Abstract
Background. Decorin adherence is crucial in the pathogenesis of Lyme borreliosis. Decorin-binding proteins (Dbp) A and B are the adhesins that mediate this interaction. DbpA and B of Borrelia garinii, Borrelia afzelii, and Borrelia burgdorferi sensu stricto (ss) differ in their amino acid sequence, but little attention has been paid to the potential difference in their decorin binding.
Methods. We expressed recombinant DbpA and DbpB of B. garinii, B. afzelii, and B. burgdorferi ss and studied their binding to decorin. We also generated recombinant Borrelia strains to study the role of DbpA and DbpB in the adhesion of live spirochetes to decorin and decorin-expressing cells.
Results. Recombinant DbpA of B. garinii and DbpB of B. garinii and B. burgdorferi ss showed strong binding to decorin, whereas DbpA of B. burgdorferi ss and both DbpA and DbpB of B. afzelii exhibited no or only minor binding activity. DbpA and DbpB of B. garinii and B. burgdorferi ss also supported the adhesion of whole spirochetes to decorin and decorin-expressing cells, whereas DbpA and DbpB of B. afzelii did not exhibit this activity.
Conclusions. Dbp A and B of B. garinii and B. burgdorferi ss mediate the interaction between the spirochete and decorin, whereas the same adhesins of B. afzelii show only negligible activity.
Lyme borreliosis (LB) is a tickborne infectious disease caused by Borrelia burgdorferi sensu lato (Bbsl) which includes B. garinii (Bg), B. afzelii (Ba), and B. burgdorferi sensu stricto (Bbss) [1]. Late manifestations of LB are associated with different tissue tropism of borrelia genospecies. Bg has the tendency to cause neuroborreliosis, whereas Ba is mostly associated with chronic skin disorders, such as acrodermatitis chronica atrophicans and Bbss with Lyme arthritis. The molecular mechanisms behind this phenomenon are unknown.
Bbsl has several surface proteins that mediate attachment to different tissues and molecules of the host. It can bind to host plasminogen and plasminogen activators [2] and to various extracellular matrix and cell surface molecules [3]. Several adhesins have been identified in Bbsl, which mediate adherence to decorin [4], glycosaminoglycans (GAGs) [5], integrins [6, 7], fibronectin [8–10], laminin [9, 11, 12], and collagen [9, 13].
Decorin-binding proteins (Dbp) A and B are 2 well-characterized adhesins of Bbss [4, 14]. DbpA and B are expressed during mammalian infection [15], and they mediate bacterial attachment to the proteoglycan decorin, which is associated with collagen fibers in the extracellular matrix [16]. The decorin binding site of DbpA has been mapped to a conserved peptide motif (EAKVRA) [17]. Decorin consists of a collagen-binding core protein and a single GAG side chain that can be either dermatan sulphate or chondroitin-6-sulphate [18]. The binding target of Dbps is the GAG side chain, especially dermatan sulphate, in decorin [19].
Decorin is widely expressed throughout the body. High concentrations are detected in the skin, joints, and endothelium [20]. Decorin binding activity has an important role in borrelial dissemination, and it is also suggested that tissues rich in decorin might serve as a protective niche for Bbss [21]. In mice, for example, mutant strains lacking either one or both of the proteins are infective, but their dissemination to distant organs is less efficient than is the dissemination of the wild-type strain [22].
Thus far, all studies concerning the biological functions of Dbps have been performed with Bbss, and little attention has been paid to the potential differences in the decorin binding activity among the 3 genospecies. In amino acid sequence comparison, only 40–60% similarity was seen among the Dbps of the genospecies [23]. Variation in decorin binding activity may be a factor that in part determines the different tissue tropism of the 3 genospecies. It may also have a role in immune evasion and persistence of LB. The aim of this study was to characterize and compare the biological activities of the Dbps of Bg, Ba, and Bbss. To accomplish that, we took 2 approaches: (1) we recombinantly expressed individual Dbps and characterized their decorin binding properties and (2) generated recombinant borrelia strains in a surface protein–deficient background strain (B313) [24] to study the role of Dbps in the adhesion of live borrelia to decorin and decorin-expressing cells.
METHODS
Bacterial Strains and Culture Conditions
Low passage wild-type Bbss N40, Bbss B31, Bbss HB-19, Bbss SH-2-82, Bg SBK40, Bg SBK46, Bg Å218, Bg 387, Ba A91, Ba 570, Ba bo23, and Ba pKo and high-passage Bbss B313 and Ba 1082 were used in this study. All Bg and Ba strains were isolated from skin biopsy samples from Finnish patients except for Bg Å218, which is a tick isolate, and Ba pKo, which was kindly provided by Volker Fingerle (National Reference Centre for Borrelia, Bavarian Food and Health Safety Authority). Bbss wild-type strains were kindly provided by Sven Bergström (University of Umeå, Sweden), and B313 by Thomas Kamradt (Deutches Rheuma-Forschungszentrum). The spirochetes were cultivated in Barbour-Stoenner-Kelly II medium [25] at 33°C and were passaged weekly. Commercial Escherichia coli host strains (INVαF′ [Invitrogen]; Novablue Giga Singles and BL21(DE3)pLysS [Novagen]; M15 [Qiagen]) were used in cloning and expression of recombinant proteins.
Cloning and Expression of Recombinant Dbps
E. coli expressing DbpA and B of Bbss N40 were constructed with pET-30 Ek/LIC vector system (Novagen) according to the manufacturer’s instructions. The primers used in polymerase chain reaction (PCR) amplification of the dbpA and B are listed in Table 1. The recombinant strains expressing DbpA and B of Bg SBK40 and Ba A91 are described elsewhere [23, 26]. The proteins were purified under native conditions with use of Ni-NTA agarose beads (Qiagen) and were dialyzed against phosphate-buffered saline (PBS).
Table 1.
Primers Used in PCR Amplification of dbpAB, dbpA or dbpB.
| Primer | Vector | Sequence (5′-3′) |
| dbpA/BbssFW | pET-30 | GAC GAC GAC AAG GGA TTA AAA GGA GAA ACA AAA ATC |
| dbpA/BbssREV | pET-30 | GAG GAG AAG CCC GGT TTA GTT ATT TTT GCA TTT TTC ATC |
| dbpB/BbssFW | pET-30 | GAC GAC GAC AAG GGA TTA GTA GAA AGA ACA AAT GC |
| dbpB/BbssREV | pET-30 | GAG GAG AAG CCC GGT TTA TTT CTT TTT TTT GCT TTT ATT AT |
| dbpAB/BbssFW | pBSV2 | AATA GCA TGC ACA AGC CAG ATT GCA TAG C |
| dbpAB/BbssREV | pBSV2 | AATA GGA TCC TTG ATT ATC GGG CGA AGA G |
| dbpAB/BgFW | pBSV2 | AATA GCA TGC ACA TTA TTT GGC AAA CTG GC |
| dbpAB/BgREV | pBSV2 | AATA GGA TCC GGT ACT TTA CGA CAG TCT TG |
| dbpAB/BaFW | pBSV2 | AATA GCA TGC CCC CTG GCA AAA TAA AAT TC |
| dbpAB/BaREV | pBSV2 | AATA GGA TCC TTA TTT TTG ATT TTT AGT TTG TTT TTC |
NOTE. Restriction enzyme sites in the primers are bold type.
Generation of Recombinant B313 Strains
Recombinant B313 strains expressing DbpA and B of Bbss N40, Bg SBK40, and Ba A91 were constructed using previously described methods [27]. Primers used in PCR amplification of dbpAB operon are listed in Table 1. The resulting recombinant borrelia strains were named B313/dbpAB/Bbss, B313/dbpAB/Bg, and B313/dbpAB/Ba, and the control strain containing only pBSV2 was named B313/pBSV2.
Characterization of the Recombinant Borrelia Strains
The presence of DbpA and B was shown by Western blot assay using polyclonal anti-Dbp antiserum (Medprobe) and anti-rabbit IgG (Santa Cruz Biotechnology). Anti-flagellin B antibody (H9724; gift from S. Bergström) with anti-mouse IgG (Santa Cruz Biotechnology) was used as a control.
Surface localization of the adhesins was shown with proteinase-K treatment. The bacteria were washed twice with PBS containing 5 mM MgCl2 and were diluted to 1 × 108 bacteria/mL. Proteinase-K was added to the cell suspension at a final concentration of 100 μg/mL, incubated at room temperature for 30 min, and washed twice with the aforementioned buffer before preparing the samples for Western blot analyses.
Western Blot Assay with Biotinylated Decorin
Decorin (from bovine cartilage; Sigma) was biotinylated with EZ-Link NHS-LC-Biotin (Pierce) according to the manufacturer’s instructions. Biotinylated decorin (1 μg/mL) was used as the primary reagent, and HRP-conjugated streptavidin (0.1 μg/mL; Pierce) was used as the secondary reagent in the Western blot assay.
Surface Plasmon Resonance Analysis
Flow cell of the CM5 chip (GE Healthcare) was coated with the recombinant Dbp (50 μg/mL) in coating buffer (10 mM sodium acetate; pH 4.5 or 5.0) with use of the amine coupling kit, according to the manufacturer’s instructions, until ∼8000 resonance units (RUs) were reached. A control cell lacking the adhesin was prepared under identical conditions. The changes in RUs caused by binding of decorin to Dbps on the chip were measured using Biacore X (Biacore AB). All binding experiments were run at room temperature. Decorin was dissolved in HBS-P running buffer (10 mM HEPES [pH, 7.4]; 0.15 M sodium chloride; 0.005% (v/v) Surfactant P20), and concentrations of 10 μg/mL, 50 μg/mL, and 100 μg/mL were used. Flow rate of 10 μL/min was applied in all measurements. The background signal from the buffer-treated reference cell was subtracted from the Dbp signal. The BIAevaluation program (version 3.0; Biacore) was used in the affinity curve calculations.
Adhesion of Recombinant Dbps to Decorin in Microtiter Plate Assay
Microtiter plates (Thermo Fisher Scientific) were coated with respective Dbp (10 μg/mL) in PBS and blocked with 1% BSA/PBS. The wells were incubated with biotinylated decorin (1 μg/mL), washed 3 times with PBS-T (PBS; 0.05% Tween 20), and incubated with 1 μg/mL alkaline phosphatase-conjugated streptavidin (Pierce). After washings, p-NPP-Na2 substrate (1 mg/mL; Reagena) was added for 25 min before the reaction was stopped with 1M sodium hydroxide, and absorbances (OD405) were measured using a Multiskan EX spectrophotometer (Thermo Fisher Scientific). All incubations were at 37 °C for 1 h, except for the substrate. Results are expressed as OD405 values subtracted with the background (a well without biotinylated decorin) absorbance. His-tag specific antibody (Penta-His biotin conjugate; Qiagen) was used to demonstrate the presence of Dbps on the wells (data not shown).
In inhibition experiments, the inhibitors were preincubated on the plates or with biotinylated decorin at 37°C for 1 h, and the assay was otherwise done as described above. Peptide LALREAKVQAIVETG (based on the DbpB sequence of Bg; 10 μg/mL; EAKVQA-peptide; Haartman Institute), dermatan sulphate (50 μg/mL; from shark cartilage; Sigma), chondroitin-6-sulphate (50 μg/mL; from shark cartilage; Sigma), and chondroitin-4-sulphate (50 μg/mL; from bovine trachea; Sigma) were used as inhibitors. Decorin (10 μg/mL) and BSA fraction V (100 μg/mL; Serological Proteins) were used as a positive and a negative control, respectively.
Dot Adhesion Assay
Wild-type and recombinant borrelia strains were washed and resuspended in PBS. One μL (5 × 105 or 1 × 106 bacteria) of the bacterial suspension was applied on a nitrocellulose membrane (Protran BA 85/20 0.45 μm; Schleicher & Schuell). The air-dried membrane was blocked with 3% nonfat dry milk in TBST (50 mM Tris-HCl [pH, 7.4]; 150 mM sodium chloride; 0.1% Tween 20) and incubated with biotinylated decorin (1 μg/mL) in TBST for 1 h at room temperature. The binding of biotinylated decorin was detected with streptavidin-HRP (0.1 μg/mL) in TBST. Detection was done as in the Western blot assay. The intensities of the dots were quantified with UVP BioSpectrum AC Imaging System. The unspecific binding of decorin to B313 was given a value of 1.
Cell Adhesion Assay
Human foreskin fibroblasts (HFFs) were cultured in Dulbecco’s modified Eagle medium (Invitrogen) containing 7% fetal calf serum (Hyclone) and gentamycin (Nalgene) in Lab-Tek 8-well chamber slide (Nunc) in a carbon dioxide incubator at 37°C for 2 days. Cells were fixed with –20°C acetone for 15 min and washed twice with PBS-T.
Recombinant borrelia strains were stained with carboxylfluorescein diacetate succinimidyl ester (CFSE; Molecular probes) as described elsewhere [28]. B313 and B313/pBSV2 were used as negative controls. CFSE-stained borrelia (107) were allowed to adhere to the cells in slide chambers at 37°C for 1 h. The slides were washed twice with PBS, and the results were imaged with confocal microscope (Zeiss LSM510 META). Pictures of 10 random fields per chamber were analyzed with Image J analyzing program, and the number of individual bacteria was counted from every field.
To confirm the presence of decorin on the fixed cells, they were stained with monoclonal anti-decorin antibody (Santa Cruz Biotechnology) in PBS at 37°C for 1 h and washed twice with PBS-T and once with PBS. HRP-conjugated anti-mouse IgG was used as a secondary antibody and incubated at 37°C for 1 h with the cells. After the washes, HRP was allowed to react with a peroxidise substrate (1 mM 3-amino-9-ethylcarbazole, 5% (v/v) dimethylformamide, 0.1% (v/v) hydrogen peroxidise in acetate buffer [pH, 5.5]) at room temperature for 30 min, and the decorin-stained cell cultures were imaged with optical microscope.
Inhibition assays were performed with B313/DbpAB/Bg and B313/DbpAB/Bbss. The inhibitors were preincubated with the CFSE-stained bacteria or the cells for at 37°C for 1 h, and the assay was otherwise performed as described above. EAKVQA-peptide (10 μg/mL), dermatan sulphate (50 μg/mL), chondroitin-6-sulphate (50 μg/mL), and chondroitin-4-sulphate (50 μg/mL) were used as inhibitors. Decorin (100 μg/mL) was used as a positive control, and BSA (100 μg/mL) was used as a negative control.
Statistical Analyses
The statistical significance of differences in the microtiter plate assay, dot adhesion assay, and cell adhesion assay were determined using analysis of variance (PASW statistics, version 18). Response variables were loge-transformed in microtiter plates and cell adhesion assay to normalize error distributions. Post hoc comparisons between means were done using Dunnett t test when there was a clear control and with Tukey's honestly significance difference test in other cases.
RESULTS
DbpB of Bg and Bbss Bind Decorin in Western Blot Assay
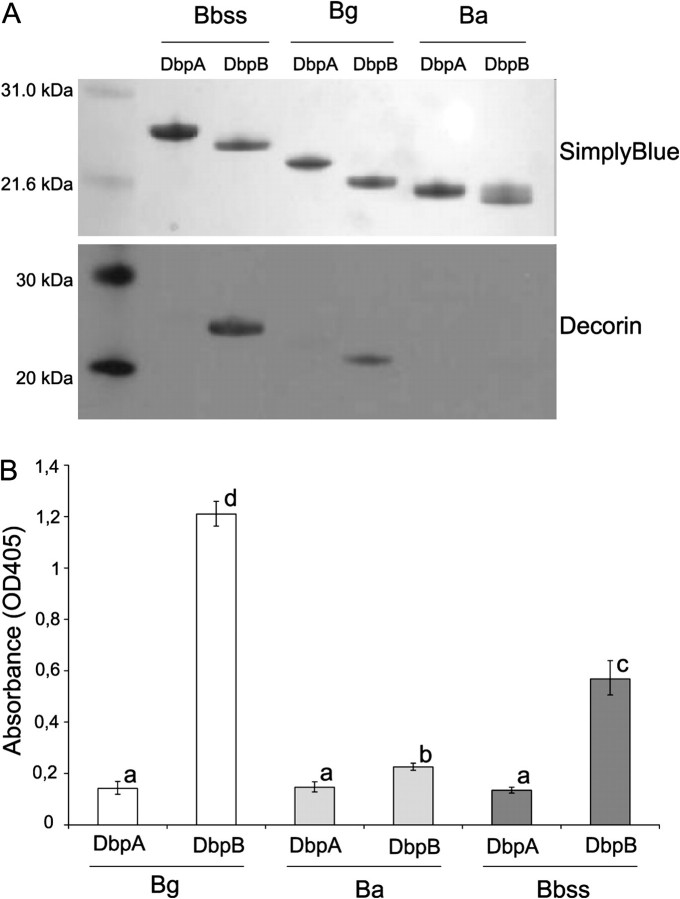
First, the binding properties of recombinant His-tagged Dbps were studied. The PCR-amplified genes were sequenced (data not shown), and proteins of correct size were identified on sodium dodecyl sulfate polyacrylamide gel electrophoresis (Figure 1A). The binding of decorin to Dbps was analyzed using a Western blot assay using biotinylated decorin as the probe. Under these conditions, only DbpB/Bg and DbpB/Bbss showed clear binding to decorin.
Figure 1.
A, Binding of decorin to recombinant Dbps in a Western blot assay. The upper panel represents SimplyBlue staining of the SDS-PAGE gel. The lower panel shows the adhesion of biotinylated decorin (1 μg/mL) to Dbps. B, Binding of decorin to recombinant Dbps in a microtiter plate assay. Recombinant Dbps (10 μg/mL) were attached on microtiter plates, and unspecific binding was blocked with BSA. The binding of biotinylated decorin (1 μg/mL) was detected with alkaline-phosphatase streptavidin and p-NPP-Na2 substrate. Results are expressed as geometric mean of OD405 values, subtracted with background, ± standard deviation of quadruplicate samples. Columns with same letter do not differ at 5% level of probability (Tukey HSD test).
DbpB of Bg and Bbss Are the Strongest Decorin Binders in a Microtiter Plate Assay
To study interaction between the Dbps and decorin under native conditions and to quantitate the binding, a microtiter plate assay was performed. This assay also showed clear binding of DbpB/Bg and DbpB/Bbss to decorin, which was statistically significantly higher than the binding of other Dbps (Figure 1B). The signals of the wells coated with DbpA/Bg, DbpA/Ba, DbpB/Ba, or DbpA/Bbss remained at a low level, although the binding of DbpB/Ba differed statistically significantly from other Dbps.
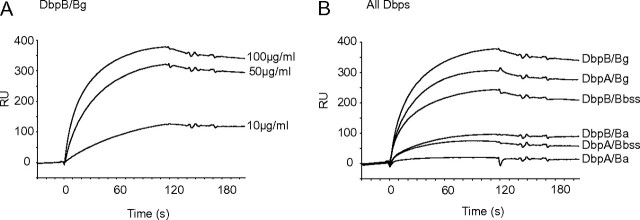
DbpA and B of Bg Have the Highest Affinities to Decorin in Surface Plasmon Resonance Assay
To characterize in more detail the affinities of individual Dbps to decorin, a surface plasmon resonance assay was performed. Decorin binding to Dbps was dose dependent (Figure 2A). DbpB/Bg had the highest affinity to decorin (Figure 2B). In contrast to Western blot and microtiter plate assays, DbpA/Bg also showed clear binding to decorin. DbpB/Bbss exhibited moderate affinity to decorin, whereas DbpA/Bbss and DbpB/Ba showed only modest binding. The binding of DbpA/Ba to decorin was negligible at the tested conditions.
Figure 2.
Surface plasmon resonance assay of decorin-Dbp interaction. Recombinant Dbps were attached covalently on CM5 chips (GE Healthcare), and unlabelled decorin was allowed to adhere to the chip. The binding was detected with Biacore 2000 equipment. A, Dose response of decorin (10, 50, and 100 μg/mL) binding to DbpB/Bg. B, Binding of decorin (100 μg/mL) to Dbps. The results are expressed as resonance units (RUs).
Taken together, the above results suggest that, depending on the mode of analysis, DbpB/Bg, DbpA/Bg, and DbpB/Bbss are the strongest decorin adhesins among the studied Dbps.
Characterization of Recombinant Borrelia Strains
Next, recombinant borrelia strains in the surface protein–deficient Bbss B313 background strain were constructed to study the role of Dbps in the adhesion of borrelia spirochetes to decorin and decorin-expressing cells. Correctness of the cloned operons was confirmed by sequencing (data not shown).
Western blot assay with specific antibodies of whole cell lysates showed that B313/DbpAB/Bg, B313/DbpAB/Ba, and B313/DbpAB/Bbss as well as wild-type Bg SBK40, Ba A91, and Bbss N40 express DbpA and B proteins (Figure 3A). Neither the background strain B313 (Figure 3A) nor B313 complemented with the pBSV2 vector only (data not shown) expressed the Dbps. Proteinase-K treatment showed the surface localization of Dbps in all recombinant strains (Figure 3B).
Figure 3.
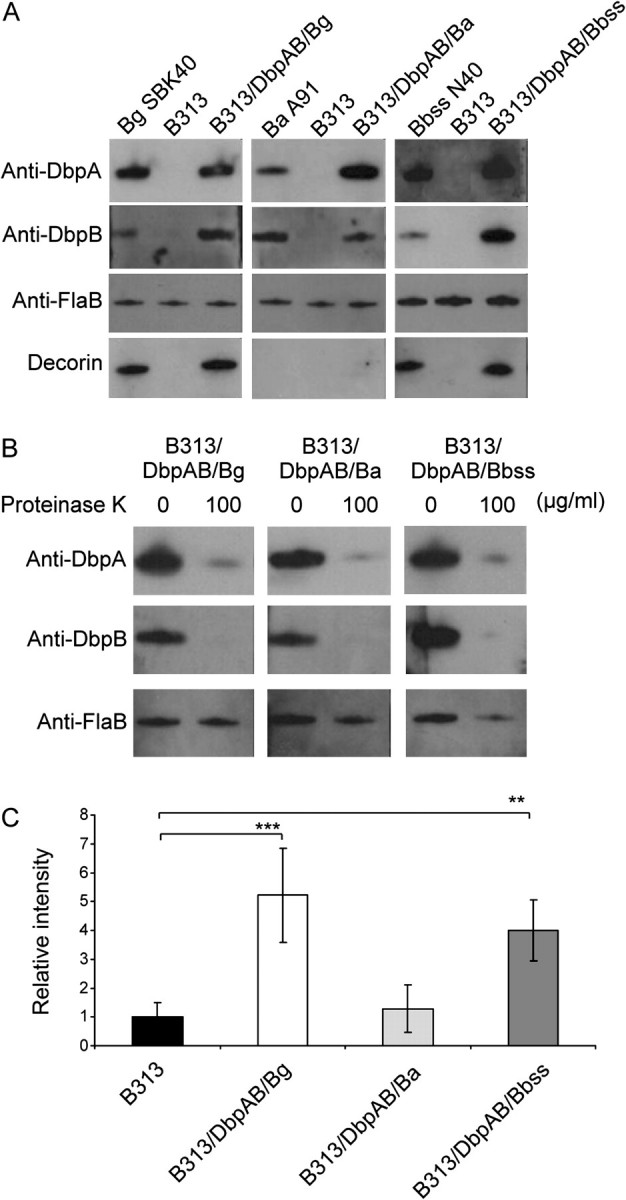
Characterization of wild-type and recombinant borrelia strains expressing the Dbps. A, Western blot assay of DbpA and DbpB proteins. The expression of dbpAB operon is shown with anti-DbpA and anti-DbpB antibodies. Anti-flagellin B (FlaB) antibody was used as a control. The lowest panel shows the adhesion of biotinylated decorin (1 μg/mL) to borrelia lysates. B, The surface localisation of Dbps in recombinant borrelia strains. Recombinant borrelia strains were proteinase-K (100 μg/mL) treated and samples were analysed using Western blotting as above. Anti-FlaB antibody was used as a control. C, Binding of decorin to intact bacteria. 5 × 105 borreliae were dotted on nitrocellulose membranes. Dried membranes were blocked with nonfat milk and probed with biotinylated decorin (1 μg/mL). Intensities of the dots were measured with a densitometer. The unspecific binding of B313 was given a value of 1. Mean absorbances ± standard deviations of four experiments are shown. **P ≤ .01, ***P ≤ .001.
In line with the Western blot results using recombinant Dbps (Figure 1A) Western analysis of cell extracts from wild-type Bg SBK40 and Bbss N40 and recombinant B313/DbpAB/Bg and B313/DbpAB/Bbss identified one band reacting with decorin in each strain (Figure 3A). With Ba A91 or B313/DbpAB/Ba, no binding to decorin was detected.
Dbps of Bg and Bbss Support the Adhesion to Decorin
A dot adhesion assay was introduced to study the binding of intact borrelia to decorin (Figure 3C). The background level binding of B313 was given the value of 1. In agreement with aforementioned results, the B313/DbpAB/Bg and B313/DbpAB/Bbss recombinant strains bound decorin statistically significantly, whereas the binding activity of B313/DbpAB/Ba was at the background level.
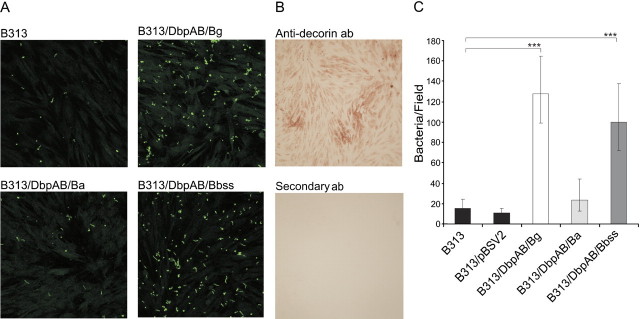
Dbps of Bg and Bbss Mediate Adhesion to Decorin-Expressing Cells
We next analyzed the ability of Dbps to support attachment of live borrelia to cells. HFF fibroblasts grown on chamber slides were shown to express decorin on their surface (Figure 4B). In the adhesion assay, CFSE-labelled borreliae were allowed to adhere to the cells, and the binding was quantitated using confocal microscopy. B313/DbpAB/Bg showed the highest adherence to the cells. B313/DbpAB/Bbss also bound well to the cells, whereas B313/DbpAB/Ba, B313/pBSV2, and B313 exhibited only background level of binding (Figure 4A and 4C). These results corroborate the unexpected finding that DbpA and DbpB of Ba, although expressed by this species, do not carry decorin binding activity.
Figure 4.
Adhesion of recombinant borrelia strains to HFF cells. A, The cells were fixed with −20°C acetone and 107 CFSE stained borrelia (green) were allowed to adhere to the cells in slide chambers and the binding was imaged with confocal microscopy. B, The presence of decorin in the cell culture was shown with monoclonal anti-decorin antibody and HRP-conjugated secondary antibody which was allowed react with a peroxidase substrate to form a brown precipitate. Lower panel shows staining with secondary antibody only. C, The mean of borrelia bound to fibroblasts per one confocal microscope field. The number of bacteria was counted from ten random fields in three independent experiments. Geometric mean ± standard deviations are shown. ***P ≤ .001.
Molecular Dissection of the Interaction Between Dbps and Decorin
It was previously shown with Bbss-derived Dbps that the decorin binding is inhibited by GAGs [19] or peptides containing the EAKVRA motif [17]. Therefore, we tested whether dermatan sulphate, chondroitin-6-sulphate, chondroitin-4-sulphate, or the EAKVQA-peptide could inhibit the interaction between decorin and DbpB/Bg. We also studied the role of these molecules in the cell adhesion assay by inhibiting the interaction between fibroblasts and B313/DbpAB/Bg or B313/DbpAB/Bbss.
In the microtiter plate assay, the binding of biotinylated decorin to DbpB/Bg was inhibited most efficiently with EAKVQA-peptide and dermatan sulphate, which both had effects similar to unlabelled decorin (P ≤ .001). Chondroitin-6-sulphate and chondroitin-4-sulphate also inhibited the decorin binding significantly (P ≤ .001), whereas BSA had no effect on the binding (Figure 5A).
Figure 5.
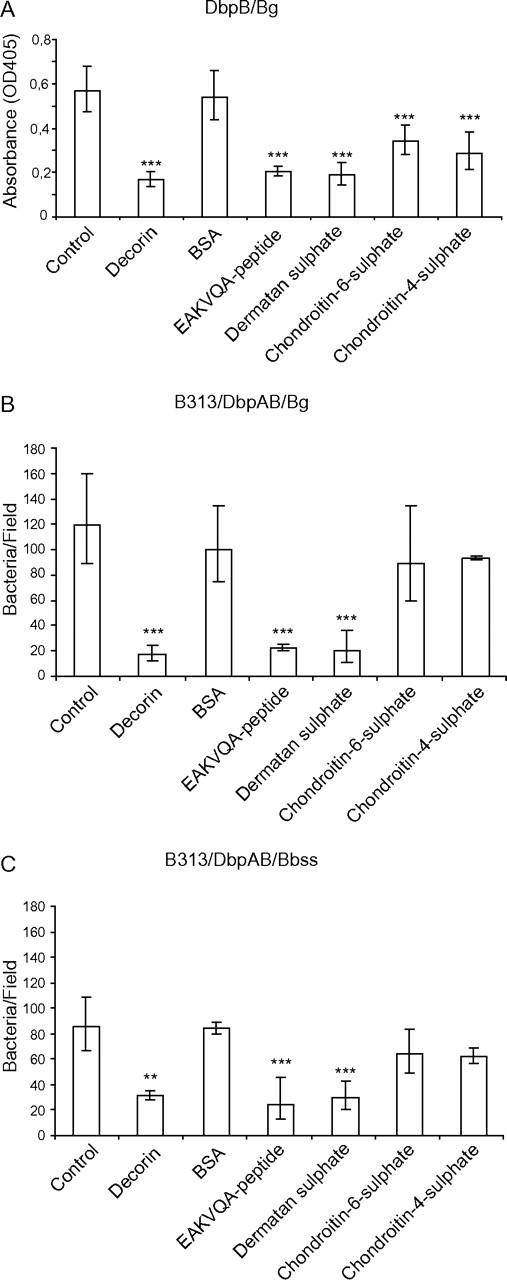
Inhibition analyses. A, Inhibition of the DbpB-decorin interaction in microtiter plate assay. The binding of decorin to DbpB/Bg was inhibited with EAKVQA-peptide, dermatan sulphate, chondroitin-6-sulphate, and chondroitin-4-sulphate. Unlabelled decorin was used as a positive and BSA as a negative control. Results are expressed as absorbances subtracted with the background absorbance. Mean absorbances ± standard deviation of three experiments are shown. B and C, Inhibitions in the cell adhesion assay. The adhesion of B313/DbpAB/Bg and B313/DbpAB/Bbss to fibroblasts was inhibited with decorin, EAKVQA-peptide, dermatan sulphate, chondroitin-6-sulphate, and chondroitin-4-sulphate. BSA was used as a negative control. The average number of borrelia bound to fibroblasts in one confocal microscope field was counted from ten random fields in three independent experiments. Geometric mean ± standard deviations are shown. **P ≤ .01, ***P ≤ .001.
In the cell adhesion assays, the adhesion of recombinant B313/DbpAB/Bg and B313/DbpAB/Bbss to fibroblasts was inhibited significantly with decorin, EAKVQA-peptide, and dermatan sulphate (P ≤ .001). Chondroitin-6-sulphate and chondroitin-4-sulphate had only a minor, statistically nonsignificant effect on the binding. BSA, which was used as a negative control, did not influence the binding (Figure 5B and C).
In conclusion, the results of the inhibition assays confirm that the binding target of DbpB/Bg and DbpB/Bbss in decorin is the GAG side chain, especially dermatan sulphate and that the EAKVQA (or EAKVQA like) motif is the structure in the adhesins carrying the activity.
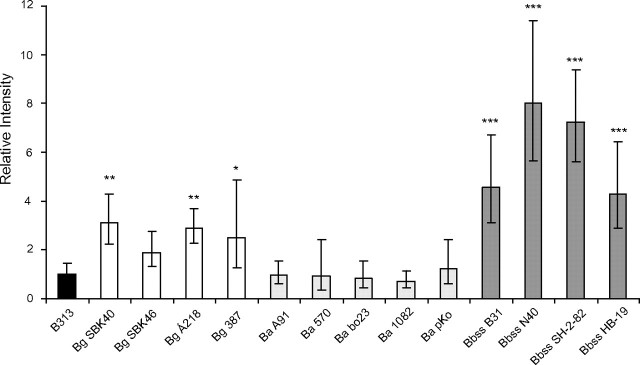
Binding of Decorin to Wild-Type Bbsl Strains
Finally, to analyze whether the decorin-binding properties of Bg SBK40, Ba A91, and Bbss N40 could be generalized to other Bg, Ba, and Bbss strains, a dot adhesion assay was performed with an array of wild-type strains. In this experiment, none of the 5 tested Ba strains exhibited decorin binding activity, whereas 8 of 9 Bg and Bbss strains showed statistically significant binding to decorin, compared with B313 (Figure 6).
Figure 6.
Binding of decorin to intact wild-type Bbsl strains. Borreliae (1 × 106) were dotted on nitrocellulose membranes. Dried membranes were blocked with nonfat milk and probed with biotinylated decorin (1 μg/mL). Intensities of the dots were measured with a densitometer. The unspecific binding of B313 was given a value of 1. Geometric mean ± standard deviations of 4 replicates are shown. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
DISCUSSION
Decorin binding activity of Bbss has an important role in borrelia infection [21, 22, 29–31]. However, all the studies concerning the decorin binding activity of borrelia have been performed using Bbss strains and Dbps derived from Bbss. Of importance, variation in the structure and also the potential variation in ligand specificity of Dbps, which may be factors that, at least in part, determine the different tissue tropism of the 3 genospecies, have drawn little attention. The results of the present study suggest that DbpA and B of different Lyme disease borrelia genospecies have different binding properties to decorin. Dbps of Bg and Bbss clearly support the adhesion of the bacteria to decorin, whereas the Dbps of Ba, although expressed on the bacterial surface, do not carry this activity.
Our Western blot assays with Bbss N40 whole cell lysates identified only one protein (DbpB) binding to decorin, which is in contrast to the seminal results of Guo et al[4], who showed, in a similar assay, that N40 expresses 2 proteins, later identified as DbpA and DbpB, with decorin binding activity. However, our Western blot results with the recombinant Dbps are in line with borrelia lysate results suggesting that, indeed, only DbpB of N40 carries significant decorin binding activity. The microtiter plate assay and SPR results further confirm this finding. The reason for this discrepancy could be random mutations in dbpA gene of the N40 strain that is in use in our laboratory, but no mutations were detected in dbpA and dbpB when they were sequenced after PCR and compared with sequences in databases. In addition, tick isolates are known to be heterogeneous, which may explain the disparate results obtained with N40 in different laboratories. A third explanation might be the different source of decorin used in our assays (bovine cartilage) and in the assays of Guo et al (bovine skin) [4]. However, whether these 2 proteoglycans are differently glycosylated or have other structural dissimilarities remains unknown.
On the basis of the current understanding that Bbss and Ba are the main genospecies associated with manifestations of the connective tissue (arthritis and acrodermatitis chronica atrophicans, respectively), it could be assumed that they would also show avid binding to decorin. This was, however, not the case because in all assays, either DbpB/Bg or B313/DbpAB/Bg bound to purified decorin or decorin-expressing cells most efficiently. Of interest, decorin is also expressed in the central nervous system [32] and cerebral endothelial cells [33], which implies that the strong affinity of Bg to decorin might have a role in the colonization of the CNS by Bg in neuroborreliosis. Another interesting observation was that DbpA/Bg showed dramatic variation in the adherence to decorin under different experimental conditions. In Western blot and microtiter plate assays (ie, under stationary incubation), DbpA/Bg showed a weak or no adherence, whereas in SPR analysis, under flow, it exhibited even higher affinity to decorin than did DbpB/Bss, which was at least a moderate binder in all assays. The high affinity of both DbpA/Bg and DbpB/Bg to decorin under constant flow could, in fact, be essential for the adherence to cerebral endothelial cells and, thus, for the initiation of central nervous system colonization. It can be hypothesized that DbpA/Bg forms a “catch bond” with decorin and needs shear force to induce the binding, as has been shown for certain other bacterial adhesins [34].
In our studies, the individual recombinant Dbps of Ba showed only little or no adherence to decorin, and the borrelia strains expressing Ba Dbps, both wild-type and recombinant (Figure 3), did not adhere to purified bovine decorin or to human cells expressing decorin (Figure 4), more than the Dbp deficient background strain B313. This was also the case with 4 other Ba wild-type strains (Figure 6). Thus, it is likely that the Dbps of Ba have biological functions and ligands other than the Dbps of Bg and Bbss. Experiments addressing this question and the role of decorin binding in central nervous system colonization by Bg have been launched.
Funding
This work was supported by the Academy of Finland, Turku Graduate School for Biomedical Sciences, the Turku University Foundation, the Finnish Cultural Foundation, the Emil Aaltonen Foundation, and the Special Governmental Fund for University Hospitals (EVO).
Acknowledgments
We thank Jarmo Käpylä, for help in SPR analysis, Seppo Neuvonen and Saija Hurme for help in statistical analyses, and Tytti Vuorinen for providing fibroblast cells.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, Benach JL. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–9. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 3.Coburn J, Fischer JR, Leong JM. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol Microbiol. 2005;57:1182–95. doi: 10.1111/j.1365-2958.2005.04759.x. [DOI] [PubMed] [Google Scholar]
- 4.Guo BP, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–72. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parveen N, Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–34. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- 6.Behera AK, Durand E, Cugini C, et al. Borrelia burgdorferi BBB07 interaction with integrin alpha3beta1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol. 2008;10:320–31. doi: 10.1111/j.1462-5822.2007.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–40. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 8.Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect Immun. 2009;77:2802–12. doi: 10.1128/IAI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallstrom T, Haupt K, Kraiczy P, et al. Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis. 2010;202:490–8. doi: 10.1086/653825. [DOI] [PubMed] [Google Scholar]
- 10.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–15. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 11.Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology. 2009;155:863–72. doi: 10.1099/mic.0.024604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Brissette CA, Bowman A, Stevenson B. burgdorferi. BmpA is a laminin-binding protein. Infect Immun. 2009;77:4940–6. doi: 10.1128/IAI.01420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambrano MC, Beklemisheva AA, Bryksin AV, Newman SA, Cabello FC. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect Immun. 2004;72:3138–46. doi: 10.1128/IAI.72.6.3138-3146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–23. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Hodzic E, Stevenson B, Barthold SW. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–35. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–97. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikas DS, Brown EL, Gurusiddappa S, Lee LY, Xu Y, Hook M. Decorin-binding sites in the adhesin DbpA from Borrelia burgdorferi: a synthetic peptide approach. J Biol Chem. 2003;278:30920–6. doi: 10.1074/jbc.M303979200. [DOI] [PubMed] [Google Scholar]
- 18.Brennan MJ, Oldberg A, Pierschbacher MD, Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. J Biol Chem. 1984;259:13742–50. [PubMed] [Google Scholar]
- 19.Fischer JR, Parveen N, Magoun L, Leong JM. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2003;100:7307–12. doi: 10.1073/pnas.1231043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Järveläinen HT, Kinsella MG, Wight TN, Sandell LJ. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991;266:23274–81. [PubMed] [Google Scholar]
- 21.Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004;165:977–85. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–46. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heikkila T, Seppala I, Saxen H, Panelius J, Yrjanainen H, Lahdenne P. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J Clin Microbiol. 2002;40:453–60. doi: 10.1128/JCM.40.2.453-460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadziene A, Wilske B, Ferdows MS, Barbour AG. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–5. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Heikkila T, Seppala I, Saxen H, et al. Cloning of the gene encoding the decorin-binding protein B (DbpB) in Borrelia burgdorferi sensu lato and characterisation of the antibody responses to DbpB in Lyme borreliosis. J Med Microbiol. 2002;51:641–8. doi: 10.1099/0022-1317-51-8-641. [DOI] [PubMed] [Google Scholar]
- 27.Hartiala P, Hytonen J, Suhonen J, Lepparanta O, Tuominen-Gustafsson H, Viljanen MK. Borrelia burgdorferi inhibits human neutrophil functions. Microbes Infect. 2008;10:60–8. doi: 10.1016/j.micinf.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Tuominen-Gustafsson H, Penttinen M, Hytonen J, Viljanen MK. Use of CFSE staining of borreliae in studies on the interaction between borreliae and human neutrophils. BMC Microbiol. 2006;6:92. doi: 10.1186/1471-2180-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown EL, Wooten RM, Johnson BJ, et al. Resistance to Lyme disease in decorin-deficient mice. J Clin Invest. 2001;107:845–52. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Xu Q, Seemanaplli SV, McShan K, Liang FT. Common and unique contributions of decorin-binding proteins A and B to the overall virulence of Borrelia burgdorferi. PLoS One. 2008;3:e3340. doi: 10.1371/journal.pone.0003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Q, McShan K, Liang FT. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol Microbiol. 2008;69:15–29. doi: 10.1111/j.1365-2958.2008.06264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanemann CO, Kuhn G, Lie A, et al. Expression of decorin mRNA in the nervous system of rat. J Histochem Cytochem. 1993;41:1383–91. doi: 10.1177/41.9.8354878. [DOI] [PubMed] [Google Scholar]
- 33.Kallmann BA, Wagner S, Hummel V, et al. Characteristic gene expression profile of primary human cerebral endothelial cells. FASEB J. 2002;16:589–91. doi: 10.1096/fj.01-0594fje. [DOI] [PubMed] [Google Scholar]
- 34.Thomas W, Forero M, Yakovenko O, et al. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys J. 2006;90:753–64. doi: 10.1529/biophysj.105.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]