
| ||||
| % of subjects (95% CI)§ | 73.4 (69.3–77.5) | 63.4 (58.9–67.9) | 74.9 (70.4–79.3) | 64.3 (59.4–69.2) |
|
| ||||
| Treatment failed — no. of subjects (%) | 119 (26.6) | 163 (36.6) | 91 (25.1) | 132 (35.7) |
|
| ||||
| Seizures not terminated, no rescue therapy given | 50 (11.2) | 64 (14.4) | 42 (11.6) | 51 (13.8) |
|
| ||||
| Seizures not terminated, rescue therapy given | 22 (4.9) | 42 (9.4) | 14 (3.9) | 38 (10.3) |
|
| ||||
| Seizures terminated, rescue therapy given | 47 (10.5) | 57 (12.8) | 35 (9.7) | 43 (11.6) |
|
| ||||
|
Secondary outcomes
| ||||
| Endotracheal intubation within 30 min after ED arrival
| ||||
| No. of subjects — % | 63 (14.1) | 64 (14.4) | 53 (14.6) | 53 (14.3) |
|
| ||||
| Relative risk (95% CI) | 0.98 (0.70–1.34) | 1.02 (0.71–1.45) | ||
|
| ||||
| Hospitalization
| ||||
| No. of subjects — % | 258 (57.6) | 292 (65.6) | 210 (58.0) | 250 (67.6) |
|
| ||||
| Relative risk (95% CI) | 0.88 (0.79–0.98) | 0.86 (0.77–0.96) | ||
|
| ||||
| ICU admission
| ||||
| No. of subjects — % | 128 (28.6) | 161 (36.2) | 102 (28.2) | 138 (37.3) |
|
| ||||
| Relative risk (95% CI) | 0.79 (0.65–0.95) | 0.76 (0.61–0.93) | ||
|
| ||||
| Recurrent seizure within 12 hr after ED arrival
| ||||
| No. of subjects — % | 51 (11.4) | 47 (10.6) | 37 (10.2) | 39 (10.5) |
|
| ||||
| Relative risk (95% CI) | 1.08 (0.74–1.56) | 0.97 (0.63–1.48) | ||
|
| ||||
| Hypotension
| ||||
| No. of subjects — % | 12 (2.7) | 13 (2.9) | 5 (1.4) | 9 (2.4) |
|
| ||||
| Relative risk (95% CI) | 0.92 (0.42–1.98) | 0.57 (0.19–1.67) | ||
|
| ||||
| IM injection-site complications
| ||||
| No. of subjects (%) | 4 (0.9) | 2 (0.5) | 4 (1.1) | 1 (0.3) |
|
| ||||
| Relative risk (95% CI) | 1.99 (0.30–10.70) | 4.09 (0.45–36.40) | ||
|
| ||||
| IV injection-site complications — no. of subjects (%) | 0 | 3 (0.7) | 0 | 3 (0.8) |
|
| ||||
| Length of ICU stay — days
| ||||
| No. of subjects with length-of-stay data | 123 | 155 | 98 | 132 |
|
| ||||
| Mean | 5.7±9.5 | 4.1±4.7 | 4.8±7.2 | 4.0±4.7 |
|
| ||||
| Median (minimum, maximum) | 3 (1, 75) | 3 (1, 31) | 3 (1, 65) | 2 (1, 31) |
|
| ||||
| P value¶ | 0.09 | 0.33 | ||
|
| ||||
| Length of hospital stay — days
| ||||
| No. of subjects with length-of-stay data | 251 | 285 | 204 | 243 |
|
| ||||
| Mean | 6.7±10.0 | 5.5±6.4 | 5.8±7.0 | 5.5±6.4 |
|
| ||||
| Median (minimum, maximum) | 4 (1, 90) | 3 (1, 58) | 3 (1, 65) | 4 (1, 58) |
|
| ||||
| P value¶ | 0.11 | 0.71 | ||
Plus–minus values are means ±SD. The relative risk is for the subjects given IM midazolam, as compared with those given IV lorazepam.
The intention-to-treat analysis included only the initial enrollment of all subjects; repeated enrollments of the same subject were not included. CI denotes confidence interval, ED emergency department, and ICU intensive care unit.
The per-protocol analysis excluded subjects with any of the following three predefined types of protocol deviations: eligibility violations, incorrect dose of study medication, or incorrect administration.
P<0.001 for noninferiority and for superiority in both the intention-to-treat and per-protocol analyses. P values for noninferiority reflect one-sided tests for differences not exceeding 10 percentage points. The primary analysis was followed by a one-sided test for superiority,7 although this was not prespecified in the protocol.
P values were calculated with the use of t-tests for the means.
SECONDARY AND SAFETY OUTCOMES
The secondary and safety outcomes were consistent with the primary outcome and reinforced the finding that intramuscular midazolam was non-inferior to intravenous lorazepam. The frequencies of endotracheal intubation, recurrent seizures, and other predefined safety outcomes were similar in the two study groups (Table 2). Among subjects admitted to the hospital, the lengths of stay in the intensive care unit and in the hospital did not differ significantly between the groups, but the proportion of subjects admitted was significantly lower (and the proportion discharged from the emergency department was significantly higher) in the intramuscular group than in the intravenous group (P = 0.01).
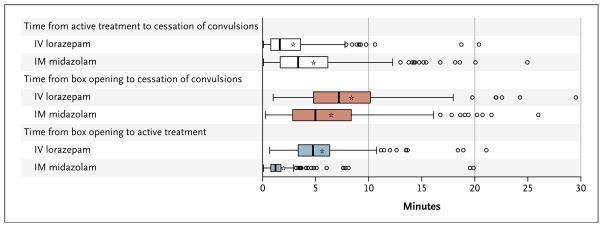
Figure 3 shows the temporal data (the times from administration of active treatment to cessation of convulsions, from box opening to cessation of convulsions, and from box opening to administration of active treatment) for the 317 subjects in the intention-to-treat analysis who met the primary outcome and for whom times of active treatment and of cessation of convulsions were recorded. The median time to administration of active treatment was significantly shorter by the intramuscular route than by the intravenous route (1.2 vs. 4.8 minutes), but the onset of action (i.e., termination of convulsions) occurred sooner after intravenous administration than after intramuscular administration (1.6 vs. 3.3 minutes). The overall interval until termination of convulsions was similar in the two treatment groups.
Figure 3. Intervals between Active Treatment and Cessation of Convulsions, Box Opening and Cessation of Convulsions, and Box Opening and Active Treatment.
The shorter time to IM drug administration was offset by the faster onset of action after IV drug administration, resulting in similar latency periods until convulsions were terminated. Time to IV administration includes the nominal time (about 20 seconds) needed to administer the drug by means of IM autoinjector. Asterisks indicate means, boxes interquartile ranges, bold vertical lines within boxes medians, I bars 1.5 times the interquartile range, and circles outliers.
DISCUSSION
This double-blind, randomized trial showed that prehospital treatment with intramuscular midazolam was at least as effective as intravenous lorazepam in subjects in status epilepticus (P<0.001 for noninferiority and for superiority). Establishing intravenous access in patients who are having seizures in the prehospital environment can be challenging and time-consuming. Since intramuscular treatments can be given more quickly and reliably than intravenous treatments and have noninferior efficacy, our data support the use of the former route of administration by EMS personnel.
The use by EMS systems of intramuscular midazolam for status epilepticus has been increasing because small studies have indicated its efficacy and because this drug is rapidly absorbed intra-muscularly. According to a meta-analysis of small trials, the use of nonintravenous midazolam in the hospital setting compared favorably with intravenous diazepam in the emergency treatment of status epilepticus.8 Furthermore, unlike lorazepam, midazolam does not have the problem of poor stability when not refrigerated. Midazolam can be administered by other nonintravenous routes as well, but the intramuscular route is more consistently effective than the intranasal or buccal routes because the drug cannot be blown or spat out by the convulsing patient.
In this noninferiority study, we used lorazepam as an active control. Inclusion of a placebo group would have been unethical, since PHTSE showed unambiguously that benzodiazepines are superior to no treatment in subjects in status epilepticus in the prehospital setting. The clinically important question is whether intramuscular midazolam works well enough for patients in status epilepticus to routinely forgo the intravenous route in order to improve the ease and speed of treatment administered by EMS personnel. The active control drug, the noninferiority margin, the trial setting, and the analysis plan were carefully chosen to avoid the known potential pitfalls and limitations of noninferiority studies.7
The doses of midazolam and lorazepam used in this trial are consistent with the most effective doses for the treatment of status epilepticus that are reported in the literature.9,10 Although these initial doses are higher than the ones used by many EMS systems and emergency physicians, they are the same as those approved for this indication and are in line with those used by epileptologists. Use of an autoinjector maximized the speed and ease of intramuscular delivery (with a nominal latency period of about 20 seconds for opening the autoinjector and administering the medication) and reduced delays in initiating intravenous access.
The relationships among benzodiazepine dose, respiratory depression, and subsequent need for endotracheal intubation are poorly characterized, but higher doses of benzodiazepines may actually reduce the number of airway interventions. Our data are consistent with the finding that endotracheal intubation is more commonly a sequela of continued seizures than it is an adverse effect of sedation from benzodiazepines.11
With regard to the mechanism of drug action, our temporal data are consistent with what would be expected: the intramuscular route delivers the medication more rapidly after the paramedics’ arrival at the scene than the intravenous route, but its onset of action is more rapid after intravenous administration than after intramuscular administration. The time saved by using the intramuscular route appears to more than offset the delay in the drug’s onset of action. It is interesting to speculate that a difference of just a few minutes with the earlier administration in the intramuscular group may have been enough to drive the slight superiority of the intramuscular route with respect to outcome. However, it is also possible that the difference in outcome between the two treatment groups reflects differences in the efficacy of the agents used rather than in the route of administration. Because this is a pragmatic clinical trial designed to inform EMS clinical practice rather than to elucidate mechanism, the effect of agent and route cannot be meaningfully separated in analyzing these data. Similarly, an autoinjector was used in this study to optimize the speed and efficiency of intramuscular delivery, but it is not possible to determine the importance of using this tool for intramuscular injections, as compared with conventional intramuscular injections.
Our data are consistent with a finding of statistical superiority of intramuscular midazolam. Regardless of whether it is noninferior or superior, this trial supports the clinical decision to use the more pragmatic intramuscular approach in the prehospital treatment of status epilepticus.
In conclusion, intramuscular midazolam is noninferior to intravenous lorazepam in stopping seizures before arrival in the emergency department in patients with status epilepticus treated by paramedics. Intramuscular midazolam is also as safe as intravenous lorazepam. The group of subjects treated with intramuscular midazolam had a higher rate of discharge from the emergency department than the group treated with intravenous lorazepam and had similar or lower rates of recurrent seizures and endotracheal intubation. The intramuscular administration of midazolam by EMS is a practical, safe, and effective alternative to the intravenous route for treating prolonged convulsive seizures in the prehospital setting.
Supplementary Material
Acknowledgments
Supported by awards from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS056975 and U01NS059041); the National Institutes of Health Office of the Director CounterACT Program; and the Biomedical Advanced Research and Development Authority of the Assistant Secretary for Preparedness and Response.
We thank Edward Jauch and Robert Woolson, clinical and statistical consultants; Ken Rockwell, central pharmacy; Henry Wang, medical safety monitor; the data and safety monitoring board: Thomas Bleck (chair), Gail Anderson, James Chamberlain, Joseph Collins, Jeffrey Saver, and Peter Gilbert (NINDS liaison); the Chemical Biological Medical Systems Joint Project Management Office, Department of Defense, for support and for providing autoinjectors through a cooperative agreement with the NINDS; and all the hardworking paramedics on the front line who made this study possible.
Footnotes
The Neurological Emergencies Treatment Trials (NETT) investigators are listed in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. doi: 10.1056/NEJMoa002141. [Erratum, N Engl J Med 2001;345:1860.] [DOI] [PubMed] [Google Scholar]
- 2.Warden CR, Frederick C. Midazolam and diazepam for pediatric seizures in the prehospital setting. Prehosp Emerg Care. 2006;10:463–7. doi: 10.1080/10903120600885126. [DOI] [PubMed] [Google Scholar]
- 3.Gottwald MD, Akers LC, Liu PK, et al. Prehospital stability of diazepam and lorazepam. Am J Emerg Med. 1999;17:333–7. doi: 10.1016/s0735-6757(99)90079-7. [DOI] [PubMed] [Google Scholar]
- 4.Exception from informed consent requirements for emergency research: Code of Federal Regulations. Washington, DC: Government Printing Office; ( http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.24) [Google Scholar]
- 5.Lowenstein DH, Alldredge BK, Allen F, et al. The Prehospital Treatment of Status Epilepticus (PHTSE) study: design and methodology. Control Clin Trials. 2001;22:290–309. doi: 10.1016/s0197-2456(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 6.Dunnett CW, Gent M. Significance testing to establish equivalence between treatments, with special reference to data in the form of 2×2 tables. Biometrics. 1977;33:593–602. [PubMed] [Google Scholar]
- 7.Guidance for industry: non-inferiority clinical trials (draft guidance) Washington, DC: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2010. ( http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf) [Google Scholar]
- 8.McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17:575–82. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 10.Millikan D, Rice B, Silbergleit R. Emergency treatment of status epilepticus: current thinking. Emerg Med Clin North Am. 2009;27:101–13. doi: 10.1016/j.emc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13:92–4. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.