Abstract
Cyclooxygenase (COX)-derived prostanoids have long been implicated in blood pressure (BP) regulation. Recently prostaglandin E2 (PGE2) and its receptor EP1R have emerged as key players in angiotensin II (Ang-II)-dependent hypertension (HTN) and related end-organ damage. However, the enzymatic source of PGE2, ie COX-1 or COX-2, and its site(s) of action are not known. The subfornical organ (SFO) is a key forebrain region that mediates systemic Ang-II-dependent HTN via reactive oxygen species (ROS). We tested the hypothesis that cross-talk between PGE2/EP1R and ROS signaling in the SFO is required for Ang-II HTN. Radiotelemetric assessment of BP revealed that HTN induced by infusion of systemic “slow-pressor” doses of Ang-II was abolished in mice with null mutations in EP1R or COX-1 but not COX-2. Slow-pressor Ang-II-evoked HTN and ROS formation in the SFO were prevented when the EP1R antagonist SC-51089 was infused directly into brains of wild-type mice, and Ang-II-induced ROS production was blunted in cells dissociated from SFO of EP1R−/− and COX-1−/− but not COX-2−/− mice. In addition, slow-pressor Ang-II infusion caused a ~3-fold increase in PGE2 levels in the SFO but not in other brain regions. Finally, genetic reconstitution of EP1R selectively in the SFO of EP1R-null mice was sufficient to rescue slow-pressor AngII-elicited HTN and ROS formation in the SFO of this model. Thus, COX-1-derived PGE2 signaling through EP1R in the SFO is required for the ROS-mediated HTN induced by systemic infusion of Ang-II, and suggests that EP1R in the SFO may provide a novel target for antihypertensive therapy.
Keywords: Prostanoids, PGE2, COX, reactive oxygen species, blood pressure, central nervous system
Introduction
Hypertension is a global health problem, afflicting nearly a third of the population and predisposing to serious diseases affecting the brain, heart and kidneys1. Cyclooxygenase (COX)-derived prostanoids, endogenous fatty acid metabolites known to play critical roles in a wide variety of biological processes, have long been implicated in blood pressure (BP) regulation2. Clinical use of prostanoid synthesis-inhibiting nonsteroidal anti-inflammatory drugs (NSAIDs) are associated with hypertension3, suggesting that endogenous prostanoids generally reduce blood BP. However, recently a more complex picture has emerged in which specific components of the prostanoid system have divergent effects and can be pro-hypertensive. For example, the major prostanoid prostaglandin E2 (PGE2) and its receptor subtype 1 (EP1R), one of four G-protein-coupled receptors (EP1–4R) mediating the effects of PGE24, are now considered key players in hypertension and related end-organ damage5–7. In particular, recent studies utilizing mice with global targeted disruption of EP1R revealed a critical role for this receptor subtype in systemic Ang-II-dependent hypertension8. However, the underlying mechanisms involved in this, including the enzymatic source of PGE2, i.e. COX-1 or COX-2 and its site(s) of action at EP1R, remain poorly defined.
There is abundant evidence that neurohumoral dysfunction is a key contributor to Ang-II-dependent hypertension9. In particular, regions devoid of a blood-brain-barrier can be activated by elevated levels of blood-borne Ang-II, triggering alterations in downstream signaling pathways and hypertension10. One of these regions, the subfornical organ (SFO), is strongly implicated in sympathoexcitation and hypertension caused by elevated levels of circulating Ang-II, particularly the model involving chronic infusion of subpressor doses of Ang-II, ie “slow-pressor”, which is thought to recapitulate key features of human essential hypertension11–13. Reactive oxygen species (ROS) signaling in the SFO is clearly involved in this model11, 14, 15; however, the factors regulating ROS production evoked by Ang-II in this brain region and contributing to neural dysregulation and hypertension remain poorly understood.
Here we sought to determine if COX-derived PGE2 and EP1R signaling in the SFO provide an essential link between Ang-II, ROS and the central neural changes that give rise to slow-pressor Ang-II hypertension. Utilizing genetic and pharmacologic tools to selectively target distinct components of the PG system in mice, we provide evidence that COX-1-derived PGE2 signaling through EP1R in the SFO is required for the ROS-mediated hypertension induced by systemic infusion of Ang-II.
Methods
An expanded Materials and Methods section is available in the Online Supplement at http://hyper.ahajournal.org/.
Animals
Adult EP1R-null, COX-1-null and COX-2-null mice (8–10 weeks old) were obtained from in-house colonies. Mice were congenic with the C57Bl/6 strain and age-matched C57Bl/6 mice were used as wild-type (WT) controls. All procedures were approved by the Animal Care and Use Committee at Cornell University. Care of the mice met or exceeded standards set forth by the NIH Guide for the Care and Use of Laboratory Animals, USDA regulations, and the AVMA Panel on Euthanasia.
Pharmacological agents
Inhibitors of EP1R (SC-51089), COX-1 (SC-560), COX-2 (NS398) and Ang-II type 1 receptors (AT1R, losartan) were utilized.
Blood pressure studies
Mice were anesthetized and instrumented with radiotelemetry devices as described11. After 7 days recovery, baseline BP measurements were taken over 3–4 days, after which mice were implanted subcutaneously with osmotic minipumps loaded with the slow-pressor dose of Ang-II (600ng/kg/min, 14 days) as described11. BP was recorded daily for 3 weeks to monitor the effects of Ang-II during the entire infusion period as well as several days post-infusion. In studies using intracerebroventricular (i.c.v) infusion of SC-51089, mice were instrumented with i.c.v. cannulae11 during the same surgical session as radiotelemeter implantation. For these studies, at the time of Ang-II pump installation, a second 14-day osmotic minipump containing SC-51089 (144µg/day) was implanted and connected to the i.c.v. cannulae and BP monitoring was carried out as described above.
Meaurement of dipsogenic responses
WT mice were instrumented with i.c.v. cannulae and allowed 7 days recovery. Mice were administered either vehicle, SC-51089 (10µg/kg), SC-560 (10mg/kg) or NS-398 (10mg/kg) by i.p. injection (200 nl) 30 minutes prior to i.c.v. bolus administration of Ang-II (200ng, 200nl). Water drinking responses were measured over 1 hour as described previously14, 16.
Quantitative real-time PCR detection of prostanoid-related transcripts
WT mice were decapitated and brains flash frozen. SFO tissue was collected by micropunch as described14. Total RNA was harvested and cDNA was generated using random hexamers. Templates (25ng) were subjected in triplicate to real-time RT-PCR using Power SYBR Green and specific primers for COX-1, COX-2, EP1–4R and PGE synthases as described14. β-actin was used for relative quantification by ΔΔCt method14.
PGE2 assay
WT mice were implanted with osmotic minipumps loaded with the 2 week slow-pressor dose of Ang-II (see above) or saline. Mice were euthanized at 3, 7 or 14 days after start of infusions and brains flash frozen. Micropunches of SFO, paraventricular nuclei (PVN), somatosensory cortex (CTX) and cerebellum (CBM) were collected from 2 mice per biological sample and weighed. Samples were processed and PGE2 concentration was determined using an enzyme immunoassay kit as described17.
ROS detection
ROS production was assessed in dissociated SFO cells and in SFO-containing tissue sections using dihydroethidium (DHE) as an indicator. For in vitro ROS detection, WT, COX-1-null, COX-2-null or EP1R-null mice were sacrificed, brains removed and coronal slices containing the SFO obtained. SFO cells were dissociated, incubated with DHE and time-resolved fluorescence was measured every 30s before (vehicle) and after addition of Ang-II (100nM) as described18. Additional in vitro studies were performed the same way except pre-treatment with SC-51089 (10µM) or losartan (3 µM) were used, and PGE2 (100nM) was also applied. For in situ ROS detection, brains were removed on day 16 of Ang-II or vehicle infusions, frozen sections were incubated with DHE and fluorescence was visualized and quantified as described11. Data are expressed as DHE fluorescence intensity relative to control samples.
Adenoviral-mediated reconstitution of EP1R in EP1R-null mice
A recombinant adenoviral vector encoding murine EP1R tagged with HA on the N-terminus (AdEP1R; Fig S4A) was engineered and then generated by the Iowa Gene Transfer Vector Core (IGTVC). AdEP1R potency and stability was validated both in vitro and in vivo (Fig S4). An Ad vector encoding GFP (AdGFP) obtained from IGTVC was used as the control vector. EP1R-null mice underwent SFO-targeted injection of AdEP1R (5×1010 pfu/mL, 500nl) or titer-matched AdGFP as described11, 14. During the same surgical session, radiotelemeters were implanted as described above. Nine days later, osmotic minipumps loaded with the 2 week slow-pressor Ang-II dose were implanted. BP recording and ROS measurements were performed as described above.
Data analysis
Data are expressed as mean ± SEM. Comparisons between two groups were evaluated using the Student’s t test. Multiple comparisons were evaluated by ANOVA followed by Dunnett’s or Tukey’s test. Differences were considered statistically significant at p<0.05.
Results
Genetic disruption of EP1R prevents hypertension during slow-pressor Ang-II infusion
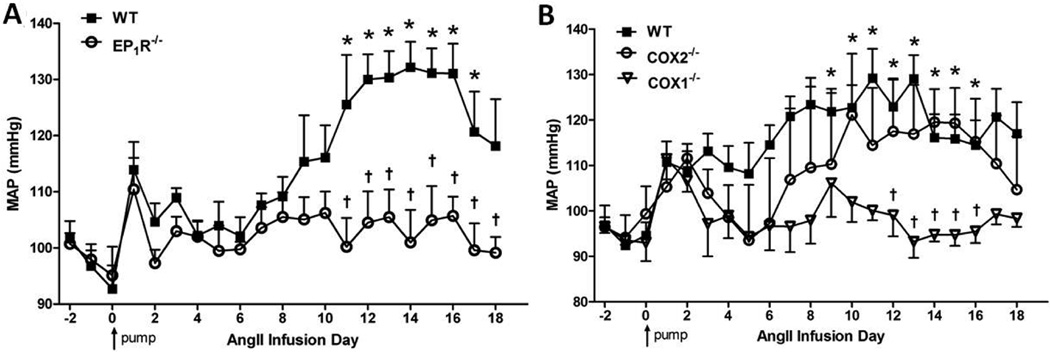
EP1R are implicated in the BP elevation induced by high doses of Ang-II8. Here, using EP1R-null and WT mice, we determined whether EP1R are involved in hypertension caused by chronic slow-pressor doses of Ang-II, a model thought to mimic human hypertension and in which there is a strong CNS component9. Baseline mean arterial pressure (MAP) was not different between the groups (EP1R−/− 98±2, WT 97±3mmHg; p>0.05). In accordance with previous studies11, 19, Ang-II induced a gradual rise in MAP in WT mice that peaked at ~30mmHg above baseline following 2 weeks Ang-II infusion (Fig 1A). In contrast, this Ang-II-induced rise in BP was absent in EP1R-null mice (Fig 1A).
Figure 1. Slow-pressor Ang-II hypertension is prevented in mice with null mutations in EP1R or COX-1 but not COX-2.
A) Summary of MAP before, during and after 2 wk slow-pressor Ang-II infusions in WT (n=5) and EP1R−/− (n=7) mice. B) MAP throughout slow-pressor Ang-II in WT (n=7), COX-2−/− (n=4) and COX-1−/− (n=5) mice. *p<0.05 vs. baseline (WT in panels A and B; COX-2 in panel B); †p<0.05 vs. WT or COX-2−/−. Arrow indicates start of Ang-II infusions.
Ang-II slow-pressor hypertension is ameliorated in COX-1-null but not COX-2-null mice
Data in Figure 1A suggest that PGE2 signaling is needed for slow-pressor Ang-II hypertension. Since PGE2 is a major reaction product of both COX isozymes20, we next sought to determine the enzymatic source of PGE2 involved in the slow-pressor effects of Ang-II using mice with null mutations in either COX-1 or COX-2. Baseline MAP did not differ between the groups (COX-1−/− 93±2, COX-2−/− 96±7, WT 98±3 mmHg; p>0.05). Similar to WT mice in Figure 1A, Ang-II induced the classic slow rise in MAP which peaked during the second week of infusion (Fig 1B). This response was abolished in COX-1-null mice, whereas it remained intact in COX-2-null mice (Fig 1B).
EP1R and COX-1 in the CNS are implicated in Ang-II-induced cardiovascular and dipsogenic effects
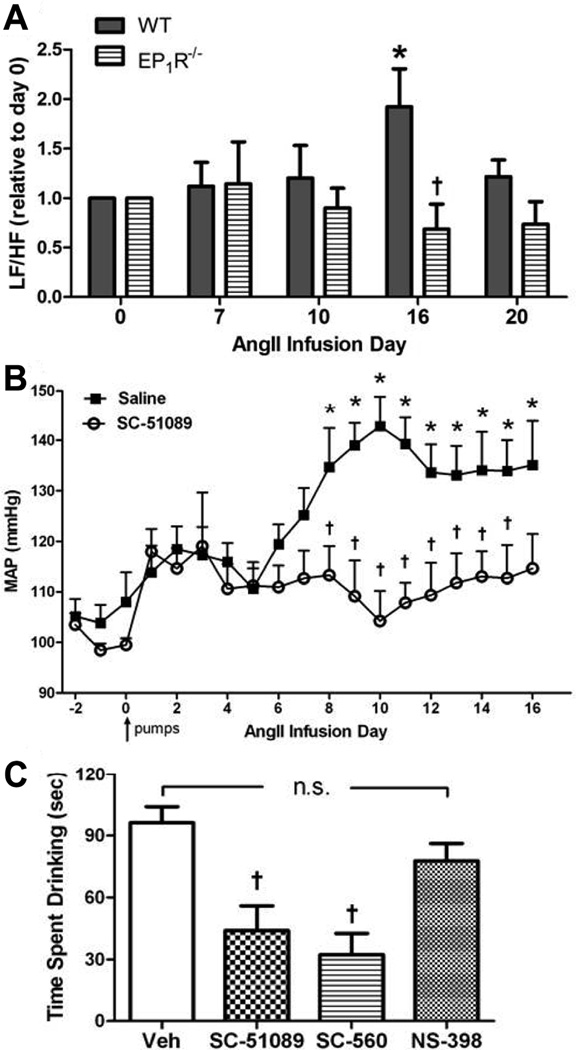
Data in Figure 1 suggest that COX-1-derived PGE2 and EP1R are involved in the rise in BP during slow-pressor Ang-II infusion, but the use of global knockouts prevents us from pinpointing the site(s) of these effects. To test the hypothesis that slow-pressor Ang-II hypertension is caused by a PGE2/EP1R mechanism operating in the CNS, we utilized three separate approaches. First, given abundant evidence that slow-pressor Ang-II hypertension is mediated via CNS-driven increases in sympathetic activity9, power spectral analysis was used to assess slow-pressor Ang-II-induced sympathetic responses in EP1R-null vs WT mice. Increased low frequency (LF)/high frequency (HF) oscillations of arterial pressure reflect increased sympathetic activity21. Consistent with previous findings, Ang-II infusions caused a doubling of the LF/HF ratio in WT mice by the end of the 2 week infusion period (pumps empty completely on day 16) (Fig 2A) when the hypertensive response is maximal (see Fig 1 and 2B). In contrast, the LF/HF ratio was unchanged in EP1R−/− mice over the course of Ang-II infusions (Fig 2A). Second, using chronic infusion of the EP1R antagonist SC51089 into brains (i.c.v.) of WT mice, data shown in Figure 2B demonstrate that the slow-pressor Ang-II-induced rise in MAP observed in i.c.v. vehicle-treated controls was prevented by blockade of EP1R in the CNS. It should be noted that i.c.v. infusions at this volume do not escape into the peripheral circulation11. Third, given the well established role of the CNS in mediating Ang-II effects on dipsogenesis16, 22, we employed the classic assay of bolus injection of Ang-II in the brain (i.c.v.) coupled with measurement of drinking responses in WT mice pre-treated (i.p.) with either SC51089 or the selective inhibitors of COX-1 (SC-560) or COX-2 (NS-398). As seen in Figure 2C, i.c.v. Ang-II elicited the well-established dipsogenic response in vehicle-treated mice. This response was intact in mice treated with the COX-2 inhibitor, whereas it was markedly attenuated in mice treated with either the COX-1 inhibitor or the EP1R antagonist.
Figure 2. EP1R and COX-1 in the CNS are involved in Ang-II-induced cardiovascular and dipsogenic effects.
A) Power spectral analysis of arterial pressure variability at several time-points before, during and after slow-pressor Ang-II infusion in WT (n=5) and EP1R−/− mice (n=7). Data are presented as the LF/HF ratio relative to day 0. *p<0.05 vs. day 0; †p<0.05 vs. WT at day 16. B) Summary of MAP in WT mice with chronic i.c.v. infusion of the EP1R antagonist SC-51089 (n=7) or saline (n=6) at the same time as systemic slow-pressor Ang-II infusions. *p<0.05 vs. baseline; †p<0.05 vs. saline. Arrow indicates start of i.c.v. and Ang-II infusions. C) Drinking responses elicited by bolus i.c.v. injection of Ang-II in WT mice treated 30 min earlier with i.p. injections of vehicle (Veh, n=11), EP1R antagonist SC51089 (n=11), COX-1 inhibitor SC560 (n=4) or COX-2 inhibitor NS398 (n=4). Data are expressed as the total time drinking (seconds) for 30 min after i.c.v. injection of Ang-II. †p<0.05 vs. vehicle; n.s., not significant.
PGE2 synthetic enzymes and receptors are expressed in the SFO under basal conditions, and PGE2 production is augmented in this brain region during slow-pressor Ang-II infusion
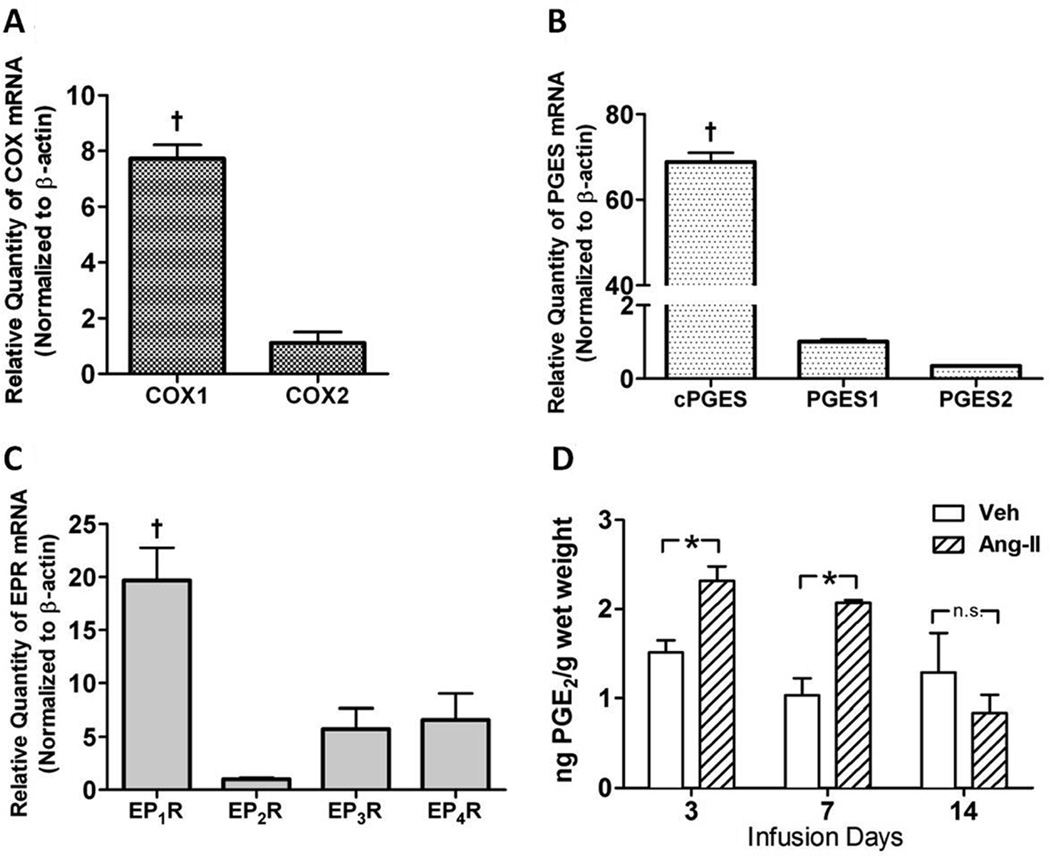
Data in Figures 1 and 2 suggest that COX-1-derived PGE2 acting at EP1R in the CNS is important in slow-pressor Ang-II hypertension. Since the SFO is a key region of the CNS mediating this form of hypertension11, 12, next we examined the capacity of the SFO for PGE2 formation and signaling. Real-time qPCR was performed to determine basal mRNA levels of COX isozymes, PGE synthases and EP receptor subtypes in SFO tissue harvested from adult WT mice. First, COX-1 was expressed at ~7-fold higher levels in SFO than COX-2 (Fig 3A). Second, of the PGE synthases that convert PGH2 to PGE2, ie PGES-1, PGES-2 or cytosolic PGES (c-PGES), the latter was expressed at much higher levels in the SFO than either of the other two isoforms (Fig 3B). Third, of the four receptor subtypes mediating PGE2 effects, mRNA levels of EP1R were more than 10-fold higher than EP2R, EP3R or EP4R in SFO tissue (Fig 3C). Importantly, all four of the EPR subtypes were expressed at very low levels in organum vaculosum of the lamina terminalis (Fig S1). Finally, we sought to directly evaluate the effects of slow-pressor Ang-II infusions on PGE2 formation in the SFO. As seen in Figure 3D, Ang-II caused a significant increase in PGE2 levels in the SFO as early as day 3 of the infusion compared to saline controls. This was sustained through 7 days of the infusion, but by day 14, PGE2 levels were not different from controls. PGE2 levels were also measured in PVN, CTX and CBM of WT mice infused with slow-pressor doses of Ang-II or vehicle, but no significant Ang-II-induced changes in PGE2 levels were observed in any of these regions compared to controls (Fig S2).
Figure 3. PGE2 synthetic enzyme and receptors are expressed at high levels in the SFO under basal conditions, and PGE2 levels are increased in the SFO early after slow-pressor Ang-II infusion.
A–C) Basal mRNA levels of EPR1–4, COX isozymes and PGE synthases in adult WT SFO tissue (n=3) as analyzed by quantitative real-time PCR. †p<0.05 vs. EP2–4R (A), vs. COX-2 (B), vs. PGES1 and PGES2. D) PGE2 levels measured by ELISA in micropunches of SFO from WT mice at day 3, 7 and 14 of slow-pressor Ang-II (n=3) or saline infusion (n=3). *p<0.05 vs. saline. In all assays, two brains were pooled for per biological sample.
PGE2/EP1R signaling is coupled to Ang-II-induced ROS accumulation in the SFO
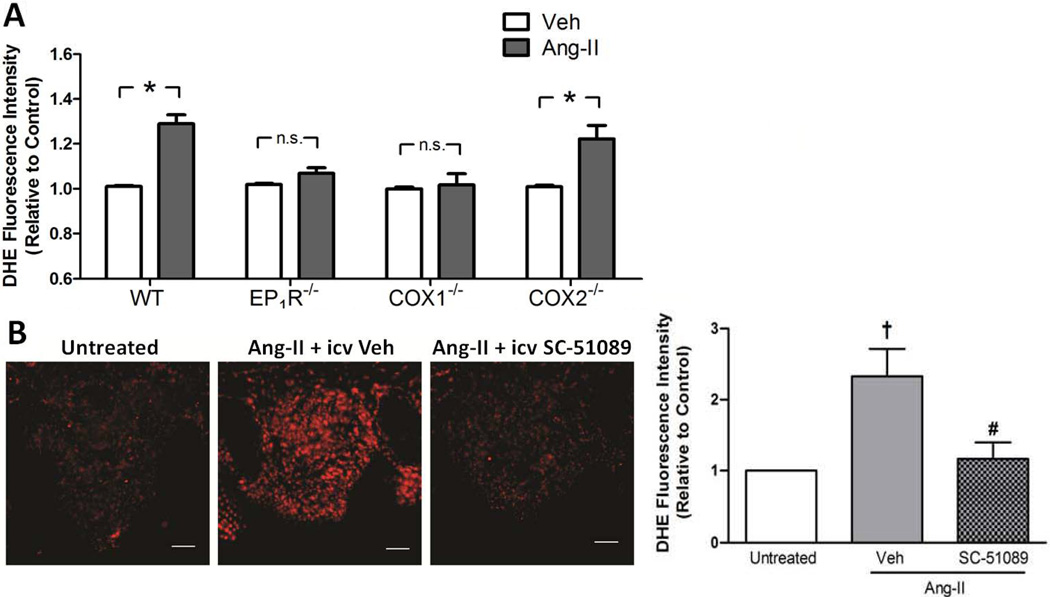
Slow-pressor Ang-II infusion causes increased ROS formation in the SFO11, 15. Thus, several experiments were performed to test the hypothesis that PGE2/EP1R are required for ROS formation in the SFO in response to Ang-II. First, ROS production was assessed in vitro in single cells dissociated from the SFO of adult WT and null mice. Consistent with earlier reports14, 16, Ang-II caused a significant increase in DHE signal in WT SFO cells compared to control (Fig 4A). This response was absent in SFO cells dissociated from either EP1R−/− or COX-1−/− mice, whereas it was intact in COX-2−/− SFO cells (Fig 4A). Pharmacological studies using the EP1R antagonist SC-51089 in SFO cells dissociated from WT mice confirmed the results observed in the EP1R−/− mice (Fig S3), and further verified earlier data16 that Ang-II-induced increases in ROS formation are sensitive to losartan (Fig S3). Interestingly, PGE2 elicited increases in DHE intensity to a similar extent as Ang-II, and while this response was inhibited by SC-51089, it was unaffected by losartan (Fig S3). Next, in situ DHE microfluorography was used to assess Ang-II-induced ROS production in the SFO of WT mice receiving systemic slow-pressor Ang-II infusions concomitant with i.c.v. infusions of either SC-51089 or vehicle. Consistent with earlier reports11, DHE fluorescence intensity was ~2.5-fold higher in the SFO of mice receiving Ang-II (day 16) compared to untreated mice (Fig 4B). This response was abolished in mice receiving i.c.v. infusions of SC-51089 (Fig 4B).
Figure 4. PGE2/EP1R signaling is required for Ang-II-induced ROS formation in the SFO.
A) Summary of the effects of Ang-II vs. vehicle (Veh) on ROS production as measured by DHE fluorescence intensity in cells dissociated from SFO of adult WT (n=24), EP1R−/− (n=9), COX1−/− (n=9) and COX2−/− (n=9) mice. *p<0.05 vs. Veh; n.s., not significant. B) Left: Representative confocal images showing DHE fluorescence in SFO tissue at day 16 of slow-pressor Ang-II infusion in mice treated concomitantly with either i.c.v. vehicle or SC-51089. Mice left untreated served as controls. Right: Summary of DHE fluorescence intensity in SFO tissue of mice with no treatment (n=7) or at day 16 of slow-pressor Ang-II infusions treated concomitantly with either i.c.v. vehicle (Veh, n=6) or SC-51089 (n=7). †p<0.05 vs.untreated; #p<0.05 vs. Ang-II + i.c.v. Veh. Scale bar: 50 µm.
Virally-mediated reconstitution of EP1R selectively in the SFO rescues slow-pressor Ang-II hypertension and ROS formation in EP1R-null mice
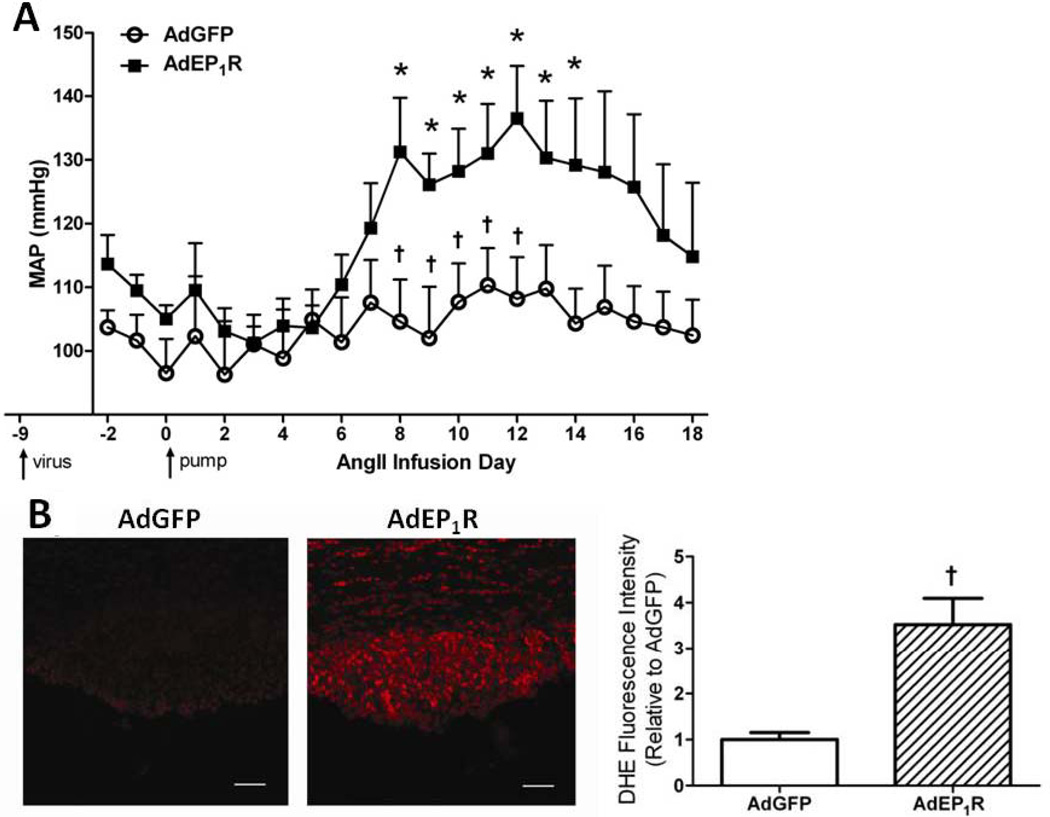
Our data thus far show that global knockout of EP1R prevents slow-pressor AngII-induced hypertension, and the CNS, particularly the SFO, may be involved. To directly test the hypothesis that SFO-selective expression of EP1R is sufficient to induce gradual hypertension elicited by slow-pressor doses of Ang-II, we utilized a genetic rescue approach to reconstitute EP1R selectively in the SFO with AdEP1R in EP1R-null mice. First, the potency and stability of AdEP1R were evaluated. AdEP1R increased exogenous EP1R mRNA levels as measured by qPCR in Neuro2A cells in a concentration-dependent manner (Fig S4B). Furthermore, in vivo targeting of the virus to the SFO induced highly robust HA-EP1R levels in the SFO as revealed by immunohistochemistry (Fig S4C). This was confirmed at the mRNA level, with qPCR showing highly abundant expression of the HA-EP1R transgene in SFO and barely detectable levels in PVN, rostral ventrolateral medulla and CTX (Fig S4D). Transgene expression was also stable over time as indicated by similar high levels of HA-EP1R mRNA in SFO at 9 and 28 days post-transduction (Fig S4D). Having verified the potency and stability of the virus, EP1R−/− mice underwent SFO-targeted injections of AdEP1R or control vector AdGFP. Prior to initiating slow-pressor Ang-II infusions 9 days after viral transduction, baseline MAP was not different in the two groups (AdGFP 97±5 mmHg, AdEP1R 105±2; p>0.05). In control AdGFP-treated EP1R−/− mice, there was no change in MAP at any time throughout the Ang-II infusion (Fig 5A), verifying data in Figure 1 showing that slow-pressor Ang-II hypertension is abolished in EP1R−/− mice. In contrast, the classic gradual rise in MAP was restored in AdEP1R-treated EP1R−/− mice (Fig 5A). This was accompanied by LF/HF ratios that were similar to those observed in WT mice at the end of the 2 week infusion period (1.72±0.6, p>0.05). To determine whether this was accompanied by restoration of Ang-II-induced ROS accumulation in the SFO, a separate cohort of EP1R−/− mice with SFO-targeted AdEP1R or AdGFP were subjected to DHE studies as described above. In AdGFP-treated EP1R−/− mice, DHE fluorescence intensity in the SFO did not change from baseline, confirming in this null strain findings in Figure 4B obtained using the EP1R antagonist. In contrast, ROS levels in the SFO of AdEP1R-treated EP1R−/− mice were re-established to that of Ang-II-treated WT mice (see Fig 4B), with a ~3-fold increase in DHE intensity compared to controls (Fig 5B).
Figure 5. Adenoviral-mediated reconstitution of EP1R selectively in the SFO rescues slow-pressor Ang-II hypertension and ROS formation in EP1R−/− mice.
A) Summary of MAP before, during and after slow-pressor Ang-II infusion in EP1R−/− mice with SFO-targeted AdGFP (n=6) or AdEP1R (n=6). *p<0.05 vs. baseline; †p<0.05 vs. AdEP1R. B) Left: Representative confocal images showing DHE fluorescence in the SFO at the end of the slow-pressor Ang-II infusion period in EP1R−/− mice with SFO-targeted AdGFP or AdEP1R. Right: Summary of DHE fluorescence intensity in the SFO of EP1R−/− mice with SFO-targeted AdGRP (n=3) or AdEP1R (n=4) at the end of the slow-pressor Ang-II infusion period. †p<0.05 vs. AdGFP. Scale bar: 50µm.
Discussion
Brain Ang-II, prostanoids and ROS have each been proposed as important mediators of BP regulation and hypertension2, 9, 23. Here we provide evidence that these factors are mechanistically linked in the pathogenesis of slow-pressor Ang-II-elicited hypertension. We show that elevations in BP during slow-pressor Ang-II infusions are abolished in mice with global null mutations of EP1R or COX-1 but not COX-2. Pharmacologic inhibition of EP1R selectively in the CNS prevents slow-pressor Ang-II hypertension, and central Ang-II-driven sympathetic and dipsogenic responses are also mediated by brain EP1R. Markedly elevated levels of COX-1, cPGES and EP1R are observed in the SFO relative to other PGE2 synthetic enzymes and receptors, making this forebrain structure an ideal platform for COX1-derived PGE2 signaling through EP1R. Indeed, slow-pressor Ang-II infusions induce early robust PGE2 production in the SFO. Both in vitro and in vivo inhibition of EP1R prevents Ang-II-induced ROS accumulation in the SFO, a response that is known to have a causative role in slow-pressor Ang-II hypertension11, 15. Finally, virally-mediated reconstitution of EP1R selectively in the SFO of EP1R-null mice restores hypertension and SFO ROS formation in response to slow-pressor Ang-II infusions. This provides the first evidence that COX-1-derived PGE2/EP1R signaling in the SFO is required for the ROS-mediated hypertension elicited in the slow-pressor Ang-II model.
The significance of these findings lies in the complex picture that has recently emerged concerning specific components of the prostanoid system and their divergent effects on BP. COX-inhibiting NSAIDs are among the most widely prescribed classes of therapeutic agents, many of the effects of which are mediated by their actions in the CNS2. Their general association with hypertension in humans has suggested that endogenous prostanoids lower BP2, 3. However, the ubiquitous tissue distribution and biological complexity of the prostanoid system2, coupled with recent evidence that certain components are pro-hypertensive6–8, 24 underscores the importance of understanding how prostanoids influence BP regulation, particularly as newer agents with a higher selectivity of action within the prostanoid system are being developed. Our data bolster the emerging concept that endogenous PGE2-mediated EP1R activation contributes to Ang-II-dependent hypertension and related end-organ damage5, 8. Although the study by Guan et al. established that the pressor effects of high-dose systemic Ang-II are blunted in EP1R−/− mice8, the enzymatic source of PGE2 and the tissue site(s) of action remained poorly defined. Here, utilizing tissue-specific reconstitution of EP1R in EP1R-null mice, our data now point to a key role for PGE2/EP1R signaling in the CNS, particularly the SFO, in mediating systemic Ang-II-dependent hypertension. Whereas these studies do not rule out the possibility that other EPR subtypes and/or other tissues sites are involved in this model of Ang-II hypertension, the complete restoration of the slow-pressor response, coupled with the markedly higher levels of EP1R expression in SFO compared to the other subtypes strongly supports this concept. This is important information in considering EP1R as a novel target for treatment of hypertension.
Another major finding of the present study is that we identified COX-1 in the SFO as the sole source of the PGE2 required for Ang-II slow-pressor hypertension. Although it is well established that Ang-II stimulates PGE2 synthesis25, the enzymatic source of PGE2 in Ang-II-evoked hypertension has remained poorly defined. Our data showing an absence of slow-pressor Ang-II-evoked hypertension in COX-1-null mice but an intact response in COX-2−/− mice implicates COX-1 as the source of PGE2 and mediator of hypertension in this model. The relative roles of COX-1 versus COX-2-derived products in Ang-II-dependent responses have been controversial, but our findings are consistent with previous studies showing that pharmacological blockade or genetic deletion of COX-1, but not COX-2, reduced the acute pressor effects of Ang-II in mice24. In addition, Capone et al. recently showed that selective pharmacological inhibition of COX-1, but not COX-2, prevented the cerebrovascular effects of Ang-II, and that COX-1 is the major source of PGE2 in the somatosensory cortex5. Our evidence that COX-1 is expressed at much higher levels than COX-2 in the SFO, and that the COX-1-coupled PGE synthase cPGES26 is the predominant isoform in the SFO suggests COX-1 as the source of increased PGE2 levels in this brain region during slow-pressor Ang-II infusion. The importance of COX-1 is further suggested by our in vitro data demonstrating that Ang-II-evoked ROS formation in dissociated SFO cells is prevented by inhibition of COX-1 but not COX-2. Thus, our data suggest that at least in the SFO, COX-1 predominates under basal conditions. Further studies will be required to define the cell-type localization of these enzymes in the SFO before and after Ang-II infusion to better understand the cellular mechanisms involved.
It is notable that systemic Ang-II induced increases in PGE2 production in the SFO but not in other brain regions including the PVN, cortex and cerebellum, despite these regions being enriched in PGE2 synthesis enzymes and receptors27, 28. This suggests that the links between Ang-II and PGE2 are specific rather than due to generalized CNS activation in this model, and is also consistent with the fact that the SFO lacks a blood-brain-barrier and can be accessed by circulating Ang-II via AT1 binding9. Indeed Ang-II has been shown to elicit PGE2 synthesis in cultured CNS cells via stimulation of AT1 receptors29, 30, and interestingly, autoradiographic studies have demonstrated intense PGE2 binding in the anteroventral region of the third ventricle (AV3V), a region that encompasses the SFO31. It is also important to note that the Ang-II-induced increase in PGE2 production in the SFO occurred early in the infusion period (3 and 7 days), prior to a significant rise in sympathetic outflow and BP. Furthermore, PGE2 levels had returned to baseline by 14 days, a time when hypertension is at its peak. This suggests that PGE2 in the SFO per se is not directly causing neural changes leading to hypertension in this model, but rather serves as a critical signaling intermediate that then triggers downstream pathways involved in central Ang-II-mediated hypertension. Indeed, essential hypertension has a slow and insidious onset, and its underlying pathophysiological mechanisms precede the elevation in BP32. Increasingly, adaptive changes in CNS neurons are considered highly relevant to hypertension9, 33. Determining whether early induction of PGE2 in the SFO by slow-pressor Ang-II is involved in such changes in CNS circuitries involved in the delayed sympathetic activation and hypertension in this model will require further investigation.
We and others have demonstrated that Ang-II induces ROS formation in the SFO via the activation of NADPH oxidase, and this is a key signaling event in the hypertensive and dipsogenic actions of Ang-II in the brain11, 14–16, 34. A key finding of the present study demonstrates that COX-1-derived PGE2 and EP1R are required for this Ang-II-evoked ROS formation in the SFO in vitro and in vivo. This is consistent with recent studies demonstrating a similar mechanism in Ang-II-mediated ROS formation in cerebral blood vessels, which likely involves NADPH oxidase5. Given that Ang-II-induced NADPH oxidase activation in neurons is Ca2+-dependent18, along with evidence that activation of EP1R results in IP3-mediated release of intracellular Ca2+35 and reduced Ca2+ efflux through the Na/Ca2+ exchanger17, it is reasonable to speculate that NADPH oxidase mediates PGE2/EP1R-mediated ROS formation in the SFO through Ca2+ signaling. Further studies will be required to elucidate the detailed cellular mechanisms linking Ang-II, prostanoids, ROS and neuronal signaling in the SFO. For example, it will be important to define the relationship between early pre-hypertensive induction of PGE2 in the SFO by slow-pressor Ang-II and ROS accumulation in this region during the later hypertensive phase in the model.
Perspectives
COX-derived prostanoid signaling has long been implicated in the pathogenesis of Ang-II-dependent hypertension, and this study provides evidence for the first time that a mechanism involving increased COX-1-dependent PGE2 formation and EP1R signaling in the SFO region of the forebrain is a key underlying mechanism. Determining how these various players are spatially and functionally linked in the SFO to provide the substrate for adaptive neural changes that lead to gradually developing Ang-II hypertension is a critical next step. However, in the meantime, this is important information as new therapeutic agents targeting the prostanoid system are being developed.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL96571, HL63887 and HL84624. XC was supported by an American Heart Association Founders Affiliate Postdoctoral Research Grant (10POST3450044). JRP was supported by NIH Medical Scientist Training Program grant GM07739 and a Ruth L. Kirschstein National Research Service Award (1F30NS060410-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among united states adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 2.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50 Suppl:S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T, Narumiya S. Function of prostanoid receptors: Studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68–69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 5.Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension. 2010;55:911–917. doi: 10.1161/HYPERTENSIONAHA.109.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suganami T, Mori K, Tanaka I, Mukoyama M, Sugawara A, Makino H, Muro S, Yahata K, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats. Hypertension. 2003;42:1183–1190. doi: 10.1161/01.HYP.0000101689.64849.97. [DOI] [PubMed] [Google Scholar]
- 7.Rutkai I, Feher A, Erdei N, Henrion D, Papp Z, Edes I, Koller A, Kaley G, Bagi Z. Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes. Cardiovasc Res. 2009;83:148–154. doi: 10.1093/cvr/cvp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: Converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell GT, Ferguson AV. Sensory circumventricular organs: Central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 12.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H680–H685. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- 13.Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state--central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2010;299:R405–R415. doi: 10.1152/ajpregu.00103.2010. [DOI] [PubMed] [Google Scholar]
- 14.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H397–H407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HF, Harris RC. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 21.Baudrie V, Laude D, Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R904–R912. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- 22.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 23.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Z, Cai H, Morrow JD, Breyer MD. Differentiation of cyclooxygenase 1- and 2-derived prostanoids in mouse kidney and aorta. Hypertension. 2006;48:323–328. doi: 10.1161/01.HYP.0000231934.67549.b7. [DOI] [PubMed] [Google Scholar]
- 26.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 27.Candelario-Jalil E, Slawik H, Ridelis I, Waschbisch A, Akundi RS, Hull M, Fiebich BL. Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: Accumulation in purkinje cells of the cerebellum. J Mol Neurosci. 2005;27:303–310. doi: 10.1385/JMN:27:3:303. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura K, Watanabe Y, Imai-Matsumura K, Connolly M, Koyama Y, Onoe H, Watanabe Y. Mapping of prostaglandin E2 binding sites in rat brain using quantitative autoradiography. Brain Res. 1992;581:292–298. doi: 10.1016/0006-8993(92)90720-t. [DOI] [PubMed] [Google Scholar]
- 29.Leung KH, Chang RS, Lotti VJ, Roscoe WA, Smith RD, Timmermans PB, Chiu AT. AT1 receptors mediate the release of prostaglandins in porcine smooth muscle cells and rat astrocytes. Am J Hypertension. 1992;5:648–656. doi: 10.1093/ajh/5.9.648. [DOI] [PubMed] [Google Scholar]
- 30.Jaiswal N, Diz DI, Tallant EA, Khosla MC, Ferrario CM. Characterization of angiotensin receptors mediating prostaglandin synthesis in C6 glioma cells. Am J Physiol. 1991;260:R1000–R1006. doi: 10.1152/ajpregu.1991.260.5.R1000. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura K, Watanabe Y, Onoe H, Watanabe Y, Hayaishi O. High density of prostaglandin E2 binding sites in the anterior wall of the 3rd ventricle: A possible site of its hyperthermic action. Brain Res. 1990;533:147–151. doi: 10.1016/0006-8993(90)91808-t. [DOI] [PubMed] [Google Scholar]
- 32.Pimenta E, Oparil S. Prehypertension: Epidemiology, consequences and treatment. Nat Rev Nephrol. 2010;6:21–30. doi: 10.1038/nrneph.2009.191. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Mifflin S. Plasticity of GABAergic mechanisms within the nucleus of the solitary tract in hypertension. Hypertension. 2010;55:201–206. doi: 10.1161/HYPERTENSIONAHA.109.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 35.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.