Abstract
Through local cell–cell interactions, Notch signaling pathway controls tissue formation and homeostasis during embryonic and adult life. In the heart, Notch1 is expressed in a variety of cell types such as cardiomyocytes, smooth muscle cells and endothelial cells. In cardiomyocytes, Notch1 is activated in proliferating embryonic and immature cardiomyocytes, and is downregulated in the myocardium during postnatal development. However, Notch signaling in the adult myocardium could be activated transiently in response to myocardial injury, suggesting that Notch signaling may contribute to cardiac repair. Indeed, activation of Notch1 intracellular domain blunts the severity of myocardial injury and improves myocardial hemodynamic function. Conversely, genetic ablation of the Notch1 gene, either systemically or in bone marrow-derived cells, leads to impaired cardiac repair following myocardial infarction. In this review, we will discuss the complex mechanisms of Notch signaling and its role in cardiac repair and regeneration after myocardial infarction.
Introduction
The Notch signaling pathway is important for multiple cellular processes, including cell fate determination, differentiation, proliferation, apoptosis, and regeneration (Miele and Osborne 1999; von Boehmer 2001). Recent studies suggest that Notch receptors are important regulators of cardiovascular development and homeostasis (Chiba 2006; del Monte et al. 2011; High and Epstein 2008; Nemir and Pedrazzini 2008; Niessen and Karsan 2008). In mammals, four Notch receptors (Notch1–4) and five structurally similar Notch ligands (Delta-like1, Delta-like3, Delta-like4, Jagged1, and Jagged2) have been identified (Bolos et al. 2007; Chiba 2006). Both Notch1 and Jagged-1 have been shown to be critical for vasculogenesis and blood flow recovery in ischemic limbs (Kwon et al. 2009; Takeshita et al. 2007). Furthermore, diminished Jagged-1 expression in endothelial cells leads to abnormal smooth muscle development, which may contribute to pulmonary artery stenosis (High et al. 2008). In the heart, Notch1 is activated in proliferating embryonic and immature cardiomyocytes (Campa et al. 2008; Collesi et al. 2008; Kratsios et al. 2010), and is downregulated in the postnatal myocardium (Collesi et al. 2008; Gude et al. 2008). However, Notch signaling in the adult myocardium could be activated transiently following myocardial infarction (Gude et al. 2008; Kratsios et al. 2010; Li et al. 2011), suggesting that Notch signaling could have a protective role following cardiac injury. Indeed, the activation of Notch signaling limits the extent of ischemic injury and improves heart function after myocardial infarction (Boni et al. 2008; Gude et al. 2008; Kratsios et al. 2010; Li et al. 2011). The mechanisms underlying Notch-mediated cardiac protection are complex and include preventing cardiomyocyte apoptosis, recruiting cardiac progenitor cells (CPCs) and immature cardiomyocytes, promoting neovascularization, and possible mediating cellular trans-differentiation. Furthermore, genetic ablation of Notch1 gene, either systemically or in bone marrow (BM)-derived cells, leads to impaired cardiac repair (Li et al. 2011), suggesting a critical role of Notch signaling in BM-derived cells.
Role of Notch1 in Bone Marrow-derived Cells in Cardiac Repair
Recruitment of BM-Derived Cells following Myocardial Injury
Following ischemic myocardial injury, systemic Notch1 deficient (N1+/−) mice (Krebs et al. 2004) developed larger myocardial infarct size and worsening heart function compared to wild-type (WT) mice, suggesting a protective role of Notch1 following myocardial infarction. When the BM of N1+/− mice were transplanted into WT mice (i.e., BM-specific Notch1-deficient mice), infarct size and heart function were worsened and neovascularization in the infarct border area was reduced compared to WT mice transplanted with WT BM. In contrast, transplantation of WT BM into N1+/− mice lessened the myocardial injury observed in N1+/− mice. These findings from reciprocal BM transplantation models suggest that Notch1 in BM-derived cells contributes to cardiac repair following myocardial injury. When GFP-labeled BM-derived cells were used, GFP-N1+/− BMT mice showed decreased GFP expression and GFP-positive cells in the infarct border area compared to GFP-WT BMT mice, suggesting that Notch1 regulates the recruitment of BM-derived cells into the injured hearts. For example, there were more Ki-67+ proliferative and less cleaved-Caspase3+ apoptotic BM-derived cells in the infarct border zone in WT BMT mice than in N1+/− BMT mice. These findings suggest that Notch1 promotes proliferation and suppresses apoptosis of BM-derived cells (Li et al. 2011).
Role of Notch1in BM-Derived Mesenchymal Stem Cells
To determine which subgroups of BM-derived cells could be regulated by Notch1, GFP+ cells in the heart were co-stained with different cellular markers. In contrast to CD34+, CD45+, c-kit+, CD4+, CD68+, CD14+, and CD11b+ cells, more CD105+ and Sca-1+ cells were found in the GFP-WT BMT mice compared to GFP-N1+/− BMT mice, suggesting that Notch1 may increase the recruitment and/or proliferation of BM-derived mesenchymal stem cells (MSC) to the injured heart. The importance of Notch signaling in MSC is underscored by the finding that injection of infarcted hearts with MSC overexpressing NICD decreases infarct size and improves heart function. Conversely, injection of Notch1-deficient MSC into the infarcted heart leads to increased infarct size and worsening of cardiac function (Li et al. 2011).
Decreased expression of Hey1 and HeyL in Notch1-deficient MSC suggests that they may be potential targets of Notch1 signaling in BM-derived MSC. Furthermore, downregulation of CSF3R and CXCR4 expression in N1+/− MSC suggests that Notch1 may mediate the mobilization and migration of BM-derived cells through CSF3R and CXCR4 signaling pathways. Indeed, G-CSF/CSF3R and SDF-1/CXCR4 signaling contribute importantly to the recruitment of BM-derived cells to injured tissues (Abbott et al. 2004; Li et al. 2006). Interestingly, Notch1 upregulates CXCR4 (Wang et al. 2009) and mediates BM-derived cells response to GCSF by its intracellular cdc10 repeat domain (Bigas et al. 1998). Taken together, these findings suggest a critical role of Notch signaling in the recruitment of BM-derived MSC.
Role of Notch1in Neovascularization
Notch signaling regulates the function of vascular cells such as endothelial and smooth muscle cells during pathological states (Kratsios et al. 2010; Kwon et al. 2009; Li et al. 2009; Takeshita et al. 2007). Previous studies reveal a potential cross-talk between Notch and other vascular signaling pathways such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), Wnt, hedgehog, and bone morphogenic protein (BMP) in regulating arteriogenesis (Espinosa et al. 2003; Lawson et al. 2002; Niessen and Karsan 2007). For the injured heart, microvessel formation in the infarct border zone as well as VEGF expression were greatly reduced in BM-specific Notch1-deficient mice, highlighting the role of Notch1 in BM-derived cells in the neovascularization process. Indeed, BM-derived cells could secrete growth factors and cytokines that promote neovascularization and prevent cardiomyocyte apoptosis in the infarct border zone (Li et al. 2006; Uemura et al. 2006). When BM-derived cells in the injured heart were co-stained with smooth muscle and endothelial cell markers, Notch1 appears to promote the differentiation of BM-derived cells into vascular-like cells (i.e., smooth muscle actin and isolectin B4 positive). These findings suggest that Notch1 in BM-derived cells may contribute to vascularization through paracrine effects as oppose to transdifferentiation of BM-derived cells into definitive vascular cells.
Notch Signaling Mediates Interaction between MSC and Cardiomyocyte
BM-derived cells could prevent cardiomyocyte apoptosis in the infarct border zone (Li et al. 2006; Li et al. 2011). When neonatal cardiomyocytes were co-cultured with mouse BM-derived MSC, MSC were found to enhance cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms (Sassoli et al. 2011). For example, the proliferative response of neonatal cardiomyocytes involved the activation of Notch-1 receptor by its ligand Jagged-1 expressed on adjacent MSC. When the cardiomyocytes were exposed to MSC-derived conditioned medium, the release of VEGF-1 and FGF was greatly potentiated, indicating the ability of MSC to stimulate growth factor production by cardiomyocytes through paracrine mechanisms.
Notch-Mediated Cardiomyocyte Protection
Notch is involved in promoting protective signaling in the myocardium following myocardial injury (Gude et al. 2008; Kratsios et al. 2010). For example, Notch1 is activated in cardiomyocytes of the infarct border zone coincident with nuclear c-Met (Gude et al. 2008). Furthermore, injured hearts injected with an adenoviral vector expressing NICD leads to improved heart function and reduced infarct size compared to injection of control adenoviral vector. In cultured cardiomyocytes, treatment with hepatic growth factor (HGF) or insulin increases the levels of Notch effector Hes1, whereas overexpression of activated NICD leads to increased Akt phosphorylation. These findings suggest that the protective effects of Notch signaling in cardiomyocytes may be mediated through HGF/c-Met and Akt survival pathway.
The activation of Notch1(Kratsios et al. 2010) leads to the upregulation of Hes-1 and Hey-1 mRNA levels after, suggesting that Hes-1 and Hey-1 may mediate some of the protective effects of Notch signaling in the adult myocardium. Activation of Notch also increased the expression of antiapoptotic gene Bcl-2, whereas the expression of heart failure markers such Myh6, Myh7, Glut-1, and proapoptotic protease caspase 9 is reduced.
Cardiac fibroblast-myofibroblast transformation (CMT) is a critical event in the initiation of myocardial fibrosis, which could further impair cardiac function following myocardial injury. A recent study suggests that Notch signaling may negatively regulate CMT (Fan et al. 2011). The study found that decreased expression of Notch1, Notch3 and Notch4 in response to TGF-β1 leads to increased α-SMA expression and collagen synthesis. Thus, Notch signaling may play an important role in reducing myocardial fibrosis after injury, by regulating the degree of CMT.
In cardiac-specific Notch1 deficient (C-N1−/−) mice, the loss of Notch1 in postnatal cardiomyocyte surprisingly did not affect the severity of myocardial injury. These findings suggest that Notch1 signaling in cardiomyocytes does not contribute to cardiac repair. In WT mice, the expression of all Notch receptors was increased following myocardial infarction. However, in C-N1−/− mice, the expression of Notch2 and Notch3 was further increased, suggesting that Notch2 and Notch3 may compensate for the loss of Notch1 in adult cardiomyocytes (Li et al. 2011).
Role of Notch1 in c-Kit+ Cells and Immature Cardiomyocytes
Nkx2.5 is a target gene of Notch1 in c-kit+ cardiac progenitor cells (CPCs) (Boni et al. 2008). The c-kit+ cells express Notch1 receptor, with supporting cells expressing the Notch ligand Jagged1. The nuclear-translocated NICD binds to RBP-Jk and form a protein complex, which in turn, binds to the Nkx2.5 promoter thereby initiating transcription and myocyte differentiation. In contrast, c-kit mutant mice (compound heterozygote KitW/KitW-ν mice) exhibit an intrinsic defect in hematopoietic stem cell mobilization. Furthermore, bone marrow-derived c-kit+ cells can lead to an improvement in cardiac function (Fazel et al. 2006). Taken together, these results suggest that Notch1 regulates the function and recruitment of either resident c-kit+ CPCs or bone marrow-derived c-kit+ precursor cells. Indeed, inhibition of Notch1 decreases the commitment of c-kit+ cells to the myocyte lineage. This may results in decreased cardiomyogenesis, myocardial regeneration, and cardiac function.
Several in vitro and in vivo studies demonstrate that cell cycle–related genes, such as c-Myc, cyclin D1, and cyclin dependent kinase inhibitors (CKIs), are transcriptional targets of Notch signaling (Carlson et al. 2008; Klinakis et al. 2006; Ronchini and Capobianco 2001). Notch activation induces cell cycle reentry in quiescent cardiomyocytes (Campa et al. 2008; Collesi et al. 2008) and plays an important role in cardiac cell differentiation (Nemir et al. 2006; Schroeder et al. 2006). The proliferative potential of immature cardiomyocytes is stimulated by the sustained activation of the Notch pathway (Collesi et al. 2008). Notch1 activation by Jagged1 or increased Notch signaling by constitutive expression of its activated form markedly stimulated proliferative signaling such as G1/S cyclins and p38 MAPK and promote immature cardiomyocytes expansion. Furthermore, the activation of Notch signaling leads to increased immature cardiomyocytes, which exhibit low or undetectable levels of the cyclin-dependent kinase inhibitors (CKIs), p27Kip1 and p21Cip1, and the Cdt1 inhibitor geminin (Collesi et al. 2008). In contrast, fully differentiated cardiomyocytes expressed abundant levels of CKIs, whereas the G1/S cyclins are almost undetectable. These features are consistent with the forced activation of Notch2, which promotes reentry of cardiomyocytes into the cell cycle (Campa et al. 2008). Indeed, the activation of Notch signaling in the adult heart inhibits cardiogenic differentiation of cardiac precursors and favors cellular proliferation by triggering incomplete cell cycle progression in cardiac myocyte (Kratsios et al. 2010; Croquelois et al. 2008). These finding suggests that the maintenance or reactivation of Notch signaling might represent an important therapeutic target to protect adult heart after myocardial damage.
Summary
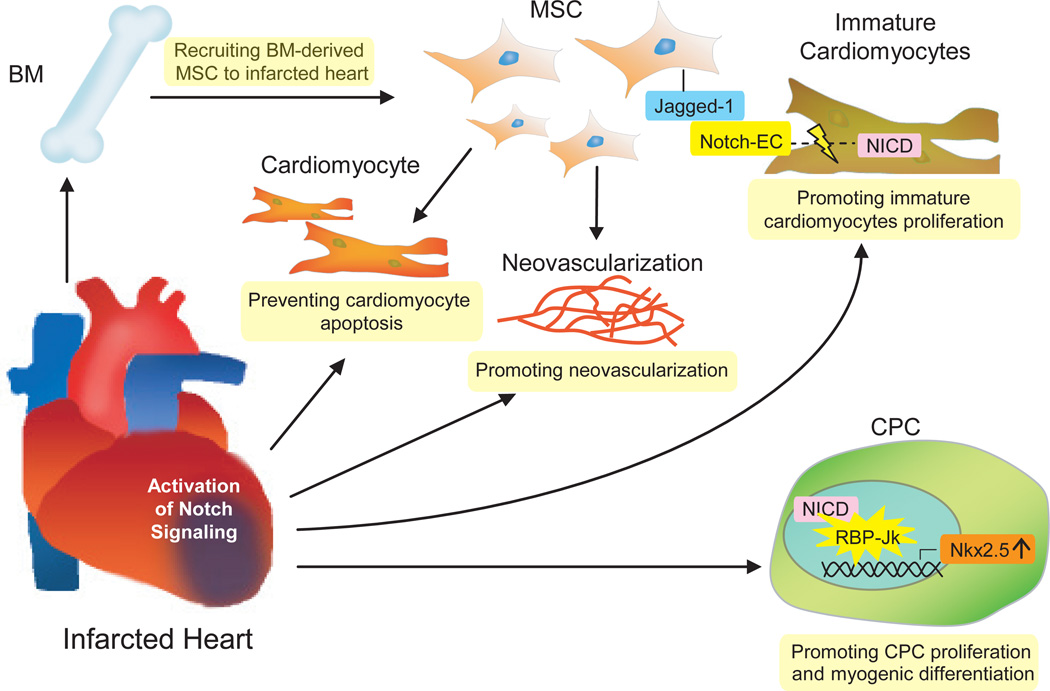
Studies using genetic ablation or activation of Notch signaling have shown that Notch signaling, especially that of Notch1, plays a critical role in cardiac repair and regeneration after myocardial injury. The activation of Notch signaling improves cardiac function, minimizes myocardial fibrosis, suppresses cardiomyocytes apoptosis, and increases neovascularization. These effects of Notch signaling provide a novel paradigm for improving outcome after myocardial infarction. The mechanisms involve a complex interplay between different cell types (Figure 1). As mentioned, Notch target cells include BM-derived cells such as MSC, immature and mature cardiomyocytes, CPC, and vascular cells. Furthermore, Notch target genes are important regulators of cell migration, differentiation, angiogenesis, proliferation, and apoptosis. Thus, Notch signaling serves to link various signaling pathways to the survival and regeneration of the injured myocardium. This coordinate response may offer a therapeutic approach for modulating cardiac repair and regeneration following myocardial infarction.
Figure 1.
Notch signaling cardiac repair and regeneration action. BM: bone marrow; MSC: mesenchymal stem cell; Notch-EC: Notch extracellular domain; NICD: Notch intracellular domain; CPC: cardiac progenitor cell.
Acknowledgement
This work was supported, in part, by grants from the National Institutes of Health (HL052233, NS07001, and DK085006).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 1998;18:2324–2333. doi: 10.1128/mcb.18.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Boni A, Urbanek K, Nascimbene A, et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Campa VM, Gutierrez-Lanza R, Cerignoli F, et al. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croquelois A, Domenighetti AA, Nemir M, et al. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway. J Exp Med. 2008;205:3173–3185. doi: 10.1084/jem.20081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte G, Casanova JC, Guadix JA, et al. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ Res. 2011;108:824–836. doi: 10.1161/CIRCRESAHA.110.229062. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- Fan Y, Dong H, Pan Q, et al. Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol Res. 2011 doi: 10.33549/physiolres.932149. [DOI] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gude NA, Emmanuel G, Wu W, et al. Activation of Notch-mediated protective signaling in the myocardium. Circ Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, et al. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Szabolcs M, Politi K, et al. Myc is a Notch1 transcriptional target and a requisite for Notch1-induced mammary tumorigenesis in mice. Proc Natl Acad Sci U S A. 2006;103:9262–9267. doi: 10.1073/pnas.0603371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratsios P, Catela C, Salimova E, et al. Distinct roles for cell-autonomous Notch signaling in cardiomyocytes of the embryonic and adult heart. Circ Res. 2010;106:559–572. doi: 10.1161/CIRCRESAHA.109.203034. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SM, Suzuki T, Kawamoto A, et al. Pivotal role of lnk adaptor protein in endothelial progenitor cell biology for vascular regeneration. Circ Res. 2009;104:969–977. doi: 10.1161/CIRCRESAHA.108.192856. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Fukuda N, Yokoyama S, et al. Effects of G-CSF on cardiac remodeling and arterial hyperplasia in rats. Eur J Pharmacol. 2006;549:98–106. doi: 10.1016/j.ejphar.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Hiroi Y, Ngoy S, et al. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123:866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Takeshita K, Liu PY, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Nemir M, Croquelois A, Pedrazzini T, Radtke F. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res. 2006;98:1471–1478. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- Nemir M, Pedrazzini T. Functional role of Notch signaling in the developing and postnatal heart. J Mol Cell Cardiol. 2008;45:495–504. doi: 10.1016/j.yjmcc.2008.02.273. [DOI] [PubMed] [Google Scholar]
- Niessen K, Karsan A. Notch signaling in the developing cardiovascular system. Am J Physiol Cell Physiol. 2007;293:C1–C11. doi: 10.1152/ajpcell.00415.2006. [DOI] [PubMed] [Google Scholar]
- Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–1181. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoli C, Pini A, Mazzanti B, et al. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: Clues for cardiac regeneration. J Mol Cell Cardiol. 2011;51:399–408. doi: 10.1016/j.yjmcc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Meier-Stiegen F, Schwanbeck R, et al. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech Dev. 2006;123:570–579. doi: 10.1016/j.mod.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Satoh M, Ii M, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Coming to grips with Notch. J Exp Med. 2001;194:F43–F46. doi: 10.1084/jem.194.7.f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Hu XB, He F, et al. Lipopolysaccharide-induced maturation of bone marrow-derived dendritic cells is regulated by notch signaling through the up-regulation of CXCR4. J Biol Chem. 2009;284:15993–16003. doi: 10.1074/jbc.M901144200. [DOI] [PMC free article] [PubMed] [Google Scholar]