Abstract
Atherosclerosis continues to be the leading cause of cardiovascular disease. Development of atherosclerosis depends on chronic inflammation in the aorta and multiple immune cells are involved in this process. Importantly, resident macrophages and dendritic cells (DCs) are present within the healthy aorta, but the functions of these cells remain poorly characterized. Local inflammation within the aortic wall promotes the recruitment of monocytes and DC precursors to the aorta and micro-environmental factors direct the differentiation of these emigrated cells into multiple subsets of macrophages and DCs. Recent data suggest that several populations of macrophages and DCs can co-exist within the aorta. Although the functions of M1, M2, Mox, and M4 macrophages are well characterized in vitro, there is a limited set of data on the role of these populations in atherogenesis in vivo. Recent studies on the origin and the potential role of aortic DCs provide novel insights into the biology of aortic DC subsets and prospective mechanisms of the immune response in atherosclerosis. This review integrates the results of experiments analyzing heterogeneity of DCs and macrophage subsets in healthy and diseased vessels and briefly discusses the known and potential functions of these cells in atherogenesis.
Keywords: atherosclerosis, immune response, monocytes, dendritic cells, macrophages
The Discovery of Macrophages and Dendritic Cells within the Aorta
For almost a century, macrophages have been known to be the cells that are involved in atherosclerosis. Anitschkow (1913) discovered that early and advanced atherosclerotic plaques are developed in direct correlation with the amount of dietary cholesterol. Anitschkow also identified some lesional leukocytes as “large cells with a foamy protoplasma” containing cholesterol derivates in small vacuoles, which he considered to be derived from blood lymphocytes or Langerhans’ cells. He named these cells “cholesterolesterphagocytes,” based on their probable etiology and assumed function. The idea that monocytes are involved in the induction and persistence of atherosclerosis was extensively studied during the following decades and this hypothesis received additional confirmation from various immunohistochemical studies. Hoff (1972) reported the existence of mononuclear cells that were ultrastructurally identified as monocytes by the presence of cytoplasmic peroxisomes in the intima, in endothelial junctions, and among the endothelium of human aortas obtained at autopsy. In an electron-microscopic survey of the aorta in the adult rat, white blood cells were found to adhere to the intima; which were invariably lymphocytes or monocytes (Joris et al., 1979). Alpha-naphtyl acetate esterase-positive (ANAE) monocytes were also detected within the central and peripheral areas of lesions within rabbit aortas (Hansson et al., 1980). Further characterization of these cells provided evidence suggesting the existence of aortic macrophages (MΦ; Gerrity, 1981; Jonasson et al., 1986) and CD1a+ dendritic cells (DCs; Bobryshev and Lord, 1998) within the subendothelial space of the intima.
Vascular Dendritic Cells in Healthy Aortas
The phenotype and markers of identification vary for MΦs and DCs within several tissues and often depend on the degree of maturation and activation (Mosser and Edwards, 2008; Geissmann et al., 2010). With respect to DCs located within the aorta, several studies have reported unique features of human aortic DCs that express CD1a+S-100+lag+CD31−CD83−CD86− and resemble Langerhan’s cells (Bobryshev, 2010). These vascular-associated DCs were named vascular DCs (Bobryshev and Lord, 1995). Additional seminal studies demonstrated the presence of resident DCs in apparently healthy, non-diseased aortas (Bobryshev and Lord, 1995; Waltner-Romen et al., 1998; Millonig et al., 2001; Ma-Krupa et al., 2004). Interestingly, DCs display distinct anatomical features when located at different layers of the aorta, and probably the adventitia as well. Structurally low-differentiated DCs are predominantly found in close proximity to the endothelium within the normal intima, whereas DCs with a moderately developed tubulovesicular system are localized throughout the thickness of the tunica intima, mostly being concentrated in the subendothelial space (Bobryshev, 2010), suggesting distinct structural and probably functional differences between subsets of DCs located at different areas of the aortic wall.
Recent studies using advanced techniques such as confocal microscopy, monocyte-labeling technique, and flow cytometry-based analysis have further provided fascinating data about the accumulation of DCs and MΦ within the healthy artery. Flow cytometric analysis of C57BL/6 aortas clearly demonstrated the presence of aortic CD11c+CD40+ cells (Galkina et al., 2006). However, as whole aortas were digested with enzymes, this approach permitted the characterization of leukocytes within the aorta, but did not provide data about the anatomical distribution of DCs within the aortic wall. Additional studies utilizing confocal microscopy revealed the presence of bone-marrow-derived CD11c+ cells within the intima of healthy aortas of C57BL/6 mice (Jongstra-Bilen et al., 2006).
Why would DCs accumulate within the healthy non-diseased artery? It is well-known that atherosclerosis is a site-specific disease characterized by the preferential development of plaques at the lesser curvature of the aorta, and that flow-dependent activation of the aortic endothelium is partially responsible for the accelerated recruitment of monocytes and DC-precursors to atherosclerosis-prone areas. Interestingly, an abundance of CD68+CD11c+ cells, but not CD68+ macrophages were detected within the lesion-susceptible lesser curvature of the healthy aortic intima (Jongstra-Bilen et al., 2006). Thus, the initial localization of intimal CD11c+ cells is determined by the micro-environment at specific anatomical locations. However, the site-specific localization of intimal CD11c+ cells occurs independent of circulating cholesterol levels, highlighting the importance of blood flow patterns rather than plasma lipid levels in the direction of DC localization within the aorta. Additional characterization of DCs by Choi et al. (2009) revealed preferential accumulation of these cells within the cardiac valve and aortic sinus of C57BL/6 mice. These aortic DCs expressed low levels of CD40 and were positive for CD1d, CD80, and CD86 antigens, suggesting that they possess an immature DC phenotype (Table 1).
Table 1.
Location and DC phenotype in healthy and atherosclerotic aortas.
| Dendritic cell phenotype | Study population | Locations | Stages of atherosclerosis (demonstrated) | Reference |
|---|---|---|---|---|
| CD1a+S-100+lag+CD31−CD83−CD86− DCs | Humans | Aortic intima | Bobryshev and Lord (1995) | |
| IFNα+ plasmacytoid DCs | Humans | Carotid and coronary arteries | Type IV–V and VI | Erbel et al. (2007), Niessner et al. (2006) |
| CD11c+CD40+ DCs | C57BL/6 mice | Aorta | Type 0-I | Galkina et al. (2006) |
| CD11c+CD68+ DCs | C57BL/6 mice | Aortic intima (lesser curvature) | Type 0-I | Jongstra-Bilen et al. (2006) |
| CD11c+CD40lowCD1d+ CD80+ CD86+ (immature DCs) | C57BL/6 mice | Aorta, aortic sinus and cardiac valve | Type 0-I | Choi et al. (2009) |
| CD11c+MHC-II+CD11b−F4/80−CD207+CD103+ DCs (Flt-3-dependent, Mn-independent precursors) | C57BL/6 mice | Aorta | Type 0-I | Choi et al. (2011) |
| CD11c+MHC-II+CD11b+F4/80+ CD14+CD103−DC-SIGN+ DCs (M-CSF-dependent, Mn-dependent) | C57BL/6 mice | Aorta | Type 0-I | Choi et al. (2011) |
| CD11c+CD11b−CD68+MHC-II+33D1+ DCs (GM-CSF-dependent proliferation in response to cholesterol) | Ldlr−/− mice (40% fat, 1.25% cholesterol, 1–3 weeks) | Aortic intima | Type 0-I | Zhu et al. (2009) |
| CD11c+CD11b+CD8α−CD115−F4/80−440c− PDCA-1− CCL17+ DCs | Apoe−/− mice (21% fat, 0.15% cholesterol, 8–12 weeks) | Aorta and aortic root | Type I–IV | Weber et al. (2011) |
| CD11clowB-220+CD11b−PDCA-1+ DCs | Ldlr−/− mice (0.25% cholesterol, 8 weeks) | Aorta | Type I–IV | Daissormont et al. (2011) |
CD11c is not a unique marker for DCs, since some subsets of MΦs are CD11c+ (Geissmann et al., 2010). Until recently, questions concerning the origin and sub-type of intimal CD11c+ cells that reside within healthy aortas were unresolved. DCs are generated at least by two major pathways that differ in their requirement for the Flt3/Flt3 ligand (Flt3L) axis. Development of DCs from monocyte-independent precursors is Flt-3/Flt3L-dependent (Naik et al., 2006; Onai et al., 2006; Liu et al., 2009), whereas the generation of DCs from monocytes is Flt3/Flt3L-independent (Cheong et al., 2010). To address the dilemma about the developmental source of aortic CD11c+ cells, Choi et al. (2011) successfully adapted a previously developed flow cytometry-based approach for the analysis of murine aortas (Galkina et al., 2006) and tested the effects of Flt3 on the expansion of aortic CD11c+ cells. Flt3 treatment resulted in an expansion of CD11c+ cells within the intima and adventitia of C57BL/6 mice suggesting a DC origin of CD11c+MHC-IIhigh cells.
Additional studies have also demonstrated the existence of two major subsets of DCs as CD11c+CD11b+F4/80+ and CD11c+CD11b−F4/80− cells within the aortas of C57BL/6 mice (Table 1). CD11c+CD11b−F4/80− cells possessed a distinct phenotype characterized by CD103 and CD207 expression, and were negative for CD8, CD205, CX3CR1, and 33D1 (Choi et al., 2011). CD11c+CD11b+F4/80+CD103− DCs expressed the CD14 co-receptor for TLR4 and DC-SIGN antigen (Table 1). Development of these two subsets of DCs was considerably different: CD11c+CD11b+F4/80+CD103− DCs were M-CSF-dependent, and likely monocyte-derived DCs. In contrast, CD11c+CD11b−F4/80−CD103+ DCs were Flt3-dependent DCs.
DC Functions within Healthy Aortas
The function of vascular DCs within healthy arteries remains unclear; however, recent data suggest that widespread distribution of HLA-DR-expressing cells within the healthy aortic intima may play a role in the maintenance of vascular homeostasis (Bobryshev et al., 2011). Similarly, CD11c+ DCs may play an active role during the initial stages of atherosclerosis. Jongstra-Bilen et al. (2006) demonstrated that aortic resident CD11c+ DCs actively uptake neutral lipids within high cholesterol diet-fed Ldlr−/− mice. Furthermore, as CD11c+ DCs are preferentially located within the lesser curvature of the healthy aortas, the initial accumulation of lipids is directed and regulated by CD11c+ intimal DCs within the atherosclerosis-prone lesser curvature of the healthy aorta. Interestingly, only a specific subset of CD11c+CD11b−33D1− DCs accumulates lipids suggesting that there is a functional complexity between DC subsets in the aorta, which is already reflected at the levels of lipid uptake within relatively non-diseased vessels.
An important question concerning DC functions in atherosclerosis is whether these professional antigen-presenting cells are capable of presenting antigens within the aortic wall. It has been shown that adoptively transferred bone marrow-derived DCs, activated with OVA peptide, induced profound proliferation of TCR-specific lymphocytes that were detected in the spleen and aortas of OT-I recipient mice (Galkina et al., 2006). These results provided initial evidence that T cells residing within the aorta can be activated by DCs, suggesting that this process may be important in the initiation and/or progression of atherosclerosis. However, this study did not directly address whether aortic DCs are capable of presenting antigens to T cells. In a subsequent study, Choi et al. (2009) convincingly showed that isolated aortic CD11c+ DCs are able to present OVA peptide to OT-I and OT-II T cells in vitro and in vivo. Importantly, these results correlate with experiments conducted using a model of engineered bioartificial human arteries that mimic the size and structural dimensions of human arteries. Human CD11c+ DCs seeded within bioartificial arteries migrated to the intima, where they triggered TLR-4-dependent T-cell activation, and initiated an adaptive immune response (Han et al., 2008), suggesting that antigen presentation by human DCs can occur in vivo.
Alteration of Dendritic Cell Populations during Atherogenesis
The number of CD11c+ cells increases with the progression of atherosclerosis in Apoe−/− mice (Galkina and Ley, 2009; Manthey and Zernecke, 2011). DCs are detected in close proximity to T cells in the zones of neovascularization within atherosclerotic plaques, and near the vasa vasorum in the adventitia. In the shoulder regions of unstable human plaques, CD83+ DCs are in close proximity to CD40L+ T cells (Erbel et al., 2007). There is an increasing number of studies that have focused on the mechanisms underlying the accumulation of MΦs within atherosclerotic aortas (Swirski et al., 2009; Ley et al., 2011); however, there is still limited information about the potential mechanisms of aortic DC accumulation in atherogenesis. Increased DC numbers may be due to increased migration from peripheral circulation, reduced emigration out of the vessel wall or increased local proliferation. A recent study clearly demonstrated that at least for a specific subset of intimal CD11c+CD11b−CD68+MHC-II+33D1+ DCs, increased granulocyte/macrophage colony-stimulating factor (GM-CSF)-dependent proliferation might be a reason for elevated levels of intimal DCs in the aorta of Ldlr−/− mice after a short-term high cholesterol diet (Zhu et al., 2009). Interestingly, aortic CD11c+CD11b− DCs proliferate independently from Ly6Chigh monocytes, as BrDU+-proliferating DCs are detected even after PTX-induced blockade of monocyte recruitment to the aorta (Zhu et al., 2009).
Additional characterization of aortas isolated from Ldlr−/− mice fed a high-fat cholesterol diet for 16 weeks further shed light on the dynamics of DC subset accumulation within the aorta (Choi et al., 2009). Unexpectedly, both subsets of aortic CD11c+MHC-II+CD11b+F4/80+ DCs and CD11c+MHC-II+CD11b−F4/80−CD103+ DCs were elevated in atherosclerotic aortas of Ldlr−/− mice (Choi et al., 2009). To further address questions about the origin of intimal DC subsets, Choi et al. (2011) generated Flt3−/−Ldlr−/− mice and investigated the distribution of DC subsets within the aortic intima of atherosclerotic aortas. The numbers of CD11c+CD11b−CD103+ DCs, but not CD11c+CD11b+CD103− DC, were reduced in Flt3−/−Ldlr−/− mice, suggesting that aortic CD11c+CD11b−CD103+ DCs are generated from a Flt3-dependent DC precursor.
Possible Implication of Dendritic Cells in Atherosclerosis
To date, there is strong evidence that DC subsets actively participate in the positive and negative regulation of the immune response. GM-CSF-dependent CD8α+33D1−DEC205+ r cells induces high levels of Th1 cytokines IFNγ and IL-2, whereas CD8α−33D1+DEC205− cells, which are FLT-3-dependent, induce also large amounts of IL-4 and IL-10 (Pulendran et al., 1999). Plasmacytoid DCs (pDCs) sense viral nucleic acids and produce a large amount of type I interferons. But also can play an important role in the induction of tolerance. Lung pDCs induce oral tolerance (Lewkowich et al., 2008), where as gut-associated CD103+ DCs play roles in the regulation of the balance between Tregs and Th1 cells (Annacker et al., 2005).
The identification of DC functions in atherosclerosis has presented a considerable challenge, as aortic DCs display molecular markers and features of several cell types and can be probably derived from multiple progenitors (Geissmann et al., 2010; Niessner and Weyand, 2010). Nevertheless, recent studies have firmly established the presence of DCs within the healthy and atherosclerosis-prone aorta, and have started to address questions about the functions of DC subsets in lymphoid organs and the aorta in atherosclerosis (Randolph, 2009).
Several lines of evidence suggest that hypercholesterolemia affects the ability of DCs to migrate from peripheral tissues to draining lymph nodes during atherogenesis (Angeli et al., 2004). Prolonged retention of MΦs and DCs might also support their pro-inflammatory status and the progression of lesions within atherosclerotic aortas (Llodra et al., 2004). However, evidence also suggests that DCs maintain their capacity to prime naïve T cells under the conditions of hypercholesterolemia (Packard et al., 2008). DCs isolated from Ldlr−/− or Apoe−/− mice efficiently induce T-cell proliferation, IFNγ, and TNFα production after ex vivo co-culture with naïve CD4+ T cells (Packard et al., 2008). It is worth noting that hyperlipidemia also increases the levels of blood circulating CD11c+ monocytes and upregulates their expression of CD11b and CD29. CD11c deficiency reduces monocyte/macrophage accumulation in atherosclerotic lesions and attenuates atherogenesis in Cd11c−/−Apoe−/− mice fed a high-fat diet. Interestingly, deficiency of CD11c decreases the firm arrest of mouse monocytes to vascular cell adhesion molecule-1 (VCAM-1) and E-selectin in a shear flow assay (Wu et al., 2009). Thus, elevated cholesterol levels not only provide possible antigens for DCs, but also regulate the accumulation of CD11c+ monocytes and potentially DC numbers in the aortic wall.
Dendritic cells are specialized in capturing, processing, and presenting antigen-derived peptides on major histocompatibility complex (MHC) molecules in vivo, thereby, allowing DCs to shape T-cell responses to modulate immunity or tolerance (Steinman, 2007). Numerous bodies of evidence have demonstrated that oxLDL plays a pivotal role in the formation of foam cells, induction of vascular dysfunction, and the support of leukocyte migration to the atherosclerosis-prone aorta (Matsuura et al., 2006). Importantly, modified lipids likely affect the maturation and phenotype of DCs. OxLDL during the late stage of monocyte differentiation gives rise to phenotypically mature DCs that secrete IL-12 but not IL-10, and support both syngeneic and allogeneic T-cell stimulation (Perrin-Cocon et al., 2001). In contrast, oxidized phospholipids (ox-PLs) alter DC activation and prevent their maturation through the blockade of TLR-3- and TLR-4-dependent induction of CD40, CD80, CD83, and CD86 (Bluml et al., 2005). It remains to be determined if only specific sets of lipids affect DC functions and whether distinct subsets of DCs respond selectively to modified lipid loading.
oxLDL serves as a potential source of antigens in atherosclerosis and DCs might present antigens derived from oxLDL to induce a specific T-cell response. Additionally, not only oxLDL, but also other antigens, including beta-2 glycoprotein I (β2GPI) and heat shock proteins HSP-60 and HSP-65 activate T cells. Recently, our understanding of the oxidation hypothesis of atherosclerosis was altered and some attention has shifted toward native LDL, as potential antigens for atherosclerosis. In the elegant and extensive work by Hermansson et al. (2010), the authors focused on identifying the potential mechanism of oxLDL recognition by T cells via generation of T-cell hybridomas from human apolipoprotein B100 transgenic mice that were immunized with human oxLDL. Unexpectedly, T-cell hybridomas responded to native and purified LDL apolipoprotein ApoB100, but not to oxLDL, suggesting the existence of an immune response against native LDL. Subsequent studies have demonstrated that immunization with lipid-loaded DCs resulted in various effects on the progression of atherosclerosis and local modulation of inflammation within the aortic wall (de Jager and Kuiper, 2011). However, despite important insights into the biology of DCs, there are a limited number of studies that have tested the effects of oxLDL and native LDL on DC functions, and the consequences of modified lipid uptake for T-cell differentiation and activation.
To investigate the role of CD11c+ DC in atherogenesis, Gautier et al. (2009) generated a mouse model with the over-expression of the anti-apoptotic gene hBcl-2 under the control of the CD11c promoter. It should be noted that Bcl2 expression was not detected on pDC and CD11b+F4/80+ monocytes indicating that in this model, the major effects of Bcl2 over-expression were directed toward CD11chighMHC-IIhigh cells. The CD11chighMHC-IIhigh DC population was increased in DC-hBcl2 mice, and was associated with enhanced T-cell activation, elevated levels of Th1, Th17-related cytokines, and increased production of Th1-driven IgG2c antibodies. Unexpectedly, increased levels of CD11c+ cells also resulted in reduced plasma cholesterol. In line with these results, the depletion of DCs in hyperlipidemic CD11c-diphtheria toxin receptor (DTR) Apoe−/− transgenic mice resulted in enhanced cholesterolemia. These data suggest a strong relationship between CD11c-expressing cells and cholesterol homeostasis. To date, the mechanisms that regulate CD11c+ cell-dependent cholesterol metabolism are unknown.
A new approach was recently taken to study the potential implication of CD11c+CD11b−CD103+ classical DCs in atherosclerosis using Flt-3−/−Ldlr−/− mice (Choi et al., 2011). Deficiency of Flt-3 and therefore, depletion of CD11c+CD11b−CD103+ DCs resulted in increased plaque burden within the aortic sinus and the aortic arch. This accelerated atherogenesis was accompanied by the reduction of Treg in the aorta and increased levels of aortic IFNγ and TNFα without significant alteration in the lipid levels (Choi et al., 2011). Thus, aortic CD11c+CD103+ DCs likely function as tolerogenic DCs during atherosclerosis. Although traditionally viewed as the main inducers of immunity, DCs are also important players in the induction and maintenance of peripheral self-tolerance (Steinman et al., 2003). As Treg cells are the key component of a suppressor anti-atherogenic arm of the immune response, aortic CD11c+CD103+ DCs may play an important role in their generation. Recently another subset of CCL17-expressing DCs was identified as an important player in atherosclerosis (Weber et al., 2011). DC-derived CCL17 limited the expansion of Tregs and increased the plaque burden. In contrast, blocking antibodies against CCL17 maintained Treg differentiation and attenuated atherosclerosis. These new results suggest the existence of a CCL17-specific DC subset that has the capacity to alter Treg homeostasis in atherogenesis. To date, the mechanisms that control the number and suppressor functions of Tregs in atherosclerosis are not well understood, but the recently discovered functions of CD11c+CD11b−CD103+ DCs and CCL17+DCs offer some evidence to suggest that DC-mediated regulation of Treg occurs in atherosclerosis.
Plasmacytoid DCs
Recent studies have further characterized several subsets of DCs and have resulted in a deeper understanding of the heterogeneity and origin of vascular DCs. pDCs are a distinct subset of DCs specialized in direct virus TLR7, TLR8, and TLR9-dependent recognition that results in rapid induction of high levels of type I interferon (interferon α/β, IFN) and other cytokines. pDCs are closely related to classical antigen-presenting DC, since these cells have some common developmental origin and genetic similarity (Reizis et al., 2011). The characterization of pDC lineage has presented a considerable challenge, as pDCs display molecular markers and features of several cell types, and can be derived from multiple progenitors. Typically, pDCs show only low expression of CD11c, while this marker is abundantly expressed by myeloid DCs (mDCs). Ironically, pDCs also express some lymphocyte antigens including CD4, Rag-1, CD45RA/B220, and pIII transcript of the MHC class II transactivator (CIITA). One of the remaining enigmas of pDC biology concerns their ability to present antigen (Villadangos and Young, 2008). pDCs are found to be weak antigen-presenting cells compared with classical DCs. Instead, pDCs are considered immunomodulating cells that can direct the immune response by tipping the balance of T helper responses via their secretion of type I interferons.
There are several lines of evidence suggesting that pDCs might have a role in atherosclerosis. IFNα+ pDCs are detected within atherosclerosis-prone vessels (Bobryshev and Lord, 1995; Niessner et al., 2006; Daissormont et al., 2011), with preferential expression within the shoulder region of atherosclerotic plaques. One of the important pieces of data to suggest a pro-inflammatory role of pDCs came from a study demonstrating that type I IFNs, released by pDCs, can induce the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on CD4+ T cells (Niessner et al., 2006). Furthermore, adoptive transfer of human plaque-isolated CD4+ T cells into immunodeficient mice that are engrafted with human atherosclerotic plaque results in apoptosis of VSMCs in a TRAIL-dependent manner (Sato et al., 2006). Altogether, these series of experiments suggest an important link between aortic pDCs, the induction of TRAIL on T cells, and possible TRAIL-dependent T cell-induced apoptosis of VSMCs.
In addition to the activities of pDCs as an immunogenic cell sentinel, pDCs can also act as tolerogeneic cells when expressing inducible tolerogenic enzyme indoleamine 2,3-dioxygenase (IDO), the inducible costimulator ligand (ICOS-L), and/or the programmed death 1 ligand (PD-L1), which mediates Treg development (Colonna et al., 2004; Matta et al., 2010). A recent study by Daissormont et al. (2011) further addressed the potential role of pDCs and reported an anti-inflammatory activity of pDCs during atherogenesis. Surprisingly, pDC depletion by 120G8 mAb, which recognizes PDCA-1 (marrow stromal cell antigen 2, BST2), a marker exclusively expressed on mouse pDC, increased plaque burden in Ldlr−/− recipient mice (Daissormont et al., 2011). This acceleration of atherosclerosis was accompanied by increased T-cell proliferation, elevated IFNγ production and reduced expression of IDO in pDCs isolated from lymphoid tissue. These results highlight the tolerogenic functions of pDCs and suggest that this subset of DCs may play an important role in maintaining the balance between Th1 and Treg cells during atherogenesis in aortas and secondary lymphoid organs. Eicosapentaenoic acid (EPA) has beneficial effects on cardiovascular diseases, although the precise mechanism is unknown. Interestingly, the treatment with EPA attenuates atherosclerosis and increases the number of CD11c+CD80-CD86- DCs expressing IDO in the spleen, therefore inducing tolerogenic phenotype of DCs (Nakajima et al., 2011).
Plasmacytoid DCs are not tolerogenic under basal, non-stimulated conditions and have shown no IDO expression. Specific engagement of B7-1 receptors by CD152-Ig (CTLA-4-Ig) or CD200R1 by CD200-Ig lead to initiating of IDO-dependent tolerance (Reizis et al., 2011). Thus, the functional relationships between pDCs roles as the inducers of the immune response versus tolerance are dependent on multiple ligands and likely cytokines that contribute to the expression of a tolerogenic phenotype by pDCs. Although the somewhat current data about the role of pDCs are debatable, they clearly emphasize the complexity of pDC functions in atherosclerosis. It will be important to understand how increased levels of potential antigens from the necrotic core and the alterations in the T-cell response affect DC functions during atherogenesis. Recent studies that have focused on the role of DCs in atherosclerosis also raise the tantalizing question of whether the antigen-presenting functions of DCs will be a key component in the regulation of the immune response in atherosclerosis.
The Role of Macrophages in Atherosclerosis
Macrophages (MΦ) were the first inflammatory cells to be associated with atherosclerosis, where they represent the major cell type in atherogenesis and play important roles in the progression of the lesions. The essential role of MΦs in the initiation and progression of atherosclerosis was initially demonstrated using M-CSF-deficient osteopetrotic (op) Apoe−/− mice, which resulted in a dramatic 86% reduction in plaque volume (Smith et al., 1995). More recently, a model of CD11b promoter-diptheria toxin transgenic Apoe−/− chimeric mice with CD11b-DTR bone marrow was used to deplete CD11b+ cells and test the role of CD11b+ cells in atherogenesis (Stoneman et al., 2007). CD11b+ cell depletion significantly affected plaque development and altered plaque composition in the early stages of atherogenesis. However, despite a 50% reduction of monocytes in mice with advanced plaques, the depletion of CD11b+ cells had no effect on the extent of plaques or the composition of advanced lesions. Because monocytes, MΦ, neutrophils, and a subset of DCs express CD11b, and have been implicated in the pathology of atherosclerosis, the specific contributions of MΦ and MΦ subsets to the observed phenotypes were uncertain. Using an analogous method, Paulson et al. (2010), recently used CD11c-DTR Ldlr−/− chimeric mice to investigate the role of CD11c+ DCs in atherogenesis (Gautier et al., 2009). CD11c-DTR Ldlr−/− chimeric mice fed a cholesterol-rich diet for 5–10 days displayed a 55% reduction in intimal lipid area in comparison to un-depleted control mice, suggesting a pro-atherogenic role for CD11c+ DCs. However, as foam cells can similarly express CD11c, it is unclear whether the observed effects are due to the removal of foam cells or the removal of DCs from the forming atherosclerotic lesions.
Macrophage Subsets
In the initial stages of atherogenesis, blood monocytes adhere to and transmigrate through the endothelial cell layer to differentiate into specialized mononuclear phagocytes. The process of deriving polarized aortic MΦ and/or DCs from monocytes involves a variety of factors including components of the extracellular matrix, pro-inflammatory cytokines/chemokines, anti-inflammatory cytokines/chemokines, oxidized or modified LDL, and CD40/CD40L (Ley et al., 2011; Moore and Tabas, 2011). Recently, the process of MΦ polarization during atherogenesis has been a subject of interest as MΦ subsets have been demonstrated to display some degree of plasticity and heterogeneity within atherosclerotic lesions (Ley et al., 2011; Moore and Tabas, 2011; Wolfs et al., 2011). Initially, a model of two modes of MΦ activation was proposed to differentiate between inflammatory MΦs (M1) and anti-inflammatory MΦs (M2; Gordon and Taylor, 2005). Pro-inflammatory M1 MΦs were originally described from studies conducted in the 1960s by Mackaness and colleagues (Blanden et al., 1969) who demonstrated that murine MΦs displayed enhanced anti-microbial activity, in a stimulus dependent manner, in response to Mycobacterium bovis bacillus Calmette–Guerin (BCG) or Listeria monocytogenes infections (Blanden et al., 1969). In contrast, Stein et al. (1992) demonstrated in 1992 that MΦs could be polarized to an “alternative” state (M2) in the presence of IL-4 characterized by expression of the macrophage mannose receptor (CD206). More recently, with the addition of the newer M2 subsets (M2a-c), Mox, and M4 subsets the paradigm of MΦ activation has been further extended (Ley et al., 2011; Wolfs et al., 2011).
M1 Macrophages
Following the discovery that M1 and M2 MΦ subsets are generated in different inflammatory conditions, the attention of the field was directed toward investigating the generation, phenotype, and possible functions of aortic MΦs during atherogenesis. In order to better understand the properties of MΦ subsets and identify new markers for the recognition of M2 MΦs, several approaches for the generation of MΦ subsets were developed. To date, both human and murine monocytes can be polarized into various MΦ subsets in vitro depending on the culture conditions used. Monocyte/macrophage colony-stimulating factor (M-CSF) is a key cytokine for the generation of un-polarized MΦs from monocytes in vitro (Stanley et al., 1978). Un-polarized MΦs can then be driven toward polarized MΦ subsets based on environmental cues. M1 MΦs were originally derived and characterized through the combined treatment of un-polarized MΦs with IFNγ and TNFα. This treatment results in the generation of M1 MΦs that strongly produce pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-12, and TNFα, and possess enhanced microbicidal activity (reviewed in Mosser and Edwards, 2008; Ley et al., 2011; Wolfs et al., 2011). Similarly, the use of MyD88-dependent TLR agonists and IFN-γ are effective in producing M1 MΦs in vitro (Mosser and Edwards, 2008). M1 MΦs have been described to exert definitive pro-inflammatory roles, thereby worsening the progression of autoimmune disorders like rheumatoid arthritis, Crohn’s disease, multiple sclerosis, or protecting the host from infectious microorganisms (Murray and Wynn, 2011).
While information on the polarization of MΦs in vitro is useful as a starting point, the situation in vivo is quite complex. Following transmigration, monocytes can differentiate into MΦs via intimal M-CSF (Smith et al., 1995). During this process, un-polarized MΦs upregulate the expression of pattern recognition receptors, including toll-like receptors, c-type lectin receptors, scavenger receptors, retinoic acid-inducible gene 1-like helicase receptors, and NOD-like receptors, in order to search the micro-environment for pathogens, foreign substances, apoptotic cells, and oxLDL (Murray and Wynn, 2011). In response to Th1, γδ T cell, NK, and NKT cell-derived IFNγ and pro-inflammatory TNFα, MΦs can polarize toward a classic M1 phenotype that is characterized by high expression of macrophage receptor with collagenous structure (Marco), suppressor of cytokine signaling 3 (Socs3), inducible nitric oxide synthase (Nos2), TNF-α, IL-1β, IL-6, IL-12, IL-23, cyclooxygenase 2 (Cox2), indoleamine 2,3-dioxygenase 1 (Ido1), and the production of reactive oxygen and nitrogen species (Murray and Wynn, 2011). In the context of atherosclerosis, several lines of evidence exist to suggest that M1 MΦs are located within both human and murine atherosclerotic plaques. Bouhlel et al. (2007) demonstrated the presence of CCL2+CD206neg M1 MΦs in human endartectomy specimens. In murine aortas, M1 MΦs are found in high-fat diet-fed Ldlr−/− aortas by immunofluorescence and flow cytometry (Kadl et al., 2010). Interestingly, Khallou-Laschet et al. (2010) demonstrated initial accumulation of M2 MΦs and further increased in M1 MΦs within the advanced lesions of Apoe−/− mice.
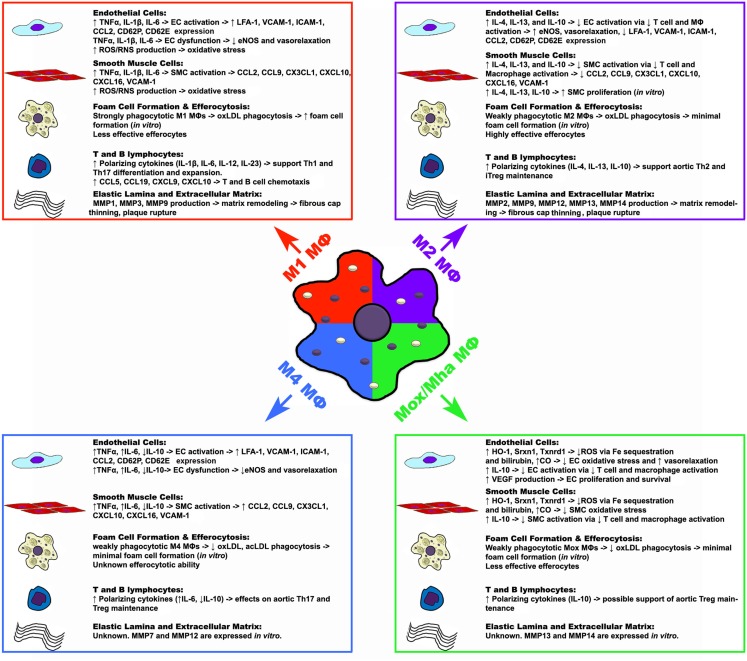
While the specific effects of M1-derived pro-inflammatory factors have not been assessed in atherosclerosis, in vitro data and global cytokine knockout studies have provided some insight concerning the functions that M1 MΦs may exert in situ. The effects of TNFα and other pro-inflammatory cytokines on the vascular endothelium and smooth muscle cell layer are well established. As M1 MΦs secrete elevated levels of TNFα, IL-1β, and IL-6, these M1-derived cytokines may play a role in further activating endothelial and smooth muscle cells, resulting in the upregulation of endothelial and smooth muscle cell chemokines; and may play a role in endothelial dysfunction via down-regulation of endothelial eNOS expression and ROS/RNS driven oxidative stress (Figure 1; Table 2). Similarly, M1 MΦs may play a role in driving the generation of the pro-inflammatory Th1 cell and controversial Th17 cell subsets, thereby promoting further inflammation (Butcher and Galkina, 2011; Murray and Wynn, 2011). In addition, as bone marrow macrophage-derived M1 MΦs strongly phagocytose oxLDL, secrete matrix metalloproteases (MMP1, MMP3, and MMP9), and are poor efferocytes in vitro, M1 MΦs may play a role in promoting the formation of a large necrotic core, plaque destabilization, and thrombus formation (Ley et al., 2011; Moore and Tabas, 2011; Murray and Wynn, 2011; Wolfs et al., 2011).
Figure 1.
Potential functions of macrophage polarization states in atherosclerosis. Upon activation, macrophages can assume different polarization states in response to environmental cues, which may have various effects on the components of atherosclerotic plaques. While M1, M2, and Mox/Mha subsets have been shown to exist in atherosclerotic plaques, the presence of M4 macrophages have yet to be shown. Macrophage-derived cytokines, chemokines, other factors, and possible effects are listed in the box. ↑, increase; ↓, decrease; →, result or effect; CO, carbon monoxide; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule 1; MΦ, macrophage; MMP, matrix metalloproteinase; SMC, smooth muscle cell; Srxn1, sulfiredoxin 1 homolog; Txnrd1, thioredoxin reductase 1; VCAM-1, vascular cell adhesion molecule 1.
Table 2.
Possible implications of MΦ subsets in atherosclerosis.
| MΦ subsets | Cytokine production | Chemokine production | Other secreted factors | Markers and enzymes | Efferocytosis | Plaque stability |
|---|---|---|---|---|---|---|
| M1 MΦ | TNFα, IL-1β, IL-6, IL-12, IL-15, IL-18, IL-23, and TRAIL (Martinez et al., 2006; Ley et al., 2011) | CCL5, 9, 15, 19, 20, CXCL1, 2, 3, 9, 10, and 11 (Gordon and Taylor, 2005; Martinez et al., 2006) | NO (Martinez et al., 2006; Murray and Wynn, 2011) | Mm – F4/80, Ly6Chigh–low, Ly6C/Ly6Glow-neg Hs – CD14high–low,CD16low-neg Both – CD45, CD11b*, CD86,CD11c*, CD68, CD115, IL-4R, MHC-I, MHC-II, Marco, Socs3, iNOS. (Murray and Wynn, 2011) |
Less effective efferocytes. IL-4, IL-10, and pro-resolving eicosanoids promote a M1→M2 transition. | ↑ MMP1, MMP3, and MMP9 expression. |
| M2 MΦ | IL-4, IL-10 and IL-13 (Martinez et al., 2006; Ley et al., 2011) | CCL13, 18, 23, 24, and CXCL13 (Gordon and Taylor, 2005; Martinez et al., 2006) | IGF-1 (Martinez et al., 2006; Murray and Wynn, 2011) | Mm – F4/80, Ly6Chigh–low, Ly6C/Ly6Glow-neg, Chi3l3, Relmα, Hs – Chi3l2 Both – CD45, CD11b*, CD11c*, CD68, CD115, CD206, Arg1, IL-4R, Klf4, Socs2, Irf4, Chia, Dectin-1 (Murray and Wynn, 2011) | Highly effective efferocytes. IL-10 and pro-resolving eicosanoids → ↑ efferocytosis (Tabas, 2010) | ↑ MMP2, MMP9, MMP12, MMP13, and MMP14 expression. |
| Mox Mha MΦ | IL-10 and IL-1β (Kadl et al., 2010) | THP-1 cells migrate to Mox supernatants (Kadl et al., 2010) | VEGF, CO, Biliverdin and Bilirubin (Kadl et al., 2010) | Both – Hmox1, Srxn1, Txnrd1, Gclm, Gclc, Trb1, Cox2, Nrf2, Klf4, Cebpb, HLA-DRlow (Kadl et al., 2010; Boyle et al., 2011) | ↓Efferocytosis of apoptotoic thymocytes in vitro (Kadl et al., 2010) | Unknown. |
| M4 MΦ | TNFα and IL-6 (Gleissner et al., 2010b) | CCL18 and CCL22 (Gleissner et al., 2010b) | Unknown | Hs – CD45, CD14, and CD86 (Gleissner et al., 2010a) | Unknown | ↑ MMP12, MMP7 expression in vitro. |
MΦ, macrophage; IL, interleukin; TNFα, tumor necrosis factor alpha; NO, nitrous oxide; IGF-1, insulin like growth factor 1; VEGF, vascular endothelial growth factor; CO, carbon monoxide; iNOS, inducible nitric oxide synthase, Arg, arginase; *, tissue location dependent; Mm, mouse-specific expression; Hs, human-specific expression.
M2 Macrophages
M2 MΦs were originally derived from monocyte-derived MΦs with M-CSF and IL-4 (Stein et al., 1992) and defined based on the expression of the macrophage mannose receptor (CD206); however, more recent studies have found that MΦ phenotypes reminiscent of M2 MΦs can also be produced in vitro. In vitro, alternative MΦs (M2a) can be polarized using M-CSF and the Th2-related cytokines IL-4 or IL-13. Similarly, the M2b phenotype can be obtained in the presence of immune complexes and IL-1β or LPS, while M2c MΦs can be derived using IL-10, TGFβ, or glucocorticoids (Mosser and Edwards, 2008; Ley et al., 2011; Wolfs et al., 2011). What would be a source of M2 cytokines within the atherosclerotic aorta? Mast cells and Th2 cells are found within atherosclerotic aortas and can serve as a potential source of IL-1β, IL-4, IL-13, and IL-10 cytokines, which may in turn polarize MΦs toward alternative activation phenotypes.
In contrast to pro-inflammatory M1, M2 MΦs have been described as wound-healing MΦs, based on their ability to promote wound healing through matrix remodeling, efferocytosis, and the recruitment of fibroblasts (Gordon and Martinez, 2010; Murray and Wynn, 2011). In general, M2 MΦs characteristically express CD206, resistin-like molecule alpha (Relma; Fizz1), suppressor of cytokine signaling 2 (Socs2), interferon regulatory factor 4 (Irf4), chitinase, acidic (Chia), chitinase 3-like protein 2 (Chi3l1; Gp39, Ykl40), chitinase 3-like protein 2 (Chi3l2; Ykl39), chitinase 3-like protein 3 (Chi3l3; Ym1), CXCL13, CCL12, CCL24, and Krüppel-like factor 4 (Klf4; Murray and Wynn, 2011), which can be used to identify them in vivo (Table 2). In the context of atherosclerosis, M2 MΦs have been observed within both human and murine atheroma. Bouhlel et al. (2007) first demonstrated the presence of CD206+ M2 MΦs within human endarterectomy specimens in the stable areas of the plaque. Chinetti-Gbaguidi et al. (2011) demonstrated that CD68+CD206+ M2 MΦs, which localized far from the lipid core in comparison to CD68+CD206− M1 MΦs, contained smaller lipid droplets as demonstrated by Oil red O staining.
Similar to M1 MΦs, while the effects of M2-secreted cytokines, chemokines, and small molecules have not been specifically assessed in atherosclerosis, in vitro data and global cytokine knockout studies have provided some insight concerning the possible functions M2 MΦs in vivo (Figure 1). Depending on the conditions in vitro, M2 MΦs may produce anti-inflammatory cytokines including, IL-4, IL-13, IL-10. Based on studies of global cytokine knockout mice in atherosclerosis (Kleemann et al., 2008), M2 MΦ-derived IL-4, IL-13, and IL-10, through their immunosuppressive effects on T cell and MΦ activation, may lead to decreased endothelial cell and smooth muscle cell activation; leading to decreased pro-inflammatory chemokine expression, increased endothelial eNOS expression, vasorelaxation, and smooth muscle cell proliferation (Figure 1; Table 2). Additionally, M2-secreted IL-4, IL-13, and IL-10 may support the generation of anti-inflammatory Th2 cells and immunosuppressive Treg, favoring alternative inflammation. In contrast to M1 MΦs, bone marrow-derived M2 MΦs are unable to phagocytose oxLDL efficiently but are highly effective efferocytes that secrete a variety of matrix metalloproteases (MMP2, MMP9, MMP12, MMP13, MMP14), suggesting that M2 MΦs may promote the clearance of apoptotic cells in early atherosclerotic plaques but could destabilize the plaque in the advanced stages of the disease.
Mox/Mha Subset of Macrophages
Recently, an additional MΦ subset, namely Mox MΦs, has been proposed (Kadl et al., 2010). Un-polarized bone marrow-derived MΦ cultures that were treated with the ox-PL 1-palmitoyl-2arachidonoyl-sn-glycero-3-phosphorylcholine generated a population of MΦs that expressed a unique profile of genes including Heme oxygenase-1 (HO-1), sufiredoxin-1 (Srnx1), and thioredoxin reductase 1 (Txnrd1) in a nuclear factor, erythroid-derived 2, like 2 (Nrf2) dependent manner (Kadl et al., 2010). This population of Mox MΦs was also found within the aortas of 30 week western diet-fed Ldlr−/− mice by immunohistochemistry and flow cytometry. In addition to their hallmark expression of HO-1, Mox MΦs expressed the anti-oxidant enzymes Txnrd1, and Srnx1 by immunofluorescence. This unique population of Mox (CD45+CD11b+F4/80+HO-1+ cells) MΦs comprised 23% of the aortic CD11b+F4/80+ population. The remaining 39% of aortic CD11b+F4/80+ cells were classified as CD11b+F4/80+CD86+ M1 MΦs and 22% expressed the M2 marker CD206 (Kadl et al., 2010). The proposed phenotype of Mox MΦs closely resembles the phenotype of the recently proposed hemorrhage-associated MΦs (Mha; Boyle et al., 2009; Boyle et al., 2011). Human monocyte-derived MΦs that are treated in vitro with hapto-hemoglobin complexes or oxidized red blood cells were found to upregulate CD163, HO-1, and IL-10 in an Nrf2-dependent manner (Boyle et al., 2009). These CD163+CD68+ MΦs were associated with the fibrous cap region and regions of hemorrhage within human coronary artery plaques. These results suggest that Mox MΦs, which are present within the atherosclerotic mouse lesions, are phenotypically similar to Mha MΦs, and underscore the heterogeneity of MΦs with atherosclerotic vessels.
At present, little is known about the functionality of Mox/Mha MΦs in vivo (Figure 1). As Mox/Mha MΦs express IL-10, VEGF, and enzymes with anti-oxidizing activities, they may exert anti-inflammatory actions on the vasculature in vivo. In this regard, HO-1, Srxn1, and Txnrd1, through the sequestration of iron, the production of bilirubin, and carbon monoxide (Otterbein et al., 2003; Wolfs et al., 2011), may protect endothelial cells and smooth muscle cells from oxidative stress and promote vasorelaxation through the production of carbon monoxide (Figure 1; Table 2). In addition, Mox/Mha-derived IL-10 and VEGF may play anti-inflammatory roles through the suppression of T-cell and MΦ activation, and the promotion of endothelial cell proliferation and survival, respectively (Kadl et al., 2010; Wolfs et al., 2011). However, data from in vitro phagocytosis assays demonstrated that Mox MΦs are poor efferocytes and are only weakly able to phagocytose oxLDL, suggesting that Mox/Mha MΦs may not be able to effectively clear apoptotic cells and resolve inflammation in vivo (Kadl et al., 2010; Wolfs et al., 2011).
M4 Macrophages
Recently, Gleissner et al. (2010b) identified a unique subset of M-CSF/CXCL4-dependent macrophages that was termed M4 MΦs. M4 MΦs expressed a unique set of transcripts, including higher levels of Cd86, tumor necrosis factor (ligand) superfamily member 10 (Tnfsf10), mannose receptor, c type 1 (Mrc1), Ccl18, Ccl22, Tnf, and lower levels of pentraxin 3 (Ptx3), Cd36, and Il10. In this study, M4 MΦs were weakly phagocytic and unable to efficiently phagocytize acLDL or oxLDL, suggesting that M4 MΦs may not readily become foam cells or function as efferocytes within atherosclerotic plaques. The functions of M4 MΦs remains poor understood. In the context of atherosclerosis, atherosclerotic lesions have been demonstrated to contain M1, M2, and M4 marker-expressing MΦs, suggesting that the pool of lesional MΦs may play different roles in the pathology of atherosclerosis (Figure 1; Table 2). For more information on M4 MΦs we refer the reader to the pertinent review by C. Gleissner in this issue.
Mixed Phenotype and Plasticity of Macrophage Subsets
Although the in vitro conditions of generation of MΦ subsets are very distinct and require specific sets of cytokines, several subsets of MΦs likely exist simultaneously within atherosclerotic aortas in vivo. As alluded to in the preceding sections, the murine and human atherosclerotic plaque MΦ pool is, in reality, comprised of a complex mixture of MΦ subsets; which work together to result in the progression and persistence of atherosclerotic plaques. There is some evidence, mainly from in vitro studies, to suggest that the phenotypes of MΦ subsets are not fixed, and that these cells possess some phenotypic plasticity in response to micro-environmental factors. For example, Khallou-Laschet et al. (2010) reported that bone marrow-derived MΦs from C57BL/6 and Apoe−/− mice driven to M1 or M2 phenotypes are able to assume the opposing phenotype (M2 or M1, respectively) when the culture conditions are switched. This idea is also supported by the work of Feig et al. (2011), in which murine aortic sections were examined for the expression of M2 MΦ markers in a model of athero-regression via laser capture microdissection. Apoe−/− aortic arches were transplanted into C57BL/6, ApoAI−/−, human apoAI Apoe−/− transgenic, and Apoe−/− mice and assessed for pro-inflammatory chemokines, MHC, class 1-related (Mr1), Cd163, C-lectin, and Fizz-1 expression 1-week post transplantation. In this model, lesional CD68+ MΦs expressed higher levels of M2 markers within athero-regressive C57BL/6 and hApoeAI Apoe−/− transgenic mice, suggesting that plaque MΦs can assume a M2 phenotype in the athero-regressive state. Recently in different system, Liao et al. (2011) examined the role of Krüppel-like factor 4 (KLF-4) in MΦ polarization in vitro, and in a model of diet-induced obesity. KLF4 was strongly induced in M2 MΦs, where it interacted with Stat-6 to reinforce the expression of M2 genes and antagonized M1 gene expression via the sequestration of co-activators of NF-kB activation in vitro. In a model of diet-induced obesity, LysM-Cre-Klf4flox/floxC57BL/6 bone marrow chimeras displayed significant increases in total body weight, subcutaneous and visceral fat depot sizes, glucose intolerance, and insulin resistance, which corresponded with elevated M1 related gene expression within the stromal vascular fractions of LysM-CreKlf4flox/floxC57BL/6 chimeras in comparison to Klf4flox/floxC57BL/6 chimeras. These results suggest that KLF4 may similarly play a role in the formation of M2 MΦs in atherosclerotic plaques.
Thus, at present, the functions and specific contributions of MΦ subsets to the pathogenesis of atherosclerosis are largely unknown. While in vitro MΦ polarization and global cytokine knockout studies have provided some mechanistic insight, novel techniques to specifically target MΦ subsets or MΦ effector molecules are necessary to further clarify the roles of MΦ subsets in atherosclerosis. In addition, anticipated follow-up studies of Mox/Mha and M4 MΦ in atherosclerosis should help to clarify the functions of these subsets in atherogenesis.
To date, it is clear that both MΦs and DCs display phenotypic and functional heterogeneity within atherosclerotic and non-diseased arteries. However, as the majority of the studies have examined either DC or macrophage heterogeneity at a single time point, it is unclear how these two populations change as atherosclerotic plaques develop and progress. According to the American Heart Association guidelines for the histological classification of atherosclerotic plaques, there are six distinct stages of atherosclerotic lesions (Stary et al., 1995). These stages range from clinically silent initial (I), fatty streak (II), and intermediate lesions (III), to clinically overt atheroma (IV), fibroatheroma (V), and complicated lesions (VI). Mouse models of atherosclerosis, including Apoe−/− and Ldlr−/− mice, develop atherosclerotic lesions in a similar manner, ranging from initial (I), fatty streak (II) lesions to intermediate (III), atheroma (IV), and fibrous plaques (V) (Nakashima et al., 1994). Thus, based on these lesion stage definitions, we have noted (Table 1) the stage(s) at which dendritic and macrophage subsets have been observed. In general, while mouse studies have primarily focused on DCs in the non-atherosclerotic C57BL/6 aorta or initial stages of atherosclerosis in Apoe−/− and Ldlr−/− mice, human studies have focused on vascular DCs within late stage symptomatic or asymptomatic endarterectomy patients. As DC subsets have been shown to exert pro-inflammatory (Weber et al., 2011) and tolerogenic (Choi et al., 2011) functions, examining the dynamics of DC subset recruitment and functions within the aorta during atherogenesis will likely result in new therapeutic opportunities for targeting pro-inflammatory DCs or developing vaccines. Similarly, while MΦs represent the majority of leukocytes within atherosclerotic plaques, the specific functions of MΦ subsets have only recently come under consideration. While macrophages are present throughout the development of atherosclerosis, little is known about the specific recruitment or egress of M1 and M2 MΦs from atherosclerotic plaques.
Concluding Remarks
Our understanding of the mechanisms of atherosclerosis has notably progressed in parallel with work adding to our understanding of immune system under homeostatic and inflammatory conditions. Recent breakthrough discoveries on the plasticity and heterogeneity of monocytes, MΦs, and DCs have opened new directions for the investigation and further understanding of the potential roles of multiple subsets of DCs and MΦs in the initiation, maintenance and resolution of atherosclerosis. To date, multiple lines of evidence clearly demonstrate that DCs and MΦs represent heterogeneous populations with distinct phenotypes in vitro and in vivo within atherosclerotic aortas. However, the functions and specific mechanisms that control the generation of distinct MΦ and DC subsets within atherosclerotic vessels are poorly understood. In this review, we have focused on the characterization and potential implications of DC and MΦ subsets within healthy and atherosclerotic aortas (Tables 1 and 2).
Our current understanding of the functions of MΦ subsets in vivo relies heavily upon their generation and functions in vitro, as well as, the effects of global cytokine, chemokine, and enzyme knockouts, rather than the effects of MΦ subset-specific knockouts. Cell-type specific knockouts using the Cre-Lox recombination strategy and transgenic mice have been used to specifically knockout genes within myeloid cells; however, as the expression profiles of different myeloid cells can be fairly similar, unintended effects can occur. Unfortunately, at present, MΦ subset-specific Cre-mice have not been reported, and the precise functions of MΦ subsets in vivo remain somewhat elusive.
What may we conclude at the present time about the effects of different subsets of DCs and MΦs on the biology of the aorta? First, distinct subsets of resident aortic DC and MΦs may be important contributors to balancing the activation and suppressor arms of the immune response within the normal, non-inflamed aorta. Additional studies that will be devoted to the biology of healthy aortas should clarify the consequences of early events during the induction of atherosclerosis, and identify unique roles for each subset of DC and MΦ for each step of atherosclerosis. As atherosclerosis is an age- and time-related disease, the kinetics of induction and the molecular and cellular events that underlie these events are important and may provide novel therapeutic opportunities. Second, several intriguing studies have suggested that MΦs and DCs are comprised of a diverse group of cells in atherosclerosis. DC subsets are found within healthy and atherosclerosis-prone aortas and understanding how different subsets of DCs respond to lipid uptake will help to advance the field. It is unclear if all DCs serve as antigen-presenting cells, and very little is currently known about a preferential subset of DCs that is responsible for T cell-induced inflammation within the aorta. Similarly, several subsets of MΦs are present within healthy and atherosclerotic arteries. While M1 MΦs likely play a critical role in the pathology of atherosclerosis, the relationship between M1 MΦs and other MΦ subsets, as well as the possibility of phenotypic plasticity within atherosclerotic plaques are unknown. Third, it would be important to identify the pathway(s) through which CD11c+ cells may modulate the levels of plasma cholesterol. Additionally, while we have considerable knowledge about the recruitment of monocyte subsets to and exit from the atherosclerotic plaques, it is still uncertain whether the efflux or retention of DCs is regulated by the plasma cholesterol levels. Finally, DC-based vaccination provides some promising data for the treatment of atherosclerosis, but several questions such as specificity of antigens, choice of adjuvants and optimal type of DCs used for vaccination remain to be better investigated.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by American Heart Association Pre-doctoral Fellowship grant 11PRE7520041 (to Matthew Butcher) and by the NHLBI HL107522 (to Elena Galkina).
References
- Angeli V., Llodra J., Rong J. X., Satoh K., Ishii S., Shimizu T., Fisher E. A., Randolph G. J. (2004). Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 10.1016/j.immuni.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Anitschkow N. (1913). Uber die veranderungen der kaninchenaorta bei experimenteller cholesterinsteatose. Beitr. Pathol. Anat. 56, 379–404 [Google Scholar]
- Annacker O., Coombes J. L., Malmstrom V., Uhlig H. H., Bourne T., Johansson-Lindbom B., Agace W. W., Parker C. M., Powrie F. (2005) Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202, 1051–1061 10.1084/jem.20040662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. (1969). The host response to Calmette-Guerin bacillus infection in mice. J. Exp. Med. 129, 1079–1107 10.1084/jem.129.5.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S., Kirchberger S., Bochkov V. N., Kronke G., Stuhlmeier K., Majdic O., Zlabinger G. J., Knapp W., Binder B. R., Stockl J., Leitinger N. (2005). Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J. Immunol. 175, 501–508 [DOI] [PubMed] [Google Scholar]
- Bobryshev Y. V. (2010). Dendritic cells and their role in atherogenesis. Lab. Invest. 90, 970–984 10.1038/labinvest.2010.94 [DOI] [PubMed] [Google Scholar]
- Bobryshev Y. V., Lord R. S. (1995). Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch. Histol. Cytol. 58, 307–322 10.1679/aohc.58.307 [DOI] [PubMed] [Google Scholar]
- Bobryshev Y. V., Lord R. S. (1998). Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc. Res. 37, 799–810 10.1016/S0008-6363(97)00229-0 [DOI] [PubMed] [Google Scholar]
- Bobryshev Y. V., Moisenovich M. M., Pustovalova O. L., Agapov I. I., Orekhov A. N. (2011). Widespread distribution of HLA-DR-expressing cells in macroscopically undiseased intima of the human aorta: A possible role in surveillance and maintenance of vascular homeostasis. Immunobiology 22. 10.1016/j.imbio.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Bouhlel M. A., Derudas B., Rigamonti E., DiFvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., Chinetti-Gbaguidi G. (2007). PPAR[gamma] Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 6, 137–143 10.1016/j.cmet.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Boyle J. J., Harrington H. A., Piper E., Elderfield K., Stark J., Landis R. C., Haskard D. O. (2009). Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am. J. Pathol. 174, 1097–1108 10.2353/ajpath.2009.080431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. J., Johns M., Lo J., Chiodini A., Ambrose N., Evans P. C., Mason J. C., Haskard D. O. (2011). Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 31, 2685–2691 10.1161/ATVBAHA.111.225813 [DOI] [PubMed] [Google Scholar]
- Butcher M., Galkina E. (2011). Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb. Haemost. 106, 787–795 10.1160/TH11-05-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C., Matos I., Choi J. H., Dandamudi D. B., Shrestha E., Longhi M. P., Jeffrey K. L., Anthony R. M., Kluger C., Nchinda G., Koh H., Rodriguez A., Idoyaga J., Pack M., Velinzon K., Park C. G., Steinman R. M. (2010). Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 143, 16–29 10.1016/j.cell.2010.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G., Baron M., Bouhlel M. A., Vanhoutte J., Copin C., Sebti Y., Derudas B., Mayi T., Bories G., Tailleux A., Haulon S., Zawadzki C., Jude B., Staels B. (2011). Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ. Res. 108, 985–995 10.1161/CIRCRESAHA.110.233775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H., Cheong C., Dandamudi D. B., Park C. G., Rodriguez A., Mehandru S., Velinzon K., Jung I. H., Yoo J. Y., Oh G. T., Steinman R. M. (2011). Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831 10.1016/j.immuni.2011.09.014 [DOI] [PubMed] [Google Scholar]
- Choi J. H., Do Y., Cheong C., Koh H., Boscardin S. B., Oh Y. S., Bozzacco L., Trumpfheller C., Park C. G., Steinman R. M. (2009). Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206, 497–505 10.1084/jem.20082129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Trinchieri G., Liu Y. J. (2004). Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 10.1038/ni1141 [DOI] [PubMed] [Google Scholar]
- Daissormont I. T., Christ A., Temmerman L., Sampedro M. S., Seijkens T., Rousch M., Poggi M., Boon L., van der Loos C., Daemen M., Lutgens E., Halvorsen B., Aukrust P., Janssen E., Biessen E. A. (2011). Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 109, 1387–1395 10.1161/CIRCRESAHA.111.256529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager S. C., Kuiper J. (2011). Vaccination strategies in atherosclerosis. Thromb. Haemost. 106, 796–803 10.1160/TH11-05-0369 [DOI] [PubMed] [Google Scholar]
- Erbel C., Sato K., Meyer F. B., Kopecky S. L., Frye R. L., Goronzy J. J., Weyand C. M. (2007). Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res. Cardiol. 102, 123–132 10.1007/s00395-006-0636-x [DOI] [PubMed] [Google Scholar]
- Feig J. E., Rong J. X., Shamir R., Sanson M., Vengrenyuk Y., Liu J., Rayner K., Moore K., Garabedian M., Fisher E. A. (2011). HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. U.S.A. 108, 7166–7171 10.1073/pnas.1016086108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E., Kadl A., Sanders J., Varughese D., Sarembock I. J., Ley K. (2006). Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 10.1084/jem.20052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E., Ley K. (2009). Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 27, 165–197 10.1146/annurev.immunol.021908.132620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier E. L., Huby T., Saint-Charles F., Ouzilleau B., Pirault J., Deswaerte V., Ginhoux F., Miller E. R., Witztum J. L., Chapman M. J., Lesnik P. (2009). Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 119, 2367–2375 10.1161/CIRCULATIONAHA.108.806158 [DOI] [PubMed] [Google Scholar]
- Geissmann F., Gordon S., Hume D. A., Mowat A. M., Randolph G. J. (2010). Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 10, 453–460 10.1038/nri2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. (1981). The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am. J. Pathol. 103, 181–190 [PMC free article] [PubMed] [Google Scholar]
- Gleissner C. A., Shaked I., Erbel C., Böckle D., Katus H. A., Ley K. (2010a). CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 106, 203–211 10.1161/CIRCRESAHA.109.199505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner C. A., Shaked I., Little K. M., Ley K. (2010b). CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J. Immunol. 184, 4810–4818 10.4049/jimmunol.0901368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Martinez F. O. (2010). Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Gordon S., Taylor P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- Han J. W., Shimada K., Ma-Krupa W., Johnson T. L., Nerem R. M., Goronzy J. J., Weyand C. M. (2008). Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ. Res. 102, 546–553 10.1161/CIRCRESAHA.107.161653 [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G., Bylock A., Hjalmarsson L. (1980). Ultrastructural studies on the localization of IgG in the aortic endothelium and subendothelial intima of atherosclerotic and nonatherosclerotic rabbits. Exp. Mol. Pathol. 33, 302–315 10.1016/0014-4800(80)90028-3 [DOI] [PubMed] [Google Scholar]
- Hermansson A., Ketelhuth D. F., Strodthoff D., Wurm M., Hansson E. M., Nicoletti A., Paulsson-Berne G., Hansson G. K. (2010). Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 207, 1081–1093 10.1084/jem.20092243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H. H. (1972). Human intracranial atherosclerosis. A histochemical and ultrastructural study of gross fatty steak lesions. Am. J. Pathol. 69, 421–438 [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. (1986). Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 10.1161/01.ATV.6.2.131 [DOI] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Haidari M., Zhu S. N., Chen M., Guha D., Cybulsky M. I. (2006). Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 10.1084/jem.20060245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Stetz E., Majno G. (1979). Lymphocytes and monocytes in the aortic intima–An electron-microscopic study in the rat. Atherosclerosis 34, 221–231 10.1016/S0021-9150(79)80003-9 [DOI] [PubMed] [Google Scholar]
- Kadl A., Meher A. K., Sharma P. R., Lee M. Y., Doran A. C., Johnstone S. R., Elliott M. R., Gruber F., Han J., Chen W., Kensler T., Ravichandran K. S., Isakson B. E., Wamhoff B. R., Leitinger N. (2010). Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 107, 737–746 10.1161/CIRCRESAHA.109.215715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khallou-Laschet J., Varthaman A., Fornasa G., Compain C., Gaston A. T., Clement M., Dussiot M., Levillain O., Graff-Dubois S., Nicoletti A., Caligiuri G. (2010). Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5, e8852. 10.1371/journal.pone.0008852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann R., Zadelaar S., Kooistra T. (2008). Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc. Res. 79, 360–376 10.1093/cvr/cvn120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Miller Y. I., Hedrick C. C. (2011). Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1506–1516 10.1161/ATVBAHA.110.221127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich I. P., Lajoie S., Clark J. R., Herman N. S., Sproles A. A., Wills-Karp M. (2008). Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE 3, e3879. 10.1371/journal.pone.0003879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Sharma N., Kapadia F., Zhou G., Lu Y., Hong H., Paruchuri K., Mahabeleshwar G. H., Dalmas E., Venteclef N., Flask C. A., Kim J., Doreian B. W., Lu K. Q., Kaestner K. H., Hamik A., Clement K., Jain M. K. (2011). Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 10.1172/JCI45444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Victora G. D., Schwickert T. A., Guermonprez P., Meredith M. M., Yao K., Chu F. F., Randolph G. J., Rudensky A. Y., Nussenzweig M. (2009). In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 10.1126/science.1171243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llodra J., Angeli V., Liu J., Trogan E., Fisher E. A., Randolph G. J. (2004). Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. U.S.A. 101, 11779–11784 10.1073/pnas.0403259101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Krupa W., Jeon M. S., Spoerl S., Tedder T. F., Goronzy J. J., Weyand C. M. (2004). Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J. Exp. Med. 199, 173–183 10.1084/jem.20030850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey H. D., Zernecke A. (2011). Dendritic cells in atherosclerosis: functions in immune regulation and beyond. Thromb. Haemost. 106, 772–778 10.1160/TH11-05-0296 [DOI] [PubMed] [Google Scholar]
- Martinez F. O., Gordon S., Locati M., Mantovani A. (2006). Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- Matsuura E., Kobayashi K., Tabuchi M., Lopez L. R. (2006). Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis. Prog. Lipid Res. 45, 466–486 10.1016/j.plipres.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Matta B. M., Castellaneta A., Thomson A. W. (2010). Tolerogenic plasmacytoid DC. Eur. J. Immunol. 40, 2667–2676 10.1002/eji.201040839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig G., Niederegger H., Rabl W., Hochleitner B. W., Hoefer D., Romani N., Wick G. (2001). Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler. Thromb. Vasc. Biol. 21, 503–508 10.1161/01.ATV.21.4.503 [DOI] [PubMed] [Google Scholar]
- Moore K. J., Tabas I. (2011). Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Edwards J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J., Wynn T. A. (2011). Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S. H., Metcalf D., van Nieuwenhuijze A., Wicks I., Wu L., O’Keeffe M., Shortman K. (2006). Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 10.1038/nrg1954 [DOI] [PubMed] [Google Scholar]
- Nakajima K., Yamashita T., Kita T., Takeda M., Sasaki N., Kasahara K., Shinohara M., Rikitake Y., Ishida T., Yokoyama M., Hirata K. (2011). Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31, 1963–1972 10.1161/ATVBAHA.111.229443 [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. (1994). ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14, 133–140 10.1161/01.ATV.14.1.133 [DOI] [PubMed] [Google Scholar]
- Niessner A., Sato K., Chaikof E. L., Colmegna I., Goronzy J. J., Weyand C. M. (2006). Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 114, 2482–2489 10.1161/CIRCULATIONAHA.106.642801 [DOI] [PubMed] [Google Scholar]
- Niessner A., Weyand C. M. (2010). Dendritic cells in atherosclerotic disease. Clin. Immunol. 134, 25–32 10.1016/j.clim.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N., Obata-Onai A., Tussiwand R., Lanzavecchia A., Manz MG. (2006) Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J. Exp. Med. 203, 227–238 10.1084/jem.20051645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein L. E., Soares M. P., Yamashita K., Bach F. H. (2003). Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 24, 449–455 10.1016/S1471-4906(03)00181-9 [DOI] [PubMed] [Google Scholar]
- Packard R. R., Maganto-Garcia E., Gotsman I., Tabas I., Libby P., Lichtman A. H. (2008). CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ. Res. 103, 965–973 10.1161/CIRCRESAHA.108.185793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Zhu S. N., Chen M., Nurmohamed S., Jongstra-Bilen J., Cybulsky M. I. (2010). Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 10.1161/CIRCRESAHA.109.210781 [DOI] [PubMed] [Google Scholar]
- Perrin-Cocon L., Coutant F., Agaugue S., Deforges S., Andre P., Lotteau V. (2001). Oxidized low-density lipoprotein promotes mature dendritic cell transition from differentiating monocyte. J. Immunol. 167, 3785–3791 [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J. L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C. R. (1999). Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. U.S.A. 96, 1036–1041 10.1073/pnas.96.3.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G. J. (2009). The fate of monocytes in atherosclerosis. J. Thromb. Haemost. 7, 28–30 10.1111/j.1538-7836.2009.03423.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B., Bunin A., Ghosh H. S., Lewis K. L., Sisirak V. (2011). Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 29, 163–183 10.1146/annurev-immunol-031210-101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Niessner A., Kopecky S. L., Frye R. L., Goronzy J. J., Weyand C. M. (2006). TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 203, 239–250 10.1084/jem.20051062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Trogan E., Ginsberg M., Grigaux C., Tian J., Miyata M. (1995). Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. U.S.A. 92, 8264–8268 10.1073/pnas.92.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Chen D. M., Lin H. S. (1978). Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature 274, 168–170 10.1038/274087a0 [DOI] [PubMed] [Google Scholar]
- Stary H. C., Chandler A. B., Dinsmore R. E., Fuster V., Glagov S., Insull W., Rosenfeld M. E., Schwartz C. J., Wagner W. D., Wissler R. W. (1995). A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15, 1512–1531 10.1161/01.ATV.15.9.1512 [DOI] [PubMed] [Google Scholar]
- Stein M., Keshav S., Harris N., Gordon S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 10.1084/jem.176.1.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. (2007). Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 37, S53-S60. 10.1002/eji.200737400 [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Hawiger D., Nussenzweig M. C. (2003). Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 10.1146/annurev.immunol.21.120601.141040 [DOI] [PubMed] [Google Scholar]
- Stoneman V., Braganza D., Figg N., Mercer J., Lang R., Goddard M., Bennett M. (2007). Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 100, 884–893 10.1161/01.RES.0000260802.75766.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F. K., Weissleder R., Pittet M. J. (2009). Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1424–1432 10.1161/ATVBAHA.108.180521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. (2010). Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 10.1038/nri2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J. A., Young L. (2008). Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 10.1016/j.immuni.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Waltner-Romen M., Falkensammer G., Rabl W., Wick G. (1998). A previously unrecognized site of local accumulation of mononuclear cells. The vascular-associated lymphoid tissue. J. Histochem. Cytochem. 46, 1347–1350 10.1177/002215549804601202 [DOI] [PubMed] [Google Scholar]
- Weber C., Meiler S., Doring Y., Koch M., Drechsler M., Megens R. T., Rowinska Z., Bidzhekov K., Fecher C., Ribechini E., van Zandvoort M. A., Binder C. J., Jelinek I., Hristov M., Boon L., Jung S., Korn T., Lutz M. B., Forster I., Zenke M., Hieronymus T., Junt T., Zernecke A. (2011). CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Invest. 121, 2898–2910 10.1172/JCI44415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs I. M., Donners M. M., de Winther M. P. (2011). Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb. Haemost. 106, 763–771 10.1160/TH11-05-0320 [DOI] [PubMed] [Google Scholar]
- Wu H., Gower R. M., Wang H., Perrard X. Y., Ma R., Bullard D. C., Burns A. R., Paul A., Smith C. W., Simon S. I., Ballantyne C. M. (2009). Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119, 2708–2717 10.1161/CIRCULATIONAHA.108.812537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. N., Chen M., Jongstra-Bilen J., Cybulsky M. I. (2009). GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J. Exp. Med. 206, 2141–2149 10.1084/jem.20081666 [DOI] [PMC free article] [PubMed] [Google Scholar]