Abstract
Background. Human immunodeficiency virus (HIV)–infected patients have decreased immune response to vaccines. Few data are available about pandemic flu vaccination in this population.
Methods. We conducted a multicenter, patient-blinded, randomized trial in a cohort of HIV-infected adults. Patients received 2 injections 21 days apart of a AS03A-adjuvanted H1N1v vaccine containing 3.75 μg hemagglutinin (HA) or a nonadjuvanted H1N1v vaccine containing 15 μg HA to assess hemagglutination inhibition (HI) response and safety.
Results. A total of 309 patients were randomized, and 306 were vaccinated. After the first vaccine dose, HI titers ≥1:40 were observed in 93.4% of the patients in the adjuvanted group (A group) (n = 155) and in 75.5% in the nonadjuvanted group (B group) (n = 151) (P < .001); seroconversion rates were 88.8% and 71.2%, and factor increases in geometric mean titers (GMT) of 21.9 and 15.1, respectively. After 2 injections, 98.6% of patients of the A group and 92.1% of the B group demonstrated HI titers ≥1:40 (P = .018); seroconversion rates were 96.5% and 87.1%, respectively, and factor increases in GMT were 45.5 and 21.2, respectively. The majority of adverse events were mild to moderate in severity; no impact on CD4+ cell count or viral load has been detected.
Conclusions. In HIV-1–infected adults, the AS03A-adjuvanted H1N1v vaccine yielded a higher immune response than did the nonadjuvanted one, with no impact on HIV infection.
Two large studies performed before the era of highly active antiretroviral therapy (HAART) evidenced high numbers of hospital admissions and high mortality related to seasonal influenza in human immunodeficiency virus (HIV)–infected patients [1,2]. In the post-HAART era, the number of influenza-associated hospitalizations has decreased in those patients, but hospitalization rates remain comparable to rates in other high-risk groups [3]. Recent observations have shown that HIV infection did not lead to a more severe infection with the novel H1N1v variant [4]. However, shedding of the influenza virus may persist >5 days in some subjects despite treatment with oseltamivir, and the coexistence of opportunistic infections may mask the diagnosis of H1N1v infection and complicate the course of the disease [5].
Previous studies have suggested that the immunogenicity of the seasonal influenza vaccine was decreased in HIV-infected patients and have shown that vaccine response was related to plasma HIV viral load rather than CD4+ cell counts [6–8]. The addition of oil-in-water adjuvant to seasonal influenza vaccine demonstrated an increased immunogenicity in HIV-infected patients, with a clinically acceptable safety profile [9, 10]. In most HIV-infected patients, neither influenza infection nor influenza vaccination induced an increase in HIV viral load or a decrease of CD4+ cell count [11, 12].
In 2009, both the seasonal and the H1N1v influenza vaccines were recommended for HIV-infected patients due to the potential risk of severe clinical forms in this immunocompromised population [13, 14]. All published trials in the general population were designed with 2 injections administered 21 days apart. The published data indicated that 1 dose of adjuvanted or nonadjuvanted monovalent influenza A H1N1v 2009 vaccine was sufficient to immunize healthy adults [15–17]. As for seasonal influenza vaccine, an adjuvanted vaccine might help to induce better protection in immunocompromised patients. Nevertheless, the safety of the AS03A adjuvant in HIV-infected patients remains insufficiently documented, and the effect of adjuvants on the balance between HIV and the host immune system, which could result in viral rebound, remains unknown.
We thus conducted a randomized, patient-blinded clinical trial to evaluate the immunogenicity and the safety of 2 doses of H1N1v vaccine adjuvanted with AS03A, compared with 2 doses of nonadjuvanted H1N1v vaccine, in HIV-infected adults.
METHODS
Study Design
The HIFLUVAC (HIV InFLUenza VACcine) trial was a phase 2, randomized, patient-blinded, multicenter clinical trial. Patients were eligible to participate if they were ≥18 years of age; had HIV-1 infection; had received HAART for at least 6 months (with viral load <50 copies/mL on at least the previous 2 visits) or were without any antiretroviral therapy for at least 6 months (and without any indication to start a treatment in the following 3 months); did not have an history of clinically or virologically confirmed influenza infection during the previous 6 months; had no febrile episode within 1 week prior to vaccination; did not receive immune-modulatory (eg, interferon alpha and interleukin 2) or immunosuppressant drugs; had no evolutive opportunistic infection within the month preceding inclusion; and had no other vaccination within 3 weeks prior to inclusion or planned within 3 weeks following the second vaccination.
The trial was performed in French National Agency for Research on AIDS and Viral Hepatitis (Agence National de Recherche contre le VIH et les hépatites virales (ANRS) ANRS-labeled sites in France. Patients were centrally randomized (1:1) to receive 2 intramuscular injections of A/H1N1v vaccine either unadjuvanted or adjuvanted with AS03A and administered 21 days apart. Randomization was stratified according to HAART versus no HAART at baseline, and 2 lists were generated with a block size of 6 using SAS, version 9.1 (SAS Institute) by the trial statistician. A central coordinating center was responsible for validation of patient eligibility, randomization, data collection, and monitoring. No center knew the vaccine allocation of any patient prior to randomization, and the result of individual randomization was faxed by the central office to the trial center.
Blood samples were planned for assessment of hemagglutination inhibition (HI) antibodies against vaccine antigen prior to vaccination, 21 days following each dose, and at months 6 and 12. Standard biochemical tests, CD4+/CD8+ cell counts, and serum HIV-1 RNA levels were planned for days 0, 21, and 42 and for months 3, 6, and 12.
Written informed consent was obtained from each patient. The protocol was conducted in accordance with the Declaration of Helsinki and French law for biomedical research and was approved by the “Ile de France III” Ethics Committee (“Comité de Protection des Personnes Ile-de-France III,” Paris).
Vaccines
The monovalent A/H1N1v inactivated split-virion vaccine was manufactured by GlaxoSmithKline (GSK) Biologicals. The vaccine seed virus was prepared from the reassortant virus NYMC X-179A (New York Medical College), generated from the A/California/7/2009 strain, as recommended by the World Health Organization (WHO) [18]. The seed virus was propagated on embryonated eggs, and the vaccine was produced using the licensed manufacturing process for Pandemrix (a trade mark of the GlaxoSmithKline group of companies). AS03A, a tocopherol oil-in-water emulsion-based adjuvant system containing 11.86 mg tocopherol was manufactured by GSK Biologicals. The AS03A-adjuvanted vaccine contained 3.75 μg hemagglutinin (HA), whereas the nonadjuvanted vaccine was formulated to contain 15 μg HA, which was the dosage used for seasonal influenza vaccine. The H1N1v vaccine was formulated in multidose vials and injected intramuscularly in a total 0.5 mL of antigen suspension, mixed with AS03A adjuvant emulsion or without adjuvant.
Safety Assessment
Patients were provided with diary cards to record the occurrence and severity of solicited local reactions at the injection site (erythema, edema, and pain), solicited general reactions (fever, headache, asthenia, shivering, increased sweating, myalgia, and arthralgia), and any unsolicited adverse events during 21 days after vaccination. Temperature was recorded daily during the 6 days after vaccination. We also collected data related to the occurrence of selected adverse events of special interest, including neurologic and immune system disorders. Subjects having an influenza-like illness (ILI) defined as an oral temperature of >37.8°C with at least 1 influenza-like symptom (cough, sore throat, rhinorrhea, and nasal obstruction) were asked to provide nasal and throat swab specimens for virological testing.
Laboratory Assays
Laboratory assays for immune response were performed in a blind way in a centralized laboratory (GSK Biologicals). The titer of antibodies against the vaccine strain was measured in all samples by a validated HI assay as described by the WHO Collaborating Center for Influenza, Centers for Diseases Control and Prevention [19]. Serum samples were treated by enzymatic treatment and heated to destroy nonspecific inhibitors. Hemagglutination was performed in a microtiter test using chicken erythrocytes with the A/California/7/2009 (H1N1v) strain used as antigen. HI assays were performed in duplicate for each sample using serial 2-fold dilutions with a starting dilution of the treated serum of 1:10. The sample titer was the highest dilution that completely inhibited hemagglutination.
Statistical Analysis
The main outcome measure was the HI response against vaccine antigen. The seroprotection rate was defined as the percentage of patients with a post-vaccination HI titer ≥ 1:40. The seroconversion rate was defined as the percentage of patients with a prevaccination HI titer < 1:10 and a post-vaccination titer ≥ 1:40 or showing a significant increase in antibody titer; a significant increase in antibody titer was defined as a prevaccination titer ≥ 1:10 and at least a 4-fold increase in post-vaccination titer. Seroconversion factor or geometric mean fold increase was defined as the geometric mean of the within-subject ratios of the post-vaccination reciprocal HI titer to the day 0 reciprocal HI titer. Safety end points were solicited adverse events and unsolicited adverse events.
Descriptive analyses are presented for vaccinated patients for safety and for vaccinated and tested patients for immunogenicity; 95% confidence intervals (CI) for rates are exact (Clopper-Pearson) CIs. Immunogenicity was analyzed by the standard HI end points (with 95% CIs) used by regulatory authorities for evaluation of influenza vaccines [20–22]. Logistic regression was used to analyze determinants of and factors associated with HI titers ≥ 1:40 after 1 and 2 doses. Odds ratios found in logistic regressions do not approximate relative risks, because HI titers ≥ 1:40 rates are high.
A sample size of 135 subjects analyzed per group was estimated to give a power of at least 88% to meet both the European Medicines Agency and US Food and Drug Administration criteria assuming 85% for seroconversion and seroprotection (HI titers ≥ 1:40) rates and 30 for geometric mean fold increase.
RESULTS
Study Patients
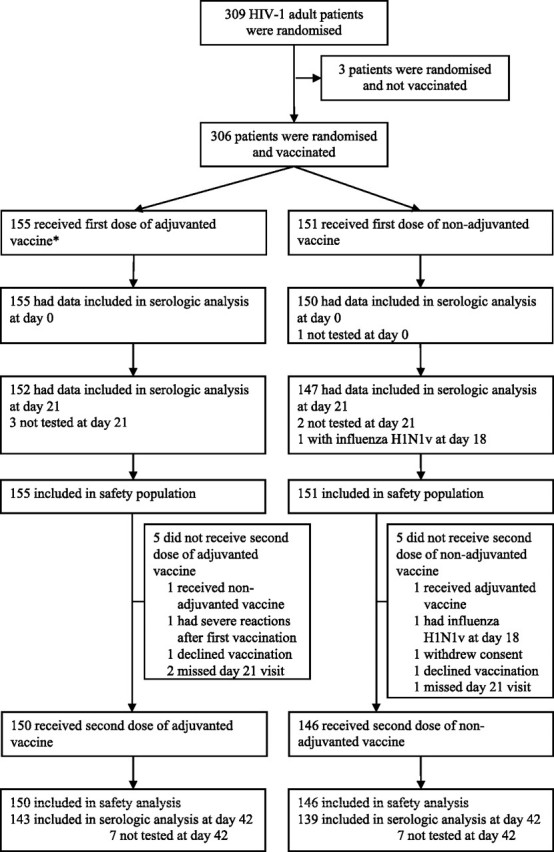
Three hundred and nine patients were randomized from 26 October and 6 November 2009, of whom 306 were vaccinated. Three patients did not receive the vaccine and were not included in the analysis. Overall, 155 patients of the adjuvanted vaccine group and 151 patients of the nonadjuvanted vaccine group were included in the safety population (Figure 1). The demographic profiles and the clinical characteristics of the 2 randomized groups were well-balanced and are described in Table 1. At inclusion, 26% of patients reported having received a 2009 seasonal influenza vaccine.
Figure 1.
Participant disposition of the HIFLUVAC (HIV InFLUenza VACcine) trial. HIV, human immunodeficiency virus.
Table 1.
Demographic and Clinical Characteristics of the Patients
| AS03A adjuvanted H1N1v vaccine(3.75 μg HA) |
Nonadjuvanted H1N1v vaccine(15 μg HA) |
With HAART | Without HAART | All | |||||
| Characteristic | WithHAART | Without HAART | All | With HAART | Without HAART | All | |||
| No. of patients | 120 | 35 | 155 | 117 | 34 | 151 | 237 | 69 | 306 |
| Female sex | 19 (16) | 4 (11) | 23 (15) | 25 (21) | 11 (32) | 36 (24) | 44 (19) | 15 (22) | 59 (19) |
| Age, median years (IQR) | 47.3 (41.7–54.7) | 42.5 (33.2–50.1) | 46.5 (38.6–54.0) | 48.8 (44.2–54.7) | 39.0 (32.6–45.7) | 47.3 (40.2–53.6) | 47.7 (42.5–54.7) | 40.7 (33.2–48.6) | 46.9 (40.0–53.8) |
| CDC stage C | 38 (32) | 2 (6) | 40 (26) | 38 (33) | 1 (3) | 39 (26) | 76 (32) | 3 (4) | 79 (26) |
| Smoker | 45 (38) | 16 (46) | 61 (39) | 37 (32) | 13 (38) | 50 (33) | 82 (34) | 29 (42) | 111 (36) |
| HCV and/or HBV coinfection | 18 (15) | 5 (14) | 23 (15) | 14 (12) | 2 (6) | 16 (11) | 32 (14) | 7 (10) | 39 (13) |
| HBsAg positive | 8 (6.7) | 2 (5.7) | 10 (6.5) | 5 (4.3) | 0 (0) | 5 (3.3) | 13 (5.5) | 2 (2.9) | 15 (4.9) |
| Anti-HCV antibody positive | 10 (8.4) | 3 (8.6) | 13 (8.4) | 10 (8.5) | 2 (5.9) | 12 (7.9) | 20 (8.5) | 5 (7.2) | 25 (8.2) |
| Nadir CD4+ cell count, median cells/mm3 (IQR) | 190 (89–278) | 435 (336–542) | 240 (107–369) | 173 (86–253) | 451 (348–541) | 204 (95–371) | 177 (86–263) | 447 (348–541) | 229 (106–369) |
| Baseline CD4+ cell count median cells/mm3 (IQR) | 537 (383–763) | 503 (364–602) | 522 (378–752) | 560 (422–712) | 522 (451–635) | 551 (428–702) | 556 (407–736) | 507 (421–611) | 536 (412–706) |
| Baseline CD4+ cell count <200 cells/mm3 | 6 (5.0) | 0 (0) | 6 (3.9) | 3 (3.4) | 0 (0) | 3 (2.6) | 9 (3.8) | 0 (0) | 9 (3.0) |
| Baseline CD4+ cell count 200–349 cells/mm3 | 21 (17.5) | 7 (20) | 28 (18.1) | 14 (12.1) | 1 (2.9) | 15 (10.0) | 35 (14.8) | 8 (11.8) | 43 (14.1) |
| Baseline CD4+ cell count 350–499 cells/mm3 | 25 (20.8) | 10 (28.6) | 35 (22.6) | 28 (24.1) | 13 (38.2) | 41 (27.3) | 53 (22.5) | 23 (33.3) | 76 (24.9) |
| Baseline CD4+ cell count ≥500 cells/mm3 | 68 (56.7) | 18 (51.4) | 86 (55.5) | 71 (61.2) | 20 (58.8) | 91 (60.7) | 139 (58.9) | 38 (55.1) | 177 (58) |
| HIV-1 viral load, median copies/mL (IQR) | 20 (20–40) | 13372 (1524–46081) | 40 (20–50) | 20 (20–40) | 8306 (3850–29048) | 40 (20–61) | 20 (20–40) | 9127 (3183–38200) | 40 (20–50) |
| HIV-1 viral load <50 copies/mL | 116 (97) | 3 (9) | 119 (77) | 112 (96) | 0 (0) | 112 (74) | 228 (96) | 3 (4) | 231 (76) |
| Received 2009 seasonal influenza vaccine | 32 (27) | 7 (20) | 39 (33) | 33 (28) | 6 (18) | 39 (26) | 65 (27) | 13 (19) | 78 (26) |
| Received 2009 pneumococcal vaccine | 36 (30) | 6 (17) | 42 (27) | 31 (27) | 8 (24) | 39 (26) | 67 (28) | 14 (20) | 81 (27) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. CDC, Centers for Disease Control and Prevention; HA, hemagglutinin; HAART, highly active antiretroviral therapy; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatits C virus; IQR, interquartile range.
One patient was not tested at day 0 (nonadjuvanted vaccine group). Seven patients were not tested at day 21 (4 in the nonadjuvanted vaccine group and 3 in the adjuvanted vaccine group), including 1 patient with virologically documented influenza H1N1v illness on day 18 after vaccination and 1 patient who withdrew consent after receipt of the first vaccine dose (both in the nonadjuvanted vaccine group). The immunogenicity after the first vaccination was thus assessed in 299 subjects.
Of the 306 patients, 296 received a second dose of vaccine, of whom 14 were not tested at day 42. Thus, the immunogenicity after the second vaccination was assessed in 282 subjects.
Immunogenicity
At baseline, 7.7% and 10.0% of patients had HI antibodies against A/California/7/2009 (H1N1v) strain with titers of 1:40 or more in the adjuvanted and nonadjuvanted groups, respectively. Baseline HI titers of 1:40 or more were observed in 13 (16.7%) of 78 patients (95% CI, 9.2%–26.8%) who had received the 2009 seasonal vaccine, as compared with 14 (6.2%) of 227 patients (95% CI, 3.4%–10.1%) who had not received it (P = .049).
A single dose of adjuvanted H1N1 vaccine produced a higher immune response than did a single dose of nonadjuvanted vaccine (Table 2). The proportion of patients with HI titers of 1:40 or greater were 93.4% (95% CI, 88.2% to 96.8%) in the adjuvanted vaccine group and 75.5% (95% CI, 67.7%–82.2%) in the nonadjuvanted vaccine group (P < .001). In the adjuvanted vaccine group, the immune response was not different between patients with HAART and patients without HAART and was consistent with the regulatory requirements for the use of vaccine in adults (Table 2). In the nonadjuvanted vaccine group, the immune response was lower for treated patients than for untreated patients, with proportion of patients with HI titers ≥ 1:40 being 70.2% and 93.9%, respectively (P = .005). A single dose of nonadjuvanted vaccine met the regulatory requirements in patients not receiving HAART but was below the Center for Biologics Evaluation and Research (CBER) requirements for seroprotection in patients receiving HAART.
Table 2.
Immune Response Stratified by HIV Treatment for Adjuvanted and Nonadjuvanted Vaccine, Hemagglutination Antibodies Against A/California/7/2009 (H1N1v)
| AS03A adjuvanted H1N1v vaccine (3.75 μg HA) |
Nonadjuvanted H1N1v vaccine (15 μg HA) |
|||||
| Variable | With HAART | Without HAART | All | With HAART | Without HAART | All |
| Prevaccination at day 0 | ||||||
| No. of patients tested | 120 | 35 | 155 | 116 | 34 | 150 |
| Geometric mean titer (95% CI) | 9.2 (7.7–10.9) | 6.4 (5.3–7.7) | 8.5 (7.3–9.8) | 8.6 (7.3–10.0) | 6.7 (5.4–8.4) | 8.1 (7.1–9.2) |
| No. (%) of patients with HI titers ≥ 1:40 (95% CI) | 12 (10.0)(5.3–16.8) | 0 (0.0)(0.0–.1) | 12 (7.7)(4.1–13.1) | 13 (11.2)(6.1–18.4) | 2 (5.9)(0.7–19.7) | 15 (10.0)(5.7–16.0) |
| Post–dose 1 at day 21 | ||||||
| No. of patients tested | 117 | 35 | 152 | 114 | 33 | 147 |
| Geometric mean titer (95% CI) | 189.4 (151.1–236.8) | 175.0 (118.6–258.2) | 186.0 (154.0–224.7) | 102.8 (77.8–137.2) | 201.6 (135.7–323.9) | 119.6 (94.0–152.1) |
| No. (%) of patients with HI titers ≥ 1:40 (95% CI) | 109 (93.2)(87.0–97.0) | 33 (94.3)(80.8–99.3) | 142 (93.4)(88.2–96.8) | 80 (70.2)(60.9–78.4) | 31 (93.9)(79.8–99.3) | 111 (75.5)(67.7–82.2) |
| Seroconversion rate, no. (%) of patients (95% CI) | 103 (88.0)(80.7–93.3) | 32 (91.4)(76.9–98.2) | 135 (88.8)(82.7–93.4) | 74 (65.5)(56.0–74.2) | 30 (90.9)(75.7–98.1) | 104 (71.2)(63.2–78.4) |
| Seroconversion factor (95% CI) | 20.5 (16.3–26.0) | 27.3 (18.7–40.0) | 21.9 (18.0–26.8) | 12.4 (9.4–16.2) | 29.7 (19.9–44.5) | 15.1 (11.9–19.0) |
| Post–dose 2 at day 42 | ||||||
| No. of patients tested | 108 | 35 | 143 | 108 | 31 | 139 |
| Geometric mean titer (95% CI) | 424.4 (358.3–502.7) | 275.7 (203.8–373.0) | 381.9 (329.0–443.3) | 164.1 (131.4–205.0) | 197.8 (141.1–277.3) | 171.1 (141.9–206.3) |
| No. (%) of patients with HI titers ≥ 1:40 (95% CI) | 107 (99.1)(95.0–100.0) | 34 (97.1)(85.1–99.9) | 141 (98.6)(95.0–99.8) | 98 (90.7)(83.6–95.5) | 30 (96.8)(83.3–99.9) | 128 (92.1)(86.3–96.0) |
| Seroconversion rate, no. (%) of patients (95% CI) | 104 (96.3)(90.8–99.0) | 34 (97.1)(85.1–99.9) | 138 (96.5)(92.0–98.9) | 92 (85.2)(77.1–91.3) | 29 (93.6)(78.6–99.2) | 121 (87.1)(80.3–92.1) |
| Seroconversion factor (95% CI) | 46.3 (36.6–58.6) | 43.1 (31.6–58.8) | 45.5 (37.6–55.1) | 19.5 (15.5–24.5) | 28.6 (20.9–39.2) | 21.2 (17.5–25.7) |
NOTE. Seroconversion rate is given as the percentage of patients with a prevaccination HI titer <1:10 and a post-vaccination titer ≥ 1:40, or showing a significant increase in antibody titer defined as a prevaccination titer ≥ 1:10 and at least a 4-fold increase in post-vaccination titer. Seroconversion factor or geometric mean fold increase is defined as the geometric mean of the within-subject ratios of the post-vaccination reciprocal HI titer to the day 0 reciprocal HI titer. According to the European Union Committee for Medicinal Products for Human Use, criteria for HI antibody response in adults 18–60 years of age are a percentage of HI titers ≥ 1:40 > 70%, a percentage of seroconversion >40%, and a geometric mean fold increase > 2.5. According to the United States Center for Biologics Evaluation and Research, criteria for HI antibody response in adults <65 years of age are a lower limit of the 95% CI for HI titers ≥ 1:40 ≥ 70% and a lower limit of the 95% CI for seroconversion ≥ 40%. CI, confidence interval; HA, hemagglutinin; HI, hemagglutination inhibition.
After the second dose of vaccine, 98.6% (95% CI, 95.0%–99.8%) and 92.1% (95% CI, 86.3%–96.0%) of patients demonstrated HI titers of 1:40 or greater in the adjuvanted and the nonadjuvanted groups, respectively (P = .018). In the 2 groups, the immune response was not different between patients with HAART and those without HAART.
In a multivariate analysis, a single dose of adjuvanted vaccine resulted in a higher proportion of patients with HI titers ≥ 1:40 than did a single dose of nonadjuvanted vaccine (odds ratio [OR], 5.33; 95% CI, 2.44–11.65; P < .001), and hepatitis C virus (HCV) and/or hepatitis B virus (HBV) coinfection was negatively associated with HI titers ≥ 1:40 (OR, 0.26; 95% CI, 0.11–0.62; P = .0023) (Table 3). HCV coinfection was observed in 24 of 39 coinfected patients and largely contributed to this effect: 15 (63%) of 24 HCV coinfected patients and 11 (73%) of 15 HBV coinfected patients had HI titers ≥1:40, as compared with 237 (87%) of 274 and 241 (85%) of 283 HIV-1 monoinfected patients. CD4+ cell count was not predictive, and no differences were detected between CD4+ cell count subgroups, defined as <200, 200–349, 350–499, and ≥500 cells/mm3 (data not shown). Seroprotection rates remained higher after 2 doses of adjuvanted vaccine than after 2 doses of unadjuvanted vaccine (OR, 6.52; 95% CI, 1.37–30.93; P = .018). Similar results were obtained for the rate of seroconversion after the first or the second injection (data not shown).
Table 3.
Univariate and Multivariate Analyses of Predictive Baseline Factors for Hemagglutination Inhibition Titers ≥ 1:40 at Day 21 After Vaccination
| Variable | No. of patients | Univariate analysis OR (95% CI)a | Pb | Multivariate analysis OR (95% CI)a | Pb |
| Male vs female | 299 | 1.218 (0.564–2.627) | .62 | ||
| Age <40 years | 299 | 1.676 (0.744–3.778) | .21 | 1.153 (.469–2.834) | .76 |
| Smoker | 299 | 0.859 (0.450–1.638) | .64 | ||
| CDC stage C | 299 | 0.907 (0.481–1.711) | .76 | ||
| HCV and/or HBV coinfection | 298 | 0.326 (0.150–.706) | .005 | 0.259 (.109–.617) | .002 |
| Nadir CD4+ cell count, per 50 cells/mm3 higher | 299 | 1.069 (0.977–1.171) | .15 | 0.976 (.875–1.089) | .66 |
| Baseline CD4+ cell count, ≥350/mm3 | 298 | 1.182 (0.532–2.629) | .68 | ||
| Seasonal influenza vaccination | 299 | 1.297 (0.610–2.757) | .50 | ||
| HAART vs without HAART | 299 | 0.281 (0.097–.815) | .02 | 0.444 (.025–7.924) | .58 |
| Baseline plasma HIV-1 viral load, log10 copies/mL | 299 | 1.601 (1.062–2.416) | .024 | 1.251 (.426–3.672) | .68 |
| AS03A adjuvanted vsnonadjuvanted H1N1v vaccine | 299 | 4.605 (2.190–9.685) | <.001 | 5.331 (2.439–11.65) | <.001 |
NOTE. CDC, Centers for Disease Control and Prevention; CI, confidence interval; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; OR, odds ratio.
Wald confidence interval.
Wald χ2 P value.
Safety
No deaths or adverse events of special interest were reported. Until day 42, 3 serious adverse events were reported: 1 hospitalization for ILI 2 days after the second unadjuvanted vaccine injection, 1 recurrent depression episode, and 1 increase in serum alanine transaminase value attributable to other drugs.
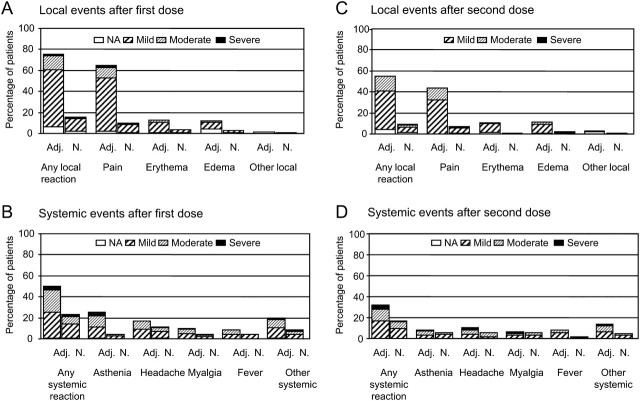
After the first or second injection, at least 1 solicited local adverse event was reported by 110 patients (71%) in the adjuvanted vaccine group and 32 patients (21%) in the nonadjuvanted vaccine group (P < .001); at least 1 solicited systemic adverse event was reported by 74 patients (48%) in the adjuvanted vaccine group and 41 patients (27%) in the nonadjuvanted vaccine group (P < .001). The most commonly reported solicited local adverse event was injection-site pain, and the most commonly reported solicited systemic events were asthenia and headache. The majority of solicited adverse events were mild to moderate in intensity. A general trend for lower frequencies of solicited adverse events was observed after the second vaccination, compared with after the first vaccination, whereas their pattern remained the same (Figure 2). Unsolicited adverse events were reported by 12% and 6% of subjects in the adjuvanted and unadjuvanted vaccine group, respectively; of these events, 93% occurred within 7 days after the first or second dose of vaccine (data not shown).
Figure 2.
Solicited reports of adverse events 21 days after the first dose and second dose of the H1N1 vaccine. Patients with at least 1 vaccine-related adverse event are reported. Severity of vaccine-related adverse events was graded as mild (mild or transient discomfort, without limitation of normal daily activities, and no medical intervention or corrective treatment required), moderate (mild to moderate limitation of normal daily activities and minimal medical intervention or corrective treatment required), or severe (marked limitation of normal daily activities, medical intervention and corrective treatment required, and possible hospitalization), except for erythema and edema at the injection site, which was graded on the basis of a measurement of the diameter of the local reaction (mild, 0–50 mm; moderate, 50–100 mm; severe, >100 mm). *Other severe systemic reactions in the adjuvanted vaccine group after receipt of the first dose: oral herpes, rhinorrhea, and arthralgia, with 1 patient who reported multiple events (arthralgia, shivering, rhinorrhea, back pain, abdominal pain, cough, dyspnea, nasal congestion, and pharyngolaryngeal pain; day 1 to day 10); other severe systemic reactions in the nonadjuvanted vaccine group: arthralgia, cough, and hot flash. †Other severe systemic reactions in the adjuvanted vaccine group after receipt of the second dose: arthralgia in 2 patients, shivering; other severe systemic reactions in the nonadjuvanted vaccine group: shivering. Adj, AS03A-adjuvanted vaccine group; N, nonadjuvanted-vaccine group; NA, not available.
There was no significant variation in the CD4+ cell counts between day 0 and day 21 or day 42 in any group: in the adjuvanted group, median changes of CD4+ cell counts were +0 cells/mm3 at day 21 and +0 cells/mm3 at day 42 for patients receiving HAART and were +5 cells/mm3 at day 21 and +39 cells/mm3 at day 42 for patients not treated for HIV infection; in the nonadjuvanted group, median changes of CD4+ cell counts were −3 cells/mm3 at day 21 and −30 cells/mm3 at day 42 for patients receiving HAART and were +5 cells/mm3 at day 21 and −21 cells/mm3 at day 42 for patients not treated for HIV infection. The viral load did not vary significantly between days 0 and 42 in both adjuvanted and nonadjuvanted vaccine groups (data not shown).
Twenty-three patients had ILI, including 7 (5%) in the adjuvanted vaccine group and 16 (11%) in the nonadjuvanted vaccine group. Nasal swab samples were available for 6 patients in the adjuvanted vaccine group and 11 patients in the nonadjuvanted vaccine group. Two patients in the nonadjuvanted group had test results that were positive for 2009 H1N1, 1 patient on day 18 after the first vaccination (onset of symptoms, day 13) and 1 patient on day 25 after the second vaccination.
DISCUSSION
Our study is the first randomized trial designed to evaluate the immunogenicity and the safety of H1N1v vaccine, unadjuvanted or adjuvanted with AS03A, in a population of HIV-1–infected adults receiving HAART or without indication for antiretroviral therapy. We observed that the adjuvanted vaccine formulated with 3.75 μg of HA antigen confers a higher immune response than does the nonadjuvanted vaccine, which contains 4 times the antigen content, especially in patients treated with HAART and after the first dose.
In the subgroup of patients receiving HAART, the administration of a single 15-μg dose of nonadjuvanted vaccine was associated with a proportion of patients with an HI titer of 1:40 or more of 70.2%, a seroconversion rate of 65.5%, and a geometric mean fold increase of 12.4. Although this met the regulatory requirements of the European Union Committee for Medicinal Products for Human Use, it did not meet the CBER requirements for seroprotection. In contrast, the treated patients who received the adjuvanted vaccine met both regulatory requirements after the first injection. Our findings are in keeping with previous results published by Iorio et al [9], who reported that patients with HIV infection receiving HAART who received a nonadjuvanted seasonal influenza vaccine had a significantly lower immune response, compared with that of patients who received the MF-59 adjuvanted vaccine.
Moreover, the high response rate obtained after a single dose of adjuvanted vaccine is equal to that obtained after 2 doses of nonadjuvanted vaccine (93.4% vs 92.1%).
In our study, the HI immune response was shown to be independent of baseline CD4+ cell counts, which is in contrast with other studies that have used either the same AS03 adjuvanted vaccine [23] or another nonadjuvanted formulation [24], which have shown lower HI responses after a single-dose vaccination and reported that baseline CD4+ cell counts were positively associated with better immune responses. In our trial, patients selected had to either have virologically controlled infection for at least 6 months or not to have met the criteria for HAART initiation. Consequently, patients with high baseline CD4+ cell counts were preferentially enrolled, with only 3% of patients having CD4+ cell counts <200 cells/mm3; therefore, the population under study may not have been diverse enough to evidence a correlation between CD4+ cell counts and immune response. This indirect selection of patients with high CD4+ cell counts, which nonetheless reflects the current status of patients with HIV infection in care in industrialized countries, could also explain the higher vaccine response rates observed in our study, compared with those of other H1N1v vaccine trials involving HIV-infected patients. However, in the study of Bickel et al [23], which used the same AS03 adjuvanted vaccine, patients had similar CD4+ cell count at baseline (mean CD4+ cell count, 514 cells/mm3). It is also possible that the observed difference in HI between studies resulted, at least in part, from technical read-out variations among the laboratories where the respective study samples were tested, as has already been observed with seasonal influenza [25].
The results of the present trial show that the 2009 A/H1N1v vaccine with or without AS03A adjuvant was associated with a clinically acceptable safety profile in HIV-infected patients. As previously observed in a cohort of healthy volunteers [26], solicited local and systemic adverse events were more frequent in patients who received the adjuvanted vaccine. These adverse events (notably, fever, headache, and asthenia) remained mostly mild to moderate in intensity and decreased in frequency between the first and the second dose, as reported in healthy adults.
Interestingly, in an H1N1v vaccine trial in a cohort of healthy adult volunteers reported by Roman et al [26], pain at the injection site was reported in 88.9% of subjects receiving the AS03A adjuvanted H1N1v vaccine (5.25 μg HA) and in 59.1% of subjects receiving the nonadjuvanted H1N1v vaccine (21 μg HA), compared with 65% and only 10%, respectively, after the first dose in our study; erythema was observed in 31.7% of subjects who received the AS03A adjuvanted vaccine and in 4.5% of subjects who received the nonadjuvanted vaccine, compared with 12.9% and 3.3%, respectively, after the first dose in our study. Also, asthenia, which was the most common systemic adverse event in both studies, was reported for 41.3% of subjects who received the adjuvanted vaccine and 27.3% of subjects who received the nonadjuvanted vaccine in the Roman et al [26] trial, whereas these frequencies were 25% and 4%, respectively, in our study. Therefore, although the quantity of antigen was slightly higher in the latter study, the intensity of local inflammation at injection site and the intensity of systemic reactions appear to be sharply decreased in HIV-infected patients, compared with healthy volunteers vaccinated with H1N1v vaccine.
Previous reports have suggested that seasonal influenza vaccines could, in some settings, induce a transient increase in viral load. Our results with H1N1v vaccine did not demonstrate any significant change in viral load or CD4+ cell counts after administration of either AS03A adjuvanted or nonadjuvanted H1N1v vaccine. Although we cannot exclude a transient increase of HIV-1 RNA replication within days of the vaccination, results do not suggest any increased risk of disease progression following vaccination with either formulation, both in treated and in untreated patients. This is in line with the report of a large study involving 36,000 HIV-infected patients, which reported the absence of long-term effects of seasonal influenza vaccine on CD4+ cell counts, viral load, or progression to AIDS or death [11].
In conclusion, in a large randomized, controlled trial, the AS03A adjuvanted H1N1v vaccine yielded a higher HI immune response than did the nonadjuvanted vaccine in a cohort of HIV-infected adults and had no impact on HIV infection. These findings encourage the further development of adjuvanted vaccines for use in the difficult-to-immunize population of people living with HIV infection, which may be of critical importance in future vaccination programs against influenza viruses with novel HAs [27].
Funding
French National Agency for Research on AIDS and Viral Hepatitis (ANRS, Paris, France) and GlaxoSmithKline Biologicals, which provided the vaccines and performed immunological tests.
Acknowledgments
We thank the study participants and the participating clinicians at each site; the French Research Program on Pandemic H1N1v Flu: Institut de Microbiologie et Maladies Infectieuses; Francis Beauvais, for his help in preparing the manuscript; and GSK Biologicals staff members Laurence Baufays, Nathalie Clyti, Sophie Muller, Marie-Hélène Chautard, and Isabelle Naeije, for support in study management, and Karl Walravens, Urban Lundberg, and Roger Bernhard, for serological testing.
Appendix 1.
ANRS 151 HIFLUVAC Study Group Members
G. Pialoux, L. Slama (Hôpital Tenon, Paris), J. F. Delfraissy, J. Ghosn, M. T. Rannou, E. Fourn, Y. Quertainmont (Hôpital de Bicêtre, Le Kremlin-Bicêtre), F. Raffi, C. Allavena, C. Biron, D. Besson, H. Hue (Hôpital de l’Hôtel-Dieu, Nantes), J. Reynes, J. M. Jacquet (Hôpital Gui de Chauliac, Montpellier), J. M. Molina, N. Colin de Verdière (Hôpital Saint-Louis, Paris), P. M. Girard, M. C. Meyohas, D. Bollens (Hôpital Saint-Antoine, Paris), P. Yéni, X. Duval, B. Phung, C. Godard, N. El Alami albi (Hôpital Bichat-Claude Bernard, Paris), O. Launay, P. Loulergue, L. Belarbi, L. Iordache (Hôpital Cochin, Paris), A. Sobel, Y. Lévy, E. Bamago (Hôpital Henri Mondor, Créteil), H. Laurichesse, J. Beytout, C. Jacomet, (Hôpital Gabriel Montpied, Clermont Ferrand), D. Rey, J. M. Lang, P. Fischer, M. Partisani (Hôpitaux Universitaires, Strasbourg), Y. Yazdanpanah, F. Ajana, T. Huleux (Hôpital Gustave Dron, Tourcoing), F. Lucht, C. Delafontaine, C. Guglielminotti, A. Frésard, P. Fouilloux, V. Ronat (Hôpital Bellevue, Saint-Etienne), T. May, S. Wassoumbou (Hôpital de Brabois, Vandoeuvre-lès-Nancy), J - F. Bergmann, A. Rami, M. Parrinello (Hôpital Lariboisière, Paris), C. Michelet, F. Fily, M. Ratajczak (Hôpital Pontchaillou, Rennes), F. Bricaire, C. Katlama, A. Chermak (Hôpital Pitié-Salpêtrière, Paris), P. Dellamonica, A. Leplatois (Hôpital de l'Archet, Nice), J. Saillard, S. Couffin-Cadiergues, A. Bouxin-Métro (ANRS, Paris), J.P. Aboulker, C. Desaint, V. Foubert, M. Resch, A. Grenier, M. Le Cornec, E. Moreau, S. Marie-Antoine, Y. Saïdi, A. Arulananthan, C. Durier (Methodology and coordinating center, INSERM SC10, Villejuif France).
REIVAC Network. O. Launay (coordinator: CIC BT505, Paris), F. Lucht (CIC CIE3 axe vaccinologie, Saint Etienne), X. Duval (CIC P - 007, Bichat-Claude Bernard, Paris), N. Colin de Verdière (Service de maladies infectieuses et tropicales de Saint-Louis, Paris), H. Laurichesse, J. Beytout (CIC P - 501, Clermont Ferrand).
Scientific committee. Odile Launay, coordinator (Université Paris Descartes, Hôpital Cochin, Inserm, Paris); Jean-Pierre Aboulker, Christine Durier, Corinne Desaint (Inserm SC10, Villejuif); Pierre Loulergue (Hôpital Cochin, Paris); Xavier Duval (Hôpital Bichat, Paris); Christine Jacomet (CHU Clermont Ferrand, Clermont Ferrand); Constance Delaugerre (Hôpital Saint-Louis, Paris); Sylvie van der Werf (Institut Pasteur, Paris); Sophie.Muller (GSK, Paris); Frédéric Lucht (Hôpital Bellevue, Saint-Etienne), Jade Gohsn, Jean-François Delfraissy (Hôpital de Bicêtre, Kremlin-Bicêtre), Marianne Lhenaff (TRT5, Paris), Juliette Saillard, Sandrine Couffin-Cadiergues (ANRS, Paris).
References
- 1.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–907. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 2.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–446. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–307. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Martinez E, Marcos M, Hoyo I, et al. Program and abstracts of the 17th Conference on Retrovirus and Opportunistic Infections (CROI). Alexandria, VA: CROI, San Francisco, 2010. 2009 H1N1 virus infection in HIV+ adults [abstract 802LB] 379. [Google Scholar]
- 5.Reyes-Terán G, de la Rosa-Zamboni D, Ormsby C, et al. Program and abstracts of the 17th Conference on Retrovirus and Opportunistic Infections. (CROI). Alexandria, VA: CROI, San Francisco, 2010. Clinical features of subjects infected with HIV and H1N1 influenza virus [abstract 803LB] 379. [Google Scholar]
- 6.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka H, Teruya K, Tanaka M, et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:167–173. [PubMed] [Google Scholar]
- 8.Evison J, Farese S, Seitz M, Uehlinger DE, Furrer H, Mühlemann K. Randomised, double-blind comparative trial of subunit and virosomal influenza vaccines for immunocompromised patients. Clin Infect Dis. 2009;48:1402–1412. doi: 10.1086/598193. [DOI] [PubMed] [Google Scholar]
- 9.Iorio AM, Francisci D, Camilloni B, et al. Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59-adjuvanted influenza vaccine or a conventional non-adjuvanted subunit vaccine during highly active antiretroviral therapy. Vaccine. 2003;21:3629–3637. doi: 10.1016/s0264-410x(03)00408-0. [DOI] [PubMed] [Google Scholar]
- 10.Durando P, Fenoglio D, Boschini A, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15:253–259. doi: 10.1128/CVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro P, Plana M, Gonzalez R, et al. Influence of a vaccination schedule on viral load Rebound and immune responses in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25:1249–1258. doi: 10.1089/aid.2009.0015. [DOI] [PubMed] [Google Scholar]
- 12.Skiest DJ, Machala T. Comparison of the effects of acute influenza infection and influenza vaccination on HIV viral load and CD4 cell counts. J Clin Virol. 2003;26:307–315. doi: 10.1016/s1386-6532(02)00047-1. [DOI] [PubMed] [Google Scholar]
- 13.Prise en charge médicale des personnes infectées par le VIH. Addendum aux recommandations 2008 du groupe d’experts lié à la pandémie grippale A(H1N1)v. 2009. http://www.sante-sports.gouv.fr/IMG//pdf/H1N1_et_VIH_Yeni_v2_160909.pdf. Accessed 18 April 2010. [Google Scholar]
- 14.Updated interim recommendations—HIV-infected adults and adolescents: considerations for clinicians regarding 2009 H1N1 influenza. http://www.cdc.gov/h1n1flu/guidance_hiv.htm. Accessed 18 April 2010. [Google Scholar]
- 15.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 16.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 17.Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Availability of a candidate reassortant vaccine virus for the novel influenza A (H1N1) vaccine development X-179A. http://www.who.int/csr/resources/publications/swineflu/candidates_X-179a/en/index.html. Accessed 18 April 2010. [Google Scholar]
- 19.Hehme NW, Künzel W, Petschke F, et al. Ten years of experience with the trivalent split-influenza vaccine. Fluarix Clin Drug Invest. 2002;22:751–769. [Google Scholar]
- 20.European Committee for Proprietary Medicinal Products. Guideline on dossier structure and content for pandemic influenza vaccine marketing authorization application (CPMP/VEG/4717/03) London: European Agency for the Evaluation of Medicinal Products; 2004. [Google Scholar]
- 21.European Committee for Proprietary Medicinal Products. Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006) London: European Agency for the Evaluation of Medicinal Products; 2007. [Google Scholar]
- 22.FDA Guidance for Industry. Clinical data needed to support the licensure of pandemic influenza vaccines. Rockville, Maryland: US Food and Drug Administration; 2007. http://www.fda.gov/cber/gdlns/panfluvac.htm. Accessed 18 April 2010. [Google Scholar]
- 23.Bickel M, Wieters I, Khaykin P, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–F35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 24.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson I, Gaines Das R, Wood JM, Katz JM. Comparison of neutralizing antibody assays for detection of antibody to A/H3N2 viruses: an international collaborative study. Vaccine. 2007;25:4056–4063. doi: 10.1016/j.vaccine.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 26.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: Preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Lambert L, Fauci A. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]