Abstract
Background. Increased susceptibility to Plasmodium falciparum infection during pregnancy has been attributed to the accumulation of infected erythrocytes in the placenta. This phenomenon is mediated by a var gene coding for VAR2CSA, which adheres to chondroitin sulphate A. However, the contribution of parasites transcribing other var genes to maternal infections has not been well characterized.
Methods. Transcription of var2csa and var groups A, B, and C was measured by real-time polymerase chain reaction in 30 placental and 21 peripheral P. falciparum isolates from pregnant women and in 42 isolates from nonpregnant adults and children. Associations of infections with non-var2csa isolates with maternal parasitemia and immune responses were assessed.
Results. Placental parasites showed the highest levels of var2csa. ABC var genes were transcribed by 20 (67%) of 30 placental isolates and were associated with higher parasitemia compared with infections by parasites only transcribing var2csa (P = .004). Peripheral isolates from pregnant women transcribed ABC var genes at levels similar to those of parasites infecting nonpregnant adults with clinical malaria (P[varA] = .420, P[varB] = .808, and P[varC] = .619).
Conclusions. Transcripts of var2csa are abundant in pregnancy-associated P. falciparum infections; however, ABC var types are also common, especially in peripheral blood, with transcription levels similar to those of infections out of pregnancy. These findings are of interest for the design of malaria vaccines for pregnant women.
Parasitic diseases constitute a great threat to the health of women living in tropical countries [1]. Disparities between sexes in the burden of infectious diseases increase during pregnancy, when women become more susceptible to several pathogens [2]. This is the case of Plasmodium falciparum infection, which is more frequent and severe among pregnant women than among nonpregnant women and men [3]. P. falciparum infection in pregnancy is characterized by the accumulation of trophozoite infected erythrocytes (IEs) in placental intervillous spaces [4], a phenomenon that has been suggested to trigger deleterious effects on the mothers and their offspring, especially in primigravidae [3].
Adhesive properties of IEs to different host receptors, including chondroitin sulphate A (CSA) in the placenta [5], are mediated by P. falciparum erythrocyte membrane protein 1 (PfEMP1) [6]. Each parasite contains ∼60 var genes that codify for different PfEMP1 variants [7]. Despite immense diversity in global var genomic repertoires, genes can be classified into 5 groups (A–E) on the basis of the position in the chromosome and 5′ upstream sequence [8]. Transcription of A and/or B groups has been associated with symptomatic and severe malaria [9–13], whereas C genes have been linked to asymptomatic infections [10, 11] and cerebral malaria [13]. The D type gene, var1csa, was initially associated with CSA binding, but was later found to be constitutively transcribed in all isolates [14, 15] and probably not exported to the erythrocyte surface [14]. Conversely, the single and relatively conserved member of group E, var2csa, is transcribed by placental isolates [15–17], expressed on the surface of placental IEs [18], and provides high-affinity binding to CSA [19]. Antibodies blocking this adhesion are developed after exposure to placental parasites [20] and are associated with reduced risk of malaria infection in multigravidae [20] and improved pregnancy outcomes [21, 22].
In the light of these experimental findings, adverse effects of malaria in pregnant women have been attributed to specific adhesion of VAR2CSA to placental tissue, and therefore this antigen constitutes an attractive vaccine candidate against malaria in pregnancy [23]. However, IEs from pregnant women have been shown to adhere other receptors [5, 24, 25], to simultaneously up-regulate other genes [26, 27], and to express non-VAR2CSA PfEMP1 in the membrane [28, 29]. Moreover, placental infection was found to boost antibody responses against IEs from nonpregnant hosts which do not express VAR2CSA [30, 31] and to persist after delivery [32], suggesting that VAR2CSA may not be the only parasite variant infecting pregnant women. All this evidence led us to hypothesize that parasites transcribing A, B, or C var genes may also contribute to malaria physiopathology in pregnancy. Importantly, transcription of these var groups has been little explored in maternal infections [26, 27]. To address this, we aimed to characterize the var gene profile of parasites infecting pregnant women by real-time quantitative polymerase chain reaction (PCR), and to analyze the association of infections with non-var2csa variants with maternal parasite densities and immune responses.
METHODS
Study Area
The study was conducted at the Centro de Investigação em Saúde da Manhiça in Manhiça district, southern Mozambique. A detailed geographic and demographic description of the area has been reported elsewhere [33]. Briefly, Manhiça is characterized by perennial malaria transmission with some seasonality of moderate intensity, mostly attributable to P. falciparum. At the time of the study, the prevalence of active P. falciparum infections detected by placental histology was 18% [34], and malaria control in pregnancy relied exclusively on case management.
Participants and Sample Collection
Pregnant women were recruited at delivery at the maternity ward of the Manhiça District Hospital (MDH), from March 2004 through November 2005. Demographic data of the mother and birth weight of the newborns were recorded. Placental and peripheral parasitemia were determined by optical examination of thick and thin blood films. If films tested positive for P. falciparum, peripheral and/or placental blood samples were withdrawn into ethylenediaminetetraacetic acid tubes after making several 1-cm–deep incisions in the endometrial side of freshly delivered placentas. After centrifugation, plasma samples were stored at −20°C. Placental IEs were snap frozen in ethanol and dry ice and stored at −80°C. Peripheral IEs were cultured to trophozoite stage and frozen as described.
Children aged 1–5 years, nonpregnant women of childbearing age, and men >15 years of age were recruited from patients attending the MDH with a primary clinical diagnosis of P. falciparum malaria and asexual peripheral parasitemia on thick blood film examination. Blood samples were collected into heparin tubes, and IE pellets were cryopreserved in glycerolyte solution. Two blood drops from each sample were spotted onto filter paper (Schleicher and Schuell; no. 903TM).
Written informed consent was obtained from participants or their respective parents or guardians before sample collection. Parasitaemic individuals were treated according to standard national guidelines at the time of the study. Approval for the protocols was obtained from the National Mozambican Ethics Review Committee and the Hospital Clínic of Barcelona Ethics Review Committee.
DNA Extraction and msp Genotyping
DNA was extracted from a 50-μL blood drop onto filter paper by use of a QIAamp DNA Mini kit (Qiagen) and resuspended in 100 μL of water. Merozoite surface protein 1 and 2 genes (msp1 and msp2, respectively) were amplified through nested PCR [35] and visualized by agarose gel electrophoresis. The multiplicity of infection (MOI) was estimated as the highest msp1 or msp2 allele number detected in each sample.
RNA Extraction and Complementary DNA Synthesis
Cryopreserved IEs were thawed and matured to pigmented parasite forms [36]. Snap frozen and matured IEs were resuspended in 20 vol of Trizol (Invitrogen), and RNA was extracted using a PureLink Micro-to-Midi RNA purification kit (Invitrogen). The quantity and integrity of the RNA was assessed in a Nanodrop spectrophotometer (Thermo Scientific) and a 2100 Bioanalyzer (Agilent), respectively. Total RNA was treated with DNAse-I (Invitrogen) for 1 h at 37°C, and reverse transcription was performed using the Superscript III First Strand synthesis system (Invitrogen). RNA samples without reverse transcriptase enzyme were processed in parallel. Reverse transcription positive and negative controls were tested by PCR of P. falciparum tubulin (PF10_0084) using primers forward 5′-GATCCAAGTGGTACCTAT-3′ and reverse 5′-GGATACTCCTCTCTTATT-3′ (sequences provided by A. Rowe, University of Edinburgh, Edinburgh, UK) to confirm the presence of complementary DNA and discard genomic DNA (gDNA) contamination. RNA was also extracted from 3 CSA-binding strains (CS2CSA [strain MRA-96 from Malaria Research and Reference Reagent Resource Centre], FCR3CSA, and 193TCSA) and the rosetting strain R29, after culture and selection for their specific cytoadhesion phenotypes [36].
Quantitative PCR of var, hprt, and sbp1
The relative copy number of target genes was determined in an ABI Prism 7500 Real-Time system (Applied Biosystems), using primers directed to the var2csa DBL3X domain [17] and var groups A (primer set A1), B (primer set B1), and C (primer set C2) [12]. Transcripts from the trophozoite up-regulated gene hypoxanthine phosphorybosyltransferase (hprt, PF10_0121; forward, 5′-GTTGCCATCGCTTGTCTTTT-3′; reverse, 5′-TTCCCTCATCATTAACCAAACA-3′) and the ring upregulated gene skeleton binding protein 1 (sbp1, PFE0065w; forward, 5′-GGCACTTGCAACTACCGAAT-3′; reverse 5′-GCTTGAAAAACCGTCATCGT-3′) were quantified to compare the intraerythrocytic developmental stage of the isolates. Reactions were performed in a final volume of 20 μL, including 5 μL of DNA and 10 μL of Power SYBR Green Master mix (Applied Biosystems). Cycling conditions were 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. Seryl-tRNA synthetase (seryl-tRS, PF07_0073) was used as the endogenous control [16]. Data were analyzed using the 7500 System SDS software (version 1.4; Applied Biosystems). Efficiencies (E), calculated by the formula E = 10−1/m from 7-log dilutions of 3D7 gDNA, where m is the slope, were 1.90 for A genes, 1.82 for B genes, 1.86 for C genes, 1.89 for var2csa, 1.91 for hprt, 1.94 for sbp1, and 1.92 for seryl-tRS. Specificity of primer pairs was ensured by melting curve analysis of final products. Target gene cycle threshold (Ct) values exceeding linearity of dilution curves (Ct > 34) were not quantified. Plate design was based on the Pfaffl method [37], using 3D7 gDNA as the reference. The formula C/EΔCt was used to estimate the number of gene copies [11], where C is the number of copies of the gene in 3D7 and ΔCt is the difference in Ct values between a sample and 3D7 gDNA in the corresponding plate. To normalize for the amount of total DNA loaded in each reaction, target gene copies were divided by seryl-tRS copies.
Flow Cytometry and Enzyme-Linked Immunosorbent Assay
Immunoglobulin G (IgG) against IE surface antigens expressed by FCR3CSA and R29 laboratory lines at trophozoite stage were measured in plasma samples from pregnant women by flow cytometry [30]. IgG levels were expressed as the difference between the mean fluorescence intensity of IEs and that of uninfected red blood cells. IgGs against the merozoite recombinant protein EBA-175 were measured by enzyme-linked immunosorbent assay and expressed as optical density at 492 nm [30]. A pool of plasma samples from hyperimmune Mozambican pregnant women and men as well as 10 plasma samples from nonexposed Europeans were included as positive and negative controls, respectively.
Definitions and Statistical Methods
Pregnant women were classified as primigravidae if they were in their first pregnancy and multigravidae if they reported having at least 1 previous pregnancy. Nonpregnant individuals were grouped into adults (men and nonpregnant women) and children. Transcription of each var group was defined as negative when no fluorescence was detected or when Ct > 34 and positive for quantifiable samples. Isolates positive for at least 1 var group other than var2csa were defined as ABC var parasites. Categorical and continuous variables were compared by the Fisher exact test and Kruskal-Wallis test, respectively. Prevalence of var transcription in matched placental and peripheral infections in pregnant women were compared by the McNemar test and reported as the number of pairs with a divergent var profile. Parasite densities, MOI, and level of var transcription in paired infections were compared by the Wilcoxon matched-pairs signed-rank test and reported as the median difference between placental and peripheral values. Data were plotted using Prism software (version 4; GraphPad), and statistical analysis was performed with Stata/SE software (version 11.0; StataCorp). P values of <.05 were considered to be statistically significant.
RESULTS
Characteristics of Study Participants and Parasite Isolates
Among 361 pregnant women recruited at the MDH, 43 presented P. falciparum parasitemia by microscopic analysis of their peripheral and/or placental blood samples. Fourteen samples had insufficient blood volume for RNA extraction, and 8 had no detectable P. falciparum RNA. Transcriptional data were finally obtained for 30 placental and 21 peripheral isolates from 32 different pregnant women, detailed as follows: 19 placental and peripheral isolate pairs from the same women, 5 placental isolates (peripheral pairs not available), 2 peripheral isolates (placental pairs not available), and 6 placental isolates from women without peripheral infection. The transcription of var was also measured in 23 isolates from nonpregnant adults and 19 from children. Characteristics of the studied populations are summarized in Table 1. The number of pregnancies in multigravidae was 2–6. The mean parasite density was higher in placenta blood samples compared with that in paired peripheral blood (difference, 3,968 parasites/μL; P = .044), whereas the median MOI was similar in both compartments (difference, 0; P = .636). Parasitemia was also higher in nonpregnant adults and children, all with clinical malaria, compared with parasitemia in pregnant women (P = .001). MOI was lower in children compared with adults (P = .011). Maternal parasitemia and MOI did not differ by parity or age and were not associated with newborns’ weight (data not shown).
Table 1.
Characteristics of the Study Population
| Parameter | Pregnant women (N = 32) | Nonpregnant adults (N = 23) | Children (N = 19) |
| Median age, years (IQR) | 20 (18–26) | 25 (14–51) | 2 (1–4) |
| Female sex | NA | 12 (52) | 4 (21) |
| Primigravidae | 16 (50) | NA | NA |
| Median newborn weight, g (IQR) | 2,910 (2,560–3,245) | NA | NA |
| Median parasite level, parasites/μL (IQR) | |||
| Peripheral blood samples | 2,266 (982–13,496)a | 65,518 (30,273–117,812) | 33,008 (21,292–53,553) |
| Placental blood samples | 7,928 (1,995–19,866)b | NA | NA |
| Median multiplicity of infection (IQR) | |||
| Peripheral blood samples | 5 (3–6)a | 4 (1–7) | 3 (2–7) |
| Placental blood samples | 4 (3–5)b | NA | NA |
NOTE. Data are no. (%) of participants, unless otherwise indicated. IQR, interquartile range; NA, not applicable.
N = 21.
N = 30.
To discard differences in the developmental stage of parasites [38], sbp1 and hprt transcription were compared between placental isolates and all in vitro matured peripheral isolates. Both isolate types showed similar relative copy numbers of sbp1 (copy no. for placental isolates, 2.3 [interquartile range {IQR}, .9–4.6]; copy no. for peripheral isolates, 3.4 [IQR, 1.7–7.8]; P = .481) and hprt (copy no. for placental isolates, 7.1 [IQR, 5.0–11.8]; copy no. for peripheral isolates, 8.7 [IQR, 3.7–18.6]; P = .127). Moreover, matured peripheral isolates differed significantly in their levels of sbp1 and hprt compared with 10 uncultured ring-stage peripheral isolates (sbp1 copy no., 102 [IQR, 74.9–157.1]; hprt copy no., 2.3 [IQR, 1.0–4.8]; P < .001 for both genes).
Transcription of var Genes
Primer coverage was assessed by determining the number of var genes contained in a subgroup of 40 field gDNA samples. The median number of genes (no. of A genes, 10 [IQR, 7–31]; no. of B genes, 23 [IQR, 19–32]; no. of C genes, 3 [IQR 2–3]; no. of var2csa genes, 2 [IQR, 1–3]) did not differ from expected numbers in 3D7 [12] (P = .851 for A genes; P = .778 for B genes; P = .134 for C genes; P = .223 for var2csa genes), confirming that primers were suitable for var gene quantification in the parasite isolates included in the study.
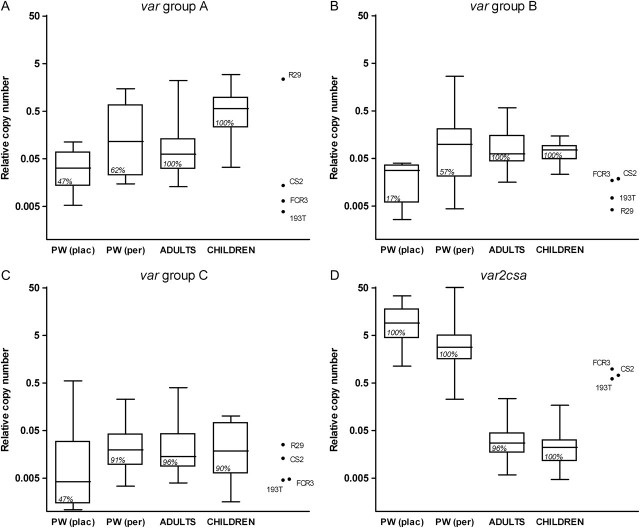
Transcripts from var groups A, B, and/or C were detected in 20 (67%) of 30 placental isolates and in all 21 (100%) peripheral isolates from pregnant women. Among placental isolates (n = 30), 14 (47%) isolates transcribed genes from var group A, 5 (17%) transcribed genes from group B, and 14 (47%) transcribed genes from group C. Among peripheral isolates (n = 21), group A transcription was detected in 13 samples (62%), group B in 12 samples (57%), and group C in 19 samples (91%). The prevalence of group A was similar between paired placental and peripheral isolates (10 [53%] and 11 [58%] isolates, respectively; P > .999 [7 divergent pairs]), whereas B and C transcript prevalence was higher in peripheral isolates (10 [53%] for B and 17 [89%] for C) than in matched placental infections (4 [21%] for B and 10 [53%] for C; P = .031 [6 divergent pairs] and P = .039 [9 divergent pairs], respectively). Two (33%) of 6 placental isolates from pregnant women without peripheral infection transcribed a C gene, but none transcribed an A or B gene.
Relative copy numbers of var genes in quantifiable samples were compared between placenta and peripheral isolates collected from pregnant women, and also between isolates from pregnant and nonpregnant individuals. Copy numbers of groups B and C were higher in peripheral isolates compared with matched placental pairs (median difference for B, −.003; P = .046; and median difference for C, −.01; P < .001) (Figure 1A–1C), and a similar trend was found for A genes (median difference, −.002; P = .091). Peripheral isolates from pregnant women transcribed similar levels of var groups A, B, and C compared with isolates from nonpregnant adults (P = .420, P = .808, and P = .619, respectively) (Figure 1A–1C). Transcript levels of group A genes were significantly higher in parasites infecting children compared with those infecting pregnant and nonpregnant adults (P < .001), whereas no differences in levels of B and C genes were found between parasite populations.
Figure 1.
Transcription of var genes of Plasmodium falciparum in pregnant women (PW), nonpregnant adults, and children, as well as in 4 laboratory strains (CS2CSA, 193TCSA, FCR3CSA, and R29). Data are shown as log-transformed relative copy numbers. Boxes delimit medians and interquartile ranges; bars indicate extreme values. Transcription prevalence is indicated inside boxes. Plac, placental isolates; per, peripheral isolates.
Transcripts of var2csa detected by quantitative PCR were present in all P. falciparum isolates collected from pregnant women and in 42 (97%) of 43 isolates from nonpregnant individuals. Levels of var2csa were higher in pregnant women than in nonpregnant hosts (P < .001), higher in placenta blood samples than in matched peripheral blood samples (median difference, 6.1; P < .001), and higher in field isolates from pregnant women than in CSA-binding laboratory lines (P = .008) (Figure 1D).
Parasitemia, IgG Responses, and var Transcription in Pregnant Women
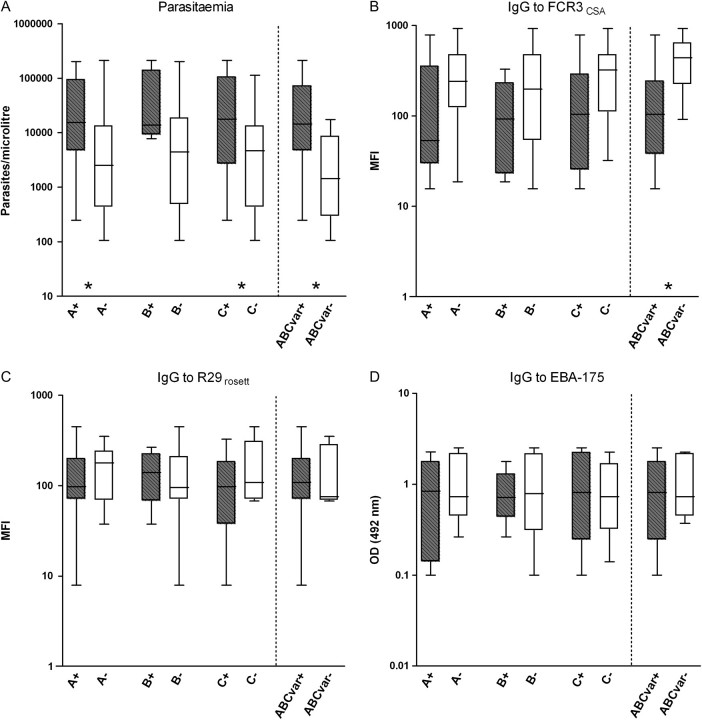
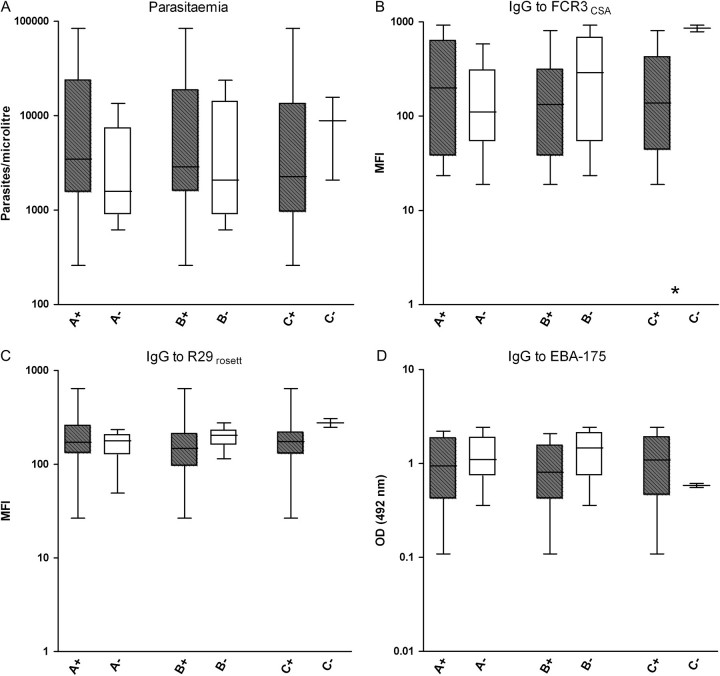
Pregnant women infected with parasites transcribing A, B, and/or C in the placenta had higher placental parasitemia than pregnant women infected with parasites only transcribing var2csa (P = .022 for A, P = .046 for C, and P = .004 for any ABC var gene) (Figure 2A). In contrast, peripheral parasitemia was not associated with the presence of A, B, or C transcripts (P = .158 for A, P = .394 for B, and P = .719 for C) (Figure 3A). On the other hand, IgG levels against FCR3CSA IEs were lower in pregnant women with ABC var placental infections compared with infections only transcribing var2csa (P = .004) (Figure 2B). Presence of peripheral parasites transcribing C genes was also associated with low levels of IgG to FCR3CSA (P = .031) (Figure 3B). No differences were found in levels of IgG against surface antigens of R29 IEs or against the merozoite recombinant protein EBA-175 (Figure 2C, Figure 2D, Figure 3C, and Figure 3D). Similarly, no differences in A, B, or C var transcription were found by age, parity, newborns’ weight, or MOI (data not shown).
Figure 2.
Parasitemia and immunoglobulin G (IgG) levels against Plasmodium falciparum antigens in pregnant women infected in their placentas with isolates transcribing A, B, or C var gene groups (plus signs, gray boxes) and isolates not transcribing that particular var subgroup (minus signs, white boxes). Placental isolates transcribing at least 1 of the A, B, or C var groups (ABCvar+) were compared with isolates exclusively transcribing var2csa (ABCvar−). Data are shown as log-transformed number of parasites per microliter, mean fluorescence intensity (MFI), or optical density (OD) values, respectively. Boxes delimit medians and interquartile ranges; bars indicate extreme values. *P < .05, Kruskal-Wallis test.
Figure 3.
Parasitemia and immunoglobulin G (IgG) levels against Plasmodium falciparum antigens in pregnant women infected in their peripheral blood with isolates transcribing A, B, or C var gene groups (plus signs, gray boxes) and isolates not transcribing that particular var subgroup (minus signs, white boxes). Data are shown as log-transformed number of parasites per microliter, mean fluorescence intensity (MFI), or optical density (OD) values, respectively. Boxes delimit medians and interquartile ranges; bars indicate extreme values. *P < .05, Kruskal-Wallis test.
DISCUSSION
The results of this study show that infection with non-var2csa P. falciparum variants is common during pregnancy, since ABC var transcripts were detected in two-thirds of placental infections and in all peripheral infections among Mozambican pregnant women. Peripheral isolates from pregnant women transcribed ABC var genes at levels similar to those of parasites infecting nonpregnant adults, and ABC var transcription by placental parasites was associated with high parasite densities in this organ. On the other hand, isolates from pregnant women were found to predominantly transcribe var2csa, in accordance with the current concept that gives to this gene a central role in placental malaria [5, 16].
Higher var2csa levels in placental isolates compared with parasites in peripheral blood samples of pregnant women supports the hypothesis of placental-mediated selection of var2csa variants. Interestingly, var2csa levels in isolates from pregnant women were found to be higher than levels in 3 P. falciparum strains selected for CSA binding. This finding could be explained by simultaneous transcription of multiple var2csa copies contained in field parasites [39], as is also suggested by the analysis of Mozambican parasite genomes, in which var2csa primers amplified a median number of 2 (IQR, 1–3) gene copies. Alternatively, reduction in overall abundance of var transcripts in parasites adapted to culture may occur [40]. Similarly, maternal parasites transcribed var2csa at higher levels than ABC var genes in parasites from nonpregnant adults and children, an observation already reported in a microarray-based study of the transcriptome of maternal parasites [26]. These results suggest a distinct transcriptional regulation of var2csa compared with other var genes [41], although heterogeneous transcription of different ABC var genes with the same binding specificities [42] in nonpregnant hosts or incomplete primer coverage of the whole var repertoire may also contribute to these differences.
In addition to transcribing var2csa, 67% of placental isolates also transcribed ABC var genes. This observation is in accordance with results of previous proteomic studies that identified non-VAR2CSA PfEMP1 in placental IEs [28, 29]. However, the transcription of non-var2csa var genes in infected placentas was the lowest when it was compared with that in peripheral isolates from pregnant women, nonpregnant adults, and children. Moreover, none of the 6 placental isolates collected from pregnant women without peripheral infection transcribed A or B gene groups. Detection of ABC var transcripts in the placenta may be explained by perfusion of the intervillous spaces with peripheral IEs transcribing these var genes. Alternatively, the association found for the presence of placental ABC var transcripts with high parasite loads and low antibody levels against FCR3CSA suggests that reduced immunity against CSA-binding strains may allow accumulation of parasites transcribing var2csa in the placenta, therefore increasing the number of parasites that can potentially switch from var2csa to an ABC var type. However, this observation should be taken with caution, since the potential confounding effect of parity, age, or parasitemia on maternal antibody responses [20, 22, 31] could not be discarded due to the limited number of isolates included in the study.
Peripheral parasites from pregnant women transcribed ABC var genes at levels similar to those of parasites from nonpregnant adults with clinical malaria. This finding indicates that ABC var transcription by parasites present in the systemic blood circulation of pregnant women does not merely result from spontaneous var switching of var2csa parasites [43], but rather represents parasite populations similar to those causing disease out of pregnancy. These results are in accordance with results of previous studies showing that, although at low intensities, 47%–71% and 60% of peripheral isolates from pregnant women can cytoadhere to CD36 [5, 24] and form rosettes [25], respectively. Observations from this study would explain the high prevalence of infection and parasitemia at first trimester [44, 45], when placental structure is incomplete [46] and CSA is not available for VAR2CSA selection. Of importance, there is some evidence that P. falciparum infection early in pregnancy may contribute to reduce the birth weight of newborns [47]. Moreover, parasites transcribing A, B, or C var genes, which have been associated with severe disease [10–13], may be able to accumulate in other organs of pregnant women, such as the brain [48], and to contribute to the development of maternal anemia, a common consequence of malaria during pregnancy that increases maternal mortality, intrauterine growth retardation, and fetal anemia [49]. Finally, ABC var gene variants may persist after delivery [32] and increase the risk of malaria during the postpartum period [50].
This study presents 2 main limitations. First, it was not possible to address whether the ABC var transcription profile found in symptomatic adults and pregnant women differed from that in asymptomatic individuals, since asymptomatic nonpregnant adults were not recruited for this study. Second, whether maternal parasites that transcribe ABC var genes can cytoadhere in the placenta or other tissues could not be determined, because P. falciparum parasites from pregnant women were not cryopreserved to allow in vitro characterization of their adhesion profile.
In conclusion, this study shows that, although var2csa transcription predominates in placental and peripheral infections during pregnancy, pregnant women are also infected in their peripheral blood by parasites transcribing A, B, and/or C var genes at levels similar to those of isolates from nonpregnant adults. Vaccines designed to block adhesion of IEs to CSA, combined with non-VAR2CSA PfEMP1 or other conserved P. falciparum antigens, may prevent accumulation of parasites in the placenta and significantly reduce maternal infections with non-var2csa parasites, especially early in pregnancy [44, 45, 47]. The contribution of var genes other than var2csa to malaria pathogenesis in pregnant women should be further evaluated.
Funding
This work was supported by the Instituto de Salud Carlos III (project PS09/01113; grant FI06/00019 to E. R.-V.; salary support CP-04/00220 to A. M.); and the Ministerio de Ciencia e Innovación (salary support RYC-2008-02631 to C. D.). The Manhiça Health Research Centre receives core support from the Spanish Agency for International Cooperation and Development.
Acknowledgments
We are very grateful to all individuals who agreed to participate in the study; the staff of the MDH and the Manhiça Health Research Centre; the clinical officers, field supervisors, and data managers; and Laura Puyol, Lázaro Mussacate, Nelito Ernesto José, Ana Rosa Manhiça, and Vania Simango, for their contribution to the collection of samples. We also thank C. Chitnis (International Centre for Genetic Engineering and Biotechnology, New Delhi, India) for providing us with FCR3CSA and R29 parasite lines, as well as EBA-175 protein; MR4 for providing us with CS2CSA malaria parasite, contributed by S.J. Rogerson (University of Melbourne, Melbourne, Australia); J. Gysin (Institut Pasteur, Marseille, France) for the 193TCSA parasite line; and A. Cortés (Institut de Recerca Biomèdica, Barcelona, Spain) for technical advice and helpful discussion.
The funding sources did not have any involvement in the study design, collection, analysis and interpretation of data, writing of the report, or in decision to submit the paper for publication. The researchers are independent from the funders.
References
- 1.Brabin L, Brabin BJ. Parasitic infections in women and their consequences. Adv Parasitol. 1992;31:1–81. doi: 10.1016/s0065-308x(08)60020-2. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 4.Beeson JG, Amin N, Kanjala M, Rogerson SJ. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect Immun. 2002;70:5412–5. doi: 10.1128/IAI.70.10.5412-5415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–10. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavstsen T, Salanti A, Jensen AT, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen ATR, Magistrado P, Sharp S, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. 2004;199:1179–90. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyriacou HM, Stone GN, Challis RJ, et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. 2006;150:211–8. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaestli M, Cockburn IA, Cortés A, Baea K, Rowe JA, Beck HP. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis. 2006;193:1567–74. doi: 10.1086/503776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rottmann M, Lavstsen T, Mugasa JP, et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–11. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalmbach Y, Rottmann M, Kombila M, Kremsner PG, Beck H-P, Kun Jürgen FJ. Differential var gene expression in children with malaria and antidromic effects on host gene expression. J Infect Dis. 2010;202:313–7. doi: 10.1086/653586. [DOI] [PubMed] [Google Scholar]
- 14.Winter G, Chen Q, Flick K, Kremsner P, Fernandez V, Wahlgren M. The 3D7var5.2 (varCOMMON) type var gene family is commonly expressed in non-placental Plasmodium falciparum malaria. Mol Biochem Parasitol. 2003;127:179–91. doi: 10.1016/s0166-6851(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 15.Tuikue Ndam NG, Salanti A, Bertin G, et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J Infect Dis. 2005;192:331–5. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 16.Salanti A, Staalsoe T, Lavstsen T, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Duffy MF, Caragounis A, Noviyanti R, et al. Transcribed var genes associated with placental malaria in Malawian women. Infect Immun. 2006;74:4875–83. doi: 10.1128/IAI.01978-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magistrado P, Salanti A, Tuikue Ndam NG, et al. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J Infect Dis. 2008;198:1071–4. doi: 10.1086/591502. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava A, Gangnard S, Round A, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci U S A. 2010;107:4884–9. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–2. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 21.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–3. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–9. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 23.Staalsoe T, Jensen AT, Theander TG, Hviid L. Novel Plasmodium falciparum malaria vaccines: evidence-based searching for variant surface antigens as candidates for vaccination against pregnancy-associated malaria. Immunol Lett. 2002;84:133–6. doi: 10.1016/s0165-2478(02)00159-1. [DOI] [PubMed] [Google Scholar]
- 24.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis. 1999;180:464–72. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogerson SJ, Beeson JG, Mhango CG, Dzinjalamala FK, Molyneux ME. Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect Immun. 2000;68:391–3. doi: 10.1128/iai.68.1.391-393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis SE, Malkov VA, Oleinikov AV, et al. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun. 2007;75:4838–50. doi: 10.1128/IAI.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuikue Ndam N, Bischoff E, Proux C, et al. Plasmodium falciparumtranscriptome analysis reveals pregnancy malaria associated gene expression. PLoS One. 2008;3:e1855. doi: 10.1371/journal.pone.0001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried M, Wendler JP, Mutabingwa TK, Duffy PE. Mass spectrometric analysis of Plasmodium falciparum erythrocyte membrane protein-1 variants expressed by placental malaria parasites. Proteomics. 2004;4:1086–93. doi: 10.1002/pmic.200300666. [DOI] [PubMed] [Google Scholar]
- 29.Fried M, Hixson KK, Anderson L, Ogata Y, Mutabingwa TK, Duffy PE. The distinct proteome of placental malaria parasites. Mol Biochem Parasitol. 2007;155:57–65. doi: 10.1016/j.molbiopara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Serra-Casas E, Menéndez C, Bardají A, et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J Infect Dis. 2010;201:123–31. doi: 10.1086/648595. [DOI] [PubMed] [Google Scholar]
- 31.Mayor A, Rovira-Vallbona E, Machevo S, et al. Parity and placental infection affect antibody responses against Plasmodium falciparum during pregnancy. Infect Immun. 2011;79:1654–59. doi: 10.1128/IAI.01000-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serra-Casas E, Menendez C, Dobano C, et al. Persistence of Plasmodium falciparum parasites in infected pregnant Mozambican women after delivery. Infect Immun. 2011;79:298–304. doi: 10.1128/IAI.00814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 34.Menéndez C, Bardají A, Sigauque B, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3:e1934. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snounou G, Zhu X, Siripoon N, et al. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999;93:369–74. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 36.Ljungström I, Perlmann H, Schlichtherle M, Scherf A, Wahlgren M, editors. Methods in malaria research. 4th ed. Manassas, VA: Malaria Research and Reference Reagent Resource Center; 2004. [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:e5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sander AF, Salanti A, Lavstsen T, et al. Multiple var2csa-type PfEMP1 genes located at different chromosomal loci occur in many Plasmodium falciparum isolates. PLoS One. 2009;4:e6667. doi: 10.1371/journal.pone.0006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters JM, Fowler EV, Krause DR, Cheng Q, Gatton ML. Differential changes in Plasmodium falciparum var transcription during adaptation to culture. J Infect Dis. 2007;195:748–55. doi: 10.1086/511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amulic B, Salanti A, Lavstsen T, Nielsen MA, Deitsch KW. An upstream open reading frame controls translation of var2csa, a gene implicated in placental malaria. PLoS Pathog. 2009;5:e1000256. doi: 10.1371/journal.ppat.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson BA, Welch TL, Smith JD. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol Microbiol. 2003;47:1265–78. doi: 10.1046/j.1365-2958.2003.03378.x. [DOI] [PubMed] [Google Scholar]
- 43.Roberts DJ, Craig AG, Berendt AR, et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–92. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 45.Coulibaly SO, Gies S, D'Alessandro U. Malaria burden among pregnant women living in the rural district of Boromo, Burkina Faso. Am J Trop Med Hyg. 2007;77:56–60. [PubMed] [Google Scholar]
- 46.Khong TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Arch Pathol Lab Med. 1991;115:722–5. [PubMed] [Google Scholar]
- 47.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg. 2007;76:849–54. [PubMed] [Google Scholar]
- 48.Menéndez C, Romagosa C, Ismail MR, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. doi: 10.1371/journal.pmed.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–14S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 50.Diagne N, Rogier C, Sokhna CS, et al. Increased susceptibility to malaria during the early postpartum period. N Engl J Med. 2000;343:598–603. doi: 10.1056/NEJM200008313430901. [DOI] [PubMed] [Google Scholar]